Abstract
Pulmonary function has circadian modulations. Variations in human pulmonary function during the daytime hours (diurnal variations) remain to be well characterized. Discerning these variations will contribute to better understanding the relationship between biorhythms and lung physiology and to improving clinical management of pulmonary diseases. The aim of this study was to determine the magnitude of pulmonary function variability during the usual daytime hours in a population of patients referred for pulmonary function testing. Diurnal fluctuations of human pulmonary function were examined by studying retrospectively a study population of 4,756 individuals with performed pulmonary function tests. We found the lowest and highest spirometric values in the 12:00–12:59 pm and 3:00–4:59 pm time intervals respectively. The difference in the forced expiratory volume in 1 second (FEV1) between the noon (12:00–12:59 pm) and afternoon (4:00–4:59 pm) intervals was 17.6% (P<0.01). Furthermore, the highest values of diffusing capacity of the lung for carbon monoxide [DLCO] and alveolar volume [Va] were identified in the 8:00–8:59 am time interval. These findings, identifying a model of diurnal variations of pulmonary function in individuals referred for pulmonary function testing, are of interest for better understanding lung physiology and human circadian rhythms and may have clinical value in managing lung disorders.
Keywords: Pulmonary function, diurnal variations, pulmonary function tests, diffusing capacity, pulmonary diseases
Introduction
Circadian rhythms – the cyclical 24h patterns of physiological functions, including the pulmonary function, have become an area of increasing interest in basic and clinically oriented research. The persistence of circadian rhythms is orchestrated by the “circadian clock”, located in the suprachiasmatic nucleus of the hypothalamus [1, 2] and regulated by the hypothalamic-pituitary axis, the autonomic nervous system and clock proteins, which form regulatory feedback loops [3, 4]. The peak and trough of these cycles are attributed to different biological requirements of daytime activities and nighttime sleep and the lack of synchronization may lead to mood disorders, gastrointestinal disturbances, headache, fatigue and even development of malignancy [5].
While the existence of a circadian rhythm in lung function is well recognized [6], the current knowledge about circadian changes in human pulmonary function is somewhat controversial and predominantly related to asthmatics [7, 8]. Previous studies, utilizing pulmonary function tests (PFTs) as a tool to evaluate respiratory status, have indicated early morning lows in the forced expiratory volume in one second (FEV1) and pulmonary flow rates of asthma patients [7, 8]. The early-morning deterioration observed in asthmatics has been associated with decreased serum epinephrine levels, increased vagus nerve tone and cholinergic activity, esophageal reflux, and increase of circulating eosinophils in the corresponding time of the day [8]. Little information is available regarding the pattern of pulmonary function fluctuations during the daytime hours (diurnal rhythm). It has been shown that healthy individuals, in contrast to asthmatics, exhibit the lowest maximal voluntary ventilation at noon compared with afternoon and morning, and display the highest flow rates in the afternoon hours [9]. Other studies have suggested that the airway resistance peaks in the morning, reaches its lowest point at noon and increases in the afternoon [10]. This pattern has been observed in healthy individuals as well as those with restrictive and stable obstructive disease [10]. Additionally, little is known about the diurnal variations in the carbon monoxide diffusing capacity of the lung (DLCO). While some studies have failed to show any circadian rhythm, others have suggested that DLCO peaks in the morning and decreases as the day advances [11–14].
Discerning the diurnal fluctuations in pulmonary function is important in order to better understand the impact of biorhythms on pulmonary physiology and to optimize the clinical management of pulmonary diseases. In this study, we show a pattern of diurnal pulmonary function variations in a population of individuals referred for pulmonary function testing.
Methods
Settings and patients
This study was approved by the Institutional Review Board of the North Shore-Long Island Jewish Health System. Pulmonary function studies of patients 16 years and older performed in the PFT Laboratory of Long Island Jewish Medical Center in New Hyde Park, NY between 3/1997 and 5/2002 were retrospectively reviewed. Pulmonary testing was completed according to protocol, and objective and standardized criteria were applied to ensure accuracy and reproducibility. All spirometric, plethysmographic and diffusion capacity measurements were obtained on Vmax22 PFT system with V6200 Autobox (SensorMedics Corp., Yorba Linda, CA). Calibration procedures were performed before each testing session according to the manufacturer's recommendations, meeting American Thoracic Society (ATS) standards [15]. Functional residual capacity (FRC) was measured using plethysmographic constant volume technique. The mass flow sensor was calibrated manually with constant and variable flow using a calibration syringe. Pressure calibration was performed with a sine-wave pump daily and anytime a leak or malfunction was suspected. At least monthly, biological calibration was performed on standard subjects. Results from the following PFTs were analyzed: FEV1, forced vital capacity (FVC), peak expiratory flow (PEF, peak flow during FVC maneuver), total lung capacity (TLC) and DLCO. DLCO was obtained by single-breath technique. Each spirometry consisted of three independent flow-volume loops and the highest FEV1 and FVC were selected following standard recommendations [15]. All volumes were standardized to BTPS (body temperature and pressure, saturated).
The data were grouped according to the time of the day the test was conducted and nine data sets were created: 8:00–8:59 am; 9:00–9:59 am; 10:00–10:59 am; 11:00–11:59 am; 12:00–12:59 pm;1:00–1:59 pm; 2:00–2:59 pm; 3:00–3:59 pm; and 4:00–4:59 pm. The results were grouped based on real time recordings by the computer. Each numerical pulmonary function value had a time stamp reflecting the time the actual maneuver was performed up to the second. In cases, where the same patient had more than one PFT performed at the same time on a different day (< 1% of all studies) only the first study was included in the statistical analysis. This rule was adopted to ensure the independence of the values within each dataset.
Statistical analysis
The mean values, standard deviations (SD) and standard errors of the mean (SEM) were calculated for each of the nine groups. One-way analysis of variance (ANOVA) was used to identify any statistical differences between the various PFT means. If the null-hypothesis was rejected, Fisher least significant difference multiple comparisons were performed. P values less than 0.05 were considered statistically significant unless otherwise specified. To eliminate the potential adverse impact of uneven age and height distribution among the nine groups, FEV1 and DLCO were also calculated as percent of predicted values by means of the commonly used Crapo equations [16, 17]. The statistical analysis was performed on SigmaStat 3.1 software package (SysStat Software Inc., Point Richmond, CA).
Results
Demographics of the study population
Total 4756 PFTs were reviewed. The demographics of the study population are presented in Table 1. The male/female ratio ranged from 0.41 to 0.52. The mean age was in the range 56.3–61.5 years, and the mean height and weight values were 166.4–168.8 cm and 76.6–80.3 kg respectively.
Table 1.
Demographic characteristics of the studied population*
| Time Groups | N** | Male/Female | Age (years) | Height (cm) | Weight (kg) |
| 8:00–8:59 am | 420 | 0.52 | 57.3 ± 16.4 | 168.8 ± 10.3 | 80.3 ± 20.9 |
| 9:00–9:59 am | 696 | 0.45 | 59.4 ± 16.0 | 166.5 ± 10.2 | 78.3 ± 19.7 |
| 10:00–10:59 am | 653 | 0.45 | 59.9 ± 16.5 | 166.8 ± 10.2 | 79.0 ± 21.0 |
| 11:00–11:59 am | 512 | 0.49 | 59.5 ± 17.6 | 167.8 ± 10.4 | 78.1 ± 20.2 |
| 12:00–12:59 pm | 410 | 0.49 | 61.5 ± 15.9 | 167.2 ± 10.5 | 76.6 ± 19.7 |
| 1:00–1:59 pm | 800 | 0.47 | 60.2 ± 17.2 | 167.1 ± 10.5 | 77.1 ± 20.1 |
| 2:00–2:59 pm | 728 | 0.44 | 59.5 ± 17.1 | 166.8 ± 10.7 | 78.9 ± 20.5 |
| 3:00–3:59 pm | 431 | 0.41 | 56.3 ± 18.2 | 166.4 ± 9.9 | 78.8 ± 21.5 |
| 4:00–4:59 pm | 106 | 0.48 | 56.9 ± 15.9 | 167.5 ± 9.4 | 78.7 ± 19.2 |
Data are expressed as mean ± SD
N-number of studies
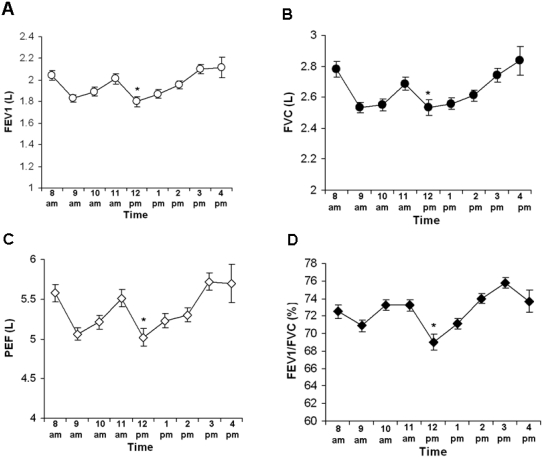
Diurnal fluctuations in FEV1, FVC, PEF and FEV1/FVC ratio
To study diurnal fluctuations of pulmonary function, as determined by spirometry, we analyzed results from 4,435 spirometry tests. Statistical analysis of FEV1, FVC and FEV1/FVC ratio and PEF demonstrated statistically significant diurnal changes for all four variables (Table 2). The distribution of the mean spirometric values followed an approximate W-shaped curve (Figure 1 A, B, C, D). The lowest mean FEV1, FVC, PEF and FEV1/FVC values were observed in the 12:00–12:59 pm interval (Figure 1 A, B, C, D). The highest mean values were identified in the 3:00–3:59 pm and 4:00–4:59 pm groups. Two additional smaller peaks (in 8:00–8:59 am and 11:00–11:59 am) and a trough (in 9:00–9:59 am) were observed. The difference between the lowest (1.80 L, in 12:00–12:59 pm) and the highest (2.12 L, 3:00–3:59 pm) FEV1 values was 17.6% (P<0.01) (Figure 1 A). Similarly, FVC and PEF peaked between 3:00–4:00 pm and had their nadir in 12:00–12:59 pm (P<0.05) (Figure 1 B, C). The variation between the highest and the lowest PEF was 0.7 L/s or 14% (P<0.001). FEV1/FVC ratio followed the same pattern. The highest mean value of 75.8% was identified in 3:00–3:59 pm, and the lowest (69%) in 12:00–12:59 pm with an absolute difference of 6.8% (P<0.001) (Figure 1 D). Considering the higher average age of the subjects in 12:00–12:59 pm, the possible confounding impact of age on the results needed to be addressed. The difference between the highest and the lowest average age across all groups is about 5 years. According to the most commonly used reference equations [16], this age variation may account for about 100–125 mL in FEV1 loss and cannot explain the observed difference, which exceeds 300 mL. Furthermore, based on the same reference [16], the impact of the uneven age distribution on FEV1/FVC ratio would be minimal as opposed to the statistically and clinically significant difference of 6.8 percentage points in our dataset. In order to further eliminate age and height as confounding factors, we performed ANOVA and pair-wise multiple comparisons on FEV1 as a percent-of-predicted-value, which confirmed the already established pattern (Figure 2).
Table 2.
Mean spirometric values by group*
| Group | N** | FEV1 (L) | FVC (L) | PEF (L) | FEV1/FVC (%) |
|---|---|---|---|---|---|
| 8 | 397 | 2.044 ±0.0451 | 2.781 ±0.0501 | 5.58 ±0.111 | 72.5 ±0.721,2 |
| 9 | 655 | 1.829 ±0.0342 | 2.534 ±0.0392 | 5.06 ±0.082 | 70.9 ±0.641,2 |
| 10 | 617 | 1.893 ±0.0372 | 2.551 ±0.0422 | 5.21 ±0.092 | 73.2 ±0.611,2 |
| 11 | 470 | 2.010 ±0.0441 | 2.688 ±0.0491 | 5.51 ±0.111 | 73.2 ±0.701,2 |
| 12 | 385 | 1.800 ±0.0492 | 2.534 ±0.0542 | 5.02 ±0.112 | 69.0 ±0.882 |
| 13 | 748 | 1.871 ±0.0372 | 2.558 ±0.0412 | 5.23 ±0.092 | 71.1 ±0.591,2 |
| 14 | 672 | 1.956 ±0.0361 | 2.611 ±0.041 | 5.30 ±0.091,2 | 74.0 ±0.561 |
| 15 | 399 | 2.101 ±0.0461 | 2.743 ±0.0531 | 5.72 ±0.111 | 75.8 ±0.601 |
| 16 | 92 | 2.117 ±0.0931 | 2.835 ±0.1091 | 5.70 ±0.241 | 73.7 ±1.271 |
Data are expressed as mean ± SE
N- number of studies
statistically different from the lowest value in the column (P<0.05)
statistically different from the highest value in the column (P<0.05)
Figure 1.
Diurnal changes in PFTs. Results from spirometry tests conducted between 8:00 am and 5:00 pm were analyzed. Tests in the following time intervals were analyzed: 8:00–8:59 am (8 am); 9:00–9:59 am (9 am); 10:00–10:59 (10 am); 11:00–11:59 am (11 am); 12:00–12:59 pm (12 pm); 1:00–1:59 pm (1pm); 2:00–2:59 pm (2 pm); 3:00–3:59 pm (3 pm); and 4:00–4:59 pm (4 pm). The asterisk notes the difference between the lowest and the highest value. For complete statistical analysis please refer to Table 2. (A) Variations in FEV1 (*P<0.01 as compared with 4 pm). (B) Variations in FVC (*P<0.05 as compared with 4 pm). (C) Variations in the peak expiratory flow (PEF) (*P<0.05 as compared with 3 pm). (D) Variations in the FEV1/FVC ratio (*P<0.001 as compared with 3 pm). Data are presented as mean ± SEM.
Figure 2.
Variation of FEV1 as a percent of predicted values for the same time intervals as described in the legend to Fig. 1. Data are presented as mean ± SEM (*P<0.001 as compared with 3 pm).
Diurnal changes in DLCO and TLC
We also analyzed 2895 DLCO tests performed during the study period. We identified the highest mean DLCO value of 18.9 mL/min/mmHg in 8:00–8:59 am (Figure 3 A). The difference between this value and the remaining 8 groups (average 16.8 mL/min/mmHg) was 12.6 % (P<0.001). The differences within those 8 groups were negligible. Substituting percentage of the predicted value for the absolute DLCO values did not change the pattern (Figure 3 B).
Figure 3.
Diurnal variations in the peak diffusing capacity of the lung for carbon monoxide (DLCO). (A) Variations in DLCO from 8:00 am to 5:00 pm. Tests in the same time intervals as described in the legend to Fig. 1 were reviewed. Statistical analysis was performed on the following numbers of tests per each time group: 287 (8:00–8:59 am); 460 (9:00–9:59 am); 409 (10:00–10:59 am); 316 (11:00–11:59 am); 212 (12:00–12:59 pm); 452 (1:00–1:59 pm); 442 (2:00–2:59 pm); 255 (3:00–3:59 pm); 62 (4:00–4:59 pm) (*P<0.001 as compared with the average value of the remaining groups) (B) Variation of DLCO as a percent of predicted values. Data are presented as mean ± SEM (*P<0.001).
One-way ANOVA performed on the 3,035 plethysmography tests failed to detect any statistically significant difference in the mean TLC values of the nine groups (data not shown, P=0.33).
Discussion
After decades of routine use of PFTs as a primary tool for pulmonary assessment, there is still some controversy related to the circadian variation of human pulmonary function. In this study, we identify patterns of diurnal changes in pulmonary function based on statistical analysis of PFTs performed over a 5-year period. Our data demonstrate an approximate W-shape distribution of the most common spirometric variables, with the lowest values around noon and a peak in the afternoon hours. Our findings suggest a model of circadian variations in human pulmonary function, which confirms some previous findings [9] but also brings some new results. The pattern of relatively high morning flows is somewhat unexpected and is likely more characteristic for non-asthmatic lung patients. The nocturnal/early morning fall in FEV1 is well documented in patients with asthma and especially labile asthma [7, 8] but may not be evident in non-asthmatics, although certain studies showed otherwise [10]. Experimental data and clinical experience also suggest that the morning flow-limitation occurs in earlier morning hours (3:00–6:00 am) and at night [8]. Our collected data were limited to the range 8:00 am-5:00 pm, so we could not confirm this hypothesis.
The present study shows a DLCO peak between 8:00 am and 9:00 am and relatively steady values after 9:00 am. Our data are consistent with previous findings demonstrating a similar morning peak with progressive decrease in the subsequent hours [12, 13]. Some authors attribute this to a progressive drop in hemoglobin and hematocrit as the day progresses [14]. However, it seems highly unlikely that the described variability of more than 10% in our study is due to hematocrit decrease alone. Higher alveolar volumes (Va) in the morning hours have also been implicated in the etiology of the circadian variation of DLCO [18]. Our results showing 7.8% lower Va in the period of time 9 am-5pm as compared with 8am-9 am (P<0.001, data not shown) support this theory. The mechanism of the change in Va was suggested to be related to changes in the lung recoil pressure [19]. DLCO has been found to increase in response to norepinephrine, which directly enhances the diffusing capacity of the alveolar membrane [20]. Therefore, increased catecholamine levels in the morning may contribute to higher DLCO.
In this retrospective study, we review data from individuals who required PFTs for any reason. The referrals often contained symptoms rather than a diagnosis and were generally considered too unreliable. The lack of relevant clinical information did not allow us to further subclassify these individuals. 34% of subjects had FEV1/FVC ratio less than 70% suggesting obstruction (data not shown). The studied database allowed controlling for age, sex, height and weight, but not for other confounding factors. The possibility of unequal prevalence of obstructive lung disorders in each of the nine patient groups presents a difficult to address problem. The gold standard of low FEV1/FVC ratio cannot be applied while examining diurnal FEV1 changes as there is no way to estimate how much FEV1 reduction is caused by circadian rhythms and how much by disease. If we assume that COPD related FEV1 reduction will be associated in most cases with some DLCO decrease and the difference of 17.6% in FEV1 between the noon and late afternoon measurements were to be explained solely by higher and, respectively, lower prevalence of COPD in those groups, one would expect to see similar changes in DLCO. As discussed above, there was no significant DLCO variation in the course of the day with the exception of the morning peak.
The physiological and molecular mechanisms underlying the relationship between diurnal variations in pulmonary function shown here and circadian rhythms remain to be elucidated. Circadian changes occur in close temporal relation with body temperature, oxygen consumption and carbon dioxide production. However, none of those variables could fully explain the diurnal pattern of ventilation [21]. Breathing is closely regulated by metabolic rate, which is influenced by multiple factors including activity and state of arousal as well as hormonal and autonomic nervous system inputs [22, 23]. Circadian oscillations in the sympathetic activity may have an impact on the airway smooth muscles [24]. Airway responsiveness to pharmacological provocation tests has also been shown to follow a daily variation pattern [25].
Our findings are of interest for better understanding the effect of biorhythms on lung physiology and pathogenesis of pulmonary diseases. Even small changes in lung dynamics in individuals with pulmonary diseases may be significant. Timing of treatment with regards to circadian changes could optimize the therapeutic effect of inhaled medications with a half-life of a couple of hours. Also, one could make an argument that scheduling elective surgical procedures or extubating patients with very limited lung function can be performed in accordance with the diurnal pulmonary clock.
Acknowledgments
The authors thank Kevin J. Tracey and Margot Gallowitsch-Puerta for critically reading this manuscript.
References
- 1.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. New York: Oxford University Press; 1991. [Google Scholar]
- 2.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 3.Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna NH, Shimomura K, King DP, Young MW, Weitz CJ, Takahashi JS. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 4.Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo JJ, Sassone-Corsi P. Circadian clock control by SUMOylation of BMAL1. Science. 2005;309:1390–1394. doi: 10.1126/science.1110689. [DOI] [PubMed] [Google Scholar]
- 5.Bovbjerg DH. Circadian disruption and cancer: sleep and immune regulation. Brain Behav Immun. 2003;17:S48–S50. doi: 10.1016/s0889-1591(02)00066-1. [DOI] [PubMed] [Google Scholar]
- 6.Hetzel MR. The pulmonary clock. Thorax. 1981;36:481–486. doi: 10.1136/thx.36.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soutar CA, Costello J, Ijaduola O, Turner-Warwick M. Nocturnal and morning asthma. Thorax. 1975;30:436–440. doi: 10.1136/thx.30.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calhoun WJ. Nocturnal asthma. Chest. 2003;123(3 Suppl):399S–405S. doi: 10.1378/chest.123.3_suppl.399s. [DOI] [PubMed] [Google Scholar]
- 9.Larsson K, Hedenstrom H, Malmberg P. Learning effects, variation during office hours and reproducibility of static and dynamic spirometry. Respiration. 1987;51:214–222. doi: 10.1159/000195204. [DOI] [PubMed] [Google Scholar]
- 10.Hruby J, Butler J. Variability of routine pulmonary function tests. Thorax. 1975;30:548–553. doi: 10.1136/thx.30.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burioka N, Suyama H, Sako T, Hoshino E, Matsumoto Y, Sasaki T. Circadian rhythms of the indices in the diffusing function of the lung in healthy men. Yonago Acta Medica. 1999;42:189–192. [Google Scholar]
- 12.Mahajan KK, Mahajan SK, Mishra N. Diurnal variations in lung transfer factor and its components. Indian J Physiol Pharmacol. 1990;34:209–211. [PubMed] [Google Scholar]
- 13.Cinkotai FF, Thomson ML. Diurnal variation in pulmonary diffusing capacity for carbon monoxide. J Appl Physiol. 1966;21:539–542. doi: 10.1152/jappl.1966.21.2.539. [DOI] [PubMed] [Google Scholar]
- 14.Frey TM, Crapo RO, Jensen RL, Elliott CG. Diurnal variation of the diffusing capacity of the lung: is it real? Am Rev Respir Dis. 1987;136:1381–1384. doi: 10.1164/ajrccm/136.6.1381. [DOI] [PubMed] [Google Scholar]
- 15.ATS guidelines: Standardization of spirometry (1994 update) Am J Respir Crit Care Med. 1995;152:1107. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 16.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equpment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 17.Crapo RO, Morris AH. Standardized single-breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 18.Panda A, McHardy G. Diurnal variation in pulmonary diffusing capacity and expiratory volumes. Indian J Physiol Pharmacol. 1980;24:112–118. [PubMed] [Google Scholar]
- 19.Saito S, Burioka N, Hoshino E. Circadian variation of the lung elastic recoil pressure in healthy male non-smokers. Yonago I gaku Zasshi. 1996;47:130–139. [Google Scholar]
- 20.Lewis BL, McElroy WT, Hayford-Welsing EJ, Samberg LC. The effect of body position, ganglionic blockade and norepinephrine on the pulmonary capillary bed. J Clin Invest. 1960;39:1345–1352. doi: 10.1172/JCI104152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortola JP. Breathing around the clock: an overview of the circadian pattern of respiration. Eur J Appl Physiol. 2004;91:119–129. doi: 10.1007/s00421-003-0978-0. [DOI] [PubMed] [Google Scholar]
- 22.Phillipson EA, Bowes G. Control of breathing during sleep. In: Cherniack NS, Widdicombe JG, editors. Handbook of physiology, section 3, The respiratory system, vol. II, Control of breathing, part 2. Bethesda, MD: American Physiological Society; 1986. pp. 649–689. [Google Scholar]
- 23.Mortola JP, Gautier H. Interaction between metabolism and ventilation: Effects of respiratory gases and temperature. In: Dempsey JA, Pack AI, editors. Regulation of breathing. 2nd edn. New York: Marcel Dekker; 1995. pp. 1011–1064. [Google Scholar]
- 24.Barnes PJ. Circadian rhythms and airway function. Bull Eur Physiopathol Respir. 1987;23:532. [PubMed] [Google Scholar]
- 25.Dreher D, Koller EA. Circadian rhythms of specific airway conductance and bronchial reactivity to histamine: the effects of parasympathetic blockade. Eur Respir J. 1990;3:414–420. [PubMed] [Google Scholar]