Abstract
Objective
We sought to assess the efficacy of preoperative autologous blood donation in reducing patient exposure to allogeneic blood products following elective cardiac surgery.
Methods
We included 48 patients in a prospective study and randomly assigned them into the control or treatment group. We excluded patients with aortic stenosis, main trunk stenosis and unstable angina. Group A (n = 23; coronary disease n = 21 and valvular disease n = 2) was the control group, and group B (n = 25; coronary disease n = 21, valvular disease n = 4) received preoperative autologous blood donation. All patients had cardiopulmonary bypass surgery, and we processed mediastinal blood with a cell-saver device before reinfusion. All patients received aprotinin, and we reinfused blood shed from the mediastinum postoperatively.
Results
No major peri- or postoperative complications occurred. We interrupted preoperative blood donation in 2 patients (8%) because of worsened angina pectoris. The mean time between the first blood donation and surgery was 22.5 (standard deviation [SD] 9.4, range 12–50) days. In group A, 9 patients (39.1%) were exposed to allogeneic blood products. In group B, 11 patients (47.8%) were exposed to blood products (p = 0.73), and 4 (16%) were exposed to allogeneic blood products (p = 0.036).
Conclusion
Preoperative blood donation was completed in 92% of the targeted low-risk population. The procedure significantly reduced exposure to perioperative allogeneic blood products.
Abstract
Objectif
Nous avons cherché à évaluer l'efficacité du prélèvement de sang autologue préopératoire lorsqu'il s'agit de réduire l'exposition du patient aux produits sanguins allogènes pendant et après une chirurgie cardiaque élective.
Méthodes
Nous avons inclus 48 patients dans une étude prospective et nous les avons affectés au hasard au groupe témoin ou au groupe des participants étudiés. Nous avons exclu les patients qui avaient une sténose de l'aorte, une sténose coronarienne du tronc commun et une angine instable. Le Groupe A (n = 23; coronaropathie n = 21 et valvulopathie n = 2) était le groupe témoin, tandis que l'on a prélevé du sang autologue avant l'opération chez les participants du Groupe B (n = 25; coronaropathie n = 21, valvulopathie n = 4). Tous les patients ont été opérés sous circulation extra-corporelle et nous avons traité le sang médiastinal au moyen d'un laveur de cellules avant la réinfusion. Tous les patients ont reçu de l'aprotinine, et nous avons retransfusé le sang qui s'est écoulé du médiastin après l'intervention.
Résultats
Il n'y a pas eu de complications périopératoires ou postopératoires majeures. Nous avons interrompu le prélèvement préopératoire de sang chez 2 patients (8 %) parce que leur angine de poitrine s'était aggravée. Le temps moyen écoulé entre le 1e prélèvement de sang et la chirurgie s'est établi à 22,5 (écart-type [ET] 9,4; intervalle de 12 à 50) jours. Chez les participants du Groupe A, 9 patients (39,1 %) ont été exposés à des produits sanguins allogènes. Chez ceux du Groupe B, 11 patients (47,8 %) ont été exposés à des produits sanguins (p = 0,73), et 4 (16 %) à des produits sanguins allogènes (p = 0,036).
Conclusion
Le prélèvement de sang préopératoire a été complété chez 92 % des participants de la population à faible risque ciblée. L'intervention réduit considérablement l'exposition aux produits sanguins allogènes au cours de la période périopératoire.
Preoperative autologous blood donation (PAD) has been used in elective cardiac surgery, particularly in stable patients with valvular or coronary disease.1–4 However, this practice has not been re-examined since the introduction of antifibrinolytic agents and reinfusion of mediastinal blood during the postoperative period. Combining the use of an antifibrinolytic agent, the reinfusion of mediastinal blood after surgery and PAD should reduce the need for transfusion; however, no study in the literature has evaluated this strategy. We sought to evaluate the impact of the use of PAD within a complete blood-sparing strategy.
Methods
Participants
We conducted our prospective randomized study between January 2001 and October 2002. The Ethical Committee of the Research Centre at the Montreal Heart Institute reviewed and approved our study protocol, and we obtained informed consent from all the patients.
We considered patients aged 18–80 years who were in the preoperative phase of an elective cardiac surgery to be eligible for this study. A total of 3100 patients underwent cardiac surgery at the Montreal Heart Institute during the recruitment period. We excluded those who had aortic stenosis, coronary disease in the left main trunk and unstable angina. We also excluded patients who lived outside the Montréal area and were unable to attend preoperative blood donation appointments, used erythropoietin preoperatively, had a hemoglobin level less than 110 g/L and required emergency surgery.
We randomly assigned patients into 2 groups: the control group or the treatment group in which patients received PAD. Neither the patient nor the surgeon was blinded to the group assignment; however, the intensive care unit (ICU) intensivist, nurses and residents were blinded. Recruitment was not randomized within individual surgeons' practices. Four surgeons participated in the study.
We aimed to discontinue patient use of ASA or plavix about 1 week before surgery.
Cardiopulmonary bypass circuit and use of aprotinin
We performed all cardiopulmonary bypasses using a solid venous reservoir with a venous filter (40 μm filter), a hollow-fibre membrane oxygenator (Sorin Biomedica), nonpulsatile roller pumps (Stöckert and Nellcor), a 32-μm arterial filter (Capiox CX AF01; Terumo) and a vacuum-assisted venous return system (Boehringer). We processed mediastinal blood with a cell-saver device before reinfusion. We used a standard Hammersmith full-dose protocol (2 × 106 kallicrein inhibitor units [KIU] in pump prime, 2 × 106 KIU loading dose, followed by 0.5 × 106 KIU/h) of aprotinin (Bayer) in all patients.
Preoperative autologous blood donation
Héma-Québec, the local administration in charge of the management of human blood products, approved our autologous blood donation protocol. The protocol consisted of harvesting 2 units of 350 mL each (or 6 mL/kg when the patient's weight was below 60 kg). We harvested 1 unit weekly (maximum 2 units). We stored the units in citrate phosphate double dextrose (CP2D) solution at 4°C for a maximum period of 35 days. One unit was stored longer than 35 days and was discarded before the surgery. All patients randomly assigned to the PAD group received 300 mg of ferrous sulfate orally, 3 times daily for the period between recruitment and surgery.
Postoperative mediastinal blood reinfusion
Postoperative mediastinal blood reinfusion occurred in both groups during the first 6 hours after surgery when postoperative bleeding was more than 100 mL/h. The reinfusion system consisted of 28 F thoracic tubes connected to a 3-chamber system: a collection chamber, underwater seal chamber and 20-cm H20 suction-control chamber. The 500 mL capacity sterile collection chamber contained 20 mL of adenine-supplemented citrate dextrose. We filtered all collected blood through an 80-μm filter and autotransfused the blood to the patient on an hourly basis until no drainage was present or for a maximum of 12 hours.
Indications for the use of blood products
We administered autologous blood units or allogeneic red blood cell units when the patient's hemoglobin level was below 60 g/L during the cardiopulmonary bypass and 80 g/L postoperatively. In group B, we always administered autologous blood units before allogeneic transfusion. We firmly applied these transfusion thresholds in all patients. We administered 2 units of fresh frozen plasma (FFP) after correction of the activated clotting time in case of moderate postoperative bleeding, defined as 100–300 mL per hour or more than 300 mL per 2 hours with an international normalized ratio of 1.8 or more. With severe postoperative bleeding (> 300 mL/h) we administered 4 units of FFP. We administered 6 units of platelets when the platelet level was below 80/L.
Statistical analysis
We used either the χ2 test or the Student t test to compare variables. We performed statistical analyses using SPSS software (SPSS). We considered p < 0.05 to be statistically significant.
Results
Study population
Of the 3100 patients who underwent cardiac surgery at the Montreal Heart Institute, 930 (30%) had elective surgery with the potential for PAD. Of these 930 patients, 300 were from the Montréal area and, therefore, able to easily attend preoperative blood donation appointments. We excluded 100 patients based on our exclusion criteria, and, of the 200 patients remaining, we recruited 48 patients for our study. We randomly assigned 23 patients to group A, the control group (coronary disease n = 23, valvular disease n = 2). Group B, the treatment group, had 25 patients (coronary disease n = 21, valvular disease n = 4). Random assignment to the treatment group was homogeneously distributed among the 4 participating surgeons (p = 0.20), and perioperative blood loss was similar among them (p = 0.64). Of the 48 included patients, 44 patients were taking an antiplatelet agent at the time of recruitment; we stopped this therapy 1 week before surgery in 20 patients. The other 4 patients were taking coumadin; we stopped this therapy 4 days before the surgery in all patients.
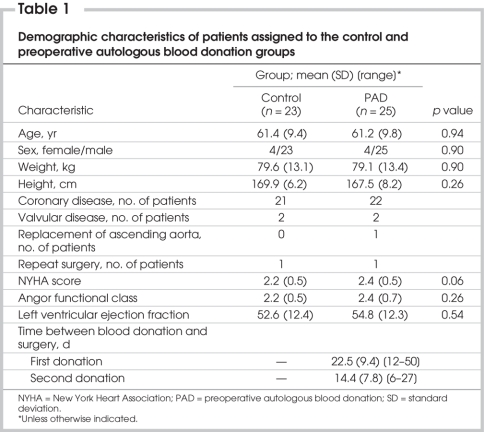
Table 1 shows the demographic characteristics of the study population. The mean age (and standard deviation [SD]) of the patients in the control group, which comprised 19 men and 4 women, was 61.4 (9.4) years. The mean age (and SD) of patients in the PAD group, which comprised 21 men and 4 women, was 61.2 (9.8) years. Weight, height and body surface area did not differ significantly between the groups. The New York Heart Association (NYHA) class, the angina functional class and the left ventricular ejection fraction did not differ significantly between the groups.
Table 1
Tolerance of PAD
We did not complete PAD in 2 patients (8%) because of worsened angina pectoris. The change in status of these patients was noted at the blood donation clinic before harvesting the first unit. The patients stabilized enough to come electively to their surgeries without donating blood. Even though they did not give blood, we included them in our analysis of the PAD group (intention-to-treat analysis). The blood donation protocol was well tolerated in all the other patients, with no change in angina following the donation episodes. The mean time between the first blood donation and surgery was 22.5 (SD 9.4, range 12–50) days, and the mean time between the second donation and surgery was 14.4 (SD 7.8, range 6–27) days. Preoperative hemoglobin levels did not differ significantly between the groups (p = 0.12).
Surgical and postoperative data
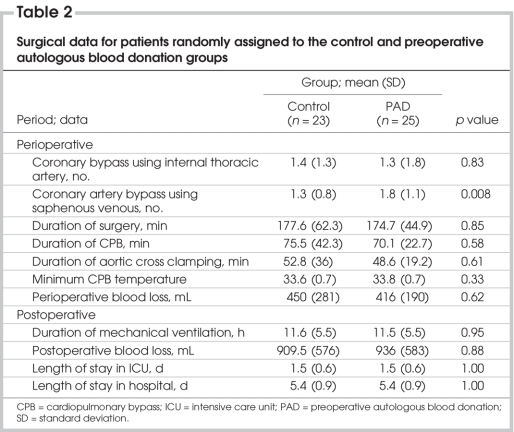
Table 1 and Table 2 depict surgical data for patients in both groups. The main indication for surgery in both groups was coronary disease. One patient in each group underwent a repeat procedure. The mean durations of surgery, cardiopulmonary bypass and aortic cross clamping did not differ between the groups. The duration of mechanical ventilation, postoperative blood loss (including recorded intraoperative blood loss and blood shed in the mediastinum in the first 24 hours in the ICU, length of stay in the ICU and length of stay in hospital did not differ significantly between the groups. Postoperatively, we had to reinfuse mediastinal blood in 5 patients in each group; the mean reinfused blood volume (and SD) was 488 (226) mL in the control group and 343 (176) mL in the PAD group (p = 0.076).
Table 2
Intraoperative exposure to blood products
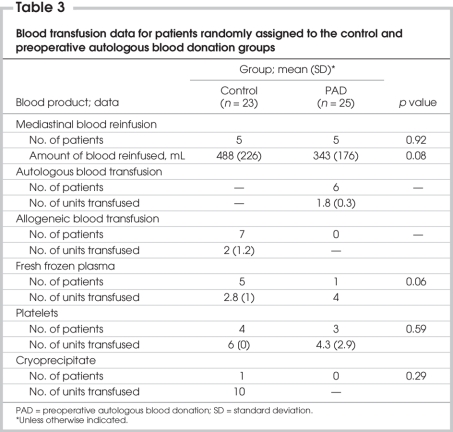
Intraoperatively, 1 patient in the control group received 3 allogeneic blood units, and another patient received 6 platelet units; in the PAD group, 1 patient received 2 autologous blood units.
Postoperative exposure to blood products
In the control group, 6 patients received allogeneic red blood cell units (mean 2, SD 1.2 units if transfused), 5 patients received FFP units (mean 2.8, SD 1 units), 4 patients received 6 platelet units and 1 patient received 10 cryoprecipitate units.
In the PAD group, 9 patients received autologous red blood cell units (mean 1.8, SD 0.3 units), 1 patient received 4 FFP units and 3 patients received platelets (mean 4.3, SD 2.9 units).
Proportion of patients exposed to blood products
In the PAD group, 11 patients (47.8%) received blood products, including the autologous units harvested preoperatively, in the perioperative period. Four of 25 (16%) patients in this group were exposed to allogeneic blood products. In the control group, 9 patients (39.1%) were exposed to allogeneic blood products (a relative risk increase of 2.25). Exposure to all blood products was comparable between the groups (47.8% v. 39.1%, p = 0.73), but exposure to allogeneic blood products was significantly decreased in the PAD group (16% v. 39.1%, p = 0.036) (Table 3).
Table 3
Postoperative biological data
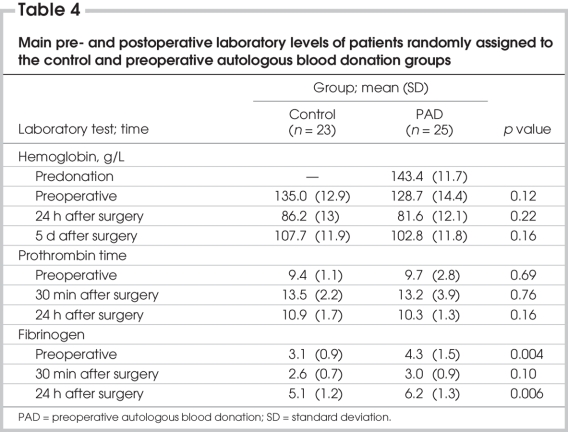
The hemoglobin and troponin rates as well prothrombin time, partial thromboplastin time and international normalized ratio did not differ significantly between the groups. The fibrinogen level in the subgroup of patients who received blood transfusions significantly increased compared with the control group (p = 0.048) (Table 4).
Table 4
Discussion
The use of PAD markedly increased in the 1980s and early 1990s but has been declining recently, mainly because of cost.5 One can also question whether physicians should stop offering PAD to their patients. Nevertheless, its systematic use, and its efficacy in a modern setting and in combination with other blood-sparing methods in a low-risk cardiac surgery population have not been well studied. We sought to assess exposure to allogeneic blood products after PAD in preparation for elective cardiac surgery associated with the systematic use of antifibrinolytic agents and postoperative mediastinal blood reinfusion.
We completed PAD in 92% of the targeted population, underlining the feasibility of this method in elective, low-risk patients of cardiac surgery. Only 2 patients (8%) sustained worsening of their angina symptoms, cancelling their planned blood donation. The PAD procedure was well tolerated in all the patients who ended up giving blood, and preoperative hemoglobin rates did not differ significantly between the control and PAD groups, underlining the capacity of quick blood regeneration in this population.
Despite strict indications for transfusion, the overall rate of blood transfusion (autologous plus allogeneic) observed in our study and others6 was not significantly different in the PAD group compared with the control group; however, PAD eliminated completely the need for allogeneic red blood cell transfusion.
Moreover, we observed no adverse event linked with the use of autologous blood, underlining the safety of this method when the rigorous rules of blood donation and storage are respected.
On the other hand, none of our patients exposed to allogeneic blood products experienced complications; however, this may be explained by our small sample size, which was an important limitation in our study. Generally, the risk of transmitting hepatitis B, hepatitis C and HIV via allogeneic blood has recently become very low owing to major improvements in donor and blood screening.7,8 The risk of transfusion-related mortality from hepatitis and HIV is now lower than that from acute hemolytic reactions, which PAD does not prevent.9
Nevertheless, allogeneic transfusion is not completely risk-free; it is associated with a substantial risk in the patients' minds, which generates anxiety.9 This point is reinforced by the emergence of numerous potentially dangerous new infectious agents that could be transmitted by transfusion.8,10
Other complications of allogeneic transfusion are less well known and probably underestimated, but they are still a reality.9 This is the case with immune-mediated transfusion-related acute lung injury (1 in 5000 transfusions), which could be the second most common cause of major morbidity and death attributable to transfusion.11 Although none of our patients experienced any postoperative infectious complications, immune suppression may also be caused by allogeneic transfusion.9 The hypothesis that immune suppression may increase the risk of postoperative bacterial infection is also a reality.12
The cost of PAD remains an important problem. Whether the systematic use of PAD programs in stable patients in large health care institutions could substantially reduce the cost remains to be demonstrated.
In conclusion, this study demonstrated that PAD can help low-risk cardiac surgery patients avoid exposure to allogeneic red blood cells. Ninety-two percent of the patients randomly assigned to blood donation withstood the harvesting of 2 units without clinical deterioration. The limitations of the clinical use of a PAD program are multiple and include the necessary waiting period for donation before surgery, the necessity of living relatively close to the blood harvesting centre, the absence of a critical cardiac condition that might cause an anemic and hypovolemic state following blood donation, and the resources required for harvesting and handling the blood units. The referral pattern at the Montreal Heart Institute, where two-thirds of patients have to travel from another city for surgery, is a considerable limitation; PAD could at best be of benefit to 10%–15% of our patient population, or 200–300 patients annually.
Contributors: Drs. Bouchard, Marcheix and Carrier designed the study. Drs. Robitaille and Pellerin acquired the data, which Drs. Al-Shamary, Vanden Eynden, Demers and Perrault analyzed. Drs. Bouchard, Marcheix, Al-Shamary, Vanden Eynden and Carrier wrote the article, which Drs. Demers, Robitaille, Pellerin and Perrault reviewed. All authors approved the final version for publication.
Competing interests: None declared.
Accepted for publication Mar. 23, 2007
Correspondence to: Dr. D. Bouchard, Department of Surgery, Montreal Heart Institute, 5000 Belanger St., Montréal QC H1T 1C8; fax 514 593-2157; denis.bouchard@icm-mhi.org
References
- 1.Love TR, Hendren WG, O'Keefe DD, et al. Transfusion of predonated autologous blood in elective cardiac surgery. Ann Thorac Surg 1987;43:508-12. [DOI] [PubMed]
- 2.Owings DV, Kruskall MS, Thurer RL, et al. Autologous blood donations prior to elective cardiac surgery. JAMA 1989;262: 1963-8. [PubMed]
- 3.Britton LW, Eastlund T, Dziuban SW, et al. Predonated autologous blood use in elective cardiac surgery. Ann Thorac Surg 1989;47:529-32. [DOI] [PubMed]
- 4.Hardy JF, Harel F, Bélisle S. Transfusions in patients undergoing cardiac surgery with autologous blood. Can J Anaesth 2000; 47:705-11. [DOI] [PubMed]
- 5.Etchason J, Petz L, Keeler E, et al. The cost-effectiveness of preoperative autologous blood donations. N Engl J Med 1995; 332:719-24. [DOI] [PubMed]
- 6.Forgie MA, Wells PS, Laupacis A, et al. Preoperative autologous donation decreases allogeneic transfusion but increases exposure to all red blood cell transfusion: results of a meta-analysis. Arch Intern Med 1998; 158:610-6. [DOI] [PubMed]
- 7.Muirhead B. Preoperative autologous donation has no role in cardiac surgery. J Cardiothorac Vasc Anesth 2003;17:126-8. [DOI] [PubMed]
- 8.Chamberland ME. Emerging infectious agents: Do they pose a risk to the safety of transfused blood and blood products? Clin Infect Dis 2002;34:797-805. [DOI] [PubMed]
- 9.Karkouti K, McCluskey S. Preoperative autologous blood donation has a role in cardiac surgery. J Cardiothorac Vasc Anesth 2003;17:121-5. [DOI] [PubMed]
- 10.Coulthart MB, Cashman NR. Variant Creutzfeldt-Jakob disease: a summary of current scientific knowledge in relation to public health. CMAJ 2001;165:51-8. [PMC free article] [PubMed]
- 11.Williamson LM, Lowe S, Love EM, et al. Serious hazards of transfusion (SHOT) initiative: analysis of the first two annual reports. BMJ 1999;319:16-9. [DOI] [PMC free article] [PubMed]
- 12.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: Fact or fiction? Blood 2001;97:1180-95. [DOI] [PubMed]