Abstract
Background
Increasing evidence indicates that brain kappa-opioid receptors (KORs) are involved in regulation of mood states. In animal models often used to study psychiatric illness, KOR agonists produce depressive-like effects (e.g., anhedonia) whereas KOR antagonists produce antidepressant- and anxiolytic-like effects. The ability of KOR agonists to produce anhedonia-like signs in laboratory animals raises the possibility that this class of drugs might be useful to ameliorate states characterized by excess reward or motivation, such as mania or stimulant intoxication.
Methods
We examined how the selective KOR agonist U69,593 affects cocaine-induced facilitation of intracranial self-stimulation (ICSS), a model of the abnormally increased reward function that characterizes mania and stimulant intoxication. Rats with stimulating electrodes implanted in the medial forebrain bundle (MFB) were tested with intraperitoneal injections of U69,593 (0.063–0.5 mg/kg) alone, cocaine (1.25–10 mg/kg) alone, and combinations of the drugs.
Results
Cocaine dose-dependently decreased ICSS thresholds, indicating that it enhanced the rewarding impact of MFB stimulation. In contrast, U69,593 dose-dependently increased ICSS thresholds, indicating that it decreased the rewarding impact of the stimulation. Pretreatment with U69,593 blocked cocaine-induced decreases in ICSS thresholds at doses that had negligible effects on their own.
Conclusions
Activation of KORs reduces the reward-related effects of cocaine. Inasmuch as cocaine-induced behavioral stimulation in rodents may model key aspects of enhanced mood in humans, these findings raise the possibility that KOR agonists might ameliorate symptoms of conditions characterized by increased motivation and hyperfunction of brain reward systems, such as mania and stimulant intoxication.
INTRODUCTION
The biological basis of mood is not understood. Most research on mood and affective states focuses on brain systems containing monoamines, such as dopamine (DA), norepinephrine (NE), and serotonin (5HT). This focus is logical, because drugs with mood-elevating effects (including stimulants, antidepressants) have prominent interactions with these systems, and tend to increase extracellular concentrations of monoamines and prolong their actions (1,2). However, there is accumulating evidence that brain opioids are also involved in the regulation of mood. As one example, we and others have found that kappa-opioid receptor (KOR) antagonists produce antidepressant-like (3–8) and anxioloytic-like (9) effects in animal models, whereas KOR agonists produce depressive-like effects (5,10,11). The molecular mechanisms by which these drugs alter mood are not understood, although KOR agonists decrease extracellular concentrations of DA within the nucleus accumbens (NAc) (1,11), a key component of the mesolimbic system. Dysregulation of the mesolimbic system is implicated in the pathophysiology of depressive conditions including bipolar disorder (12,13). Drugs that reduce the activity of brain reward systems may have utility in studying and altering the symptoms of mania, the defining state of bipolar disorder that is characterized by excessive involvement in rewarding or pleasurable activities (14).
Preclinical research on the biological basis of mania and bipolar disorder is complicated by an incomplete understanding of their pathophysiology. This has made it difficult to design models that recapitulate the behavioral symptoms of these conditions while ensuring construct validity. However, intracranial self-stimulation (ICSS) may be a useful paradigm with which to model certain aspects of mania. ICSS is an operant paradigm in which rodents respond at high rates to self-administer rewarding electrical stimulation through electrodes implanted into the brain areas including medial forebrain bundle (MFB) (15). The ICSS behavior fulfills several key diagnostic criteria used for mania in people (14). For example, rats show increases in a goal-directed activity (lever-pressing for brain stimulation) and excessive involvement in this activity even under conditions where there is a high potential for painful consequences: food-deprived rats choose to respond at a lever that produces stimulation rather than one that produces food (16), and rats tested in sub-freezing conditions choose to respond at a lever that produces stimulation rather than one the produces heat (17). Drugs that reduce symptoms of mania (e.g., antipsychotics, mood stabilizers) attenuate ICSS (18,19), indicating that these agents produce anhedonia. This common effect raises the possibility that production of anhedonia-like states may contribute to (or at least predict) the efficacy of these drugs in treating mania. Drugs that trigger mania in humans or cause mania-like behaviors in laboratory animals (e.g., cocaine) produce a profound facilitation of ICSS, reflecting hyperfunction of brain reward systems (15,20). Genetic manipulations that cause mania-like signs in mice (including sleep disruptions) similarly facilitate ICSS (21). Thus even if ICSS does not produce mania-like behaviors in rodents through the same mechanisms that produce them in humans, it has predictive validity as a test with which to model aspects of bipolar disorder and identify new classes of agents that might ameliorate key symptoms of mania.
The present studies were designed to determine if a prototypical KOR agonist (U69,593) affects the reward-related effects of cocaine in the ICSS test. Previous work indicates that interactions between KOR agonists and cocaine are complex, and depend upon the timing and context of the drug treatments. Although KOR agonists appear to block the development or expression of cocaine-induced conditioned place preferences (22,23), it has also been reported that exposure to KOR agonists can subsequently increase cocaine effects (24,25). ICSS offers several advantages that enable detailed analysis of acute interactions between KOR agonists and reward states. It is a highly trained behavior that is relatively impervious to treatments that might disrupt memory or cause anxiety, each of which could affect the outcome of place conditioning studies. It also enables real-time studies of drug interactions, whereas place conditioning studies test the memory of previously established associations.
Finally, the “curve-shift” variant of the ICSS test enables distinctions between treatment effects on reward function and response capabilities (14,15). We report that the KOR agonist U69,593 blocks the reward-related effects of cocaine in the ICSS test at doses below those that have non-specific effects on responding, providing support for the idea that this class of agents might ameliorate key symptoms of conditions characterized by heightened motivation (e.g., mania, stimulant intoxication).
METHODS
Rats
Seven male Sprague-Dawley rats (Charles River Laboratories; Raleigh, NC) were used. Rats were housed singly and maintained on a 12 h light (0700–1900 h)-12 h dark cycle with free access to food and water except during testing. Experiments were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington D.C., USA, 1996) and McLean Hospital policies.
Drugs
Cocaine hydrochloride and U69,593 (5a,7a,8b)-N-methyl-N-(7-[1-pyrrolidinyl]-1-oxaspiro[4.5]dec8-yl)-benzenacetamide) were purchased from Sigma (St. Louis, MO). Cocaine was dissolved in 0.9% saline, whereas U69,593 was dissolved in 0.1 N acetic acid diluted with distilled water. Drugs were administered by intraperitoneal (IP) injection in a volume of 1 ml/kg. Dosages of the drugs were based on their salt form.
ICSS
Rats (350–375 g) were anesthetized with pentobarbital (65 mg/kg, IP), and given subcutaneous (SC) atropine sulfate (0.25 mg/kg) to reduce bronchial secretions. Each rat was implanted with a monopolar, stainless steel electrode (0.250-mm diameter; Plastics One, Roanoke, VA) aimed at the left medial forebrain bundle (MFB), at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral from the midsaggital suture and 7.8 mm below dura; 26). The electrodes were coated with polyamide insulation except at the flattened tip. Skull screws (one of which served as the ground) and the electrode were secured to the skull with dental acrylic.
After one week of recovery, the rats learned to respond for brain stimulation as described previously (15). Each lever-press earned a 0.5-sec train of square-wave cathodal pulses (0.1-msec pulse duration) at a frequency of 141 Hz. The stimulation current (100–300 µA) was adjusted gradually to the lowest value that would sustain reliable responding (at least 40 rewards per min). Once the minimal effective current was found for each rat, it was held constant.
Each rat was then adapted to tests at its minimal effective current with a descending series of 15 stimulation frequencies. Each series comprised 1-min test trials at each frequency. For each frequency, there was an initial 5-sec "priming" phase during which non-contingent stimulation was given, followed by a 50-sec test phase during which the number of responses was counted, followed by a 5 sec time out period during which no stimulation was available. The stimulation frequency was then lowered by 10% (0.05 log10 units), and another trial was started. After responding had been evaluated at each of the 15 frequencies, the procedure was repeated such that each rat was given 6 such series per day (90 minutes of training). Minor adjustments were made to the current for each rat so that only the highest 7–8 frequencies would sustain responding. To characterize the functions relating response strength to reward magnitude, a least-squares line of best fit was plotted across the frequencies that sustained responding at 20, 30, 40, 50 and 60% of the maximum rate. ICSS threshold was defined as the frequency at which the line intersected the x-axis (theta-0; 27). Drug testing started when mean ICSS thresholds varied by less than 10% over 3 consecutive sessions.
For drug testing, three rate-frequency functions (“curves”) were determined immediately prior to drug treatment. The second and third curves were averaged to obtain the baseline (threshold and maximal response rates) parameters. After obtaining baselines for each rat on each day, the rats received drug treatments, and four more 15-min rate-frequency curves were obtained (1 hr of testing). There were 3 phases of drug testing: a phase where the rats were tested with U69,593 alone (0.063–0.5 mg/kg), a phase where they were tested with cocaine alone (1.25–10 mg/kg) alone, and a phase where they were tested with U69,593 (0.063–0.5 mg/kg) plus cocaine (5.0 mg/kg). Doses of U69,593 and cocaine are similar to those with effects in the forced swim test (5). During the U69,593 alone phase, rats received an injection of the drug followed 10 min later by an injection of saline. Testing began immediately after the second injection. The doses were given in ascending and then descending order, such that each rat received vehicle and each dose of the drug twice. This experimental design was utilized to determine if tolerance or sensitization would occur in response to repeated drug treatment. On alternate days rats were tested after injections of saline, to ensure that they had recovered from prior treatment and to minimize the possibility of conditioned drug effects. During the cocaine alone phase, rats received an injection of acid vehicle followed 10 min later by cocaine. As was the case with the U69,593 alone studies, the doses were given in ascending and then descending order, with saline treatment on alternating days. Approximately half of the rats were tested first with U69,593 (n=4), whereas the others were tested first with cocaine (n=3). During the U69,593 plus cocaine phase, rats received an injection of U69,593 (0.063, 0.125, 0.25, and 0.5 mg/kg) followed 10 min later by cocaine (5.0 mg/kg). This phase occurred last for all rats, and each dose of U69,593 was tested once.
Statistics
To determine if there were differences between the first and second test with each treatment, the effects of U69,593 and cocaine on ICSS thresholds and maximal response rates over the entire 1-hr test period were evaluated in separate two-way analyses of variance (ANOVAs) (drug dose × test number) with repeated measures. The first and second tests at each dose were then combined into single means. To determine if there were differences in responsiveness to U69,593 or cocaine that depended on which drug the rats received first, ICSS thresholds and maximal response rates were analyzed using separate three-way (test order × treatment × dose) ANOVAs with repeated measures.
The effects of U69,593 alone, cocaine alone, or U69,593 plus cocaine on thresholds and maximum rates were evaluated with separate one-way ANOVAs with repeated measures. The time course of drug effects in the test involving 0.25 mg/kg U69,593 plus 5.0 mg/kg cocaine was analyzed using a two-way (treatment × time) ANOVA with repeated measures. All significant effects and interactions were analyzed further using post hoc Newman-Keuls tests.
Histology
Rats were overdosed with pentobarbital (130 mg/kg, IP) and perfused with 4% paraformaldehyde. Brains were sliced in 40-µm sections for cresyl violet staining to localize electrode placements.
RESULTS
There were no differences in responsiveness to U69,593 or cocaine between the first and second time that these drugs were administered (data not shown), consistent with previous reports that experience-dependent changes in drug sensitivity are rarely detectable in ICSS tests (see 28). As such, the data from the two tests at each dose were combined into single means. Likewise, there were no order-effects of treatment: exposure to U69,593 first did not alter subsequent responsiveness to cocaine (F1,5=0.01, not significant [n.s.]), nor did exposure to cocaine first alter subsequent responsiveness to U69,593 (F1,5=0.37, n.s.). The electrode assembly became dislodged on one rat during the phase of the study when both drugs were given together. For this rat, only data from the tests with each of the individual drugs were retained in the analyses.
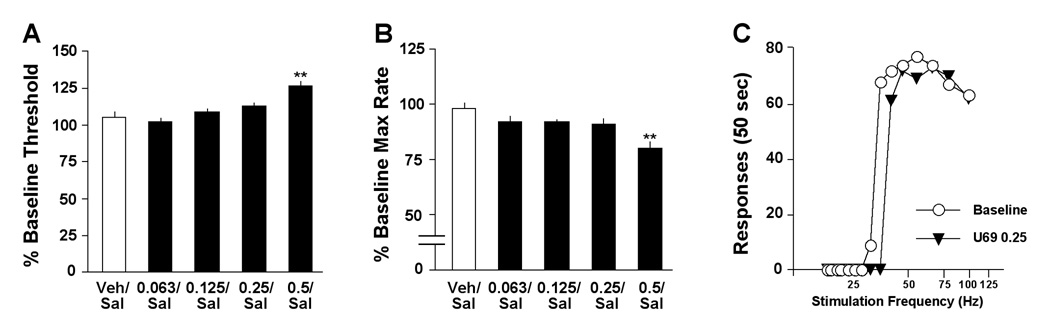
U69,593 (followed 10 min later by saline) had effects on ICSS thresholds that depended upon dose (F4,24=10.1, P<0.01) (Fig. 1A). When data were expressed as single means for the entire 1-hr test session, thresholds were significantly elevated only at 0.5 mg/kg (P<0.01, Newman-Keuls tests). Similarly, the drug had effects on maximal response rates that depended upon dose (F4,24=11.9, P<0.01) (Fig. 1B). When data were expressed as single means for the entire 1-hr test session, response rates were significantly decreased only at 0.5 mg/kg (P<0.01). Raw data from a representative rat (Fig. 1C) illustrate how an intermediate dose of U69,593 (0.25 mg/kg) increases the “dose” of stimulation that is required to sustain responding while having negligible effects on response rates. These data are consistent with those from a previous report in which ICSS testing began immediately after administration of U69,593 (9).
Figure 1.
Effects of U69,593 (U69) on ICSS. The drug or vehicle (Veh) was administered 10 min before an injection of saline (Sal), followed immediately by testing. (A) The effects of U69,593 on thresholds were dose-dependent: 0.5 mg/kg significantly increased thresholds over the course of the 60-min test session, whereas lower doses (0.063–0.25 mg/kg) did not have statistically significant effects. Bars represent the mean (± SEM) change from pretreatment baseline. (B) 0.5 mg/kg U69,593 also significantly reduced response capabilities (maximal rates). (C) U69,593 (0.25 mg/kg) caused parallel rightward shifts in rate-frequency functions. Data are from a single representative rat; for clarity, only the second of 4 rate-frequency determinations is presented. **P<0.01 compared to control (Veh/Sal), Newman-Keul’s tests.
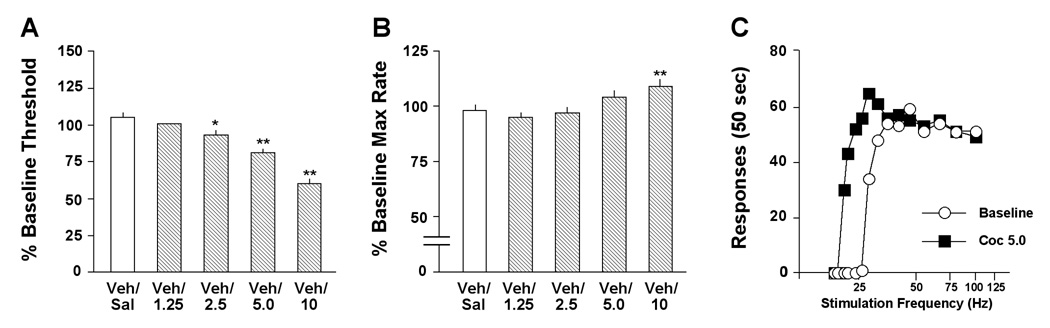
Cocaine (preceded 10 min earlier by acid vehicle) also had effects on ICSS thresholds that depended upon dose (F4,24=35.8, P<0.01) (Fig. 2A). When data were expressed as means for the 1-hr test session, thresholds were significantly decreased at 2.5 (P<0.05), 5.0 (P<0.01), and 10 mg/kg (P<0.01). The drug had effects on maximal response rates that also depended upon dose (F4,24=7.46, P<0.01) (Fig. 2B). When data were expressed as means for the 1-hr test session, response rates were significantly increased at 10 mg/kg (P<0.01). Raw data from a representative rat (Fig. 2C) illustrate how an intermediate dose of cocaine (5.0 mg/kg) reduces the “dose” of stimulation that is required to sustain responding while simultaneously causing negligible (non-significant) elevations in maximal response rates. These data are consistent those from a previous report in which 30-min ICSS test sessions began immediately after administration of cocaine (27).
Figure 2.
Effects of cocaine (Coc) on ICSS. The drug or saline (Sal) was administered 10 min after an injection of the vehicle (Veh) used for U69,593, followed immediately by testing. (A) The effects of cocaine on thresholds were dose-dependent: 2.5, 5.0 and 10 mg/kg significantly decreased thresholds over the course of the 60-min test session, whereas a lower doses (1.25 mg/kg) did not have statistically significant effects. (B) 10 mg/kg cocaine also significantly increased response capabilities (maximal rates). (C) Cocaine (5.0 mg/kg) caused parallel rightward shifts in rate-frequency functions. Data are from a single representative rat; for clarity, only the second of 4 rate-frequency determinations is presented. *P<0.05, **P<0.01 compared to control (Veh/Sal), Newman-Keul’s tests.
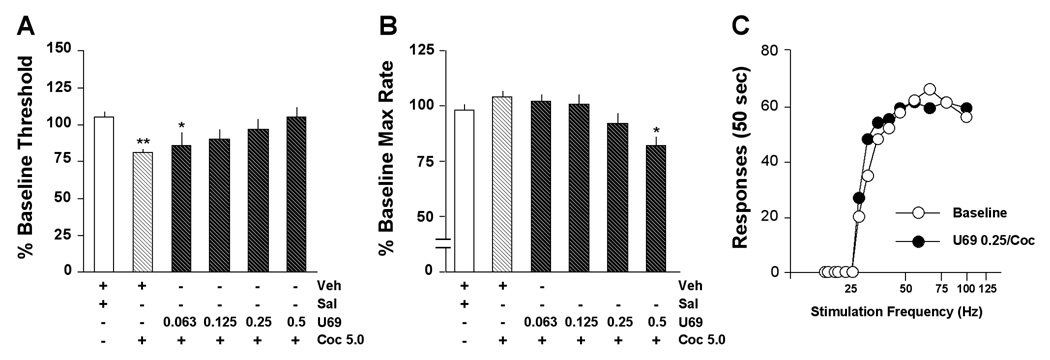
For the drug interaction studies, we selected 5.0 mg/kg cocaine because it caused a significant but not maximal decrease in ICSS thresholds in the cocaine dose-effect function (i.e., Fig. 2A). Pretreatment with U69,593 dose-dependently affected the threshold-reducing effects of 5.0 mg/kg cocaine (F5,30=4.67, P<0.01) (Fig. 3A). When data were expressed as means for the 1-hr test session, the ability of this dose of cocaine to significantly reduce ICSS thresholds (P<0.01) was attenuated by pretreatment with 0.125, 0.25, or 0.5 mg/kg U69,593. Similarly, pretreatment with U69,593 dose-dependently affected maximal response rates (F5,30=5.63, P<0.01) (Fig. 3B). When data were expressed as means for the 1-hr test session, maximal response rates after pretreatment with 0.5 mg/kg U69,593 followed by cocaine were significantly lower than those after pretreatment with vehicle followed by saline (P<0.01). Raw data from a representative rat (Fig. 3C) illustrate how pretreatment with an intermediate dose of U69,593 (0.25 mg/kg) reduces the threshold-lowering effects of 5.0 mg/kg cocaine, yielding ICSS behavior that is virtually indistinguishable from that seen during baseline testing.
Figure 3.
Effects of combined treatment with U69,593 (U69) and cocaine (Coc) on ICSS. U69,593 was administered 10 min before an injection of cocaine (5.0 mg/kg), followed immediately by testing. (A) The effects of U69,593 on cocaine-induced decreases in thresholds were dose-dependent: 0.125, 0.25 and 0.5 mg/kg U69,593 blocked cocaine effects, whereas a lower dose (0.063 mg/kg) did not have statistically significant effects. (B) 0.5 mg/kg U69,593 significantly decreased response capabilities (maximal rates) even in the presence of cocaine. (C) An intermediate dose of U69,593 (0.25 mg/kg) blocked the leftward shifts in rate-frequency functions normally seen after 5.0 mg/kg cocaine. Data are from a single representative rat; for clarity, only the second of 4 rate-frequency determinations is presented. *P<0.05, **P<0.01 compared to control (Veh/Sal), Newman-Keul’s tests.
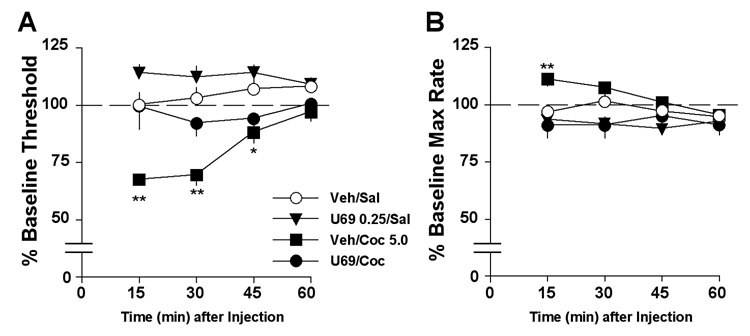
To examine KOR agonist effects on cocaine-induced decreases in ICSS thresholds in more detail, we analyzed time-course data from tests involving intermediate doses of U69,593 (0.25 mg/kg) and cocaine (5.0 mg/kg). This was the highest dose of U69,593 that did not suppress ICSS response rates on its own (i.e., Fig. 1B–C). Time course analysis revealed an interaction between treatment condition and time (F9,69=3.29, P<0.01) (Fig. 4A). Cocaine caused significant decreases in ICSS thresholds at the 15-min (P<0.01), 30-min (P<0.01) and 45-min (P<0.05) time points, all of which were blocked by U69,593. On its own, this dose of U69,593 caused nominal (non-significant) increases in ICSS thresholds. Analysis of maximal response rates also revealed an interaction between treatment condition and time (F9,69=2.27, P<0.05) (Fig. 4B). Cocaine caused a significant but transient elevation of maximal response rates at the 15-min time point (P<0.01), and this effect was blocked by U69,593.
Figure 4.
Time course of effects of combined treatment with U69,593 (U69) and cocaine (Coc) on ICSS. U69,593 (0.25 mg/kg) was administered 10 min before an injection of cocaine (5.0 mg/kg), followed immediately by testing. (A) Cocaine alone significantly decreased thresholds for the first 45 min of testing, and pretreatment with U69,593 blocked this effect. This dose of U69,593 alone caused negligible elevations in ICSS thresholds that did not differ from the control condition (Veh/Sal) at any time point. (B) Cocaine alone caused a brief increase in response capabilities (maximal rates), and this effect was blocked by U69,593. *P<0.05, **P<0.01 compared to control (Veh/Sal), Newman-Keul’s tests.
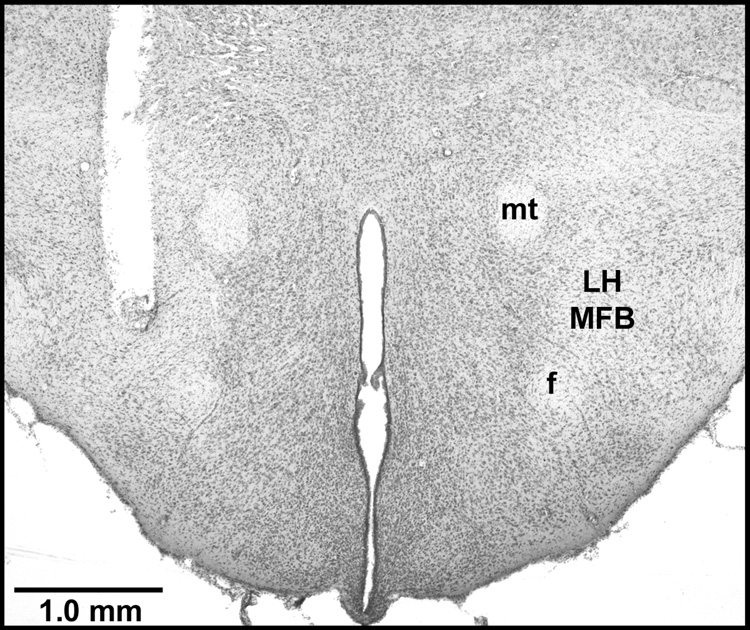
ICSS electrodes were located in the MFB at the level of the lateral hypothalamus (Fig. 5). The placements were indistinguishable from those depicted previously (10,28,29).
Figure 5.
Electrode track in a cresyl violet-stained section from a representative rat. LH, lateral hypothalamus; MFB, medial forebrain bundle; mt, mammillothalamic tract; f, fornix
DISCUSSION
The KOR agonist U69,593 blocked cocaine effects in the ICSS test. Administration of U69,593 (0.063–0.5 mg/kg, IP) alone 10 min before testing caused dose-dependent rightward shifts in the ICSS response-frequency curves, indicating that more stimulation was required to elicit pretreatment levels of responding. When expressed as the mean change in ICSS thresholds over the 1-hr test session, only the highest dose tested significantly elevated thresholds; lower doses did not affect thresholds, either when expressed as the mean of the entire test session or as each individual 15-min rate-frequency determination (“curve”). In contrast, administration of cocaine (1.25–10 mg/kg, IP) alone immediately before ICSS testing caused dose-dependent leftward shifts in the response-frequency curves, indicating that less stimulation was required to elicit pretreatment levels of responding. When expressed as the mean change in ICSS threshold over the course of the 1-hr test session, 3 doses of cocaine (2.5, 5.0, and 10 mg/kg, IP) significantly decreased ICSS thresholds. We selected the 5.0 mg/kg dose for the interaction studies so that we would have sufficient sensitivity to determine if the KOR agonist blocks (22,23) or enhances (24,25) the effects of cocaine. When U69,593 was administered 10 min before cocaine, the KOR agonist attenuated cocaine-induced leftward shifts and reductions in ICSS thresholds. This effect was evident at several doses (0.125, 0.25 mg/kg, IP) that did not affect ICSS thresholds on their own. Time course analysis demonstrates that 0.25 mg/kg U69,593 under these testing conditions (10 min pretreatment) is sufficient to block the threshold-reducing effects of 5.0 mg/kg cocaine for the entire period that they are normally evident (~45 min). The finding that KOR agonists block (in real time) the reward-related effects of cocaine adds detail to previous reports that KOR agonists block the rewarding effects of cocaine in place conditioning tests (22,23), which occur in the absence of drug treatment and depe3nd upon the memory of previous associations of treatment and environment (30). It also suggests that the ability of prior KOR agonist exposure to enhance cocaine reward may depend upon KOR-induced neuroadaptations, including alterations in DA receptors or uptake transporters (24, 31) that require hours or days to develop.
This is the first report in which interactions between a KOR agonist and cocaine were examined using ICSS. We reported that U69,593 and salvinorin A (salvA, a highly selective KOR agonist) elevate ICSS thresholds (10,11), although testing began immediately (rather than after a 10-min pretreatment) in previous studies. In the present study, as well as in these previous studies, high doses of KOR agonists decrease maximum rates of ICSS responding. Such data might indicate that KOR stimulation has non-specific (e.g., sedative) effects on the ability to respond. Large reductions in maximal response rates make ICSS data difficult to interpret, and tend to limit the size of threshold elevations that are reported (15). It is conceivable that the apparent ability of U69,593 to block the reward-related effects of cocaine is an artifact of diminished response capabilities. However, several lines of evidence suggest that U69,593 reduces reward regardless of any effects on the capacity to respond. First, the ability of U69,593 to block the reward-related effects of cocaine is detectable at doses below those with any significant effects of their own (0.125, 0.25 mg/kg). Second, in the “curve-shift” variant of the ICSS paradigm, lateral (leftward-rightward) shifts are independent of vertical (upward-downward) shifts (15,18,27,32) when the lateral shifts are parallel. The fact that U69,593 causes parallel rightward shifts in ICSS functions on its own (Fig. 1C) and does not affect the shape of cocaine-induced shifts (Fig. 3C) is evidence that this KOR agonist affects reward function independently of effects of performance capabilities.
The mechanisms by which U69,593 affects reward function are unknown, but might involve mesolimbic DA systems. Cocaine increases extracellular concentrations of DA in the NAc (1), an effect often associated with reward (33). In contrast, KOR agonists decrease extracellular concentrations of DA in this region (1,11). Decreased function of midbrain DA systems has been associated with depressive states including anhedonia in rodents (34) and dysphoria (aversion) in humans (35). Indeed, KOR agonists cause depressive states (including dysphoria) in humans (36). Thus it is possible that, in the present studies, KOR-induced decreases in mesolimbic DA activity canceled the effects of cocaine-induced increases in mesolimbic DA activity. This certainly appears to be the case with the behavior: when rats are treated with both U69,593 and cocaine, the resulting ICSS thresholds are lower than those seen with U69,593 alone and higher than those seen with cocaine alone. The fact that one effect does not predominate (or mask the other) in the ICSS test is potentially important because this may indicate that KOR agonists given to patients with abnormally elevated mood might restore euthymia without inducing depression. For example, if the anhedonia- or dysphoria-producing effects of KOR agonists were to predominate, then co-administration of cocaine would not alter U69,593-induced elevations of ICSS. On the other hand, if a drug such as cocaine were more rewarding because of its ability to counteract aversive states, then pretreatment with U69,593 might enhance cocaine effects. Our data suggest that, when administered together, the effects of U69,593 cancel those of cocaine. If this relationship were true in humans, then one way to rapidly ameliorate or reverse some symptoms of mania—specifically, pathologically enhanced motivation and reward—might be with an agent that tends to induce an opposing anhedonia-like effect without causing non-specific alterations (sedation).
Prior exposure to (rather than concomitant treatment with) a KOR agonist can increase the rewarding effects of cocaine (24). The mechanism of this effect is unknown, but might involve the ability of KOR agonists to mimic stress, which induces behavioral (37) and molecular (38) indices of cross-sensitization with psychomotor stimulants. Although our studies were designed to examine acute interactions between KOR agonists and cocaine, early phases of the study—when the rats received each individual drug alone—enabled us to examine in a small group of rats (n=4) if prior exposure to U69,593 subsequently affected sensitivity to the reward-related actions of cocaine in the ICSS test. Under the narrow set of conditions used, we found no evidence that U69,593 exposure affected the ability of cocaine to decrease ICSS thresholds. However, we have recently demonstrated that pre-exposure to salvA can enhance or attenuate subsequent sensitivity to the stimulant effects of cocaine, and that the pattern of results depends primarily upon whether the KOR agonist is given in a familiar or novel environment (25). Future studies designed to use ICSS as an endpoint may provide a clearer characterization of the conditions under which prior exposure to a KOR agonist affects cocaine reward.
Because ICSS reflects compulsive involvement in reward-related behaviors even in the presence of adverse effects (16,17), it fulfills key diagnostic criteria for mania (14). Psychostimulants such as cocaine often cause pathological and dangerous elevations of mood and trigger (or exacerbate) mania in humans, just as these drugs facilitate ICSS behavior in rodents (15). As such, ICSS may be a model with utility for studies of the neurobiology of mania and bipolar disorder. Like U69,593, drugs often used to manage bipolar disorder (e.g., antipsychotic medications, mood-stabilizers such as lithium and valproate) attenuate ICSS and the effects of psychostimulants on this behavior (18,19). Moreover, the effects of genetic manipulations that cause assorted mania-like signs in mice (including facilitated ICSS) are attenuated by lithium (21) Such findings raise the possibility that KOR agonists might ameliorate some symptoms of mania or stimulant intoxication. Indeed, preclinical studies suggest that antipsychotic drugs (FDA-approved to treat mania) may produce some of their effects through dynorphin, the endogenous agonist at KORs (37). Despite their efficacy in treating the symptoms of mania, long-term use of antipsychotics to manage this condition is limited by the development of dyskinesia with many older agents and substantial weight gain with many newer ones. Currently, no selective KOR agonists are available for human use. A recent study indicates that pentazocine (chosen because it is an FDA-approved drug with potent KOR agonist actions) rapidly reduces the symptoms of euphoric mania in clinical populations (40). Similarly, small-scale studies in humans suggest that KOR agonists have some ability to antagonize the effects of cocaine (41,42). Still other studies demonstrate that KOR agonists reduce symptoms of Tourette’s Syndrome (43,44). Caution is warranted, however, because KOR agonists can cause dysphoria in humans (36). In addition, salvA is used recreationally (45), and is emerging as a potential drug of abuse. Thus the effects of KOR agonists may be critically dependent upon dynamic interactions of neurobiological and environmental factors; their effects may be vastly different in brains with or without pathophysiology. The development of improved KOR agonists that can be tested under controlled clinical conditions might reveal new indications in the treatment of currently intractable psychiatric illness or other conditions (including stimulant intoxication) characterized by heightened motivation.
Acknowledgements
Supported by the National Institute of Mental Health (MH063266 to WAC) and the Stanley Medical Research Institute (to BMC).
Competing Interests Statement:
Dr. Carlezon has a patent (US 6,528,518; Assignee: McLean Hospital) related to the use of kappa-opioid antagonists for the treatment of depressive disorders. In addition, Dr. Carlezon and Dr. Cohen are part of a larger group (Assignees: McLean Hospital and Temple University) that has applied for a patent related to the use of compounds derived from a naturally occurring kappa-opioid agonist in the treatment of psychiatric and addictive disorders. Dr. Todtenkopf became an employee of Alkermes, Inc. after this research was completed. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
“In This Issue” Statement
There is increasing evidence that brain kappa-opioid receptors (KORs) play an important role in the regulation of mood states. Using the intracranial self-stimulation (ICSS) test, Tomasiewicz et al. demonstrate that acute administration of a KOR agonist blocks the rewarding effects of cocaine. Their findings raise the possibility that KOR agonists might ameliorate symptoms of conditions characterized by increased motivation and hyperfunction of brain reward systems, such as stimulant intoxication and mania.
REFERENCES
- 1.DiChiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan S, Kramer GL, Zukas PK, Moeller M, Petty F. In vivo biogenic amine efflux in medial prefrontal cortex with imipramine, fluoxetine, and fluvoxamine. Synapse. 1994;18:294–297. doi: 10.1002/syn.890180404. [DOI] [PubMed] [Google Scholar]
- 3.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 8.Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacol. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 9.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of k-opioid receptor antagonists in behavioral models of unlearned and learned fear in rats. J Pharmacol Exper Ther. 2007 doi: 10.1124/jpet.107.127415. in press. [DOI] [PubMed] [Google Scholar]
- 10.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacol. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 11.Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 12.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Mah L, Zarate CA, Jr, Singh J, Duan YF, Luckenbaugh DA, Manji HK, Drevets WC. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 15.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 16.Routtenberg A, Lindy J. Effects of availability of rewarding septal and hypothalamic stimulation on bar pressing for food under conditions of deprivation. J Compar Physiolog Psychol. 1965;60:158–161. doi: 10.1037/h0022365. [DOI] [PubMed] [Google Scholar]
- 17.Carlisle HJ, Snyder E. The interaction of hypothalamic self-stimulation and temperature regulation. Experentia. 1970;26:1092–1093. doi: 10.1007/BF02112693. [DOI] [PubMed] [Google Scholar]
- 18.Gallistel CR, Freyd G. Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol Biochem Behav. 1987;26:731–741. doi: 10.1016/0091-3057(87)90605-8. [DOI] [PubMed] [Google Scholar]
- 19.Tomasiewicz HC, Mague SD, Cohen BM, Carlezon WA., Jr Behavioral effects of acute and sub-acute administration of lithium and valproic acid in rats. Brain Res. 2006;1093:83–94. doi: 10.1016/j.brainres.2006.03.102. [DOI] [PubMed] [Google Scholar]
- 20.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 21.Roybal K, Theobold D, Birnbaum S, DiNieri JA, Graham A, Russo S, Vaishnav K, Kumar A, Peevey J, Oehrlein N, Vitaterna M, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK function. Proc Nat Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacol. 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- 23.Shippenberg TS, LeFevour A, Heidbreder C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- 24.McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacol. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacol. 2007 doi: 10.1038/sj.npp.1301659. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. San Diego, CA: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- 27.Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiol Behav. 1986;37:85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- 28.Carlezon WA, Jr, Todtenkopf M, McPhie DL, Pimentel P, Pliakas AM, Stellar J, Trzcinska M. Downregulation of GluR1 expression in the ventral tegmental area following repeated rewarding brain stimulation. Neuropsychopharmacol. 2001;25:234–241. doi: 10.1016/S0893-133X(01)00232-9. [DOI] [PubMed] [Google Scholar]
- 29.Mague SD, Andersen SL, Carlezon WA., Jr Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57:120–125. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 30.Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Molec Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- 31.Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edmonds DE, Gallistel CR. Parametric analysis of brain stimulation reward in the rat: III. Effect of performance variables on the reward summation function. J Comp Physiol Psychol. 1974;87:876–883. doi: 10.1037/h0037217. [DOI] [PubMed] [Google Scholar]
- 33.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1987;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 34.Wise RA. Neuroleptics and operant behavior: The anhedonia hypothesis. The Behavioral and Brain Sciences. 1982;5:39–87. [Google Scholar]
- 35.Mizrahi R, Rusjan P, Agid O, Graff A, Mamo DC, Zipursky RB, Kapur S. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. Am J Psychiatry. 2007;164:630–637. doi: 10.1176/ajp.2007.164.4.630. [DOI] [PubMed] [Google Scholar]
- 36.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 37.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, Ye N, Lange N, Cohen BM. Dynorphinergic GABA neurons are a target of both typical and atypical antipsychotic drugs in the nucleus accumbens shell, central amygdaloid nucleus and thalamic central medial nucleus. Neuroscience. 2003;121:991–998. doi: 10.1016/s0306-4522(03)00397-x. [DOI] [PubMed] [Google Scholar]
- 40.Cohen BM, Murphy B. The effects of pentazocine, a kappa agonist, in patients with mania. Int J Neuropsychopharmacol. 2007 doi: 10.1017/S1461145707008073. in press. [DOI] [PubMed] [Google Scholar]
- 41.Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- 42.Preston KL, Umbricht A, Schroeder JR, Abreu ME, Epstein DH, Pickworth WB. Cyclazocine: comparison to hydromorphone and interaction with cocaine. Behav Pharmacol. 2004;15:91–102. doi: 10.1097/00008877-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Chappell PB, Leckman JF, Scahill LD, Hardin MT, Anderson G, Cohen DJ. Neuroendocrine and behavioral effects of the selective kappa agonist spiradoline in Tourette' syndrome: a pilot study. Psychiatry Res. 1993;47:267–280. doi: 10.1016/0165-1781(93)90084-t. [DOI] [PubMed] [Google Scholar]
- 44.Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacol. 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- 45.Vortherms TA, Roth BL. Salvinorin A: from natural product to human therapeutics. Mol Interv. 2006;6:257–265. doi: 10.1124/mi.6.5.7. [DOI] [PubMed] [Google Scholar]