Abstract
Tobacco is an important cash crop of Pakistan and tremendous amount of irrational pesticides are being used to control insect growth. The frequency of plasma pesticide residues above acceptable daily intake (ADI) and its correlation with biochemical markers for assessment of adverse health effects in the tobacco farmers at district Sawabi, Pakistan was determined. Total 109 adult males consisting of 55 tobacco farmers exposed to pesticides and 54 controls were included. Pesticides residues in blood were analyzed on HPLC and GC-NPD. Plasma butyrylcholinesterase (BChE) was analyzed by Ellman's method. Biochemical markers including serum calcium, phosphorus, urea, creatinine, bilirubin and liver enzymes were measured on Selectra-E auto analyzer. The tobacco farmers had multiple pesticides residues above ADI in their blood consisting of 35 (63%) methomyl; 31 (56%) thiodicarb; 34(62%) cypermethrin; 27 (49%) Imidacloprid; 18 (32%) Methamidophos and 15 (27%) endosulfan. BChE activity was significantly decreased in the pesticides exposed farmers as compared to controls (P<0.001). Plasma biochemical markers including ALT, AST, CK, LDH and phosphate were significantly raised in the pesticides exposed farmers as compared to control group (P<0.001). Total pesticides residues revealed a significant positive correlation with AST (r=0.42), LDH(r= 0.47), ALT (r=0.20) and phosphorus (r=0.51). Excessive exposure to pesticide caused cytotoxic changes in the hepatic and renal biochemical markers which were positively correlated with pesticide residue. Hence these biomarkers might be used in addition to BChE activity for monitoring of adverse effects of pesticides on the health of farm workers.
Keywords: Pesticides residue, adverse effects, butyrylcholinesterase, aminotransferase, biochemical markers, tobacco farmers
Introduction
Pesticides are widely used through out the world in agriculture to protect crops. Tobacco is one of the important cash crops and approximately 73% of the total tobacco is cultivated in district Swabi of North West Frontier Province (NWFP), Pakistan [1]. Tobacco is a pesticides dependent crop as its broad and succulent leaves provide favourable conditions for the development of many pests and diseases [2]. To safeguard the crop from damage, tremendous amounts of pesticides are being used in the tobacco growing areas. Although it has beneficial effects on tobacco yield but lack of observance and unavailability of protective measures to counter harmful effects of pesticide have posed a real threat to the health of farmers [3].
Prolonged exposure to pesticides & then elevated concentration in the body causes adverse effects. Pesticides differ greatly in their mode of action, uptake by the body, metabolism and toxicity to humans. WHO (2001) has classified pesticides according to their toxic effects as class I (extremely hazardous) to class III (slightly hazardous) [4]. Depending upon the toxicity of the compound and exposure time, the symptoms of pesticide exposure vary from headache, vomiting, skin rash, respiratory problems, and convulsions [5]. Several studies have shown that farmers exposed to pesticide for prolonged period are more likely to develop leukemia's, brain and prostrate cancers than the general population [6–7].
Pakistan is an agricultural based country. Lack of knowledge, careless attitude and appalling safety practices in handling of pesticides pose a serious health risk to our farmers. Pesticide residues including cypermethrin, deltamethrin, diazinon, monocrotophos were reported in the blood of farm workers from Gadap, Karachi [8]. Pesticide exposure may produce biochemical changes even before adverse clinical health effects are manifested in these farmers. Prolonged exposures to pesticide affect multiple organs including liver and kidney which can be detected by serum enzymes and other biochemical parameters among farm workers [9]. It is important to identify susceptible groups or individuals who are at risk of exposure to certain types of environmental and occupational agents [10]. The acceptable daily intake (ADI) for human is considered to be a level of intake of a chemical that can be ingested daily over an entire lifetime without any appreciable risk to health [11]. Blood concentration of pesticides and different biomarkers can be used for the risk assessment, biological monitoring and estimation damage caused by pesticides residues in the farm workers [12].
Tobacco farmers in our study were involved in cultivation, pesticides application, picking and drying of its leaves in the field. The tobacco farmers were over exposed to different types of pesticide, either simultaneously or serially during work in the field. Unfortunately, very little work has been done on pesticide residues in blood and the biochemical changes at cellular level in pesticide exposed occupational workers. Thus the study was designed to determine pesticide residues in blood and their correlation with biochemical markers for the assessment of adverse health effects in the tobacco farmers of Sawabi district, Pakistan. This study paved the way for early diagnosis and monitoring of pesticide induced adverse effects among farmers by various biochemical markers.
Materials and Methods
The study was conducted in district Swabi, North West Frontier Province (NWFP) of Pakistan. Two tobacco growing villages Chota Lahore and Jangen Nath were randomly selected. This study proposal was approved by institutional review committee of AM College, Rawalpindi and Higher Education Commission (HEC) Pakistan.
Subjects
Total 109 adult males consisting of 55 tobacco farmers and 54 control subjects who were not farmers but were the residents of the same area were included in the study after an informed consent. The potentially pesticide exposed tobacco farmers were randomly selected from the farms on the basis of their full time active involvement in preparation, storage and spraying of the pesticides on the tobacco crop. All participants were males. The pesticide exposed farmer and control group were age matched and ranged from 16–72 years. Medical history and physical examination of the subjects was carried out before the start of the study. The farmers suffering from diabetes mellitus, hypertension, chronic renal failure, viral hepatitis or any other chronic illness not related to pesticide exposure were excluded from the study.
Sample collection
Blood samples (5ml) were collected in heparanized tubes and transported to laboratory. Plasma was separated by centrifugation at 1500g for 15 minutes. Biochemical analysis was carried out at the laboratory of AM College, Rawalpindi.
Pesticide residue analysis
Blood pesticides extraction was carried out by method described by Agarwal et al., (1976) [13]. Clean up was made by USEPA Method 3620B [14]. Florisil was activated at 130°C overnight and cooled in a desiccator before use. Column was packed with 1g of florisil. Extract was transferred to the column and eluted with hexane and diethyl ether. Eluent was collected and evaporated to dryness. Final samples were prepared in 2 ml acetonitrile. The residues of methomyl, thiodicarb, imidacloprid, cypermethrin and endosulfan were determined by using reverse phase C-18 column (VA-ODS 150 × 4.6 Shimadzu, Japan) at wavelength of 254 nm with flow rate of 1 ml/min on HPLC (Shimadzu, Japan). Analysis of methamidophos was done by using Elite -5 column (HP-5ms, 30 m × 0.32 mm × 0.25 um) and NPD detector on GC Model Clarus -500 (Perkin. USA) with oven temp of 50°C (1 min) Ramp 120°C/min to 100°C Ramp 25°C/min to 150°C (5min) Ramp 310 °C/min to 200°C.
Biochemical analysis
Serum enzymes and biochemical analysis was carried out on Selectra E auto analyzer (Vita lab, Netherlands) following standard procedures of biochemistry by using Pioneer diagnostic kits (USA). Plasma ALT [15], AST [16], ALP [17], CK [18] were determined according to IFCC method whereas LDH [19], was estimated as per DGKC method. Serum total bilirubin was estimated by Jendrassik and Grof method [20], Plasma total protein and albumin were assayed by Biurate [21] and BCG methods [22] respectively. Serum urea was estimated by kinetic urease/glutamate dehydrogenase method [23] and creatinine by Jaffe reaction [24]. Serum phosphorous and total calcium were measured by using phosphomolybdic [25] and cresolphthalein complexone [26] colorimetric methods respectively. Plasma BChE activity was measured as per Ellman's colorimetric method respectively. Plasma BChE activity was measured as per Ellman's colorimetric method [27] by using GD kits (Italy). C.V. of the method was found to be 3–5%.
Statistical analysis
The data was analyzed by using standard SPSS software version-15 (SPSS Inc, Chicago). Descriptive and frequency distribution of BChE biochemical markers and pesticides residues was expressed as mean (SD) and median (range) respectively. Comparison of mean biochemical changes in pesticides exposed farmers and control subjects were analyzed with independent t-test. The Spearman's coefficient correlation was calculated among plasma pesticides residues, BChE and biochemical parameters including serum AST, ALT, CPK, LDH and phosphorous. p-value of <0.05 was ed to be significant.
Results
Age of pesticide exposed farmers and controls were 28 + 9 SD years and 27 + 8 SD years respectively. All the tobacco farmers involved in pesticide handling and spraying were male and earning all their income from small scale crops. Five different classes of pesticides were used in this tobacco growing area. The main complaints of the farmers reported were nausea followed by vomiting, headache, muscle weakness, cough, shortness of breath, skin rash, diarrohea, and eye problems.
Pesticides residues analysis
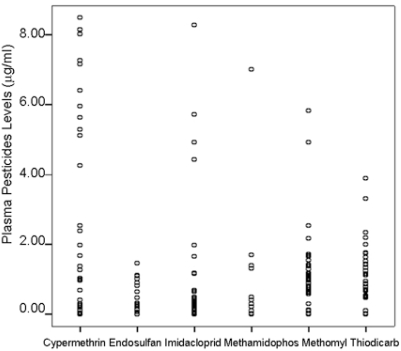
Tobacco farmers had multiple pesticides residues above the acceptable daily intake in their blood. Most of the tobacco farmers had Carbamate (CM) residues consisting of Methomyl and Thiodicarb followed by Cypermethrin, Organophosphate (OP) and Organochlorinated c A residues consisting of Methomyl and Thiodicarb followed by Cypermethrin, Organophosphate (OP) and Organochlorinated compounds (OC) above the ADI (Table 1). Scatter plot of various pesticide residues in the plasma of tobacco farmers is shown in Figure 1.
Table 1.
The number (%) of tobacco farmers having pesticides residues above the ADI (mg/kg) at Sawabi NWFP, Pakistan (n=55)
| Parameters | ADI | Farmers (%) | Pesticides Median | Range |
|---|---|---|---|---|
| Methomyl | 0.01 | 35 (63) | .74 | .01 – 5.83 |
| Thiodicarb | 0.03 | 31 (56) | .51 | .01 – 3.90 |
| Imidacloprid | 0.06 | 25 (45) | .01 | .01 – 5.70 |
| Cypermethrin | 0.05 | 34 (62) | .31 | .01 – 8.49 |
| Methamidophos | 0.0003 | 20 (36) | .30 | .01 – 7.00 |
| Endosulfan | 0.006 | 16 (27) | .39 | .01 – 1.46 |
Figure 1.
Scatter plot of different serum pesticides residues in tobacco farmers (n=55).
Biochemical analysis
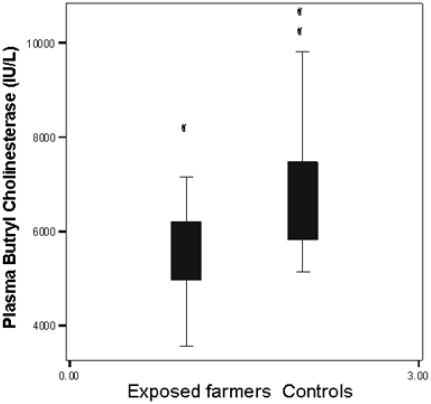
The present study demonstrated a significant decrease in plasma level of BChE 5596 U/L (929) mean (SD) in tobacco farmers as compared to 6821 U/l (1365) in the control (P<0.001). Box plot of plasma BChE levels in pesticides exposed farmers and healthy controls are illustrated in Figure 2.
Figure 2.
The boxplots of plasma Butryl cholinesterase in pesticides exposed tobacco farmers and controls (n=109).
The enzyme activity of AST, ALT, CPK, and LDH was significantly raised (p <0.001) among the pesticide exposed tobacco farmers as compared to control group. Serum urea, creatinine and phosphorous were also significantly raised in farmers as compared to controls (Table 2). The other biochemical markers did not exhibit any significant changes with pesticide residues. The correlation study also revealed that Methomyl, Thiodicarb and Methamidophos had inverse correlation with plasma BChE. The study revealed a positive correlation between residue with AST, ALT, CPK, LDH and phosphorous in the pesticides exposed farmers (Table 3).
Table 2.
Biochemical markers in pesticides exposed tobacco farmers as compared to controls (n=109)
| Parameter | Tobacco farmers (n=55) Mean + SD | Controls (n=54) Mean + SD | p Value |
|---|---|---|---|
| Total Bilirubin | 9 + 2 | 9 + 2 | 0.448 |
| Alanine Aminotransferase | 30 + 9 | 24 + 7 | 0.001 |
| Asparate Aminotransferase | 39 + 14 | 27 + 7 | 0.001 |
| Alkaline phosphate | 218 + 90 | 242 + 88 | 0.230 |
| Albumin | 43 + 3 | 43 + 4 | 0.912 |
| Protein | 75 + 4 | 74 + 3 | 0.506 |
| Creatine Kinase | 155 + 65 | 91 + 36 | 0.000 |
| Lactate Dehydrogenase | 459 + 108 | 285 + 110 | 0.000 |
| Creatinine | 91 + 16 | 83 + 13 | 0.014 |
| Urea | 5.14 + 1.24 | 4.45 + 1.07 | 0.011 |
| Uric Acid | 297 + 62 | 318 + 60 | 0.130 |
| Phosphorus | 1.95 + 0.18 | 1.78 + 0.23 | 0.000 |
| Calcium | 2.36 + 0.19 | 2.31 + 0.17 | 0.200 |
Table 3.
Coefficient Correlations (Spearman's) of various pesticides residues in plasma with change in biochemical markers in pesticides exposed tobacco farmers (n=55)
| Biochemical Parameters | Methomyl (n=35) | Thiodicarb (n=31) | Imidacloprid (n=25) | Cypermethrin (n=34) | Endosulfan (n=16) | Methamidophos (n=20) | Total pesticides |
|---|---|---|---|---|---|---|---|
| Cholinesterase | −0.64** | −0.58** | −0.18 | 0–.15 | 0.04 | −0.60** | −0.46** |
| ALT | 0.49** | 0.31* | 0.39** | 0.33* | 0.33 | 0.15 | 0.66** |
| AST | 0.40** | 0.25 | 0.28* | 0.45** | 0.13 | 0.43** | 0.71** |
| LDH | 0.36** | 0.16 | 0.22 | 0.26 | 0.02 | 0.25 | 0.51** |
| CK | 0.25 | 0.13 | 0.11 | −0.10 | 0.04 | 0.09 | 0.10 |
| Creatinine | 0.14 | 0.10 | 0.13 | 0.36** | 0.09 | 0.43** | 0.49** |
| Urea | −0.32* | −0.28* | −0.02 | 0.17 | −0.20 | 0.04 | 0.01 |
| Phosphate | 0.35** | 0.31* | 0.43** | 0.31* | −0.01 | 0.28* | 0.61** |
| Calcium | 0.15 | −0.06 | −0.03 | −0.12 | −0.09 | 0.01 | 0.06 |
| Albumin | −0.11 | 0.08 | 0.000 | −0.12 | −0.03 | −0.10 | −0.07 |
| Protein | 0.03 | 0.07 | −0.19 | −0.31* | 0.30* | −0.02 | −0.16 |
P <0.05;
p <0.01
Discussion
Tobacco is cultivated over a large area in NWFP, Pakistan and most of the tobacco farm workers are at risk due to careless handling of pesticides on the crop. Extensive use of pesticides not only affects our ecosystem but also affects the health of the farm workers. Farmers had complaints of headache, dizziness, nausea, vomiting and respiratory problems as reported by other researchers after exposure to pesticide [28–29]. However, these are generalized symptoms and mimic other common health problems. Dasgupta and co-workers (2007) reported poor correlation of self reported symptoms with blood cholinesterase among rice farmers in Vietnam [5].
In our study most of the tobacco farmers had multiple pesticide residues above the allowable daily intake (ADI) in their blood which is injurious to health. Most of the farmers had Methomyl and Thiodicarb residues above the recommended ADI levels because of their extensive use in tobacco fields without observing proper safety practices during spraying and mixing of these pesticides. Cypermethrin was the second highest residue found in our farmers where as in another study it was reported as the fourth most common cause of pesticide related illnesses [30]. Cypermethrin is a synthetic pyrethroid, toxic for the nervous system and is also carcinogenic. A study reported 25% of workers in Chinese cotton fields exhibited symptoms of cypermethrin poisoning [31]. Imidacloprid was detected in many of our farmers. Feng and co-workers (2005) reported that when concentration of imidacloprid reaches 0.1 mg/l, it significantly affects the frequency of micronucleus and sister chromatid exchange [32]. Methamidophos, one of the Organophosphate compounds was also reported in few farm workers in our study.
Our Study revealed that only a few farmers had endosulfan residues above the ADI and the maximum concentration observed was 1.46 mg/Kg. Similar levels were also reported by ther studies [33–34]. Endosulfan was detected at the highest concentration of about 90mg/kg in cotton farmers of Multan, Pakistan [35].
Plasma BChE activity has been used for several years to estimate the risk associated with pesticide induced toxicity in occupationally exposed workers. In this study we found that plasma BChE activity was significantly low in exposed farmers than the controls, indicating that our tobacco farmers had significant OP and CM residues in their body. Similar results were noted among the cotton growers in India [36] and the tobacco farmers of many other countries [1, 6]. Mc Connell and Magrotti (1994) reported significantly lower levels of cholinesterase among farmers who were involved in spraying of pesticides as compared to non-farm workers [37]. It has been reported that the plasma AChE activity is a better indicator of pesticide toxicity than erythrocyte AChE for the assessment of acute exposures to OPs, since a cumulative inhibition is observed due to the lower recovery rate of erythrocyte AChE [38]. For monitoring the exposure pesticides various analytical parameters are used. Inhibition of BChE in plasma can be used as a diagnostic tool OPs and CM [39] as the analysis of pesticides residues is expensive and its facility is not available everywhere in our country.
Liver dysfunction with raised AST, ALT and LDH was observed in most of the tobacco farmers. A similar increase in the AST and ALT levels was reported in agricultural workers in Israel [9], India [40] and selected farm workers in Gadap Karachi, Pakistan [8]. Tomei et al (1998) also reported considerable liver damage with significant increase in AST, ALP and total bilirubin among environmental disinfectant workers in Italy [41]. High degree of abnormal liver function in workers may indicate toxic effects of pesticides and the presence of pesticide residues in blood [42]. Pesticides residues and their metabolites in various human tissues and fluid are indicative of past and present exposure. Altered liver enzyme activities have been reported among occupational workers exposed to Organophosphorus pesticides alone or in combination with Organochlorine [43]. A study reported early biochemical changes in serum enzymes after exposure to mixture of pesticides in agricultural farmers [9]. Altuntus et al., (2002) reported increased activity of ALT and AST in workers engaged in agricultural and public health programs due to the effects of Methidathion, one of the most widely used Organophosphorus compound in this program [44]. Goel et al., (2000) also demonstrated a significant increase in the level of various serum and liver markers such as ALP, ALT and AST due to the effects of chloriphyrifos [45]. Greater the degree of pesticide exposure greater would be the levels of liver enzymes as reported in two cases of acute endosulfan toxicity [46]. Synthetic pyrethroid cypermethrin also showed a positive correlation with ALT, AST and LDH due to the sub cellular effects of pesticides [47]. However, further study is required in this regard. Pesticide exposure causes leakage of cytosolic enzymes from hepatocytes and other body organs [48]. It may also be due to increased gene expression due to long term requirement of detoxification of pesticides. CK in our exposed farmers was also raised. Friedman et al., (2003) reported similar elevations in CK in patients after acute exposure to pesticides [49].
Our study revealed significantly elevated levels of serum urea & creatinine among exposed farmers. Previous studies have reported subtle nephrotoxic changes in workers occupationally exposed to pesticides with higher levels of serum creatinine and urea [50]. Exposure to pesticide in a chemical plant also showed a significantly higher serum creatinine concentration which supports the subclinica kidney impairment [51]. Attia (2006) reported serum creatinine and urea in upper reference range in the pesticide applicators which is similar to our present research finding [52].
The present study also revealed that pesticide exposure increase serum phosphorous levels in pesticide exposed tobacco farmers. This finding is consistent with other researches [53]. Hyperphosphatemia has also been reported in fish and other animals [54–55] after exposure to various toxicants, like endosulfan and aldrin.
The study demonstrated that prolonged exposure to pesticides leads to pesticide residues in exposed farmers which inturn leads to renal and hepatic dysfunction because of their cytotoxic effects. Biomarkers are used to detect the hazardous health effects of pesticides before adverse clinical health effects occur. The study therefore highlights the need to properly evaluate and control the potential health effects due to exposure to toxic substances among farmers employed in pesticide handling and spray on the crop.
Conclusion
The study demonstrated that the pesticide exposed tobacco farmers had multiple pesticide residues above the allowable daily intake at district Sawabi, Pakistan. Excessive exposure to pesticide caused cytotoxic changes in the hepatic and renal biochemical markers which were positively correlated with pesticide residues. Hence these biomarkers might be used for monitoring of adverse effects of pesticides on the health of farm workers.
Acknowledgments
This study was a part of the Project No. 20-736/R & D/06/2119 entitled “The Effects of Environment Pesticides Residues on the Health of Farmers in Tobacco growing areas of NWFP, Pakistan” supported by Higher Education Commission (HEC) Pakistan.
References
- 1.Pakistan Tobacco Board. Ministry of commerce Govt of Pakistan Peshawar Tobacco Statistical Bulletin. 2004. p. 29. [Google Scholar]
- 2.McDaniel PA, Solomon G, Malone RE. The tobacco industry and pesticide regulations: case studies from tobacco industry archives. Environ Health Perspect. 2005;113(12):1659–65. doi: 10.1289/ehp.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damalas CA, Georgiou EB, Theodorou MG. Pesticide use and safety practices among Greek tobacco farmers: a survey. Int J Environ Health Res. 2006;16(5):339–48. doi: 10.1080/09603120600869190. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization; 2001. WHO recommended classification of pesticides by hazard and guidelines to classification. 2000–01 Geneva. (document reference WHO/PCS/01.4) [Google Scholar]
- 5.Dasgupta S, Meisner C, Wheeler D, Xuyen K, Thi Lam N. Pesticide poisoning of farm workers-implications of blood test results from Vietnam. Int J Hyg Environ Health. 2007;210(2):121–32. doi: 10.1016/j.ijheh.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson E, Levi PE. Pesticides: an important but underused model for the environmental health sciences. Environ Health Persp. 1996;104(1):97–106. doi: 10.1289/ehp.96104s197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabello G, Valenzuela M, Vilaxa A, Durán V, Rudolph I, Hrepic N, Calaf G. A ratmammary tumor model induced by the organophosphorus pesticides parathion and malathion, possibly through acetylcholinesterase inhibition. Environ Health Perspect. 2001;109(5):471–479. doi: 10.1289/ehp.01109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azmi MA, Naqvi SN, Azmi MA, Aslam M. Effect of pesticide residues on health and different enzyme levels in the blood of farm workers from Gadap (rural area) Karachi-Pakistan. Chemo. 2006;64(10):1739–44. doi: 10.1016/j.chemosphere.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez FA, Gomez MA, Perez VG, Lario VJ, Pena G, Gill F, Lopez O, Rodrigo L, Pino G, Pla A. Influence of exposure to pesticides on serum components and enzyme activities of cytotoxicity among intensive agricultural farmers. Environmental Research. 2006;102:70–76. doi: 10.1016/j.envres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 10.WHO/IPCS. Biomarkers and risk assessment: Concepts and Principles. Geneva: World Health Organization; 1993. Environmental health criteria 155. [Google Scholar]
- 11.Australian Government, Department of Health and Ageing, Office of Chemical Safety. Acceptable daily intake for agricultural and veterinary chemicals. Web site. http//www.ag.gov.au/cca. Accessed Current to 31 December 2007
- 12.Coye MJ, Lowe JA, Maddy KJ. Biological monitoring of agricultural workers exposed to pesticides. 11: monitoring of intact pesticides and their metabolites. J Occup Med. 1986;28:628–636. doi: 10.1097/00043764-198608000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal HC, Pillai M.K., Yadav D.V., Menon K.B., Gupta R.K. Residues of DDT and its metabolites in human blood samples in Dehli, India. Bull. WHO. 1976;54:349–351. [PMC free article] [PubMed] [Google Scholar]
- 14.USEPA. Method 3620B: Florisil Cleanup. Washington, DC: US Environmental Protection Agency; 1996. [Google Scholar]
- 15.Bergmeyer HU, Herder M, Rej R. Approved recommendation 1985 on IFCC methods for the measurement of catalytic concentration of enzymes Part 3. (IFCC Method for Alanine aminotransferase. J Clin Chem Clin Biochem. 1998;24:481–489. [PubMed] [Google Scholar]
- 16.Bergmeyer H.U, Herder M, Rej R. Approved recommendation 1985 on IFCC methods for the measurement of catalytic concentration of enzymes Part 2. IFCC Method for asparate aminotransferase. J Clin Chem Clin Biochem. 1998:24–49. [Google Scholar]
- 17.Expert Panel on enzymes: I, IFCC methods for the measurement of catalytic concentration of enzymes V: IFCC method for alkaline phosphatase. Clin Chin Acta. 1983;135:339–367. doi: 10.1016/0009-8981(83)90294-2. [DOI] [PubMed] [Google Scholar]
- 18.Hurder M, Elser RC, Gerhardt W, Mathiev M, Sampson EJ. Approved IFCC recommendation on methods for the measurement of catalytic concentration of enzymes: Part 7. IFCC method for creatine kinase (ATP: Creatine N-Phosphotransferase, EC 2.7 3.2) Eur J Clin Chem Clin Biochem. 1991;29:435–456. [PubMed] [Google Scholar]
- 19.Bais R, Philox Approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes. Part 8 IFCC method for lactate dehydrogenase (1-lactate; NAD + Oxidoreductase, EC 1.1.1.27). International Federation of Clinical Chemistry (IFCC) Eur J Clin Chem Biochem. 1994;32:639–655. [PubMed] [Google Scholar]
- 20.Perry BW, Doumas BT, Bayse DD. A candidate reference method for determination of bilirubin in serum: Test for transferability. Clin Chem. 1983;29:297–301. [PubMed] [Google Scholar]
- 21.Kingsley GR. The direct biuret method for the determination of serum proteins as applied to photoelectric and visual colorimetry. J Lab Clin Med. 1942;27:840–845. [Google Scholar]
- 22.Doumas BT, Watson WA, Bigg HG. Albumin standards and the measurement of serum albumin with Bromocresol green. Clin Chim Acta. 1997;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 23.Sampson EJ, Baird MA. Chemical inhibition used in a kinetic urease/glutamate dehydrogenase method for urea in serum. Clin Chem. 1979;25:1729–1979. [PubMed] [Google Scholar]
- 24.Spencer K. Analytical reviews in clinical biochemistry: The estimation of creatinine. Ann Clin Biochem. 1986;23:1–25. doi: 10.1177/000456328602300101. [DOI] [PubMed] [Google Scholar]
- 25.Garber CC, Miller RC. Revision of the 1963 semidine HCL standard method for inorganic phosphorous. Clin Chem. 1983;29:184–188. [PubMed] [Google Scholar]
- 26.Lorentz K. Improved determination of serum calcium with 2-cresolphthalein complexone. Clin Chem Acta. 1982;126:327–334. doi: 10.1016/0009-8981(82)90308-4. [DOI] [PubMed] [Google Scholar]
- 27.Ellmann GL, Courney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Parmacol. 1961;7:88–75. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 28.Wesseling C, McConnell R, Partanen T, Hogstedt C. Agricultural pesticide use in developing countries: health effects and research needs. Int J Health Serv. 1997;27(2):273–308. doi: 10.2190/E259-N3AH-TA1Y-H591. [DOI] [PubMed] [Google Scholar]
- 29.Kishi M, Hirschhorn N, Ojajadisastra M, Satterfee LN, Stroman S, Dilts R. Relationship of pesticide spraying to signs and symptom in Indonesian farmers. Scand. J Environ Health. 1995;21:124–133. doi: 10.5271/sjweh.19. [DOI] [PubMed] [Google Scholar]
- 30.Caroline Cox. Cypermethrin: Insecticide factsheet. J Pest Ref. 1996;16(2):15–20. [Google Scholar]
- 31.Chen S Y, Zhang Z W, He F S, Yao P P, Wu Y Q, Sun J X, Liu L H, Li Q G. An epidemiological study on occupational acute pyrethroid poisoning in cotton farmers. Brit J Occup Med. 1991;48:77–81. doi: 10.1136/oem.48.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng S, Kong Z, Wang X, Peng P, Zeng EY. Assessing the genotoxicity of imidacloprid and RH-5849 in human peripheral blood lymphocytes in vitro with comet assay and cytogenetic tests. Ecotoxicol and Environ Safety. 2005;61:239–246. doi: 10.1016/j.ecoenv.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Waliszewski SM, Aguirre AA, Benitez A, Infanzon RM, Infanzon R, Rivera J. Organochlorine Pesticide Residues in Human Blood Serum of Inhabitants of Veracruz, Mexico. Earth Environ Sci. 2004;62(4):397–402. doi: 10.1007/s001289900888. [DOI] [PubMed] [Google Scholar]
- 34.Saiyed H, Dewan A, Bhatnagar V, Shenoy U, Shenoy R, Rajmohan H, Patel K, Kashyap R, Kulkarni P, Rajan B, Lakkad B. Effect of endosulfan on male reproductive development. Environ Health Perspect. 2003;111(16):1958–62. doi: 10.1289/ehp.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansari MT, Iqbal Z, Ahmed B. Organochlorine pesticide residues in human blood in the population of Multan (Pakistan) Pak J Pharm Sci. 1997;10(1):19–28. [PubMed] [Google Scholar]
- 36.Mancini F, Van Bruggen AH, Jiggins JL, Ambatipudi AC, Murphy H. Acute pesticide poisoning among female and male cotton growers in India. Int J Occup Environ Health. 2005;11(3):221–232. doi: 10.1179/107735205800246064. [DOI] [PubMed] [Google Scholar]
- 37.Mc Connell R, Magnotti R. Screening for insecticides over under field condition : a reevalution of iontometric cholinesterase kit. Am J Pub Health. 1994;84:479–481. doi: 10.2105/ajph.84.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jors E, Morant RE, Aguilar GC, Huici, Lander F, Baelum J, Konradsen F. occupational pesticides intoxications among farmers in Bolivia a cross-sectional study. Environ Health A Global Sciences Source. 2006;5:10. doi: 10.1186/1476-069X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anitai G, Moorad D, Adani R, Doctor BP. Inhibition of acetylcholinesterase and butyl cholinesterase by chlor pyrifos – Oyon. Biochem Pharmacol. 1998;56:293–9. doi: 10.1016/s0006-2952(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 40.Patil J A, Patil A J, Gowindwar S P. Biochemical effects of various pesticides on sprayers of grape gardens. Ind J Clin Biochem. 2003;18(12):16–22. doi: 10.1007/BF02867362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomei F, Biagi M, Baccolo T P, Tomao E, Guintoli P, Rosati MV. Liver damage among environmental disinfestations workers. J Occup Health. 1998;40:193–197. [Google Scholar]
- 42.M.M Amr pesticides monitoring and its health problems in Egypt, a third world country. Toxicol Lett. 1999;107:1–13. doi: 10.1016/s0378-4274(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 43.Anwar WA. Biomarkers of human exposure to pesticides. Environ Health Prest. 1997;105(4):801–806. doi: 10.1289/ehp.97105s4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altuntas I, Delibas N, Demirci M, Kilinc I, Tamer N. The effects of methidathion on lipid peroxidation and some liver enzymes: role of vitamins E and C. Arch Toxicol. 2002;76(8):470–473. doi: 10.1007/s00204-002-0359-1. [DOI] [PubMed] [Google Scholar]
- 45.Goel A, Chanuhan DP, Dhawan DK. Protective effects of zinc in chlorpyrifos induced hepatoxicity; a biochemical and trace element study. Bio Trace Elemen Res. 2000;74:171–183. doi: 10.1385/BTER:74:2:171. [DOI] [PubMed] [Google Scholar]
- 46.Yavuz Y, Yurumez Y, Kücüker H, Ela Y, Yüksel S. Two cases of acute endosulfan toxicity. Clin Toxicol (Phila) 2007;45(5):530–2. doi: 10.1080/15563650701365909. [DOI] [PubMed] [Google Scholar]
- 47.Jee JH, Masroor F, Kang JC. Responses of cypermethrin-induced stress in haematological parameters of Korean rockfish, Sebastes schlegeli (Hilgendorf) Agriculture Research. 2005;36:898–905. [Google Scholar]
- 48.Dewan A, Bhatnager V K, Mathur M, Chakma T, Kashyap R, Sadhu HG, Sinha SK, Saiyed HN. Repeated episodes of endosulphan poisoning. Clini toxi. 2004;42(4):363–369. doi: 10.1081/clt-120039542. [DOI] [PubMed] [Google Scholar]
- 49.Friedman LS, Brautbar N, Barach P, Wolfe A, Richter ED. Creatine phosphate kinase elevations signaling muscle damage following exposures to anticholinesterases: 2 sentinel patients. Arch Environ Health. 2003;58(3):167–71. doi: 10.3200/AEOH.58.3.167-171. [DOI] [PubMed] [Google Scholar]
- 50.Yousaf M, El-Demerdash F, Kamel K, Al-Salhen K. Changes in some haematological and biochemical indices of rabbits induced by isoflavones and cypermethrin. Toxicol. 2003;189:223–234. doi: 10.1016/s0300-483x(03)00145-8. [DOI] [PubMed] [Google Scholar]
- 51.Kossmann S, Tustanowski J, Kolodziej B. Renal dysfunction in chemical plant workers producing dust pesticides. Med Pr. 2001;52(4):253–6. [PubMed] [Google Scholar]
- 52.Attia MA. Risk assessment of occupational exposure to pesticides. Earth Environ Sci. 2006;3:349–362. [Google Scholar]
- 53.Parron T, Hernandez AF, Pla A, Villanueva E. Clinical and biochemical changes in greenhouse sprayers chronically exposed to pesticides. Hum Exp Toxicol. 1996;15(12):957–63. doi: 10.1177/096032719601501203. [DOI] [PubMed] [Google Scholar]
- 54.Gill TS, Pande J, Tewari H. Effects of endosulfan on the blood and organ chemistry of freshwater fish, Barbus conchonius Hamilton. Ecotoxicol Environ Safety. 1991;21(1):80–91. doi: 10.1016/0147-6513(91)90010-m. [DOI] [PubMed] [Google Scholar]
- 55.Singh NN, Das VK, Singh S. Effect of aldrin on carbohydrate, protein, and ionic metabolism of a freshwater catfish, Heteropneustes fossils. Bull Environ Contam Toxicol. 1996;57(2):204–10. doi: 10.1007/s001289900176. [DOI] [PubMed] [Google Scholar]