Abstract
Background
There is limited evidence regarding the effectiveness and complications of mesenteric angiography in the diagnosis and management of acute lower gastrointestinal bleeding (ALGIB). Our objective was to determine the complications and outcomes of mesenteric angiography in patients with ALGIB and to identify predictors of a positive result at angiography.
Methods
We identified and reviewed the records of all patients who underwent mesenteric angiography for ALGIB at our institution during a 10-year period. We compared potential predictors of positive versus negative angiograms.
Results
Of 47 mesenteric angiograms in 35 patients, 22 (47%, 95% confidence interval [CI] 33%–61%) revealed a source of bleeding, most commonly the colon. Hematomas developed in the groins of 3 patients (6.4%, 95% CI 0%–18%), and 1 of these patients also experienced a myocardial infarction during the procedure. None of the potential predictors were significantly associated with a positive result at angiography, although the confidence intervals were wide. Twenty patients (57%, 95% CI 41%–74%) continued to bleed after the angiogram, and 18 of the patients (51%, 95% CI 35%–68%) were discharged without a definitive diagnosis.
Conclusion
With a diagnostic success of about 50%, mesenteric angiography may play an important part in the diagnosis and management of patients with ALGIB; however, one or more large, prospective multicentre studies are needed to more clearly define its role. Canadian surgeons have the opportunity to initiate collaborative multicentre studies to address such diagnostic and therapeutic clinical questions.
Abstract
Contexte
Les données probantes sur l'efficacité et les complications de l'angiographie du mésentère dans le diagnostic et la prise en charge du saignement gastro-intestinal inférieur aigu (SGIIA) sont limitées. Nous voulions cerner les complications et les résultats de l'angiographie du mésentère chez les patients atteints de SGIIA ainsi que les prédicteurs de résultats positifs de l'angiographie.
Méthodes
Nous avons repéré et étudié les dossiers de tous les patients ayant subi une angiographie du mésentère pour un SGIIA à notre établissement au cours d'une période de 10 ans. Nous avons comparé les prédicteurs possibles d'une issue positive et négative de l'angiographie.
Résultats
Sur 47 angiogrammes du mésentère subis par 35 patients, 22 (47 %, intervalle de confiance [IC] à 95 %, 33 %–61 %) ont révélé une source de saignement, le plus souvent le côlon. Trois patients (6,4 %, IC à 95 %, 0 %–18 %) ont eu un hématome à l'aine et un de ces patients a aussi subi un infarctus du myocarde au cours de l'intervention. Aucun des prédicteurs possibles n'était associé significativement à un résultat positif de l'angiographie, même si les intervalles de confiance étaient importants. Vingt patients (57 %, IC à 95 %, 41 %–74 %) ont continué de saigner après l'angiographie et 18 des patients (51 %, IC à 95 %, 35 %–68 %) ont obtenu leur congé sans que l'on pose de diagnostic définitif.
Conclusion
Avec un taux de réussite diagnostique d'environ 50 %, l'angiographie du mésentère peut jouer un rôle important dans le diagnostic et la prise en charge du SGIIA, mais il faudra toutefois procéder à une ou plusieurs études multicentriques prospectives d'envergure pour en définir le rôle plus clairement. Les chirurgiens canadiens ont l'occasion de lancer des études multicentriques en collaboration afin de répondre à ces questions cliniques sur le diagnostic et le traitement.
Acute lower gastrointestinal bleeding (ALGIB) is a common, potentially life-threatening clinical problem that leads to an estimated 20–27 hospital admissions per 100 000 people each year in the United States.1 Although most bleeding stops spontaneously, 10%–15% of patients require urgent surgery, with a mortality rate varying from 4% to 21%.2,3
Clinicians use several diagnostic methods to investigate the source of the bleeding, including colonoscopy, nuclear scintigraphy, mesenteric angiography, helical computed tomography (CT) and wireless capsule endoscopy. Despite the use of a combination of these tests, the diagnosis of the source and localization of ALGIB remains a challenge; 35%–42% of patients are discharged without a definitive diagnosis.4,5 The difficulty may be related to the broad differential diagnosis of ALGIB, the frequently intermittent nature of the bleeding and the lack of a standardized diagnostic approach to investigation.3
Although experts have suggested several different algorithms for investigating ALGIB, little data exist to support their validity.4,6–9 As a result, the decision to use colonoscopy, nuclear scintigraphy or mesenteric angiography as the initial diagnostic method remains controversial and varies depending on the physician and medical centre. In particular, there is limited evidence regarding the effectiveness of mesenteric angiography in the localization and treatment of ALGIB or the frequency of complications following this procedure. Our objective was to identify the outcomes of patients with ALGIB who underwent mesenteric angiography and the predictors of a positive result at angiography.
Methods
Patient population
We identified all patients who underwent mesenteric angiography at the London Health Sciences Centre (LHSC) or St. Joseph's Healthcare Centre in London, Ontario, from Nov. 1, 1993, to Oct. 31, 2003. One of us (P.J.K.) reviewed the medical records of each patient. We included only patients who underwent angiography within 3 days of the documented onset of ALGIB. For the purposes of this study we defined ALGIB as red blood in the rectum or melena stool with a negative gastroduodenoscopy or nasogastgric lavage among patients who presented less than 1 week after the bleeding began.
Data extraction
One of us (P.J.K.) reviewed the chart of each patient and extracted data regarding potential predictors of a positive angiography, selected based on a literature review and consultation with experts in the field. Potentially important features in the patients' histories included comorbid conditions (measured with the Charlson Comorbidity Index, a weighted index of 19 comorbid conditions10), prior gastrointestinal bleeding, diverticulosis, inflammatory bowel disease, daily use of acetylsalicylic acid (ASA, any dosage), use during the previous week of at least 2 doses of a nonsteroidal anti-inflammatory agent, daily use of an anticoagulant medication, bright red bleeding in the rectum, diarrhea, time since onset of bleeding, syncope, abdominal pain and the number of blood transfusions. We extracted data on the following physical findings: abdominal tenderness, heart rate, blood pressure, orthostatic changes in vital signs and presence of gross blood on rectal examination. Prior diagnostic investigations included hemoglobin concentration, hematocrit levels, platelet count, International Normalized Ratio (INR), nasogastric lavage, gastroduodenoscopy, colonoscopy and nuclear scintigraphy.
For each angiogram, we recorded the time from admission, the location of the bleeding (if identified), any therapeutic interventions performed and complications following the procedure. Finally, we documented whether patients experienced further bleeding during that hospital admission, what additional interventions were conducted, the final diagnosis and the total length of stay in hospital.
Statistical analysis
We calculated the mean and standard deviation of normally distributed continuous variables and the median and interquartile range of continuous variables with skewed distributions. We used independent t tests to compare means of potential predictors in patients with a positive result on first angiography with those of patients with a negative result. We performed Fisher exact tests to detect between-group differences for categorical variables. We elected not to conduct a logistic regression analysis because of the relatively low ratio of events to variables. We interpreted a result of p < 0.05 as suggestive of a difference between groups, but accepted that inferences from this data would be limited by multiplicity in the analysis.
Results
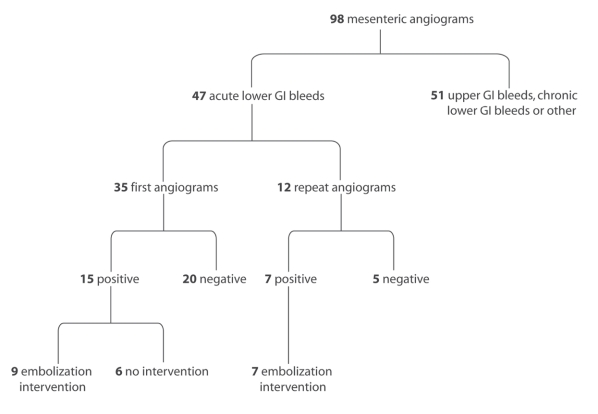
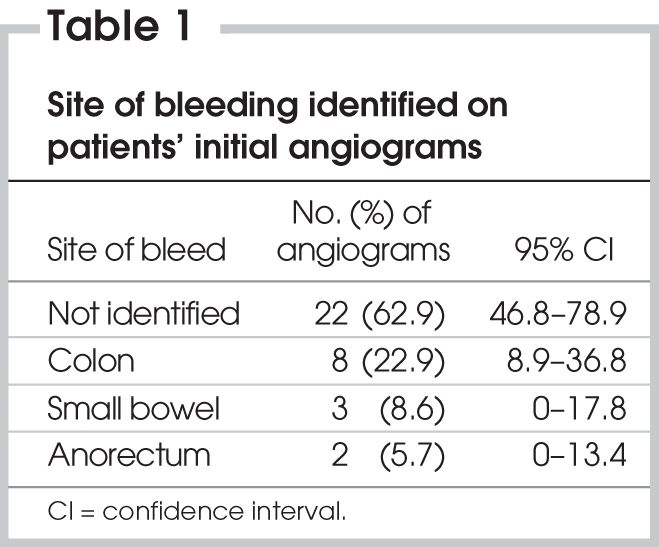
During the 10-year study period, radiologists performed 98 mesenteric angiograms, of which 47 were for patients with ALGIB (Fig. 1). Of the ALGIB angiograms, 35 were from the initial angiography, and the remaining 12 were from follow-up investigations for continued bleeding. Overall, 22 of the 47 angiograms revealed a source of bleeding (47%, 95% confidence interval [CI] 33%–61%). The results of 16 of these 22 angiograms (73%) led us to attempt a therapeutic intervention. The rate of positive angiography was lower in the initial angiograms than in the follow-up angiograms (43% v. 58%). In the positive initial angiograms, the colon was the most common site of bleeding (Table 1).
FIG. 1. Summary of mesenteric angiograms performed during the study period. GI = gastrointestinal.
Table 1
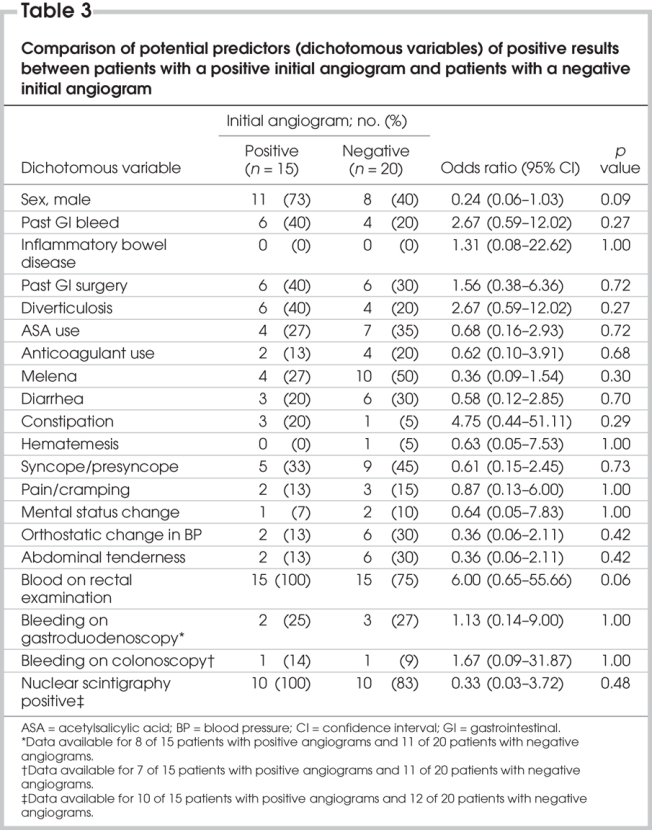
None of the potential predictors of positive angiography reached statistical significance in the comparative analyses; however, the confidence intervals included important differences for many of the variables (Table 2 and Table 3).
Table 2
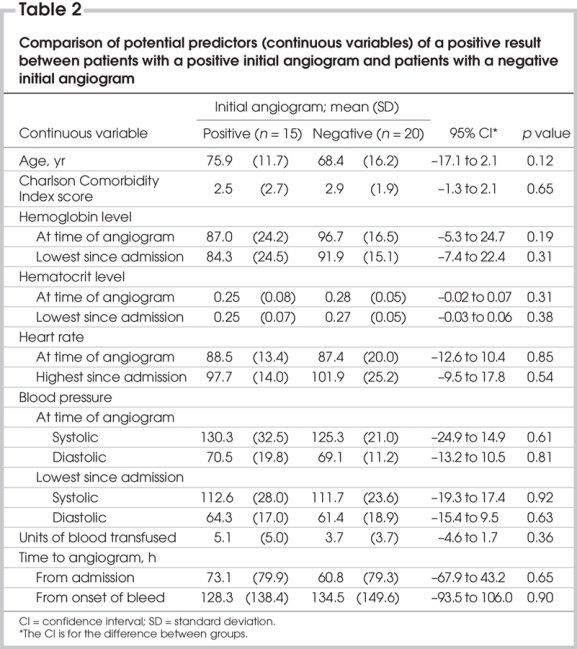
Table 3
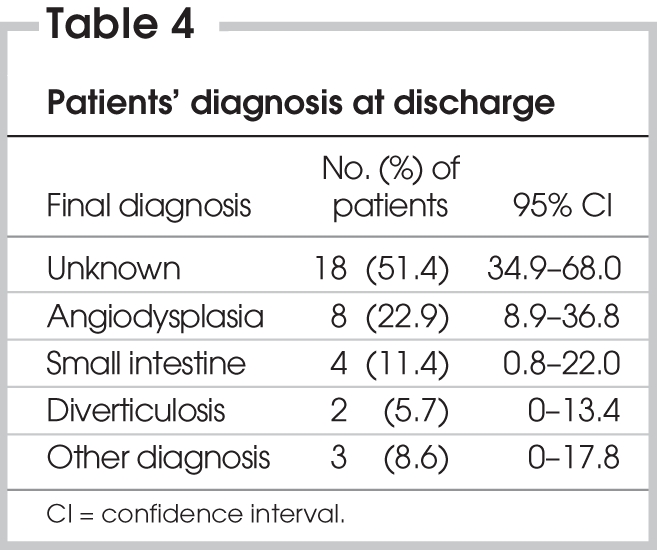
Hematomas in the groin occurred following 3 of the angiograms (6.4%, 95% CI 0%–13%); 1 of these patients also experienced a myocardial infarction during the procedure. No other complications occurred as a result of the angiography. Twenty of the 35 patients (57%, 95% CI 41%–74%) continued to bleed after the angiogram. Of the 16 patients who underwent therapeutic intervention at the time of angiography, 6 (37.5%) continued to bleed after the procedure. Five of the patients (14%) underwent one or more repeat angiograms, and 6 of the patients (17%) ultimately required a surgical procedure. No patient died during their hospital admission. Angiodysplasia was the most common cause of the bleed, although more than half of the patients were discharged without a final diagnosis (Table 4).
Table 4
Discussion
In this case series exploring the use of mesenteric angiography in the localization of ALGIB, we report the following:
1) Almost half of angiograms revealed a source of bleeding.
2) Complications during angiography were uncommon.
3) More than half of the patients experienced continued bleeding while in hospital.
4) Half of the patients were discharged without a final diagnosis.
Many clinicians hesitate to perform mesenteric angiography as the initial test in patients with ALGIB because of the uncertain rate of positivity (20%–86%) and the potential for complications.5,11,12 The rate of positive findings in our study fell in the middle of the reported range: 43% in initial angiograms and 47% overall. Given the potentially serious complications of angiography, it would be helpful to clinicians if a strategy existed to select patients most likely to have a positive result at angiography. Several investigators have attempted to identify factors that predict a positive result at angiography, with conflicting results.
Pennoyer and colleagues13 studied 131 angiograms over a 12-year period and concluded that increasing age and lower hematocrit levels were positively correlated with the results of angiography. Another retrospective review determined that only patients' transfusion requirements were predictive of a positive finding at angiography.14 More recently, a group of investigators studied a series of 88 patients and found that those who were hemodynamically unstable (defined by blood transfusions of 5 units or more in 24 hours) were more likely to have positive results with selective mesenteric angiography than those who were stable.15
Unfortunately, all of these studies had the same important limitation: far too few events (positive angiograms) for the number of predictor variables included in the analysis. Based on rigorous simulation studies, the minimum ratio of events to variables needed to confidently conduct a regression analysis is 10.16 In these 3 studies, the number of positive angiograms ranged from 30 to 45, yielding a maximum of 4 variables for inclusion in the analyses. In our background review, we identified more than 30 factors that we felt could potentially be associated with a positive finding at angiography (Table 2 and Table 3). Based on the number of positive angiograms in our series, a rigorous regression analysis would have forced us to select only 2 of these variables for analysis. Therefore, we elected not to conduct a regression analysis and instead compared each variable independently. This approach introduced a new limitation into the interpretation of our findings: owing to the multiple testing, we would expect some of the comparisons to be positive based on chance alone. Ultimately, we were not faced with this dilemma, because even with our liberal analysis there were no significant between-group differences.
The simplest solution to avoid overfitting in a regression analysis is to increase the number of events in the study. There are obvious practical difficulties with doing this, particularly when studying uncommon conditions or treatments such as mesenteric angiography in ALGIB. Investigators can overcome this problem by collaborating and pooling the data obtained at individual centres. For example, if we combined the data from the 3 studies cited earlier with the data from our centre, we would assemble a cohort with more than 130 positive angiograms. This would allow us to confidently perform a regression analysis with 13 predictor variables in the model, which would be far more powerful than any of the analyses we were able to conduct independently. Collaboration among centres provides an additional methodologic benefit: the findings are more generalizable to different practice settings than the results of a single-centre study.
Our study and the others that we identified are also limited by the retrospective nature of the data collection. Although we took precautions to extract data from the charts in a systematic manner, the quality of the data were ultimately dependent upon the accuracy of the chart recordings. A substantial portion of the data that we wished to collect was not recorded in the charts (e.g., orthostatic blood pressure changes). This missing data effectively reduced our sample size further and limited the power of the analyses to detect between-group differences.
In summary, the use of mesenteric angiography for ALGIB at our centre identified the site of bleeding about half of the time, with a relatively low rate of complications. Half of the patients experienced further episodes of bleeding during their stays in hospital, and half were discharged without a firm diagnosis. None of the factors we examined was associated with a positive result at angiography, although our findings were limited by the small sample size. Ultimately, defining the appropriate role for mesenteric angiography in the investigation and management of ALGIB will require one or more large, prospective multicentre studies. Canadian surgeons have the opportunity to initiate collaborative multicentre studies to address such diagnostic and therapeutic clinical questions.
Acknowledgments
Dr. Karanicolas is supported by a Canadian Institutes of Health Research (CIHR) Canada Graduate Scholarship.
Contributors: Drs. Karanicolas, Colquhoun and Guyatt designed the study. Drs. Karanicolas and Guyatt acquired the data, which all authors analyzed. All authors wrote and reviewed the article and approved the final version for publication.
Competing interests: None declared.
Accepted for publication Sep. 22, 2007
Correspondence to: Dr. P.J. Karanicolas, University Hospital, Rm. C8-114, Windermere Rd., London ON N6A 5A5; fax 519 663-3068; pjkarani@uwo.ca
References
- 1.Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1997;92:419-24. [PubMed]
- 2.Peura DA, Lanza FL, Goustout CJ, et al. The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol 1997;92:924-8. [PubMed]
- 3.Billingham RP. The conundrum of lower gastrointestinal bleeding. Surg Clin North Am 1997;77:241-52. [DOI] [PubMed]
- 4.Al Qahtani AR, Satin R, Stern J, et al. Investigative modalities for massive lower gastrointestinal bleeding. World J Surg 2002; 26:620-5. [DOI] [PubMed]
- 5.Brackman MR, Gushchin VV, Smith L, et al. Acute lower gastroenteric bleeding retrospective analysis (the ALGEBRA study): an analysis of the triage, management and outcomes of patients with acute lower gastrointestinal bleeding. Am Surg 2003;69:145-9. [PubMed]
- 6.Enns R. Acute lower gastrointestinal bleeding: part 2. Can J Gastroenterol 2001; 15:517-21. [DOI] [PubMed]
- 7.American Gastroenterological Association Medical Position Statement. Evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology 2000; 118:197-200. [DOI] [PubMed]
- 8.Vernava AM, Moore BA, Longo WE, et al. Lower gastrointestinal bleeding. Dis Colon Rectum 1997;40:846-58. [DOI] [PubMed]
- 9.Hoedema RE, Luchtefeld MA. The management of lower gastrointestinal hemorrhage. Dis Colon Rectum 2005;48:2010-24. [DOI] [PubMed]
- 10.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373-83. [DOI] [PubMed]
- 11.Farrell JJ, Friedman LS. Review article: the management of lower gastrointestinal bleeding. Aliment Pharmacol Ther 2005; 21: 1281-98. [DOI] [PubMed]
- 12.Hoedema RE, Luchtefeld MA. The management of lower gastrointestinal hemorrhage. Dis Colon Rectum 2005;48:2010-24. [DOI] [PubMed]
- 13.Pennoyer WP, Vignati PV, Cohen JL. Mesenteric angiography for lower gastrointestinal hemorrhage: Are there predictors for a positive study? Dis Colon Rectum 1997;40:1014-8. [DOI] [PubMed]
- 14.Garofalo TE, Abdu RA. Accuracy and efficacy of nuclear scintigraphy for the detection of gastrointestinal bleeding. Arch Surg 1997; 132:196-9. [DOI] [PubMed]
- 15.Abbas SM, Bisset IP, Holden A, et al. Clinical variables associated with positive angiographic localization of lower gastrointestinal bleeding. ANZ J Surg 2005;75:953-7. [DOI] [PubMed]
- 16.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 2004;66:411-21. [DOI] [PubMed]