Abstract
Background
Preoperative diagnosis of breast cancer is a standard of care. We conducted a population-based study to determine the factors associated with the use of percutaneous needle biopsy to diagnose breast cancer in Ontario.
Methods
We identified a total of 3644 women who underwent breast tissue sampling (percutaneous needle biopsy or surgical excision) that yielded a diagnosis of cancer between Apr. 1, 2002, and Dec. 31, 2002, and for whom we were able to obtain complete data. We performed univariate and multivariate analyses to examine the association between a number of variables and the use of percutaneous biopsy or surgery for diagnosis and the performance of biopsy with or without image guidance. The variables were age, local health integration network (LHIN), income quintile, urban or rural residence, access to a primary care provider, prior mammogram, prior regular screening mammography, screen-initiated biopsy, and surgeon and radiologist specialization in breast disease.
Results
A total of 2374 women (65%) underwent percutaneous biopsy to diagnose breast cancer. The use of percutaneous biopsy varied from 22% to 81% among LHINs. On multivariate analysis, no patient variables were associated with the use of percutaneous biopsy for diagnosis. Only the LHIN and surgeon and radiologist specialization were predictive of whether a woman received a percutaneous biopsy. These 2 variables, along with income quintile and screen-initiated biopsy, were associated with the use of image-guided biopsy as the method of choice.
Conclusion
Geographic variation in the use of percutaneous biopsy, particularly image-guided biopsy, for the diagnosis of breast cancer exists across Ontario. The frequency of such biopsies may be a useful quality indicator. Strategies to improve uptake of organized evidence-based care may increase the use of percutaneous biopsy.
Abstract
Contexte
Le diagnostic préopératoire du cancer du sein constitue une norme de soin. Nous avons effectué une étude représentative pour déterminer les facteurs associés à l'utilisation de la biopsie percutanée à l'aiguille pour le diagnostic du cancer du sein en Ontario.
Méthodes
Nous avons repéré au total 3644 femmes qui ont subi un prélèvement d'échantillon de tissu mammaire (biopsie percutanée à l'aiguille ou excision chirurgicale) ayant abouti à un diagnostic de cancer entre le 1er avril 2002 et le 31 décembre 2002 et pour lesquelles nous avons pu obtenir des données complètes. Nous avons procédé à des analyses à variable unique et à variables multiples pour étudier le lien entre un certain nombre de variables et l'utilisation de la biopsie percutanée ou de l'intervention chirurgicale pour poser le diagnostic et l'exécution de la biopsie avec ou sans guidage par imagerie. Les variables ont été l'âge, le réseau local d'intégration aux services de santé (RLISS), le quintile de revenu, la résidence en milieu urbain ou rural, l'accès à un fournisseur de soins primaires, une mammographie antérieure, une mammographie de dépistage périodique antérieure, une biopsie effectuée suite à un dépistage et la spécialisation en mammopathie du chirurgien et du radiologiste.
Résultats
Au total, 2374 femmes (65 %) ont subi une biopsie percutanée visant à diagnostiquer le cancer du sein. Le recours à la biopsie percutanée a varié de 22 % à 81 % entre les RLISS. Dans le contexte de l'analyse à variables multiples, on n'a pas établi de lien entre les variables des patientes et l'utilisation de la biopsie percutanée pour poser le diagnostic. Seul le RLISS et la spécialisation du chirurgien et du radiologiste étaient des prédicteurs susceptibles d'indiquer si une femme a subi une biopsie percutanée. Ces 2 variables, ainsi que le quintile de revenu et la biopsie effectuée suite à un dépistage, étaient associées à la biopsie à guidage par imagerie comme méthode de choix.
Conclusion
L'utilisation de la biopsie percutanée, et en particulier la biopsie à guidage par imagerie, pour poser le diagnostic de cancer du sein varie selon la région géographique en Ontario. La fréquence de ces biopsies peut constituer un indicateur de qualité utile. Des stratégies visant à améliorer l'adoption des soins factuels organisés pourraient accroître l'utilisation de la biopsie percutanée.
The advent of minimally invasive biopsy techniques for the diagnosis of breast abnormalities has been an important advance in patient management. Confirmation of a benign diagnosis can often be achieved without surgery.1 The use of percutaneous biopsy techniques has improved the accuracy and cost-effectiveness of breast cancer diagnosis after screening mammography.2,3
Breast cancer is frequently suspected preoperatively based on clinical and radiologic assessment, and the diagnosis is then confirmed by tissue sampling. Fine-needle aspiration biopsy (FNAB) of palpable masses is a widely available technique that provides material for cytologic examination. Insufficient material is obtained for diagnosis in about 15% of patients.4 Among those samples with adequate cellular material, reported sensitivity levels range from 72% to 99%.5 Fine-needle aspiration biopsy performed under image guidance has also been applied to the evaluation of nonpalpable lesions with similar sensitivity levels (68%–100%).5 The variability in reported sensitivity levels has been attributed to variation in the cellularity of the lesions sampled and the small size of the needle used.6
More recently, 14-gauge core needle biopsy (CNB) has been adopted to obtain tissue samples for histology. It has a reported sensitivity of 89%–97% and specificity of 96%–99% in large series.1,7,8 The use of CNB to obtain a preoperative diagnosis in women with cancer facilitates surgical planning and shared decision-making. The larger tissue sample that permits the detection of invasive disease and preoperatively establishes the indication for axillary staging is an important advantage of CNB over FNAB, particularly in the era of sentinel lymph node biopsy. Core needle biopsy can be used not only to identify the presence of cancer, but also to define the extent of disease when multiple biopsies are taken from the periphery of larger lesions.9 These features lead to lower rates of positive resection margins at first excision,1,10,11 fewer operative procedures to complete definite surgical therapy1,11–15 and improved cosmetic outcomes owing to a reduction in the total volume of tissue excised.16
Improvements in the equipment available and the ability to use CNB techniques under both stereotactic and ultrasound guidance have promoted the adoption of their use such that, in dedicated breast diagnostic centres, surgical biopsy is rarely used as a diagnostic procedure. As the equipment has become more user-friendly, CNB techniques have also become widely available in community health facilities. Moreover, because CNB is well tolerated and associated with minimal morbidity, it has garnered increased acceptance among patients.
Despite the many advantages attributable to the use of FNAB or CNB for the diagnosis of breast abnormalities, these techniques have not been universally adopted. We previously reported that the use of percutaneous biopsy for tissue diagnosis of breast abnormalities in Ontario varied according to patient age, local health integration network (LHIN), urban residence, access to a primary care provider and Ontario Health Insurance Plan (OHIP) screen-initiated biopsy.17 Although women with a diagnosis of cancer were slightly more likely to receive a percutaneous biopsy than those with benign disease (64.7% v. 60.3%), one-third of women with breast cancer still have the diagnosis confirmed by open biopsy. In the present report, we describe the factors associated with the use of percutaneous biopsy for diagnosis among women with breast cancer in Ontario. We also examine variables associated with the use of image guidance for the performance of percutaneous biopsy. In particular, we evaluate the effects of surgeon and radiologist experience with breast disease on the use of percutaneous biopsy in women with breast cancer.
Methods
Study population
The Institute for Clinical Evaluative Sciences approved our study in keeping with its requirements for patient confidentiality, and ethical and scientific integrity. Using the Canadian Institute for Health Information records and/or OHIP physician billing records, we identified all women in Ontario who had a tissue diagnosis of a breast abnormality between Apr. 1, 2002, and Dec. 31, 2002. We selected our study timeframe because 2002 was the most recent year after the introduction of the International classification of diseases, version 10 codes18 for which complete data were available. Methods of tissue diagnosis may have included percutaneous biopsy (FNAB or CNB, with or without image guidance) and/or partial mastectomy or mastectomy. We linked the patient records to those of the Ontario Cancer Registry to identify all women with a diagnosis of breast cancer. To ensure that the biopsy and surgery performed during the study period were likely for the same lesion, we excluded women who received a diagnosis of breast cancer more than 3 months before or 6 months after their percutaneous needle biopsies or surgical procedures, whichever came first.
Billing records for the time period covered in our study did not distinguish between FNAB and CNB, therefore we describe the application of percutaneous biopsy (FNAB or CNB) with or without image guidance in the diagnosis of breast abnormalities. Similarly, billing records did not provide details about the intended purpose or extent of surgery on the breast, therefore we refer to all surgical procedures on the breast as “surgery.”
We recorded the age group, urban or rural residence, income quintile and residence as indicated by LHIN for each woman in the cohort. We defined rural residence as residence in towns and municipalities outside the commuting zone of urban centres with population of 10 000 or more. A LHIN is a group of health care facilities and services responsible for the provision of health care to a population in a defined geographic area. We also examined the effect of access to a primary care provider, prior mammography, regular mammography screening and screen-initiated biopsy on the probability of a patient receiving a percutaneous biopsy.
A substantial proportion of mammography screening in Ontario is conducted within the Ontario Breast Screening Program (OBSP). The OBSP performs 2-view screening mammography only. Subsequent evaluation of screen-detected abnormalities is standardized and almost always involves additional imaging and/or surgical consultation. Mammography screening conducted outside of the provincial screening program is billed to OHIP. We defined a history of regular mammography screening as 2 or more mammograms between 9 and 60 months before tissue diagnosis during the study period, with no 2 mammograms performed within 11 months of each other through OBSP or OHIP.
Since the decision to proceed with percutaneous biopsy may be influenced by the consulting surgeon or the radiologist reporting the mammogram results, we also determined the degree of specialization in breast disease of the surgeon(s) consulted for patient management and/or the radiologist(s) reading the mammograms. We divided surgeons into quintiles in 2 scales based on the number of patient assessments with a breast-related diagnosis (breast consultation quintile) or the number of surgical procedures (exclusive of cosmetic procedures) performed on the breast (breast surgery quintile) during the study period. We assigned radiologists to quintiles in 2 scales based on the number of mammograms read (mammography quintile) and the number of image-guided biopsies performed (image-guided biopsy quintile) during the study period.
We categorized the women based on the type of procedure they had: percutaneous biopsy in the absence of concomitant breast imaging (FNAB) and without subsequent surgery, image-guided biopsy without subsequent surgery, FNAB followed by surgery, image-guided biopsy followed by surgery, or surgery without a preoperative percutaneous biopsy. We compared women who had any percutaneous biopsy (with or without image guidance, and with or without subsequent surgery) with those whose tissue diagnoses were confirmed by surgery as the initial procedure.
Statistical analysis
We performed univariate logistic regression analyses of patient, institution and provider variables. We performed a 2-way analysis of all variable combinations to identify interaction terms. We then developed a multivariate logistic regression model. To examine variables associated with the use of image-guided biopsy rather than FNAB without image guidance, we performed a subset analysis of women who had percutaneous biopsies.
Results
Study population
We identified 3755 women in Ontario without a previous diagnosis of breast cancer and who had a percutaneous biopsy and/or breast surgery for the diagnosis and/or treatment of a primary breast cancer between Apr. 1, 2002, and Dec. 31, 2002. There were 7368 breast cancer diagnoses in Ontario during the calendar year 2002. Of the women in whom breast cancer was diagnosed during the 9-month study period, we excluded 701 women because they had a previous diagnosis of breast cancer or lived in the Southeast LHIN where physician billing data were unavailable. After excluding women who received a diagnosis of breast cancer more than 3 months before or 6 months after percutaneous biopsy, 3755 women remained. Of these, complete data were available for 3644 women, who we included in our study.
Factors affecting the use of any percutaneous biopsy for the diagnosis of breast cancer
Percutaneous biopsy confirmed diagnoses of cancer in 2374 of 3644 women (65.1%). The age distribution, LHIN, income quintile, urban or rural residence, primary care provider, history of mammography or mammography screening, evidence of OHIP or OBSP screen-initiated biopsy, surgeon consultation quintile, surgeon breast surgery quintile, radiologist mammography quintile and radiologist image-guided biopsy quintile associated with the women in both groups are presented in Table 1. The use of percutaneous biopsy for the diagnosis of breast cancer varied markedly from 22.1% in the Northwest LHIN to 80.6% in the Southwest LHIN. No patient variables (income quintile, rural residence, primary care provider, prior mammography) differed between women who did or did not have a percutaneous biopsy. However, institutional/treatment variables were significantly different between groups. Women treated by surgeons who saw the fewest patients with breast disease or performed the fewest breast surgical procedures were much less likely to undergo percutaneous biopsy as the first diagnostic procedure compared with women treated by surgeons with higher volumes (40% v. 69% for patient volume and 48% v. 67% for surgical volume). Similarly, women whose mammograms were read by radiologists who performed the most image-guided breast biopsies were slightly more likely to have a percutaneous biopsy for diagnosis than those whose mammograms were read by radiologists who performed the fewest image-guided biopsies (70% v. 64%).
Table 1
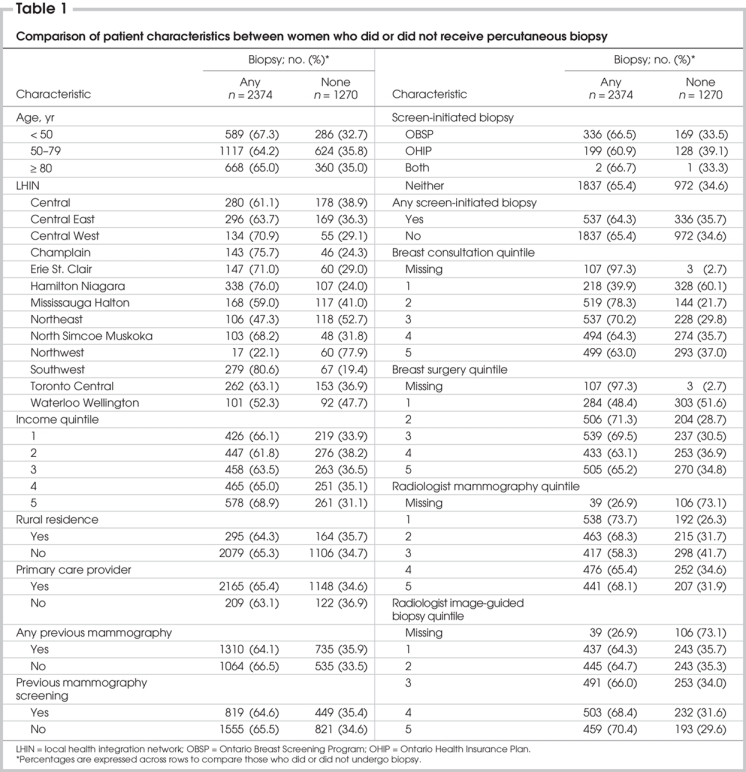
Our univariate logistic regression analysis revealed that women who received a percutaneous biopsy as the first step in diagnosis were more likely to live in the Central West, Champlain, Erie St. Clair, Hamilton Niagara and Southwest LHINs. They were also more likely to have had the mammograms preceding their diagnoses read by radiologists who performed the most image-guided breast biopsies or read more mammograms. Women seen by surgeons in the lowest breast consultation or surgery quintiles were much more likely to undergo surgery for diagnosis than women seen by other surgeons. None of the following variables was significantly associated with having a percutaneous biopsy for diagnosis: age, income quintile, rural residence, history of mammography, access to a primary care provider, or tissue diagnosis preceded by a screening mammogram (Table 2).
Table 2
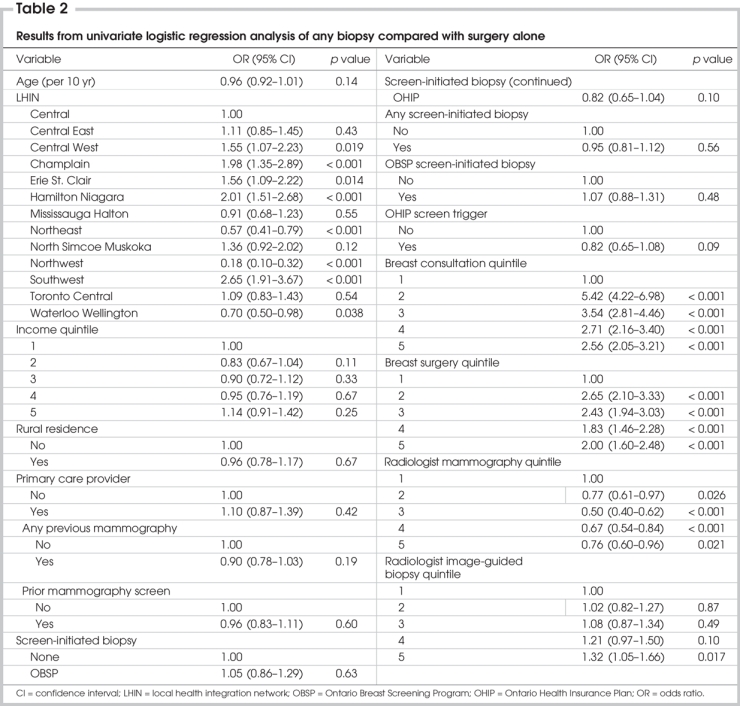
The variables we selected for inclusion in our final multivariate model were those significantly associated with the use of percutaneous biopsy on univariate analysis and those found to be associated with variation in the use of percutaneous biopsy in women with benign disease or cancer in our previous analysis.17 We performed multiple bivariate analyses, and we included all significant interaction terms in the model. Among surgeons associated with the cohort, the number of consultations for breast disease was highly correlated with the number of surgeries performed on the breast. We observed a similar, although less strong, correlation between the number of image-guided biopsies performed and mammograms read by radiologists. Thus, the variables that we selected for inclusion in the multivariate model were surgeon consultation quintile and radiologist biopsy quintile.
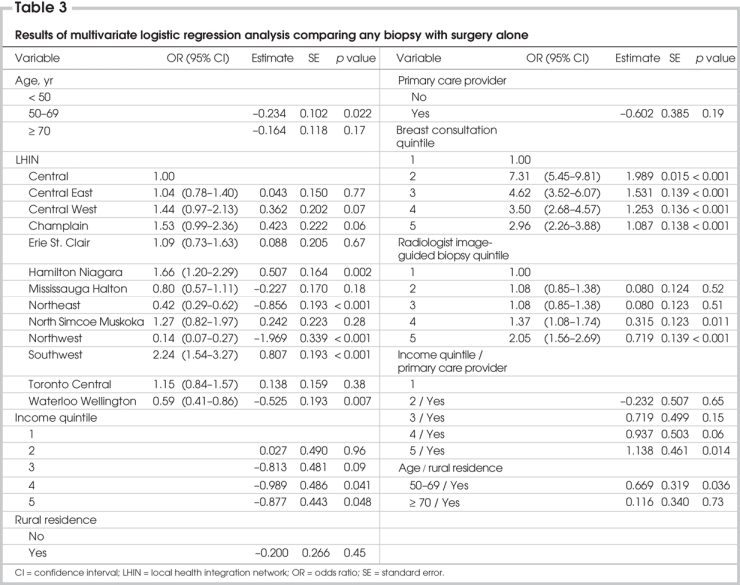
The only variables predictive of the use of percutaneous biopsy on multivariate analysis were LHIN, surgeon consultation quintile and radiologist biopsy quintile (Table 3). We observed strong associations between surgeon and radiologist quintiles and patient LHIN (p < 0.001, χ2, data not shown). Results of our univariate analysis suggested a general trend toward increasing use of percutaneous biopsy with increased image-guided biopsy experience among the radiologists reading the mammograms performed before tissue diagnosis. Surgeon experience had a more marked but dichotomous effect on the probability of a woman having a percutaneous biopsy for diagnosis. Women under the care of surgeons who conducted at least 204 assessments per year for breast disease or performed at least 196 breast surgeries per year were more likely to receive a percutaneous biopsy than those under the care of surgeons who conducted fewer assessments.
Table 3
Factors affecting the use of image-guided biopsy or FNAB alone for percutaneous biopsy
Percutaneous biopsy was used for diagnosis before surgery in 2374 women. Fine-needle aspiration biopsy alone was more frequently used than image-guided biopsy (56% v. 44%).
The age distribution, LHIN, income quintile, urban or rural residence, history of mammography or mammography screening, evidence of OHIP or OBSP screen-initiated biopsy, primary care provider, surgeon consultation quintile, surgeon breast surgery quintile, radiologist mammography quintile and radiologist image-guided biopsy quintile for women who received either image-guided biopsy or FNAB alone are presented in Table 4.
Table 4
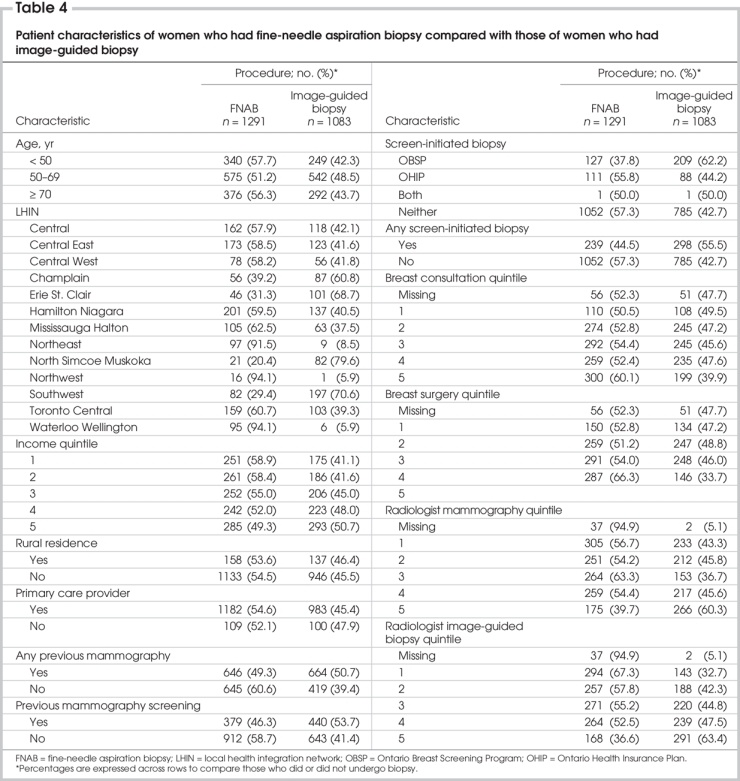
Women who received an image-guided biopsy were more likely than women who did not undergo the procedure to have had a mammogram or regular mammography screening, or had the biopsy initiated by an OBSP screen. They were also more likely to have had their mammograms reported by radiologists who read the most mammograms or performed the most image-guided biopsies. Surgeons whose practices had the highest volumes of patients with breast disease were more likely to perform FNAB than other surgeons. Use of FNAB alone varied by region (20% in the North Simcoe Muskoka LHIN to 94% in the Northwest LHIN). There was even more marked regional variation in the use of image-guided biopsy, ranging from a low of 6% in the Northwest and Waterloo Wellington LHINs, to 80% in the North Simcoe Muskoka LHIN. Women aged 50–69 years were more likely to undergo image-guided biopsy than younger or older women, and there was a trend toward greater use of image-guided biopsies among wealthier women.
The use of image-guided biopsy as the method of choice was in part correlated with regional variation in the use of percutaneous biopsy for cancer diagnosis. In LHINs with the lowest frequency of percutaneous biopsy for diagnosis, FNAB was the method of choice in more than 90% of patients, and this association was independent of the number of breast cancers diagnosed.
On univariate analysis, image-guided biopsy was more frequently associated with higher patient income quintile; a prior mammogram or regular mammography screening; OBSP screening; an OBSP screen-initiated biopsy; and residence in the Erie St. Clair, North Simcoe Muskoka and Southwest LHINs. Surgeon specialization had little effect on the use of image-guided biopsy over FNAB alone, but image-guided biopsies were much more likely than FNAB to be performed when the original mammogram was read by the radiologists who read the most mammograms (odds ratio [OR] 0.5, p < 0.001) or performed greater numbers of image-guided biopsies (more than 200/yr) (OR 0.28, p < 0.001) (Table 5).
Table 5
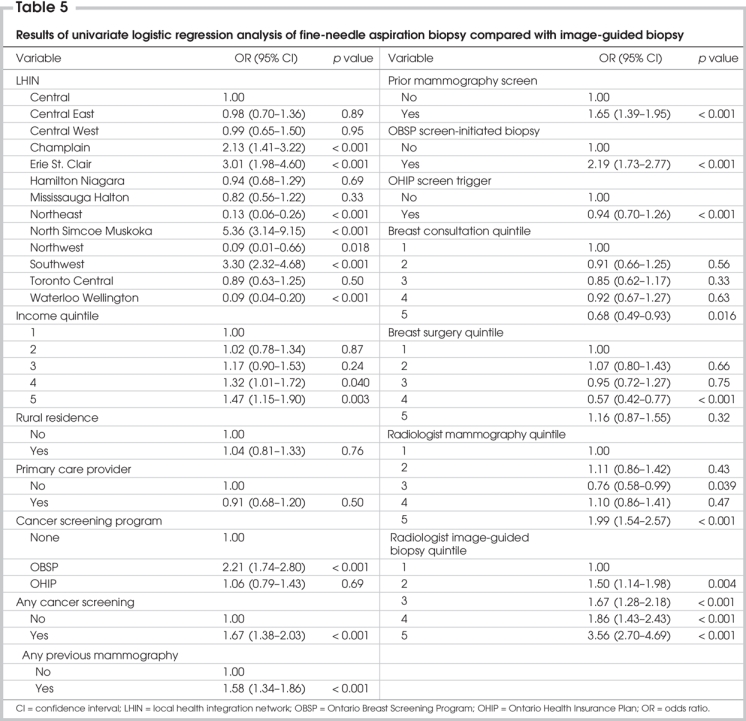
Variables included in the final multivariate model were LHIN, income quintile, a history of screening mammography, breast consultation quintile and image-guided biopsy quintile. Analysis of 2-way interactions revealed no significant interaction terms. Multivariate analysis revealed that LHIN, income quintile, a history of cancer screening, surgeon breast consultation quintile and radiologist image-guided biopsy quintile were all significantly associated with the probability of a woman having an image-guided biopsy for diagnosis (Table 6).
Table 6
Discussion
Our study demonstrates that surgery remains a widely used method for tissue diagnosis of breast cancer in Ontario, despite the introduction of minimally invasive breast biopsy techniques and the publication of a consensus statement advocating such techniques as the preferred approach to tissue diagnosis.3 To our knowledge, ours is the first population-based study to describe some of the factors associated with different methods of tissue diagnosis of breast cancer. By studying all women undergoing biopsy of breast cancers in a short time period, selection bias in the application of diagnostic techniques was minimized in our study.
In women with cancer, preoperative diagnosis reduces the number of operations required for definitive treatment.1,11,19 This reduction appears to be attributable to the identification of women who need axillary staging at the time of tumour excision and/or the planning of a more extensive resection in the presence of a known cancer diagnosis to ensure clear margins. However, some have suggested that not all patient populations benefit from a preoperative diagnosis. Morrow et al14 found no reduction in the number of surgeries required for definitive therapy among women who underwent lumpectomy alone: women with obviously cancerous mass lesions in whom axillary staging was not planned were able to undergo lumpectomy as definitive surgery. With the advent of sentinel lymph node biopsy for axillary staging, however, even those with a low probability of axillary metastases would likely undergo this procedure at the time of lumpectomy, necessitating a preoperative diagnosis of invasive cancer. Facilitation of shared decision-making is another important benefit of preoperative diagnosis. Thus, although the ideal proportion of women whose breast cancers should be diagnosed preoperatively is unknown, preoperative diagnosis is likely to be beneficial for most women.
The most influential determinant of the use of percutaneous biopsy for breast cancer diagnosis in Ontario is the LHIN in which the patient resides. Although our previous study suggested that patient age, urban residence and access to a primary care provider affected the probability of women receiving percutaneous biopsy for the diagnosis of breast abnormalities,17 the observation that patient demographics had no effect on the receipt of percutaneous biopsy for tissue diagnosis of breast cancer may reflect consistent access to care for patients with urgent conditions in a single-payer system that provides services regardless of individual circumstances. Nonetheless, the variation in the use of percutaneous biopsy across LHINs suggests differential availability of some services according to geography. The fact that surgeon and radiology quintiles are highly correlated with LHINs further implies that uneven distribution of surgeons who have higher volumes of patients with breast abnormalities or radiologists who perform more image-guided breast biopsies may also account for some of the regional variation. Finally, further study is needed to investigate the possibility that the effect of surgeon and radiology quintiles, which are highly correlated with LHINs, on the use of percutaneous (particularly image-guided) biopsy may be partly attributable to differing resource allocation across LHINs. The strong preference for surgical biopsy over percutaneous biopsy in LHINs in which the method of percutaneous biopsy was most likely to be FNAB alone rather than image-guided biopsy supports this hypothesis.
An important finding from our study was that surgeon and radiologist experience with breast assessment and breast procedures was associated with the use of percutaneous biopsy before surgery and the method of biopsy chosen, regardless of the LHIN in which care was provided. Although the relation between surgeon experience and use of percutaneous breast biopsy is nonlinear, it appears to be strong. The data suggest that there is a threshold of breast disease volume in a surgical practice that is associated with greater use of percutaneous biopsy. Because the surgeon consultation quintile variable was significantly associated with the use of percutaneous biopsy on multivariate analysis, this effect would appear to be real and largely independent of other variables in our model. The reasons for the association cannot be determined by the present study, but the observation is hypothesis-generating.
The opposite effects of radiological mammography and image-guided biopsy quintiles on the use of percutaneous biopsy are apparently paradoxical, but they may be explained by the organization of mammography facilities and by practice patterns. Mammography is performed in hospitals and free-standing radiology facilities, and through the provincial screening program. Although practitioners reading mammograms outside of hospitals conduct high volumes of screening, they do not necessarily perform breast biopsies. Additional diagnostic evaluation, including further imaging and biopsy, may be performed by a different radiologist at a different facility. Thus those radiologists who read the most mammograms may be less likely to direct the subsequent diagnostic evaluations than radiologists who read fewer mammograms but coordinate and perform subsequent imaging and biopsies. This hypothesis is supported by the linear relation that we observed between radiologist volume and percutaneous biopsy when we sorted the radiologists reading the mammograms into quintiles based on the volume of image-guided biopsies they performed. Similarly, the magnitude of effect observed with radiologist quintiles was less than that seen with surgeon quintiles, probably because of the surgeons' more direct role in coordinating patient management.
Other authors have observed that surgeon experience and volume influence their adherence to guidelines in the technical performance of cancer surgeries.20–22 To our knowledge, ours is the first study to describe an association between surgeon or radiologist volume and decision-making about diagnostic approaches. This finding may be particularly relevant to the management of breast cancer where diagnostic and treatment options are many. It is likely that radiologists who perform more image-guided breast biopsies would specifically recommend image-guided biopsies in their reports of abnormal mammograms; presumably the attending physicians who receive these reports frequently follow the recommendations. In this way, radiologists familiar with the practice of preoperative diagnosis may influence and guide management.
Although our study could not distinguish between FNAB and CNB, we were able to differentiate needle biopsies performed with image guidance from those without. The finding that radiologist and surgeon experience with breast disease and procedures would be associated with increased use of image-guided and non–image-guided biopsies respectively may be explained by the fact that image-guided biopsies are performed by radiologists, whereas FNAB without image guidance is likely performed by surgeons. Furthermore, women aged 50–69 years are offered screening through the OBSP, which provides specific recommendations for further investigation of abnormal screens, likely accounting for the increased use of image-guided biopsy in this age group and among women whose biopsies were initiated by OBSP screens. Our finding that women with any history of screening mammography were more likely to have their cancer diagnosis confirmed using image guidance is consistent with the findings of our earlier study indicating that evidence of regular primary care and a history of mammography screening were associated with the use of percutaneous biopsy. Such women, particularly those screened through the OBSP, would be more exposed to organized care. Radiologists with particular experience in image-guided biopsies are more likely than others to participate in such standardized processes for breast care.
Differences in availability of preoperative biopsy techniques may account for some of the regional variation in their use. Both FNAB and image-guided biopsy require access to specialized expertise such as cytopathology (FNAB) and image-guided biopsy techniques. The observations that variation in the use of image-guided biopsy is more marked than that in the use of FNAB and that FNAB is the predominant technique used in LHINs with lower utilization rates of percutaneous biopsy for diagnosis further suggest that limited access may play a role in the use of preoperative biopsy techniques. Our study could not determine whether variation in the availability of specialized equipment or services such as cytopathology and expert breast pathology, knowledge of current recommendations for preoperative diagnosis of cancer, or other factors may contribute to the irregular application of percutaneous biopsy across the province; however, these important questions deserve further study.
Olivotto and colleagues23 reported variation in the time to definitive diagnosis after an abnormal mammogram across different parts of British Columbia, regardless of whether the diagnosis was confirmed by percutaneous biopsy or surgery. Their results indicated that factors other than the availability of technology contribute to geographic variability in patterns of care. Other authors have also described regional variation in the provision of treatment for breast cancer.24–26 Craft and colleagues25 noted the effect of residence in different parts of Australia as well as an additional effect of rural residence on receipt of surgical care. Laliberte and colleagues26 reported that treatment in facilities that were members of National Cancer Institute–funded cancer networks was an independent predictor of compliance with guidelines for the primary treatment of early breast cancer, suggesting that organizational approaches can improve the quality of care for breast cancer patients, at least in some settings.
Our findings suggest that the proportion of women with breast cancer who undergo percutaneous biopsy may be useful as a quality indicator in the diagnosis and surgical treatment of breast cancer. To the extent that such a measurement reflects surgical decision-making in keeping with current guidelines and the availability of percutaneous (particularly image-guided) biopsy, it may be used to identify areas that could benefit from targeted approaches to increase the uptake of preoperative breast biopsy. Sixty-five percent of Ontario women with breast cancer diagnosed in 2002 received a percutaneous biopsy before definitive surgical treatment. Given the wide regional variation in the application of percutaneous biopsy, it is probable that this proportion of women could be increased with organizational strategies to address regional disparities.
Issues unrelated to indications for biopsy were associated with variation in the use of percutaneous biopsy to diagnose breast cancer. Location of health care delivery and practitioner experience with breast disease were important determinants of the use of current minimally invasive biopsy techniques. A system-wide approach to reduce barriers to the use of percutaneous biopsy, particularly image-guided CNB, is needed to optimize care for women with breast cancer.
Contributors: Drs. Holloway and Paszat designed the study. Mr. Saskin and Dr. Paszat acquired the data, which all authors analyzed. Dr. Holloway wrote the article. All authors reviewed the article and gave final approval for its publication.
Competing interests: None declared.
Accepted for publication Nov. 29, 2007
Correspondence to: Dr. C.M.B. Holloway, Division of Surgical Oncology, Sunnybrook Health Sciences Centre, 2075 Bayview Ave., Rm. T2-015, Toronto ON M4N 3M5; fax 416 480-6002; claire.holloway@sunnybrook.ca
References
- 1.White RR, Halperin TJ, Olson JA Jr, et al. Impact of core-needle breast biopsy on the surgical management of mammographic abnormalities. Ann Surg 2001; 233: 769-77. [DOI] [PMC free article] [PubMed]
- 2.Rubin E, Mennemeyer ST, Desmond RA, et al. Reducing the cost of diagnosis of breast carcinoma: impact of ultrasound and imaging-guided biopsies on a clinical breast practice. Cancer 2001;91:324-32. [DOI] [PubMed]
- 3.Silverstein MJ, Lagios MD, Recht A, et al. Image-detected breast cancer: state of the art diagnosis and treatment. J Am Coll Surg 2005;201:586-97. [DOI] [PubMed]
- 4.Berner A, Davidson B, Sigstad E, et al. Fine-needle aspiration cytology vs. core biopsy in the diagnosis of breast lesions. Diagn Cytopathol 2003;29:344-8. [DOI] [PubMed]
- 5.Masood S. Occult breast lesions and aspiration biopsy: a new challenge. Diagn Cytopathol 1993;9:613-4. [DOI] [PubMed]
- 6.Wilkinson EJ, Masood S. Stereotactic imaging and breast biopsy. In: Bland KI, Copeland EM, editors. The breast: comprehensive management of benign and malignant diseases. Vol 36, 2nd ed. Philadelphia: W.B. Saunders; 1998. p. 698-735.
- 7.Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology 1994; 193:359-64. [DOI] [PubMed]
- 8.Kaye MD, Vicinanza-Adami CA, Sullivan ML. Mammographic findings after stereotaxic biopsy of the breast performed with large-core needles. Radiology 1994;192: 149-51. [DOI] [PubMed]
- 9.Soo MS, Baker JA, Rosen EL. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol 2003;180: 941-8. [DOI] [PubMed]
- 10.Whitten TM, Wallace TW, Bird RE, et al. Image-guided core biopsy has advantages over needle localization biopsy for the diagnosis of nonpalpable breast cancer. Am Surg 1997;63:1072-7. [PubMed]
- 11.Yim JH, Barton P, Weber B, et al. Mammographically detected breast cancer: benefits of stereotactic core versus wire localization biopsy. Ann Surg 1996; 223: 688-700. [DOI] [PMC free article] [PubMed]
- 12.Smith DN, Christian R, Meyer JE. Large-core needle biopsy of nonpalpable breast cancers. The impact on subsequent surgical excisions. Arch Surg 1997;132:256-9. [DOI] [PubMed]
- 13.Butler WM, Cunningham JE, Bull D, et al. Breast cancer care: changing community standards. J Healthc Qual 2004; 26: 22-8. [DOI] [PubMed]
- 14.Morrow M, Venta L, Stinson T, et al. Prospective comparison of stereotactic core biopsy and surgical excision as diagnostic procedures for breast cancer patients [see comment]. Ann Surg 2001; 233: 537-41. Comment in: Ann Surg 2002; 235:605. [DOI] [PMC free article] [PubMed]
- 15.Lind DS, Minter R, Steinbach B, et al. Stereotactic core biopsy reduces the reexcision rate and the cost of mammographically detected cancer. J Surg Res 1998; 78: 23-6. [DOI] [PubMed]
- 16.Al-Sobhi SS, Helvie MA, Pass HA, et al. Extent of lumpectomy for breast cancer after diagnosis by stereotactic core versus wire localization biopsy. Ann Surg Oncol 1999; 6:330-5. [DOI] [PubMed]
- 17.Holloway CMB, Saskin R, Brackstone M, et al. Variation in the use of percutaneous biopsy for diagnosis of breast abnormalities in Ontario. Ann Surg Oncol 2007; 14: 2932-9. [DOI] [PubMed]
- 18.World Health Organization (WHO). International classification of diseases (ICD). 10th rev. Geneva: WHO; 2007.
- 19.Kaufman CS, Delbecq R, Jacobson L. Excising the reexcision: stereotactic core-needle biopsy decreases need for reexcision of breast cancer. World J Surg 1998;22: 1023-7. [DOI] [PubMed]
- 20.Young JM, Leong DC, Armstrong K, et al. Concordance with national guidelines for colorectal cancer care in new south wales: a population-based patterns of care study. Med J Aust 2007;186:292-5. [DOI] [PubMed]
- 21.Gilligan MA, Neuner J, Sparapani R, et al. Surgeon characteristics and variations in treatment for early-stage breast cancer. Arch Surg 2007;142:17-22. [DOI] [PubMed]
- 22.Lerut T, Nafteux P, Moons J, et al. Quality in the surgical treatment of cancer of the esophagus and gastroesophageal junction. Eur J Surg Oncol 2005;31:587-94. [DOI] [PubMed]
- 23.Olivotto IA, Kan L, King S. Waiting for a diagnosis after an abnormal screening mammogram. SMPBC diagnostic process workgroup. screening mammography program of British Columbia. Can J Public Health 2000;91:113-7. [DOI] [PMC free article] [PubMed]
- 24.Beaulieu J, Galland J, Fleming S, et al. Geographic variation in breast-conserving surgery in Kentucky's medicare population. J Ky Med Assoc 2002;100:99-103. [PubMed]
- 25.Craft PS, Primrose JG, Lindner JA, et al. Surgical management of breast cancer in Australian women in 1993: analysis of medicare statistics [see comment]. Med J Aust 1997;166:626-9. Comment in: Med J Aust 1997;166:620-1. [DOI] [PubMed]
- 26.Laliberte L, Fennell ML, Papandonatos G. The relationship of membership in research networks to compliance with treatment guidelines for early-stage breast cancer. Med Care 2005;43:471-9. [DOI] [PubMed]