Abstract
Idiopathic congenital nystagmus (ICN) is characterised by involuntary, periodic, predominantly horizontal, oscillations of both eyes. We identified 22 mutations in FRMD7 in 26 families with X-linked idiopathic congenital nystagmus. Screening of 42 ICN singleton cases (28 male, 14 females) yielded three mutations (7%). We found restricted expression of FRMD7 in human embryonic brain and developing neural retina suggesting a specific role in the control of eye movement and gaze stability.
The prevalence of idiopathic congenital nystagmus (ICN) is estimated to be 1 in 1000. In ICN visual function can be significantly reduced due to constant eye movement, but the degree of visual impairment varies1,2. The disease is likely to be due to abnormal development of areas in the brain controlling eye movements and gaze stability3. ICN is distinct from other hereditary causes of nystagmus and ocular pathology, including ocular albinism, congenital stationary night blindness, achromatopsia, blue cone monochromatism and sensory visual defects of early childhood such as congenital cataract, retinitis pigmentosa, cone-rod dystrophy and optic nerve hypoplasia 4.
ICN is usually inherited as an X-linked trait with incomplete penetrance in females. Most families map to Xq26-q27 and the locus (known as NYS1) has previously been mapped to a ∼12 Mb interval between markers DXS9909 and DXS12115,6. Zhang et al (2005) proposed further reduction of the candidate region to an interval between DXS8033 and DXS8043 based on a recombination event in a clinically unaffected female7. X-linked genetic heterogeneity has been suggested on the basis of a single ICN family that is reported to map to Xp11.4- Xp11.38.
We screened 16 families with X-linked ICN using 17 markers extending from Xq26-Xq27 (see supplementary figure 1) 6. In these families the disease was fully penetrant in males and ∼50 % penetrant in females. The phenotype was variable even within families (see supplementary figure 2 and supplementary video 1,2 and 3). In all 16 families marker haplotypes were compatible with linkage to Xq26-q27. Recombinant events in affected males in family N1 refined the location of NYS to a ∼7.5 Mb interval between markers DXS1047 and DXS1041 (see figure 1a and 1b).
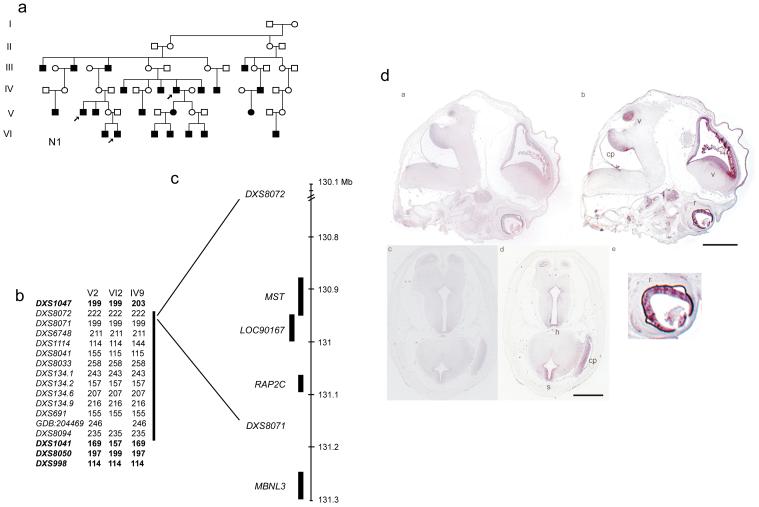
Figure 1. Refinement of the linkage interval to Xq26 and the pattern of expression of FRMD7 in brain of human embryos ∼56 dpo by in situ hybridization.
(a) shows the pedigree N1 with the critical individuals marked with an arrow whose haplotype define the minimum critical linkage interval (b) the allele size for marker DXS1047 is discordant in individual IV9 with the other affected males, V2 and VI2 and the allele size at markers DXS1041 and DXS8050 in individual VI2 is discordant as compared to individuals V2 and IV9 (c) the location of LOC90167 is shown relative to the linkage markers DXS8072 and DXS807 (d) A 599 bp and a 681 bp probe from unique sequence in the 3′UTR of FRMD7 were used as probes for in situ hybridization. Panels a and c show in situ hybridization results using sense probes, b and d use antisense probes in similar anatomical locations. Panels a and b are sagittal sections showing the lateral ventricle(v), cerebellar peduncle (cp) and developing neural retina(r). Panel e is an enlargement of panel b to illustrate the retina. Panels c and d are transverse sections showing the spinal cord (s), hypothalamus (h) and cerebellar peduncle. In panel b the scale bar = 2000μm and in panel d =1800μm.
The candidate interval contained >80 genes and high throughput DNA sequence analysis was performed of all coding exons of all genes within this interval 9. DNA from one affected male individual from each of the 16 linked families was screened for mutations.
Mutations were detected in 15/16 of the linked families in FRMD7 (FERM domain containing 7, previously known as LOC90167) at Xq26.2 having screened >40 genes by sequence analysis (see figure 1c). FRMD7 has 12 exons and encodes a novel member of the protein 4.1 superfamily (RefSeq DNA: NM_194277), (http://www.gene.ucl.ac.uk/nomenclature/). All mutations identified in FRMD7 co-segregated with disease in the linked families and were absent from 300 male control chromosomes (see table 1). The nonsense mutations Q201X and R335X predict truncated proteins containing 28% and 47% of the protein respectively. Four of the five splice site mutations were at conserved splice donor residues (position +1 and +2) and are thus predicted to be pathological by classical exon skipping and nonsense mediated decay. For the mutation IVS2 +5G>A, in family N7, compared to controls negligible amounts of transcript could be detected through amplification of exons 1-5 in lymphocytes suggesting that this is also disease-associated (see supplementary figure 3a). The silent variant, G252A, V84V in family N5, created a novel splice acceptor site within exon 4 which results in loss of transcript containing exon 1-5 sequence and the rare presence of a transcript with exon 4 skipped in lymphocytes (see Figure 3a and b supplementary data).
Table 1. Mutations in FRMD7.
Families where linkage was performed are prefixed by N, those that are familial but no linkage data was available are prefixed F, and singleton males are prefixed SM and singleton females SF. The class of mutation is categorized as T=truncating, M= missense, S=silent and del= deletion. The country of origin is denoted. All mutations identified were not found in 300 control male chromosomes. The reference cDNA sequence NM_194277 is used as a basis for numbering the nucleotide of the mutation. All mutations are located relative to the A of the first coding ATG at position 179. The reference protein sequence NP_919253 is used as the basis for numbering the amino acid mutation starting from the first methionine at position 1. The reference sequence for the genomic sequence is AL49792.
| Sample | Class | Mutation | Origin |
|---|---|---|---|
| N15 | M | G70A, G24R | Ireland |
| N7 | T | IVS2+5G>A | England |
| N4 | T | IVS3+2 T>G | England |
| N5 | S | G252A, V84V | England |
| N1, F26 | T | IVS4+1G>A | England, England |
| N16 | M | T425G, L142R | Ireland |
| N13 | T | IVS7+1G>C | Madagascar |
| N2 | T | C601T, Q201X | Italy-Germany |
| N 3 | M | T691G, L231V | Ireland -Germany |
| N6, SF21 | M | G796C, A266P | England, England |
| N11 | M | G812A, C271Y | Scotland |
| N14 | T | 887delG, G296fs | Austria |
| N12 | M | A902G, Y301C | England |
| N10, F24, SM08 | T | C1003T, R335X | England, India, England |
| N9 | T | IVS11+1G>C | Germany |
| F31 | del | 41_43delAGA, 14delI | England |
| F21 | M | G71A, G24E | Austria |
| F28 | T | 479insT, 160fs | England |
| F15 | M | A661G, N221D | England |
| F16 | M | G676A, A226T | England |
| F20 | M | C1019T, S340L | Romania |
| SM10 | T | 1262delC, 421fs | England |
The seven missense mutations at amino acid positions 24, 142, 231, 266, 271 and 301 in the linked families not only involve highly conserved residues that are invariant in Rattus, Mus, Gallus, Xenopus but are also located within invariant blocks of highly conserved residues suggesting that mutations at these locations are critical to the normal function of the protein. Residues at position 142, 231, 271 and 301 are further conserved in Tetraodon. Furthermore, with the exception of L231V the mutations are largely non-conservative in function: G24R, L142R, A266P, C271Y, Y301C. We modelled the effects of these mutations on the three dimensional structure of the protein by mapping them onto the closest orthologue of known structure which is the core domain of the cytoskeletal protein 4.1R (1GG3 in the Protein Data Bank)10,11. The crystal structure extends from residues 1-279 and therefore all but Y301C of the missense mutations in FRMD7 in the linked families could be mapped. The structural environment of the disease-associated missense mutations was inspected using the program Coot, which allows the identity and conformation of residues to be manipulated easily12. Although the effect on the structure of L231V is not clearly apparent, mutations G24R, L142R and C271 are likely to destabilise the protein by the introduction of larger amino acids within restricted areas of the protein and the introduction of a proline residue at position 266 (A266P) will disrupt a helical domain in the wild-type structure.
From our study we conclude that mutations in FRMD7 are a major cause of familial X-linked congenital motor nystagmus. We then assessed the prevalence of mutations in FRMD7 in smaller families where linkage data was not available and in a cohort of males and females with ICN but with no family history of the condition. We screened 14 families with two or more affected individuals of either sex and found mutations in 8/14 (57%). We also identified mutations in 3/42 (7%) patients without a family history that had undergone careful clinical and electrophysiological investigation to exclude other causes of inherited congenital nystagmus (see Table 1). Mutations were identified in 1/14 female singletons and 2/28 male singletons and none of the novel mutations were found in 300 male control samples. The results suggest that mutation analysis of FRMD7 may be considered of diagnostic value even in isolated cases of either sex.
Expression analysis of FRMD7 shows that the mRNA is present in most tissues but at low levels (http://symatlas.gnf.org/SymAtlas/). This was confirmed by RT-PCR with expression detected in human adult kidney, liver, pancreas and at low levels in heart and brain (data not shown). In human fetal tissue the transcript was only detectable in kidney by this method.
We then performed in situ hybridisation experiments in human embryo brain to investigate whether expression of FRMD7 was localised or restricted. In embryos ∼56 dpo there is expression in the ventricular layer of the forebrain, midbrain and cerebellar primordium, spinal cord and in the developing neural retina. In earlier embryos (∼37 dpo) the expression is restricted to the mid and hind-brain, regions known to be involved in motor control of eye movement (see figure 1d).
The functional role of FRMD7 protein is unknown but BLAST (http://www.ncbi.nlm.nih.gov/) search analysis detected close amino acid sequence homology to FARP1 (FERM, RhoGEF and pleckstrin domain protein 1; chondrocyte-derived ezrin-like protein) (AB018336) and FARP2 (AB008430). The homology is concentrated at the N terminus of the protein where a B41 and a FERM-C domain are present. The B41 domain extends from residues 1-182 and the FERM-C domain is located from residue 175-268 in FRMD7. The location of mutations relative to these domains is shown in supplementary figure 3c. The homologous protein FARP2 modulates the length and the degree of branching of neurites in rat embryonic cortical neurons and reorganises the cytoskeleton. Overexpression of FARP2 results in increased numbers of lateral growth cones extending from neurites and associated decrease in total length of the neurites per neuron 13,14. Whether the function of FRMD7 is similar to FARP2 in specialised neuronal pathways governing integration and coordination of eye movements remains to be proved. The hypothesis that null mutations in FRMD7, as found in families with X-linked congenital motor nystagmus, alter the neurite length and degree of branching of neurons as they develop in the midbrain, cerebellum and retina is a plausible explanation of how defects in the protein coded for by FRMD7 causes disease.
Supplementary Material
Acknowledgements
This project was funded by the Wellcome Trust, Medisearch Leicester and The Ulverscroft Foundation. The human embryonic material was provided by the Joint MRC-Wellcome Trust Human Developmental Biology Resource at IHG, Newcastle upon Tyne, UK (http://www.hdbr.org).
Footnotes
No conflict of interest
URLs
Online Mendelian Inheritance in Man and BLAST are found at http://wwww.ncbi.nlm.nih.gov/. The HUGO nomenclature site is at http://www.gene.ucl.ac.uk/nomenclature/). The electronic expression profile of LOC90167 is at http://symatlas.gnf.org/SymAtlas/). LOC90167 RefSeq DNA: NM_194277, FARP1 AB018336 and FARP2 AB008430.
References
- 1.Stayte M, Reeves B, Wortham C. Ocular and vision defects in preschool children. Br J Ophthalmol. 1993;77:228–32. doi: 10.1136/bjo.77.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilling RF, Thompson JR, Gottlob I. Social and visual function in nystagmus. Br J Ophthalmol. 2005;89:1278–81. doi: 10.1136/bjo.2005.070045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs JB, Dell’Osso LF. Congenital nystagmus: hypotheses for its genesis and complex waveforms within a behavioral ocular motor system model. J Vis. 2004;4:604–25. doi: 10.1167/4.7.7. [DOI] [PubMed] [Google Scholar]
- 4.Gottlob I. Nystagmus. Curr Opin Ophthalmol. 2001;12:378–83. doi: 10.1097/00055735-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Kerrison JB, Vagefi MR, Barmada MM, Maumenee IH. Congenital motor nystagmus linked to Xq26-q27. Am J Hum Genet. 1999;64:600–7. doi: 10.1086/302244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerrison JB, Giorda R, Lenart TD, Drack AV, Maumenee IH. Clinical and genetic analysis of a family with X-linked congenital nystagmus (NYS1) Ophthalmic Genet. 2001;22:241–8. doi: 10.1076/opge.22.4.241.2216. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, et al. Confirmation and refinement of a genetic locus of congenital motor nystagmus in Xq26.3-q27.1 in a Chinese family. Hum Genet. 2005;116:128–31. doi: 10.1007/s00439-004-1188-5. [DOI] [PubMed] [Google Scholar]
- 8.Cabot A, et al. A gene for X-linked idiopathic congenital nystagmus (NYS1) maps to chromosome Xp11.4-p11.3. Am J Hum Genet. 1999;64:1141–6. doi: 10.1086/302324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarpey P, et al. Mutations in the DLG3 Gene Cause Nonsyndromic X-Linked Mental Retardation. Am J Hum Genet. 2004;75:218–324. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman H, Henrick K, Nakamura H. Announcing the worldwide Protein Data Bank. Nat Struct Biol. 2003;10:980. doi: 10.1038/nsb1203-980. [DOI] [PubMed] [Google Scholar]
- 11.Han BG, Nunomura W, Takakuwa Y, Mohandas N, Jap BK. Protein 4.1R core domain structure and insights into regulation of cytoskeletal organization. Nat Struct Biol. 2000;7:871–5. doi: 10.1038/82819. [DOI] [PubMed] [Google Scholar]
- 12.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 13.Kubo T, et al. A novel FERM domain including guanine nucleotide exchange factor is involved in Rac signaling and regulates neurite remodeling. J Neurosci. 2002;22:8504–13. doi: 10.1523/JNEUROSCI.22-19-08504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyofuku T, et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–9. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.