Abstract
Foxp3+T regulatory cell (Treg) subsets play a crucial role in the maintenance of immune homeostasis against self-antigen. The lack or dysfunction of these cells is responsible for the pathogenesis and development of many autoimmune diseases. Therefore, manipulation of these cells may provide a novel therapeutic approach to treat autoimmune diseases and prevent allograft rejection during organ transplantation. In the article, we will provide current opinions concerning the classification, developmental and functional characterizations of Treg subsets. A particular emphasis will be focused on transforming cell growth factor beta (TGF-β) and its role in the differentiation and development of induced regulatory T cells (iTregs) in the periphery. Moreover, the similarity and disparity of iTregs and naturally occurring, thymus-derived CD4+CD25+Foxp3+ regulatory T cells (nTregs) will also be discussed. While proinflammatory cytokine IL-6 can convert nTregs to IL-17-producing cells, peripheral Tregs induced by TGF-β are resistant to this cytokine. This difference may affect the role of each in the adaptive immune response.
Keywords: Immunoregulation, regulatory T cells, TGF-β, Foxp3, Th17 cells
Introduction
It is now widely accepted that a cell population called “regulatory or suppressor cells” are critically involved in immune tolerance and homeostasis although clonal deletion and anergy are still thought to serve as key mechanisms of immunologic central tolerance. In the early 1970s, Gershon and Kondo observed that adoptive transfer of thymocytes from a sheep red blood cell-primed mouse to another naïve mouse prevented the latter from developing the anti-sheep red blood antibodies. They therefore suggested that in addition to the recognized effector T cell population, the transferred thymocytes contained a population of suppressor T cells [1]. Other data supporting the existence of a suppressor T cell population in normal thymocytes came from experiments in which neonatal thymectomy (NTx) of normal mice between day 2 and 4 after birth resulted in the development of thyroiditis, gastritis, orchitis, prostatitis, and sialoadenitis [2]. Although early studies concluded that the suppressor cells phenotypically consisted of CD8+ cells, both Sakaguchi et al and Powrie et al found CD5+CD4+ cells or memory CD4+ cells prevented autoimmunity in their respective models [3, 4]. In 1995, Sakaguchi et al identified the CD25 molecule (the IL-2 receptor α-chain) as a much better marker to represent suppressor/regulatory T (Treg) cells compared with CD5 and CD45RB (or CD45RC in rat). These cells constituted 5–10% of peripheral CD4+ T cells and less than 1% of peripheral CD8+ T cells in normal naïve mice, and were confined to the CD5high and CD45RBlow fraction of CD4+ T cells. Reconstitution with normal CD4+CD25+ T cells within a limited period after NTx prevented the development of autoimmunity. Removal of CD25+CD4+ T cells elicited autoimmune diseases and co-transfer of a small number of CD25+CD4+ T cells with CD25− T cells significantly inhibited the autoimmunity in the various animal models [5].
Within the last decade the transcription factor Foxp3 has been identified as a much more specific marker for Treg cells. This protein is also critically involved in the development and function of Treg cells [6–8]. Despite the fact that Foxp3-GFP “knockin” studies clearly demonstrate that there is a very broad spectrum of CD25 expression on Treg cells, without using genetically modified tissues, current techniques still preclude the isolation of live CD4+Foxp3+ cells, therefore CD4+CD25+ cells are widely used in the field of the biology of Treg cells.
CD4+CD25+ cells also exist in humans although only the CD4+CD25bright cell population displayed the immune suppressive effect [9]. Recent evidence suggests that although Foxp3 expression in murine lymphocytes appears to be a definitive marker for Treg cells, this may not be the case for human Tregs. New data demonstrate that FOXP3 (the ortholog of murine Foxp3) may be upregulated in rapidly proliferating human T cells and might be viewed as an activation marker for human T cells [10–12]. Nonetheless, mutations of the human FOXP3 gene cause disease called IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome), which is characterized by autoimmune disease in multiple endocrine organs [13–15]. In addition, a decreased frequency and suppressive activity of CD4+CD25+ cells and CD4+FOXP3+ cells has been observed in many patients with autoimmune diseases [16–20].
Although the Treg cell family is predominantly comprised of CD4+ subsets, it also contains multiple heterogeneous subsets that include CD4+CD25+Foxp3+ cells, IL-10-producing CD4+ Tr1 cells, TGF-β-producing Th3 cells, CD8+ cells, NK T cells, CD4−CD8− T cells and γδ T cells [21, 22]. CD4+ Treg subsets can be further classified into two main populations, thymus-derived, naturally occurring CD4+CD25+Foxp3+ cells (nTregs) described as above and induced Tregs (iTregs) generated from CD25− precursors in peripheral lymphoid organs. Although IL-10-induced Tr1 cells and Th3 cells induced by the oral administration of peptide also represent some of induced CD4+ subsets, they do not express Foxp3 [23, 24]. Conversely, iTregs induced ex vivo by TGF-β express Foxp3 and makers are not available to distinguish iTregs from nTregs at present [25–28]. TGF-β-induced Tregs will be defined as iTregs in this review.
Many studies have revealed that both nTregs and iTregs share the similar phenotypic and functional characteristics. Most are previously activated cells that express CD25, CTLA-4, GITR, CCR4, CD62L and Foxp3, and express CD45RBlow in mice and CD45RO in humans. Both iTregs and nTregs similarly suppress T cell activation and proliferation in vitro through cell-cell contact and membrane-bound TGF-β although they are also thought to exert their suppressive activity via suppressive cytokines in vivo [29–32]. Adoptive transfer of either variety of Treg can inhibit the development of autoimmune disease and prolong allograft survival in MHC-mismatched organ transplantation [5, 25, 31–39]. In addition, there is an interactive role between nTregs and iTregs that hints both Treg subsets may have different targets or they may have a synergic role in regulating immune tolerance [26]. Despite the fact that both Treg classes have a distinct conversion to Th17 cells when stimulated with pre-inflammatory cytokine, IL-6, there are some substantial differences regarding the differentiation and functional properties of each class. We will discuss these differences in this review.
TGF-β belongs to a family of evolutionarily conserved molecules that is an important immunoregulatory cytokine involved in the maintenance of self-tolerance and T-cell homeostasis [40]. It is produced by several immune and non-immune cell types and functions in both an autocrine and paracrine manner [41, 42]. TGF-β members consist of TGF-β1, TGF-β2, and TGF-β3. TGF-β1 is the major form expressed in the immune system in mammals. TGF-β is synthesized as a precursor protein and released in an inactive form as either a small or large latent complex [43]. To elicit its biological activity, TGF-β has to be converted from inactive form to active one [41]. Active TGF-β combines the TGF-β type I (TGF-βRI) and type II (TGF-βRII) receptors and signals through SMAD proteins or through a SMAD-independent pathway [44].
TGF-β can suppress T cell responses either through a direct or an indirect pathway. For instance, TGF-β can directly exert its antiproliferative effects on CD4+ T cells due to its ability to inhibit IL-2 production and to upregulate cell cycle inhibitors [45]. TGF-β can also directly inhibit the differentiation of Th1 and Th2 cells via a downregulation of the transcription factors T-bet and GATA-3 that are required for the expression of IFN-γ and IL-4 [46, 47], respectively. In addition, TGF-β can inhibit the activation of macrophages and their ability to produce pro-inflammatory cytokines. Moreover, TGF-β prevents the maturation of DCs and decreases MHC-class II expression by DCs, and subsequently decreases the ability of DCs to present antigens to T cells [45].
TGF-β's pivotal functions in suppressing T cell responses and maintaining immune tolerance can be further documented in studies using TGF-β1-deficient mice. These mice develop an early and fatal lymphoproliferative and inflammatory disease [49, 50]. Other studies have employed transgenic mice expressing dominant-negative mutants of TGF-βRII in T cells and ruled out T cells as a principal direct TGF-β1 target in vivo [50–52]. In the absence of TGF-β signaling, T cells undergo hyperproliferation, activation, and effector T cell differentiation that results in infiltration of leukocytes into multiple vital organs and a neonatal lethal phenotype as severe as that of TGF-β1-deficient mice [51, 52].
Of note, TGF-β can also exert a positive effect on T cells. While TGF-β suppresses Th1 and Th2 differentiation, conversely, it also induces the differentiation and development of Foxp3+ regulatory T cells. It was initially believed that TGF-β selectively expands only endogenous nTregs [53], however, further research revealed that TGF-β can indeed induce CD4+CD25− non-regulatory T cells to become regulatory T cells [54]. When Foxp3 was identified as Treg marker, several groups rapidly found TGF-β was able to induce CD4+CD25−Foxp3− cells to express Foxp3 in mice [25, 27, 28] and humans [26]. Although some investigators suspected CD4+CD25− precursor cells in this TGF-β-driven conversion were possibly CD4+CD25+ cells but lost CD25 expression, current several studies using transgenic mice and GFP Foxp3 knock-in mice have clearly demonstrated that TGF-β is capable of inducing CD4+CD25−Foxp3− cells to become Foxp3+ Treg cells (55, 56). In contrast to nTregs, one group has observed the expression of Foxp3 by iTregs is not stable and incomplete demethylation in conserved region in intron one of the Foxp3 gene is responsible for this transient Foxp3 expression in vitro [57]. Our research has also revealed that Foxp3 expression and maintenance by iTregs is transient and rapidly decreases without this cytokine in vitro. However, we have also demonstrated that the addition of IL-2 to the TGF-β-driven cultures can sustain the iTreg's Foxp3 expression, and conversely the addition of anti-IL-2 or anti-TGF-β will decrease Foxp3 expression [58]. Whether IL-2 affects the demethylation in Foxp3 locus is under investigation in our group. Of special note is that Foxp3 expression by iTreg generated in the laboratory of Ethan Shevach is stable, and they have been successful at maintaining this expression more than >50 days in vivo (59). We have also observed that when adoptively transferred to syngeneic mice, >80% of both nTregs and iTregs sustained expression of Foxp3 for one month following injection (unpublished data). We hypothesize that cytokines and/or self-antigenic stimulation promotes the maintenance of Foxp3 expression in the transferred Treg subsets in vivo.
The therapeutic effect of TGF-β-induced iTregs on autoimmune diseases and transplantation tolerance
Compelling evidence has revealed that the manipulation of nTreg cell-based therapy can control autoimmune disease and improve the survival of allografts in organ transplantation [5, 25, 31–39]. Similarly, adoptive transfer of iTregs has also been developed into a strategy that may lead to the restoration of immune tolerance in the treatment of autoimmune diseases and in the prevention of transplantation rejection. Due to the low frequency of nTregs in normal humans and to the dysfunction of nTregs in patients with some autoimmune diseases, it will be very significant to develop iTreg cells using an alternative approach.
Research in the laboratory of Sharon Wahl has demonstrated that adoptive transfer of TGF-β-converted/induced iTregs prevented house dust mite–induced allergic pathogenesis and inflammation in lungs in an asthmatic mouse model [25]. Weber et al observed that injection of murine islet-specific CD4+ iTregs generated by TCR stimulation with IL-2 and TGF-β prevented spontaneous development of type 1 diabetes and inhibited development of pancreatic infiltrates and disease onset orchestrated by Th1 effectors in NOD mice [60]. DiPaolo et al have also reported similar therapeutic potential of Ag-specific iTregs to prevent autoimmune diseases. In this study, they found iTregs are effective as nTregs at preventing organ-specific autoimmunity in a murine model of autoimmune gastritis. They also provided evidence that iTregs decreased B7 expression by DC and this iTregs-DC interaction possibly contributes to the suppressive effect of iTregs on autoimmunity [59]. Similarly, iTreg cells also significantly suppressed Th1 mediated colitis on CD4+CD62L+ T cell transfer in vivo [61]. Not only do iTregs prevent the appearance of autoimmune disease, we have also demonstrated that injection of iTregs can ameliorate established autoimmune disease. We have developed a chronic graft-vs-host disease model characterized by rapid and vigorous formation of SLE-like autoantibodies and the formation of severe immune-complex glomerulonephritis. DBA/2 mouse T cells induce this syndrome when injected into (DBA/2 × C57BL/6) F1 mice. We found TGF-β-treated DBA/2 T cells not only lost their ability to induce graft-vs-host disease but also prevented other parental T cells from inducing lymphoid hyperplasia, B cell activation, and an immune complex glomerulonephritis. Moreover, a single transfer of TGF-β-conditioned T cells to animals that had already developed anti-dsDNA Abs decreased the titer, suppressed proteinuria, and doubled survival [36]. We recently also observed injected iTregs suppressed DC accumulation and maturation in spleen and lymph nodes, and even induced tolerogenic or regulatory DC formation. It has been documented by an experiment where injection of DCs isolated from GVHD (DBA/2 × C57BL/6) F1 mice that had received iTregs but not control cells was able to prevent other parental T cells from inducing typical lupus syndromes in (DBA/2 × C57BL/6) F1 mice (manuscript in preparation).
It has been also widely accepted that Tregs play a pivotal role in transplantation tolerance. Many studies have implicated Tregs in the maintenance of transplantation tolerance to donor antigens in bone marrow and solid organ transplantation [62–64]. For instance, intrathymic antigen inoculation or monoclonal antibodies (mAbs) against CD4, CD8 or CD154 induced transplantation tolerance through the in vivo generation of Tregs (65). While removal of Tregs enhanced the allograft rejection, conversely, enrichment of Tregs promoted the allograft survival [66, 67]. In addition, evidence also exists that administration of nTregs to the recipients can target or prevent solid organ transplantation rejection [68, 69], even in inducible liver transplantation tolerance models [70].
We and others have reported iTregs generated ex vivo with TGF-β also had the potential to protect MHC-mismatched organ grafts from rejection. Watanabe et al showed that intravenous administration of TGF-β-induced CD4+CD25+ iTregs resulted in a significant effect on cardiac allograft survival in rat model [71]. We also developed a mouse cardiac allograft model to test the functional capacity of TGF-β-induced iTregs. We first observed that recipient mice developed a T cell non-responsiveness to donor alloantigens when they received iTregs but not control cells. An antigen-dependent increase in splenic CD4+CD25+ cells derived from the recipient mice contributed to this tolerance. We have also demonstrated that the transfer of TGF-β-induced alloantigen-specific iTregs co-incident with transplantation of a histoincompatible heart results in a marked extension of allograft survival [39]. This study raises the possibility that natural and induced regulatory T cells generated ex vivo have the potential to be used as an adoptive immunotherapy to control autoimmunity and induce allograft tolerance.
The distinct role of co-stimulatory molecules and IL-2 in the development of both Treg subsets
Although both nTreg and iTreg subsets share similar phenotypes and display comparable suppressive activity, several factors distinctly affect their development. First, nTregs develop in the thymus through recognition of self antigens. A high affinity cognate interaction between self-peptide:MHC complex and T cell receptor is required for this process. They also require CD28 co-stimulation because they poorly develop in CD28 deficient mice [72]. Although IL-2 and TGF-β play an important role in the maintenance of the pool size of nTregs, both cytokines are redundant for their development since both IL-2 and TGF-β knock out mice contain CD4+CD25+Foxp3+ regulatory T cells in the thymus [73, 74]. By contrast, the generation of iTregs is dependant upon the presence of both TGF-β and TGF-β receptor signals since the absence of TGF-β or blocking the TGF-β receptor signal prevents the induction of Foxp3 expression and the subsequent functional suppressive capacity. The conversion of CD4+CD25− cells in the periphery to CD25+ iTregs require weaker, suboptimal TCR stimulation and thus environmental antigens may sufficiently trigger iTreg development. However, the lack of CD28 co-stimulatory molecules does not affect the differentiation of iTregs (unpublished data), but inhibitory CTLA-4 co-stimulation and CTLA-4/B7.1 signaling are absolute requirements for the generation of iTregs. These iTregs can not be induced from naïve CD4+CD25− precursor cells in CTLA-4 deficient mice although these mice contain normal CD4+CD25+Foxp3+ nTregs [75]. This conclusion is further documented by an observation that the blocking of CTLA-4/B7.1 signal abolished the capacity of TGF-β to induce iTregs in wild type mice [76]. Ox40/Ox40L, an alternate CD28/B7-independent costimulatory pathway, also negatively regulates the development and function of both nTregs and iTregs. While stimulation of mature nTregs by Ox40 results in the loss of suppression of T cell proliferation and cytokine production [77], the generation of iTreg is completely abolished by Ox40 although Ox40 does not affect the generation of nTregs [78].
Although interleukin-2 (IL-2) was originally described as a potent T cell growth factor in vitro, recent studies have suggested that this cytokine is also critically involved in the maintenance of peripheral T cell tolerance. Mice deficient in IL-2 production or the IL-2 receptor α or β chains develop a lethal autoimmune disease [79, 80]. It is apparent that the decreased frequency of nTregs is responsible for this lethal autoimmune disease in these mice since adoptive transfer of wild type nTregs that do not produce IL-2 prevent the disease [81]. To directly assess the effect of IL-2 signaling on Treg cell development and function, Fontenot et al analyzed mice containing the Foxp3(gfp) knock-in allele that were genetically deficient in either IL-2-/- or CD25 (II2Ra-/-). They found that IL-2 signaling was dispensable for the induction of Foxp3 expression in thymocytes and nTregs from IL-2 or IL-2 receptor deficient mice were fully able to suppress T cell proliferation in vitro. However they also found IL-2 signaling was required for maintenance of pool sizes of nTregs in the periphery [73].
We initially considered that IL-2 possibly overcame the inhibitory effect of TGF-β on T cells. Unexpectedly, we also identified a specific dose of anti-IL-2 antibody that did not block the CD25 expression but completely abolished the Foxp3 expression and suppressive activity by CD4+ cells. We then found TGF-β failed to induce Foxp3+ iTregs from naïve CD4+CD25− precursor cells in IL-2 deficient mice. Addition of exogenous IL-2 but not other common gamma chain cytokines was able to rescue the ability of TGF-β to induce Foxp3+ iTreg cells by naïve CD4+CD25− cells in these mice [58]. Davidson et al also simultaneously reported a similar observation [55]. Differing IL-2 and co-stimulatory molecule requirements for the development of both nTregs and iTregs suggests that nTregs and iTregs are possibly heterogeneous populations and that integration of both Treg subsets is required for the maintenance of normal immune homeostasis. It is also likely that both nTreg and iTreg subsets can either work in concert or can work separately on different targets.
Disparity between the developmental and functional fates of Treg subsets when stimulated with the proinflammatory cytokine IL-6
A positive role for TGF-β in T cells is exemplified by the recent finding that the combination of IL-6 and TGF-β can induce mouse T cells to become proinflammatory effector cells that produce IL-17 (Th17 cells) [56, 82, 83]. As nTreg cells express a membrane-bound form of TGF-β and this TGF-β has functional activities, it is reasonable that IL-6 can convert nTregs to become Th17 cells [84]. To demonstrate this, Xu et al used purified nTregs from Foxp3 GFP knock in mice to exclude the possibility that CD4+CD25+Foxp3− non-Tregs made this conversion. We have also used both wild type and Foxp3 GFP knock in mice to confirm this observation. We believe that endogenous TGF-β produced by nTregs is critically required for this conversion since we observed the blocking TGF-β receptor I signal or using nTregs from TGF-β receptor II dominant mice resulted in the failure of Th17 conversion [85]. Moreover, we found that activation of nTregs with IL-6 resulted in decreased Foxp3 expression and suppressive activity both in vitro and in vivo. Furthermore, when used in adoptive transfer experiments nTregs treated with IL-6 ex vivo lost their ability to protect mice from a lupus-like disease [85]. Thus, in an IL-6 rich inflammatory milieu, there appears to be a major difference in the functional stability of Foxp3+ nTregs and iTregs. Moreover, recently it has been demonstrated that nTregs can be converted into Th17 cells in an in vivo model [86].
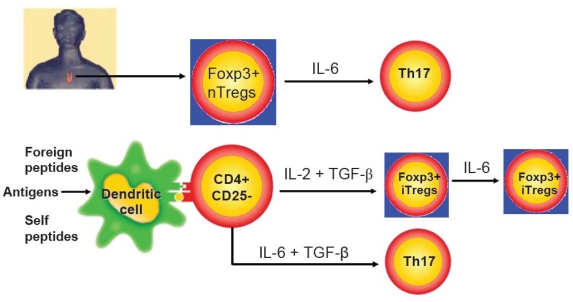
In sharp contrast, TGF-β induced iTregs were completely resistant to the Th17 conversion when similarly stimulated with IL-6. This difference can not be explained by the insufficient production of TGF-β in iTregs since both nTregs and iTregs expressed similar levels of membrane-bound TGF-β (20–25%) and secreted similar levels of active TGF-β (about 40 ng/ml). The resistance of iTregs to Th17 conversion also cannot be explained by alterations in TCR stimulation since anti-CD3/CD28 activated nTregs can still become Th17 cells when stimulated with IL-6 [85]. To account for this difference between nTregs and iTregs, we found that the combination of IL-2 and TGF-β down-regulated IL-6 receptor expression and function by activated T cells. We have observed that both cytokines markedly decreased IL-6 receptor alpha-chain (CD126) and beta-chain (CD132) expression on CD4+ cells and these cells expressed significantly lower level of phosphorylated STAT3 expression when stimulated with IL-6 (85). The difference between both nTregs and iTregs in the differentiation and function when stimulated with IL-6 has been summarized as Figure 1. We are currently investigating whether this distinct conversion of both Treg subsets to Th17 cells will occur in autoimmunity mice model in which high levels of IL-6 have been identified. If it is case, this will indicate iTregs have a greater advantage to treat autoimmune and inflammatory diseases compared with nTregs.
Figure 1.
The difference between nTregs and iTregs in the Th17 conversion when stimulated with IL-6. CD4+CD25+Foxp3+ nTregs derived from thymus convert into IL-17-producing cells and decrease Foxp3 expression when stimulated with IL-6. Conversely, similarly stimulated CD4+CD25+Foxp3+ iTregs induced with IL-2 and TGF-β from CD4+CD25− in the periphery are resistant to converting of Th17 cells and they also sustain Foxp3 phenotype and suppressive function although IL-6 and TGF-β can induce the development of Th17 cells.
These studies raise the possibility that nTregs and iTregs may have distinct roles in the adaptive immune response. In response to microbial infections some nTregs could possibly serve as a first line of host defense. If some are converted to IL-17 producing cells, these cells could contribute to neutrophil mobilization and have other pro-inflammatory effects. After subsequent eradication of the invaders, the later appearing TGF-β-induced iTregs would not only terminate the antigen-specific response, but also prevent the emergence of non-specifically stimulated or cross-reactive self-reactive T cells. Accordingly, failure of this terminal mechanism could result in an immune-mediated disease.
Differences between TGF-β induced iTregs in mice and humans
Unlike mouse T cells, conventional anti-CD3 stimulation of human CD4+ in the absence of TGF-β results in a small transient expression of Foxp3 [10, 11] although not all groups support this observation [87]. However, Treg generation from anti-CD3-stimulated human CD4+ cells is controversial. Both Shevach and our groups consistently observed TGF-β markedly increased Foxp3 expression in human CD4+ cells [26, 87]. It is notable these TGF-β induced CD4+Foxp3+ cells in human are not anergic cells. They produce ample IL-2 and other cytokines when restimulated with anti-CD3/CD28 beads. Addition of these cells to other T cells fails to suppress the T responder cell proliferation in vitro. Therefore, one concludes that TGF-β is unable to induce human T cells to develop the suppressive activity despite their high level of FOXP3 expression [87].
Nevertheless, we have previously observed TGF-β can indeed induce human CD4+ cells to become suppressor cells. When naïve human CD4+ cells were stimulated with alloantigen plus TGF-β for one week, the TGF-β-treated but not control cells (without TGF-β) prevented CD8+ T cells from proliferating in response to alloantigens and from becoming cytotoxic effector cells. Moreover, these regulatory cells exerted their suppressive activities in remarkably low numbers and maintained these effects even after they were expanded [53]. We subsequently also reported that with a low-dose Staphylococcal Enterotoxin B (SEB) stimulation, TGF-β did convert human CD4+CD25− cells to become Th3-like suppressor cells. Similarly, these cells also had potent suppressive activity since adding as few as 1% of these TGF-β-primed CD4+ T cells to fresh CD4+ cells and B cells markedly suppressed IgG production. The inhibitory effect was mediated by TGF-β and was also partially contact dependent [54].
Despite the above data on the different role of TGF-β in the induction of suppressor cells in mice and humans, several issues remain to be further investigated. First, the standard to judge whether FOXP3+ cells are Treg cells in humans needs to be further defined. Polyclonally activated, TGF-β primed CD4+ cells are unable to suppress T cell proliferation in vitro due to their non-anergic phenotype, but suppressive activity in vitro does not always accurately reflect the suppressive capacity of Treg cells in vivo. It is therefore necessary to directly determine the suppressive ability of TGF-β-induced human CD4+ cells in vivo. Our group is currently developing a xeno-GVHD model in which we will adoptively transfer human PBMC into SCID common-γ chain knock-out mice. Previous work has described that xeno-GVHD can be induced in RAG2-/-gamma c-/- mice by i.v. administration of human peripheral blood mononuclear cells (88). We will learn whether co-transfer of TGF-β-induced human CD4+ cells can suppress the expansion and cytokine production by human T cells in these mice. This experiment will likely provide a better approach to determine the suppressive characteristics of TGF-β-induced human CD4+ cells. Second, TGF-β-induced human CD4+ cells express high level of FOXP3, however, this FOXP3 is methylated and inactive. Janson et al have recently described that human nTregs display a demethylated FOXP3 promoter, in contrast, CD4+CD25low T cells and stimulated CD4+CD25− T cells remained partially methylated although the latter transiently expressed FOXP3. In addition, they also observed TGF-β and/or IL-10 does not induce any additional change in methylation level [89]. We are currently investigating whether demethylation in TGF-β-induced human CD4+ cells can restore or enhance the suppressive activity of these cells. Lastly, it is possible that different stimulatory patterns or strengths may influence the development of TGF-β-induced regulatory T cells in humans. Polyclonal mitogens may result in too strong of stimulation and this overexuberant stimulation might fail to drive the differentiation of Treg cells in the presence of TGF-β. However, low-dose SEB and alloantigen stimulation may lead to an optimal stimulatory strength that sufficiently enables TGF-β to drive Treg cells induction in humans. Our recent unpublished observation that only low dose but not high dose of SEB favors the induction of TGF-β-iTreg cells in human further supports this hypothesis.
Acknowledgments
This work was supported by grants from the Arthritis Foundation, the Arthritis National Research Foundation, the Clinical Research Feasibility Fund, the James H. Zumberge Faculty Research and Innovation Fund, the Outstanding Youth Scientist Award from National Nature Science Foundation of China (30728007), and the American College of Rheumatology Research and Education's Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign.
References
- 1.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 2.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 3.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990;172:1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T-cell subset. I. Evidence for the active participation of T cells in natural self-tolerance: deficit of a T-cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 6.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 9.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 10.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW, Ottenhoff TH, Toes RE. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 12.Yu JN, Ma JL, Wang JH, Tao XJ, Li XP, Zou HJ, Zheng SG. FOXP3, is still a specific marker for human regulatory T cells? Chin J Allergy Clin Immunol. 2008;2:5–10. [Google Scholar]
- 13.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 16.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 17.Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 18.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, Vandenbark AA. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 20.Balandina A, Lécart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz DA, Zheng SG, Gray JD, Wang JH, Ohtsuka K, Yamagiwa S. Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease. Semin Immunol. 2004;16:135–143. doi: 10.1016/j.smim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 24.Gandhi R, Anderson DE, Weiner HL. Cutting Edge: Immature human dendritic cells express latency-associated peptide and inhibit T cell activation in a TGF-beta-dependent manner. J Immunol. 2007;178:4017–4021. doi: 10.4049/jimmunol.178.7.4017. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 27.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 28.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 31.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 34.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective Treatment Of Collagen-Induced Arthritis By Adoptive Transfer Of Cd25+ Regulatory T Cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 35.Dipaolo RJ, Glass DD, Bijwaard KE, Shevach EM. CD4+CD25+ T cells prevent the development of organ-specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J Immunol. 2005;175:7135–7142. doi: 10.4049/jimmunol.175.11.7135. [DOI] [PubMed] [Google Scholar]
- 36.Zheng SG, Wang JH, Koss MN, Quismorio F, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 37.van Maurik A, Herber M, Wood KJ, Jones ND. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J Immunol. 2002;169:5401–5404. doi: 10.4049/jimmunol.169.10.5401. [DOI] [PubMed] [Google Scholar]
- 38.Xia G, He J, Zhang Z, Leventhal JR. Targeting acute allograft rejection by immunotherapy with ex vivo-expanded natural CD4+ CD25+ regulatory T cells. Transplantation. 2006;82:1749–1755. doi: 10.1097/01.tp.0000250731.44913.ee. [DOI] [PubMed] [Google Scholar]
- 39.Zheng SG, Meng L, Wang JH, Watanabe M, Barr ML, Cramer DV, Gray JD, Horwitz DA. Transfer of regulatory T cells generated ex vivo modifies graft rejection through induction of tolerogenic CD4+CD25+ cells in the recipient. Int Immunol. 2006;18:279–289. doi: 10.1093/intimm/dxh368. [DOI] [PubMed] [Google Scholar]
- 40.Bommireddy R, Doetschman T. TGFβ, T-cell tolerance and anti-CD3 therapy. Trends Mol Med. 2004;10:3–9. doi: 10.1016/j.molmed.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt-Weber, Blaser K. Regulation and role of transforming growth factor-β in immune tolerance induction and inflammation. Curr Opin Immunol. 2004;16:709–716. doi: 10.1016/j.coi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I, Rifkin DB. TGF-beta latency: Biological significance and mechanisms of activation. Stem Cells. 1997;15:190–197. doi: 10.1002/stem.150190. [DOI] [PubMed] [Google Scholar]
- 44.Bommireddy R, Ormsby I, Yin M, Boivin GP, Babcock GF, Doetschman T. TGF beta 1 inhibits Ca2+-calcineurin-mediated activation in thymocytes. J Immunol. 2003;170:3645–3652. doi: 10.4049/jimmunol.170.7.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 46.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heath VL, Murphy EE, Crain C, Tomlinson MG, O'Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 51.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 54.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 55.Davidson TS, Dipaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 56.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 57.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 59.Dipaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 60.Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, Ziegler SF, Bradley LM. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- 61.Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, Neurath MF. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai Z, Li Q, Wang Y, Gao G, Diggs LS, Tellides G, Lakkis FG. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 64.Cohen JL, Salomon BL. Therapeutic potential of CD4+ CD25+ regulatory T cells in allogeneic transplantation. Cytotherapy. 2005;7:166–170. doi: 10.1080/14653240510018145. [DOI] [PubMed] [Google Scholar]
- 65.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 66.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha, 25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 68.Xia G, He J, Zhang Z, Leventhal JR. Targeting acute allograft rejection by immunotherapy with ex vivo-expanded natural CD4+ CD25+ regulatory T cells. Transplantation. 2006;82:1749–1755. doi: 10.1097/01.tp.0000250731.44913.ee. [DOI] [PubMed] [Google Scholar]
- 69.Kitazawa Y, Fujino M, Sakai T, Azuma H, Kimura H, Isaka Y, Takahara S, Hünig T, Abe R, Li XK. Foxp3-expressing regulatory T cells expanded with CD28 superagonist antibody can prevent rat cardiac allograft rejection. J Heart Lung Transplant. 2008;27:362–371. doi: 10.1016/j.healun.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 70.Pu LY, Wang XH, Zhang F, Li XC, Yao AH, Yu Y, Lv L, Li GQ. Adoptive transfusion of ex vivo donor alloantigen-stimulated CD4(+)CD25(+) regulatory T cells ameliorates rejection of DAto-Lewis rat liver transplantation. Surgery. 2007;142:67–73. doi: 10.1016/j.surg.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe M, Mencel RL, Cramer DV, Starnes VA, Barr ML. Transforming growth factor-beta/interleukin-2-induced regulatory CD4+ T cells prolong cardiac allograft survival in rats. J Heart Lung Transplant. 2005;24:2153–2159. doi: 10.1016/j.healun.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 73.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 74.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 76.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 79.Sadlack B, Löhler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 80.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolf M, Schimpl A, Hünig T. Control of T cell hyperactivation in IL-2-deficient mice by CD4(+)CD25(−) and CD4(+)CD25(+) T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol. 2001;31:1637–1645. doi: 10.1002/1521-4141(200106)31:6<1637::aid-immu1637>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 82.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 83.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor- induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 84.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 85.Zheng SG, Wang JH, Horwitz DA. Cutting Edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-β are resistant to Th17 conversion by IL-6. J Immunol. 2008 doi: 10.4049/jimmunol.180.11.7112. in press. [DOI] [PubMed] [Google Scholar]
- 86.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, Weijer K, Spits H, Storm G, van Bloois L, Rijkers G, Martens AC, Ebeling SB. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2-/- gammac-/-double-mutant mice. Blood. 2003;102:2522–2531. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 89.Janson PC, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS ONE. 2008;3:e1612. doi: 10.1371/journal.pone.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]