Abstract
In regulated vesicle exocytosis, SNARE protein complexes drive membrane fusion to connect the vesicle lumen with the extracellular space. The triggering of fusion pore formation by Ca2+ is mediated by specific isoforms of synaptotagmin (Syt), which employ both SNARE complex and membrane binding. Ca2+ also promotes fusion pore expansion and Syts have been implicated in this process but the mechanisms involved are unclear. We determined the role of Ca2+-dependent Syt-effector interactions in fusion pore expansion by expressing Syt-1 mutants selectively altered in Ca2+-dependent SNARE binding or in Ca2+-dependent membrane insertion in PC12 cells that lack vesicle Syts. The release of different-sized fluorescent peptide-EGFP vesicle cargo or the vesicle capture of different-sized external fluorescent probes was used to assess the extent of fusion pore dilation. We found that PC12 cells expressing partial loss-of-function Syt-1 mutants impaired in Ca2+-dependent SNARE binding exhibited reduced fusion pore opening probabilities and reduced fusion pore expansion. Cells with gain-of-function Syt-1 mutants for Ca2+-dependent membrane insertion exhibited normal fusion pore opening probabilities but the fusion pores dilated extensively. The results indicate that Syt-1 uses both Ca2+-dependent membrane insertion and SNARE binding to drive fusion pore expansion.
INTRODUCTION
Neurotransmitter and peptide hormone secretion is mediated by the Ca2+-dependent exocytosis of vesicles at the plasma membrane. Vesicle fusion proceeds through membrane intermediates of stalk formation, fusion pore opening, and fusion pore expansion (Lindau and Alvarez de Toledo, 2003). In classical modes of vesicle exocytosis, fusion pores expand to the point where the vesicle membrane flattens on the plasma membrane (full fusion), leading to complete luminal contents release (full release). However, vesicle exocytosis can utilize alternative modes in which the fusion pore either abruptly closes (kiss-and-run) or in which the fusion pore dilates but subsequently recloses (cavicapture; Henkel and Almers, 1996). These transient modes of vesicle exocytosis lead to the partial release of luminal contents depending on the size and diffusibility of the cargo (Alvarez de Toledo et al., 1993; Barg et al., 2002; Taraska et al., 2003). Ca2+ levels regulate fusion pore expansion as well as fusion pore formation (Fernandez-Chacon and Alvarez de Toledo, 1995; Hartmann and Lindau, 1995; Wang et al., 2006), but the mechanisms underlying fusion pore expansion, which is the more energetically demanding step, are poorly understood (Cohen and Melikyan, 2004).
A Ca2+-dependent fusion machinery mediates the triggered formation of fusion pores. Three SNARE proteins (VAMP-2 on the vesicle with SNAP25 and syntaxin-1 on the plasma membrane) form trans complexes that promote close membrane apposition and membrane fusion (Weber et al., 1998). Members of the synaptotagmin (Syt) protein family that localize to vesicles function as Ca2+ sensors that couple Ca2+ rises to vesicle exocytosis (Chapman, 2002; Sudhof, 2004; Rizo et al., 2006). Syt-1 is the best understood isoform and functions as a synaptic vesicle Ca2+ sensor for the rapid synchronous release (Geppert et al., 1994) but not for the slow asynchronous release of neurotransmitter (Sun et al., 2007) in hippocampal neurons. Syt-2 and -9 likely play similar roles at other synapses (Xu et al., 2007). Syt-1 and -9 also cofunction as essential Ca2+ sensors for dense-core vesicle exocytosis in PC12 cells (Lynch and Martin, 2007), whereas Syt-1 and -7 contribute to Ca2+-triggered dense-core vesicle exocytosis in chromaffin cells (Schonn et al., 2008). Syts-1, -7, and -9 have also been reported to mediate Ca2+-triggered dense-core vesicle exocytosis in pancreatic β-cells (Iezzi et al., 2005; Xiong et al., 2006; Gustavsson et al., 2008). The function of most other members of the 17 member Syt family remains to be clarified (Craxton, 2007).
Syts contain two cytoplasmic C2 domains, C2A and C2B, each of which are eight-stranded β-sandwiches connected by three flexible loops. The Ca2+-binding Syt isoforms such as Syt-1 contain conserved Ca2+-binding aspartate residues in loops 1 and 3 of the C2 domains. Ca2+ binding imparts a positive electrostatic potential to the C2 domain surface that enables the binding of Syt-1 to its effectors by neutralizing acidic residues in the Ca2+-binding loops (Rizo and Sudhof, 1998; Bai and Chapman, 2004). The two major effectors for Syt-1 are acidic residues on the surface of SNARE complexes and anionic phospholipids in the plasma membrane.
Ca2+ binding to Syt-1 promotes the binding of tandem C2 domains to syntaxin-1 and SNAP25 as well as to SNARE protein complexes (Davis et al., 1999; Gerona et al., 2000; Earles et al., 2001; Bai et al., 2004). Ca2+-dependent Syt-1–SNARE interactions are mediated in part by acidic residues in the SNAP25 C-terminus binding to basic residues in Syt-1 C2A loop 2 (Zhang et al., 2002; Lynch et al., 2007). Syt-1 mutations that eliminate Ca2+-dependent SNARE binding abrogate Ca2+-triggered dense-core vesicle exocytosis in PC12 cells (Lynch et al., 2007), whereas mutations that enhance SNARE binding facilitate Ca2+-triggered synaptic vesicle exocytosis in neurons (Pang et al., 2006). The Ca2+ regulation of liposome fusion mediated by Syt-1 requires Syt-1–SNARE interactions (Bhalla et al., 2006). These findings indicate an essential role for SNARE binding in determining Ca2+-dependent vesicle fusion probabilities.
Anionic phospholipids are a second important Syt-1 effector for Ca2+-triggered vesicle fusion. The Ca2+-dependent switch in electrostatic surface potential of Syt-1 promotes interactions with acidic phospholipid headgroups (Zhang et al., 1998) and results in the partial insertion of hydrophobic residues at the tips of C2 domain loops 1 and 3 (M173/F234/V304/I367) into the lipid bilayer (Bai et al., 2002; Herrick et al., 2006). Bilayer penetration by the tandem C2 domains induces positive curvature in membranes, which may lower the activation energy required for fusion (Martens et al., 2007). Syt-1 mutants with alanines at the tips of loops 1 and 3 fail to induce membrane curvature or to confer Ca2+ regulation on SNARE-mediated liposome fusion (Martens et al., 2007). Conversely, tryptophan replacements enhance Ca2+-dependent lipid binding and increase the Ca2+-dependent probability of synaptic vesicle fusion in neurons (Rhee et al., 2005).
Beyond their role in determining Ca2+-dependent vesicle fusion probabilities, Syts also regulate fusion pore expansion (Jackson and Chapman, 2006). Syt-1 overexpression increases the lifespan of dense-core vesicle fusion pores inferred from measurements of a prespike foot (PSF) in amperometric recordings of catecholamine release from PC12 cells (Wang et al., 2001). These overexpression effects of Syt-1 require Ca2+ binding residues and proper inter-C2 domain spacing (Wang et al., 2003, 2006; Bai et al., 2004). It remains to be determined whether endogenous Syt-1 regulates dense-core vesicle fusion pore expansion and if so, whether Syt-1 utilizes membrane binding and curvature induction, or interactions with SNAREs, or both. To address these issues, we introduced Syt-1 with mutations that selectively alter Ca2+-dependent membrane insertion or SNARE interactions into PC12 cells that lack vesicle Syts (Lynch and Martin, 2007). We used fluorescent probes of distinct sizes to assess the extent of fusion pore dilation. The data indicate that reduced Syt-1–SNARE interactions markedly decrease normal fusion pore dilation, whereas increased Syt-1-membrane insertion drives extensive fusion pore opening. We conclude that Syt-1 uses both Ca2+-dependent membrane bending and SNARE binding to drive fusion pore expansion.
MATERIALS AND METHODS
Antibodies and DNA Constructs
Antibodies used were Syt-1 monoclonal (clone 604.1; Synaptic Systems, Göttingen, Germany), Syt-1 N-terminal polyclonal (Sigma-Aldrich, St. Louis, MO) and a Syt-1 polyclonal antibody generated using a Syt-1 C2AB fusion protein. The plasmid encoding atrial natriuretic factor (ANF)-enhanced green fluorescent protein (EGFP) was provided by E. Levitan (University of Pittsburgh School of Medicine, Pittsburgh, PA) and the plasmid encoding brain-derived neurotrophic factor (BDNF)-EGFP by V. Lessmann (Johannes Gutenberg Universität, Mainz, Germany). Oligonucleotides encoding Syt-1/9 shRNAs (Lynch and Martin, 2007) were ligated into pSHAG-1 vector (Paddison et al., 2002) via BamHI and BseRI sites. The open reading frame of Syt-1 was reverse-transcribed and PCR-amplified from rat PC12 cells and ligated into pcDNA3.1 (Invitrogen, Carlsbad, CA). The QuickChange Site-Directed Mutagenesis method (Stratagene, La Jolla, CA) was used to generate the Syt-1 silent mutant, pcDNA3-syt I sm (Lynch and Martin, 2007), and the R199A/K200A, M173A/F234A/V304A/I367A and M173W/F234W/V304W/I367W mutations were introduced into the pcDNA3-syt I sm construct.
Fusion Proteins and Liposome-binding Assays
Constructs encoding C2AB fusion proteins for production in E. coli corresponding to Syt-1RK, Syt-14A, and Syt-14W have been described (Lynch et al., 2007; Martens et al., 2007). The preparation of liposomes containing ∼80 copies of SNAP25/syntaxin-1 in 85% 1,2-dioleoyl-sn-glycero-phosphatidylcholine (DOPC) and 15% 1,2-dioleoyl-sn-glycero-phosphatidylserine (DOPS) from Avanti Polar Lipids (Alabaster, AL) has been described (Weber et al., 1998; Lynch et al., 2007). Protein-free liposomes of indicated composition were similarly prepared by detergent dialysis. Binding studies were conducted with 10 μM wild-type or mutant C2AB incubated with ∼1.25 mM liposomes in 25 mM HEPES, pH 7.2, 100 mM KCl, and 0.2 mM EGTA plus CaCl2 for 30 min at room temperature. Free ionic Ca2+ concentrations were calculated with the CHELATOR program (Schoenmakers et al., 1992). Bound C2AB was isolated by buoyant density flotation on Accudenz gradients (40, 30, and 0% vol/vol) by centrifugation at 190,000 × g at 4°C for 4 h (Lynch et al., 2007). Material at the 0/30% interface was collected, analyzed by SDS gel electrophoresis, and stained with colloidal Commassie Blue for quantification with a Molecular Dynamics SI Densitometer using Image Quant software (Sunnyvale, CA).
Cell Culture, Transfection, Immunoblot Analysis, and Immunocytochemistry
PC12 cells were cultured in DMEM supplemented with 5% horse serum and 5% calf serum. Transfection was conducted by electroporation using an Electroporator II (Invitrogen). The Syt-1/9-null PC12 cell line was isolated as described previously (Lynch and Martin, 2007). Syt-1 and -9 are the major Syt isoforms on dense-core vesicles in PC12 cells and their elimination by short hairpin RNA (shRNA) knock down results in a full loss of Ca2+-triggered vesicle exocytosis (Lynch and Martin, 2007). Although Syt-7 overexpression affects Ca2+-dependent vesicle exocytosis in PC12 cells (Fukuda et al., 2004; Wang et al., 2005), Syt-7 is present endogenously at very low levels (37- and 21-fold less than Syt-1 and -9, respectively; Tucker et al., 2003) and contributes very little to Ca2+-triggered dense-core vesicle exocytosis based on shRNA knock down (Supplemental Figure S1).
Protein expression levels were determined from total cell lysates prepared in 1 mM PMSF and 1% Triton X-100 and clarified by centrifugation at 16,000 × g for 5 min. Twenty micrograms of total protein determined by bicinchoninic acid (BCA) assay (Pierce Chemical, Rockford, IL) was loaded per lane for gel electrophoresis. Immunoblot analysis was conducted by standard methods. For immunocytochemistry, cells were plated on poly-dl-lysine- and collagen-coated coverslips. Cells were washed with PBS, fixed with 4% formaldehyde (wt/vol), permeabilized with 0.3% Triton X-100 in PBS, and blocked in 10% FBS in PBS. Primary and secondary antibodies were diluted in FBS blocking solution. Coverslips were mounted on slides with Mowiol 4-88 Reagent (Calbiochem, EMD Chemicals, La Jolla, CA), and cells were imaged on a C1 laser scanning confocal microscope with a 60× oil immersion objective with NA 1.4 (Nikon Instruments, Melville, NY).
Exocytosis and Dextran Uptake Assays
Cells were transiently transfected and plated on 35-mm glass-bottom dishes (MatTek, Ashland, MA) coated with poly-dl-lysine and collagen. After 48 h, cells were imaged on a Nikon TIRF microscope evanescent wave imaging system on a TE2000-U inverted microscope (Nikon Instruments) with an Apo TIRF 100×, NA 1.45 objective lens. EGFP fluorescence was excited with the 488-nm laser line of an argon ion laser. Calibration studies indicated that the exponential evanescent field of the total internal reflection fluorescence (TIRF) optical system had a penetration depth (1/e) of ∼80 nm in 1.37 refractive index medium equivalent to cytosol. Cells were imaged in basal media (15 mM HEPES, pH 7.4, 145 mM NaCl, 5.6 mM KCl, 2.2 mM CaCl2, 0.5 mM MgCl2, 5.6 mM glucose, 0.5 mM ascorbic acid, and 0.1% BSA) or depolarization medium (same as basal medium with 95 mM NaCl and 56 mM KCl). Images were acquired at 75- or 250-ms intervals with a CoolSNAP-ES digital monochrome CCD camera system (Photometrics, Tucson, AZ) controlled by Metamorph software (Molecular Devices, Downingtown, PA). Vesicles were identified manually in sequence stacks of images, and their fluorescence determined as circular regions of interest using Metamorph software. All fluorescence values were corrected by background subtraction using circular regions of interest in regions of the image lacking vesicles.
For dextran dye uptake, transfected cells expressing BDNF-EGFP were incubated for 5 min at room temperature in depolarization medium containing Texas red dextrans (50 μM) from Molecular Probes (Invitrogen). Cells were washed with PBS and imaged on a Nikon C1 laser scanning confocal microscope with a 100× oil immersion objective with NA 1.4. The number of BDNF-EGFP–containing vesicles that captured 10-, 40-, and 70-kDa Texas red dextrans was determined from a bottom confocal section of the cell. The average number of exocytic events occurring in 5-min stimulations was determined by TIRF microscopy as described above and was used to normalize the dextran capture results.
Structures for ANF-EGFP and BDNF-EGFP are not available. We estimated a molecular diameter for an ANF-EGFP monomer of ∼6 nm and for a BDNF-EGFP dimer of ∼8 nm based on empirical plots of Stokes radius versus molecular mass, assuming that these are globular proteins. Molecular diameters for Texas red dextrans were estimated with the equation: r (in nm) = 0.033 (MW in Da)0.463 (Granath and Kvist, 1967).
RESULTS
Syt-1 Mutations Selectively Alter Ca2+-dependent SNARE or Membrane Interactions In Vitro
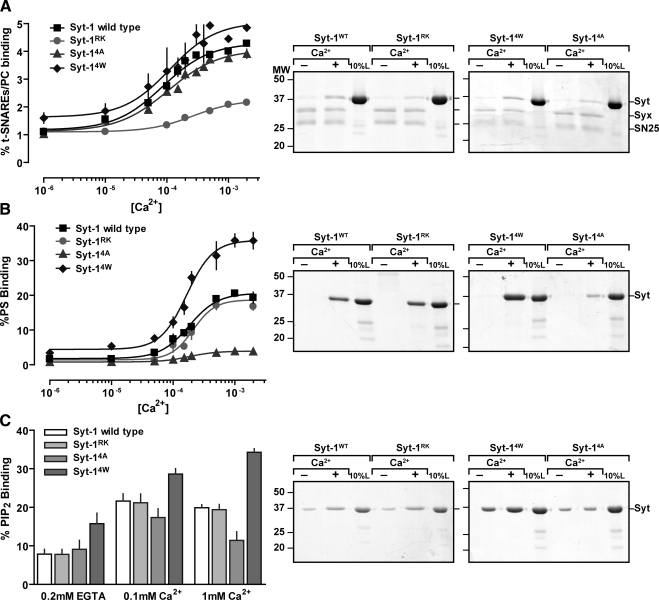
Our strategy was to examine vesicle fusion pore dilation in PC12 cells that harbored mutant versions of Syt-1 that are selectively altered in Ca2+-dependent SNARE or Ca2+-dependent membrane interactions. To selectively alter Syt-1–membrane interactions, we used Syt-1 mutants with tryptophan (called Syt-14W) or alanine (called Syt-14A) substitutions for the hydrophobic residues (M173, F234, V304, and I367) at the tips of loops 1 and 3 of the C2 domains (Martens et al., 2007). A Syt-1 C2AB protein with 4W mutations exhibited enhanced Ca2+-dependent PC/PS liposome binding, whereas binding to PC/PS liposomes by C2AB with 4A mutations was reduced (Figure 1B) as previously reported (Martens et al., 2007). Similarly, Ca2+-dependent binding of C2AB to PC/PIP2 liposomes was enhanced by 4W mutations and reduced by 4A mutations (Figure 1C). Syt-14A C2AB also fails to promote membrane curvature on liposomes in the presence of Ca2+, whereas Syt-14W C2AB causes extensive liposome tubulation (Martens et al., 2007). By contrast, the Ca2+-dependent binding of either C2AB with 4A mutations or C2AB with 4W mutations to SNARE-containing PC liposomes was indistinguishable from binding by wild-type C2AB (Figure 1A). The results indicated that Syt-14A and -14W are selective loss-of-function and gain-of-function mutants, respectively, for Ca2+-dependent membrane interactions and curvature induction, but they retain wild-type Ca2+-dependent SNARE-binding properties.
Figure 1.
Syt-1 mutants exhibit selective alterations in either Ca2+-dependent SNARE interactions or membrane insertion. Syt-1 C2AB fusion proteins, either wild-type or the indicated mutant versions (Syt-1RK, -14A, -14W), were incubated at 10 μM with the indicated Ca2+ concentrations with (A) PC liposomes containing syntaxin-1 and SNAP25, (B) protein-free PC/PS (85:15) liposomes, or (C) protein-free PC/PIP2 (95:5) liposomes. Liposome-bound C2AB was isolated from Accudenz gradients and analyzed by SDS-PAGE and Coomassie staining to determine % bound C2AB, indicated as means (±SE, n = 3). Representative gels from Syt-1 C2AB binding studies conducted with EGTA (−) or 1 mM Ca2+ (+) are shown in the right-hand panels comparing 25% of bound samples with 10% of input Syt-1 C2AB.
To selectively reduce Syt-1–SNARE interactions, we used previously characterized Syt-1 mutants (Lynch et al., 2007). Syt-1 C2A loop 2 mutations (R199A/K200A) combined with C2B loop 1 mutations (K297A/K301A; called Syt-1RK/KK) completely abolish Ca2+-dependent binding to SNARE complexes in vitro and eliminate Ca2+-triggered vesicle exocytosis in cells (Lynch et al., 2007). To reduce, but not eliminate, Ca2+-triggered vesicle exocytosis, we used a partial loss-of-function Syt-1 mutant (called Syt-1RK) that contains the C2A loop 2 mutations (R199A/K200A). A C2AB fusion protein with RK mutations exhibited strongly attenuated Ca2+-dependent binding to SNARE-containing PC liposomes in vitro (Figure 1A). By contrast, C2AB with RK mutations exhibited full wild-type Ca2+-dependent binding to PC/PS (85:15) liposomes (Figure 1B) or to PC/PIP2 (95:5) liposomes (Figure 1C). The results confirmed that Syt-1RK is a partial loss-of-function mutant that is selectively altered in Ca2+-dependent SNARE binding but not Ca2+-dependent membrane interactions (Lynch et al., 2007).
Syt Effector Interactions Regulate Fusion Pore Opening
To determine the function of Syt-1 mutant proteins, we expressed them in substitution for endogenous vesicle Syts in PC12 cells. shRNA-expressing PC12 cell lines that lack vesicle Syts (Syt-1 and -9) do not exhibit Ca2+-triggered vesicle exocytosis but can be fully rescued by expression of Syt-1 or -9 constructs with silent mutations that by-pass the shRNA targeting (Lynch and Martin, 2007). Thus, we tested Syt-1 rescue constructs that encode Syt-1RK, -14A, and -14W. Wild-type and mutant Syts were expressed at levels comparable to those in wild-type PC12 cells and were targeted to dense-core vesicles in the Syt-1/9-null cells (Supplemental Figure S2; Lynch et al., 2007).
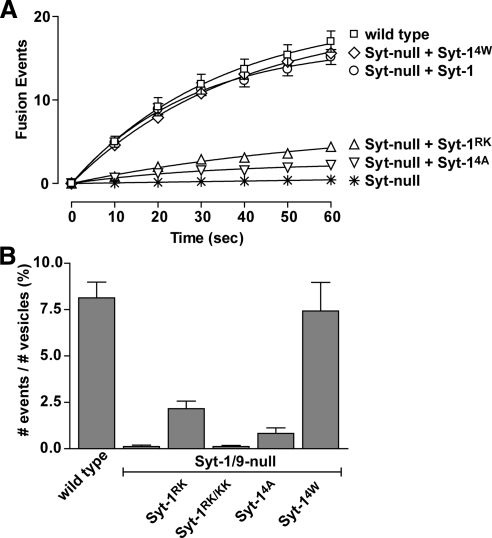
Our initial studies of dense-core vesicle exocytosis used ANF-EGFP cargo, which was similarly expressed and packaged into the dense-core vesicles in wild-type and Syt mutant-expressing cells (Supplemental Figure S3A). Incubation in depolarizing high K+ buffer was used to promote Ca2+ influx and trigger dense-core vesicle exocytosis. Exocytic events were detected as fluorescence changes in ANF-EGFP by TIRF microscopy (Lynch et al., 2007; Lynch and Martin, 2007). The time course of averaged cumulative fusion events over the first 60 s of incubation in high K+ buffer revealed that wild-type PC12 cells exhibited 17.0 ± 1.4 (±SE, n = 20) events within the adherent cell footprint (Figure 2A). Syt-1/9-null cells exhibited only 0.4 ± 0.2 (±SE, n = 20) events but Syt-1 expression in Syt-1/9-null cells fully restored Ca2+-triggered vesicle exocytosis with an average of 15.2 ± 1.5 (±SE, n = 20) fusion events (Figure 2A), confirming previous results (Lynch et al., 2007). Expression of Syt-14A, which is loss-of-function for promoting membrane curvature, largely failed to restore Ca2+-triggered vesicle exocytosis (2.2 ± 0.1 fusion events, ±SE, n = 18; Figure 2A). By contrast, expression of Syt-14W, which is gain-of-function for promoting membrane curvature, fully rescued Ca2+-triggered exocytosis (15.6 ± 0.3 fusion events, ±SE, n = 10; Figure 2A). Expression of Syt-1RK, which is partial loss-of-function for SNARE binding, only partially restored Ca2+-triggered vesicle exocytosis (4.4 ± 1.1 fusion events, ±SE, n = 10; Figure 2A) similar to previous results (Lynch et al., 2007).
Figure 2.
Ca2+-dependent SNARE binding and membrane insertion by Syt-1 regulate fusion pore opening probabilities. (A) Cells expressing ANF-EGFP were stimulated with depolarization medium and TIRF images were acquired at 0.25-s intervals. Fusion events were counted as a flash of vesicle fluorescence in adherent cell footprints, and the cumulative sum of fusion events in 10-s intervals was determined. The sums of the average number of fusion events per cell over time (mean value ±SE) for wild-type cells (n = 20), Syt-null cells (n = 20), Syt-null cells expressing Syt-1RK (n = 20), Syt-null cells expressing Syt-1RK/KK (n = 10), Syt-null cells expressing Syt-14A (n = 18), and Syt-null cells expressing Syt-14W (n = 10) were plotted. Syt-null corresponds to cells down regulated for vesicle Syt-1 and -9. (B) Fusion probabilities after stimulation were calculated as the number of release events in 60 s divided by the number of plasma membrane-proximal vesicles (mean value ±SE) for each cell type.
Vesicle fusion probabilities after stimulation were calculated as the percent of plasma membrane-proximal resident vesicles in the evanescent field that exhibited exocytosis in 60 s (Figure 2B). The number of plasma membrane-proximal resident vesicles was the same for cells expressing wild-type or mutant Syts (Supplemental Figure S3C) and most (≥75%) of the exocytic events occurred from the plasma membrane–resident vesicles (not shown). Vesicle release probabilities were close to zero in cells lacking vesicle Syts (Figure 2B) and were only partially restored in cells expressing the SNARE-binding–deficient mutant Syt-1RK (Figure 2B). No restoration of vesicle release probabilities was observed for the stronger loss-of-function SNARE-binding mutant Syt-1RK/KK. Cells expressing the Syt-14A mutant, which is impaired in Ca2+-stimulated membrane interactions, also exhibited release probabilities that were very low (Figure 2B). By contrast, the Syt-14W mutant, which exhibits enhanced Ca2+-dependent membrane binding, was able to fully restore vesicle release probabilities (Figure 2B). The results indicated that Syt-1 regulates the probability of fusion pore formation through both Ca2+-dependent SNARE binding and Ca2+-dependent membrane insertion. Neither Ca2+-dependent Syt-1–SNARE interactions nor Ca2+-dependent Syt-1-membrane interactions could compensate for the loss of the other in initiating fusion pore opening.
Syt-1 Membrane Interactions Regulate the Extent of Release from Individual Dense-Core Vesicles
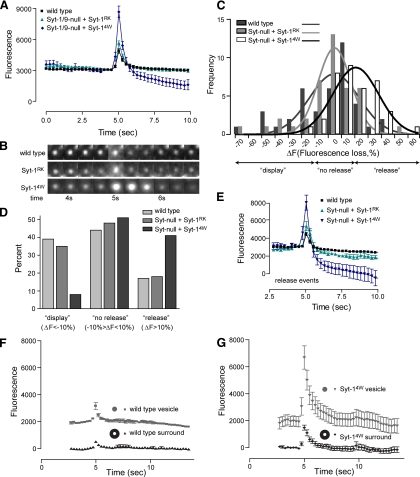
To characterize the role of Syt-1–effector interactions in regulating fusion pore expansion, we examined the kinetics of individual depolarization-evoked fusion events in Syt-1/9-null cells expressing loss-of-function Syt-1RK or gain-of-function Syt-14W mutants. ANF-GFP is a moderate-sized molecule (∼6 nm) whose release by exocytosis requires expansion of a fusion pore that is initially 1–2 nm in diameter (Breckenridge and Almers, 1987; Albillos et al., 1997). Individual exocytic events for ANF-EGFP, which were aligned by centering their peaks at 5 s, were characterized by a transient increase in fluorescence followed by a rapid decrease (Figure 3A). Increased fluorescence at the start of an exocytic event (at 4.25 s in Figure 3A) results from fusion pore formation and expansion with efflux of protons from the acidic dense-core vesicles (Barg et al., 2002; Taraska et al., 2003), which leads to enhanced EGFP fluorescence due to its pH dependence (Sawano and Miyawaki, 2000). Increases in fluorescence (to 5 s in Figure 3A) also occur as ANF-EGFP is released into the evanescent field. The ANF-EGFP quickly diffuses away from the site of release resulting in a subsequent decrease in fluorescence (5–5.75 s in Figure 3A). The majority of release events in wild-type PC12 cells terminate by cavicapture in which the fusion pore recloses with only partial release of cargo (Taraska et al., 2003). For such events, the fluorescence of ANF-EGFP remaining in the vesicle undergoes a slow further decrease due to vesicle reacidification (>6 s in Figure 3A).
Figure 3.
Syt-14W drives increased ANF-EGFP cargo release. (A) Single evoked dense-core vesicle exocytic events were imaged at a time resolution of 0.25 s in cells expressing ANF-EGFP after depolarization. The average vesicle fluorescence over time was plotted for wild-type cells (n = 40), Syt-null cells expressing Syt-1RK (n = 40), and Syt-null cells expressing Syt-14W (n = 40) by centering peak fluorescence at 5 s. Vesicle fluorescence was obtained by subtracting the background fluorescence. (B) Images of typical vesicles representing the conditions shown in A centered around 5 s are shown. (C) Distribution of vesicle fluorescence losses. The fluorescence values of ∼40 exocytosed vesicles from each of the conditions shown in A was determined at t = 0 and at t = 10 s. Changes in fluorescence (ΔF) were determined by subtraction and plotted as a frequency distribution. Minus values correspond to “display” events in which fluorescence at t = 10 s exceeded that at t = 0. Positive values correspond to “release” events in which fluorescence at t = 10 s decreased relative to that at t = 0. Mean ΔF values (±SD) for wild-type (−3.9 ± 24.9) and Syt-1RK (−2.8 ± 21.6) did not differ, whereas those for Syt-14W (+15.1 ± 16.8) differed significantly (p < 0.002) from wild-type and Syt-1RK by a general linear model analysis of variance using the Tukey post hoc test of values. (D) The percent of exocytic events from each of the conditions shown in A (for n = 40) was determined for events in which ΔF <−10% (display), ΔF = −10–10% (no release), and ΔF >10%. (E) The average fluorescence over time for all release events, where ΔF >10% was plotted for wild-type cells, Syt-null cells expressing Syt-1RK, and Syt-null cells expressing Syt-14W. Average fluorescence intensity before fusion corresponded to vesicle fluorescence with background subtracted. (F and G) The fluorescence of individual vesicle exocytic events (n = 20) from wild-type cells (F) or cells expressing Syt-14W (G) were analyzed by drawing two concentric circles, one encompassing the vesicle and the other encompassing vesicle plus surround. Fluorescence in the surround (annulus) obtained by subtraction of fluorescence in outer circle from that of inner circle depicted the diffusional spread of released ANF-EGFP.
Averaged time courses for individual exocytic events with ANF-EGFP cargo did not differ significantly for wild-type cells and cells expressing Syt-1RK (Figure 3A). For each, the fluorescence intensity after the event (at 10 s in Figure 3A) decreased to only a small extent, which indicated incomplete ANF-EGFP release that results from fusion pore reclosure. That fusion pore reclosure and vesicle reacidification had occurred was indicated by the ability of NH4Cl treatment, which dissipates the vesicle pH gradient, to enhance the fluorescence of exocytosed vesicles after they had dimmed (see below).
By contrast, cells expressing Syt-14W exhibited marked differences from wild-type cells in the averaged time courses for individual exocytic events (Figure 3A). Most prominently, there was a greater increase in ANF-EGFP fluorescence at the peak (at 5 s) of the event (Figure 3A). This enhanced fluorescence was due to much greater ANF-EGFP release into the evanescent field because it was accompanied by larger “puffs” of fluorescence near the vesicle (Figure 3B) and was followed by a substantial postfusion reduction in fluorescence (Figure 3A at 10 s). The greater reduction in postfusion vesicle ANF-EGFP fluorescence in Syt-14W–expressing cells was evident in a comparison of fluorescence loss (ΔF) values across all events in wild-type and cells expressing Syt-1RK or -14W (Figure 3C, open vs. shaded bars).
We binned exocytic events based on decreases in fluorescence at 5 s after the peak (Figure 3C) and termed these “display,” “no release” and “release” events (Figure 3, C and D). Seventeen percent of events in wild-type cells exhibited postfusion fluorescence that decreased by >10% compared with prefusion fluorescence (Figure 3D; termed “release”). Forty-four percent of wild-type events exhibited postfusion fluorescence that was similar to that of prefusion fluorescence (−10% > ΔF > 10%; termed “no release”), which correspond to vesicles that exhibit very partial content release before fusion pore reclosure. Lastly, 39% of wild-type events exhibited increased fluorescence (ΔF <−10%), which were previously termed “display” events (Perrais et al., 2004) that involve limited cargo release and persistent fusion pore opening. Syt-14W–expressing cells exhibited a marked shift in the distribution of events by reducing “display” events (from 39 to 8%) and increasing “release” events (from 17 to 41%; Figure 3D). When we compared “release” events across cell types (Figure 3E), it was apparent that these exocytic events in cells expressing Syt-14W exhibited fluorescence losses corresponding to the full release of ANF-EGFP.
The category termed “release” that exhibits fluorescence loss could include vesicles that leave the plasma membrane after exocytosis, and Syt-14W might increase such events. However, we did not detect vesicle departures when we imaged exocytosing vesicles with fluorescent cargo, BDNF-EGFP, that was not released (see below). To determine whether reductions in vesicle ANF-EGFP fluorescence were accompanied by the release of ANF-EGFP, we quantified fluorescence changes using two concentric circular regions of interest, one surrounding the vesicle and the other in the surround. An increase in fluorescence in the annulus surrounding the vesicle with its subsequent decline indicated that ANF-EGFP was released and then diffused away (Figure 3, F and G; black symbols). The release of ANF-EGFP was substantially greater for cells expressing Syt-14W compared with wild-type cells (Figure 3, F vs. G; black symbols). Overall, the results indicated that Syt-14W, a gain-of-function mutant for Ca2+-dependent membrane insertion, greatly increased the release of ANF-EGFP, a cargo molecule of ∼6 nm, which suggests that increased fusion pore dilation occurred.
Ca2+-dependent Interactions Regulate Fusion Pore Dilation
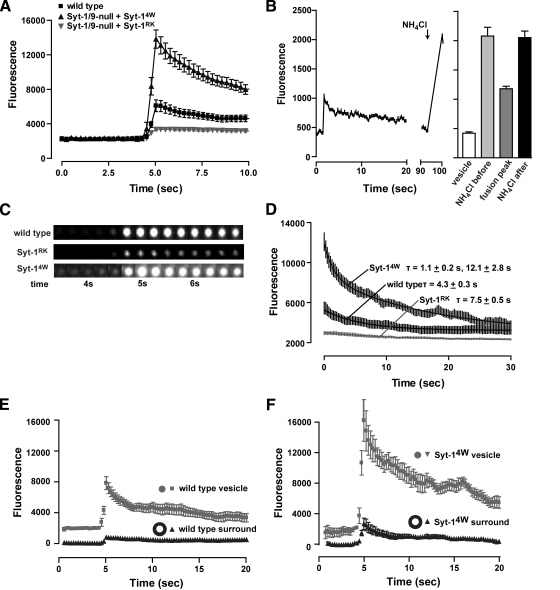
The analysis of individual exocytic events by fluorescence with ANF-EGFP cargo was complicated by its release into and diffusion out of the evanescent field. Thus, we next utilized cargo that was larger and released to a much lesser extent. A brain-derived neurotrophic factor fusion to EGFP (BDNF-EGFP) is somewhat larger than ANF-EGFP (∼8 nm as a dimer) but is also strongly hindered in its release from vesicles because of condensation in the vesicle core (Brigadski et al., 2005). BDNF-EGFP was uniformly packaged into dense-core vesicles independent of Syt wild-type or mutant status (Supplemental Figure S3B). We found that BDNF-EGFP was never fully released from vesicles in wild-type cells, which supported the conclusion that most exocytic events in PC12 cells terminate in cavicapture by fusion pore reclosure rather than in full fusion (Taraska et al., 2003). Depolarization of wild-type cells resulted in rapid increases in BDNF-EGFP fluorescence followed by a very slow decline (Figure 4A, ▪, and C). The brightening of vesicles resulted from proton efflux that was not complicated by the release of BDNF-EGFP. The subsequent very slow decline in fluorescence represented vesicle reacidification after fusion pore reclosure. This was indicated by the delayed application of NH4Cl to dissipate proton gradients in postfusion vesicles that had fully dimmed (Figure 4B). NH4Cl treatment increased the fluorescence of postfusion vesicles to an extent similar to that of NH4Cl-treated prefusion vesicles, which indicated that a proton gradient had been reestablished after fusion pore reclosure. Thus, except where noted below, vesicle BDNF-EGFP served as unreleased cargo whose fluorescence increases reported proton flux out of the vesicle.
Figure 4.
Syt-1–effector interactions regulate expansion of the fusion pore. (A) Single vesicle exocytic events were imaged by TIRF in cells expressing BDNF-EGFP at a time resolution of 0.25 s. The average vesicle fluorescence with time was plotted for wild-type cells (n = 40), Syt-null cells expressing Syt-1RK (n = 40), and Syt-null cells expressing Syt-14W (n = 40) by centering peak fluorescence at 5 s. Average vesicle fluorescence intensity was determined by subtraction of background. (B) Dense-core vesicles reacidify after exocytosis. Left panel, the fluorescence of a representative vesicle was plotted during an exocytic event and at a later time after it had dimmed in response to 50 mM NH4Cl treatment. Right panel, the fluorescence intensity (mean ±SE) of individual vesicles in cells expressing BDNF-EGFP was determined without treatment, after treatment with 50 mM NH4Cl (prefusion, n = 66), with depolarization buffer to induce exocytosis (fusion, n = 65), or with depolarization buffer followed in 10 min by NH4Cl treatment (postfusion, n = 38). (C) Images of typical vesicles representing the conditions shown in A centered around 5 s are shown. The diffuse fluorescence increase around Syt-14W vesicles indicated release of BDNF-EGFP. (D) The rate of the exponential decrease in fluorescence of the average individual fusion event for wild-type cells, Syt-null cells expressing Syt-1RK, and Syt-null cells expressing Syt-14W was determined by fitting a one- or two-component exponential decay curve to the data shown in A. Similar τ values were obtained for experiments with 13.3-Hz recordings. (E and F) The fluorescence of individual vesicle exocytic events (n = 20) from wild-type cells (E) or cells expressing Syt-14W (F) were analyzed by drawing two concentric circles, one encompassing the vesicle and the other encompassing vesicle plus surround. Fluorescence in the surround (annulus) obtained by subtraction of fluorescence in outer circle from that of inner circle depicted the diffusional spread of released BDNF-EGFP in Syt-14W–expressing but not wild-type cells.
Exocytic events in cells expressing Syt-1RK exhibited much smaller fluorescence increases than wild-type cells (Figure 4A, closed inverted triangles, and C). This was not due to decreased BDNF-EGFP content per vesicle in Syt-1RK–expressing cells (Supplemental Figure S3B). Instead, the reduced brightening of exocytosing vesicles in Syt-1RK–expressing cells appeared to be due to decreased proton efflux. That fusion pore dilation may be limiting for proton efflux was suggested by the finding that exocytic events in wild-type cells did not brighten vesicles as fully as NH4Cl-induced proton gradient dissipation (Figure 4B; Obermuller et al., 2005). Previous studies found that proton efflux is delayed relative to fusion pore formation and requires pore dilation (Barg et al., 2002). Moreover, dense-core vesicles contain substantial pH-buffering capacity, which slows the outward diffusion of protons through the pore (Wu et al., 2001). Thus, the reduced brightening of vesicles in Syt-1RK–expressing cells (Figure 4, A and C) may indicate that fusion pore dilation was reduced or shorter in duration. These results suggested that loss-of-function in SNARE binding impairs fusion pore expansion mediated by Syt-1.
By contrast, cells expressing Syt-14W exhibited fluorescence increases that were much greater than in wild-type cells (Figure 4A, ▴, and C). We interpreted this to indicate that Syt-14W markedly enhanced fusion pore expansion, which allowed greater proton efflux. However, a second contribution to the increased fluorescence in Syt-14W cells was from the partial release of BDNF-EGFP into the evanescent field as the result of extensive fusion pore dilation. This was indicated by three observations. First, cells expressing Syt-14W, but not those expressing Syt-1RK or wild-type, exhibited “puffs” of fluorescence near the vesicle indicating BDNF-EGFP release (Figure 4C). Second, studies of the fluorescence increases using two concentric circular regions of interest indicated that wild-type cells failed to release a diffusible puff of BDNF-EGFP (Figure 4E, ▴), whereas cells expressing Syt-14W released BDNF-EGFP into the annulus surrounding the vesicle (Figure 4F, ▴). Third, an analysis of fluorescence decreases after the peak (Figure 4D), indicated that decreases for wild-type and Syt-1RK cells exhibited a single exponential decay (τ = 4.3–7.5s) that approximated rates of vesicle reacidification (Fernandez-Alfonso and Ryan, 2006). By contrast, vesicles in Syt-14W cells exhibited a two-phase exponential decay (τ1 = 1.1 ± 0.2 s; τ2 = 12.1 ± 2.8 s; Figure 4D) in which the fast kinetic component likely represented diffusion of released BDNF-EGFP from sites of release. Overall, the results indicated that Syt-14W, a gain-of-function mutant for membrane insertion, strongly promoted fusion pore expansion that enabled release of BDNF-EGFP.
Measurement of Fusion Pore Dilation with Sized External Markers
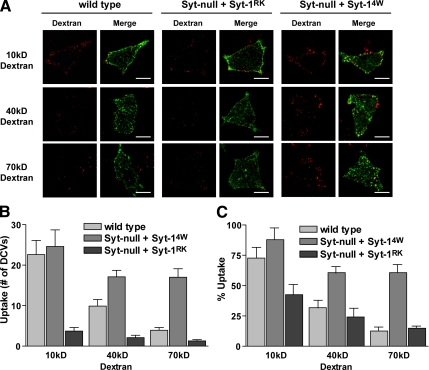
To determine the extent of fusion pore dilation in cells expressing Syt-14W and -1RK compared with wild-type cells, we took advantage of the finding that most exocytic events in PC12 cells terminated in cavicapture with fusion pore reclosure. Hence, a reclosed vesicle will capture external probes of different sizes based on the extent of fusion pore dilation before reclosure. We selectively monitored the capture of 10-, 40-, and 70-kDa Texas red dextrans by exocytosed vesicles that underwent cavicapture. Because exocytosed vesicles retain some BDNF-EGFP, we quantified red dextran uptake only into BDNF-EGFP–containing vesicles. In the absence of depolarizing stimulus, there was no dextran uptake into vesicles. During a 5-min incubation in depolarizing medium, an average of 22.6 ± 3.5 vesicles in wild-type cells and 24.6 ± 4.1 vesicles in Syt-14W–expressing cells captured a 10-kDa dextran (∼6 nm) during exocytosis (Figure 5, A and B). These numbers were similar to the number of exocytic events observed over a 5-min stimulation period, which were used to normalize the dextran uptake results (Figure 5C). The results indicated that most exocytic events in wild-type and Syt-14W–expressing cells had fusion pores that expanded to a diameter of at least ∼6 nm (Figure 5C). By contrast, only 3.7 ± 0.9 exocytosed vesicles captured 10-kDa dextran in cells expressing Syt-1RK (Figure 5, A and B). This was significantly smaller than the number of exocytic events observed for cells expressing Syt-1RK (Figure 5C) and indicated that not all fusion pores in these cells dilate to ∼6 nm. These results showed directly that Syt-1RK with reduced Ca2+-dependent SNARE binding exhibits impaired fusion pore expansion relative to wild-type cells.
Figure 5.
Syt-1-effector interactions regulate fusion pore dilation. (A) Wild-type cells, Syt-null cells expressing Syt-1RK, and Syt-null cells expressing Syt-14W that were also expressing BDNF-EGFP were incubated with dextrans (10, 40, and 70 kDa) in depolarization medium for 5 min. Representative images for the uptake of dextrans (red) into the BDNF-EGFP-containing (green) vesicles of the three cells types are shown. Scale bar, 10 μm. (B) The average number of dense-core vesicles that captured 10-, 40-, or 70-kDa dextran in a 5-min stimulation was determined for wild-type cells (n = 15 for 10 kDa, n = 35 for 40 kDa, n = 28 for 70 kDa), Syt–null cells expressing Syt-1RK (n = 18 for 10 kDa, n = 16 for 40 kDa, and n = 18 for 70 kDa), and Syt-null cells expressing Syt-14W (n = 15 for 10 kDa, n = 20 for 40 kDa, and n = 25 for 70 kDa) and expressed as the mean ±SE. (C) The data in B were normalized to the average number of fusion events that occur in each cell type in 5 min and expressed as a percentage.
In wild-type cells, only 9.9 ± 1.6 and 3.9 ± 0.7 vesicles captured 40- and 70-kDa dextrans during exocytosis, respectively (Figure 5, A and B). These results indicated that in wild-type cells only a small subset of fusion pores dilate to ∼9 nm (40-kDa dextran) and an even smaller subset to ∼12 nm (70-kDa dextran). By contrast, a much larger number of vesicles (17.1 ± 1.6 and 17.0 ± 2.1, respectively) captured 40- and 70-kDa dextrans in cells expressing Syt-14W (Figure 5, A–C). This indicated that the majority of fusion pores in Syt-14W–expressing cells dilate to or beyond ∼12 nm (70-kDa dextran). The results showed directly that a gain-of-function Syt-1 mutant that exhibits strong Ca2+-dependent membrane binding and promotes membrane curvature has a dramatic effect in promoting fusion pore expansion.
DISCUSSION
Capacitance studies revealed that elevated Ca2+ accelerates fusion pore expansion (Fernandez-Chacon and Alvarez de Toledo, 1995; Hartmann and Lindau, 1995), which was confirmed in amperometry studies in permeable PC12 cells, showing that Ca2+ reduces the lifetime of the PSF (Wang et al., 2006). That Syts regulate fusion pore expansion was suggested by the finding that Syt-1 overexpression in PC12 cells increased the lifetime of PSFs (Wang et al., 2001; Jackson and Chapman, 2006). Our studies focused on determining which Ca2+-dependent properties of Syt-1 mediate its role in promoting expansion of the fusion pore. We replaced endogenous vesicle Syts in PC12 cells with defined Syt-1 point mutants and assessed the extent to which fusion pore dilation occurred in evoked exocytic events. The major conclusion from this work was that Syt-1 utilizes both Ca2+-dependent SNARE interactions as well as Ca2+-triggered membrane insertion to drive expansion of the fusion pore. Both of these properties of Syt-1 are also essential for Ca2+-triggered fusion pore opening, which implies that the mechanisms underlying pore expansion by Ca2+-bound Syt-1 are a dynamic extension of those utilized for initial pore formation. Thus, Ca2+-bound Syt-1 drives a continuum of fusion pore opening followed by dilation through its interactions with SNAREs and target membrane.
The initial fusion pore for dense-core vesicles detected in capacitance studies exhibits a fluctuating conductance of ∼200 pS, which is equivalent to an aqueous channel of ∼1–2-nm diameter (Breckenridge and Almers, 1987; Albillos et al., 1997; Lindau and Alvarez de Toledo, 2003). Small molecules such as catecholamines experience limited release through the fusion pore, which has been detected as a PSF in amperometric recordings (Chow et al., 1992). Expansion of the pore to enable rapid further release of vesicle content has been detected by electrophysiological methods (Lindau and Alvarez de Toledo, 2003). Expansion of the pore has also been imaged by the differential release or uptake of different-sized fluorescent probes (Barg et al., 2002; Takahashi et al., 2002, 2003). In the current work, we utilized the release of ANF-EGFP, a cargo molecule of ∼6 nm, or of BDNF-EGFP, a larger cargo molecule that is inefficiently released. Because of its very limited release from vesicles, BDNF-EGFP effectively served as a probe for the movement of protons, which are very small (∼0.1 nm) but strongly buffered vesicle constituents. Because most dense-core vesicle exocytic events in PC12 cells occur by cavicapture, we also took advantage of the capture of external fluorescent probes to assess the extent to which fusion pore dilation occurred before reclosure.
The current work utilized Syt-1RK, a Syt-1 with mutations of basic residues R199 and K200 in C2A that specifically disrupt Ca2+-dependent SNARE binding without altering Syt-1 membrane interactions (Lynch et al., 2007). Cells expressing the partial loss-of-function Syt-1RK exhibited strongly reduced Ca2+-dependent probabilities for fusion pore opening (Figure 2B), but a sufficient number of evoked exocytic events remained to analyze the kinetics of individual events in detail. A key finding was that cells expressing Syt-1RK exhibited dramatic reductions in the initial brightening of BDNF-EGFP (Figure 4A) in evoked exocytic events. Because BDNF-EGFP release did not occur, this reduction of the initial brightening of BDNF-EGFP–containing vesicles likely indicated reduced proton efflux and reduced fusion pore expansion in cells expressing Syt-1RK. Some of the reduced brightening of exocytosing vesicles in Syt-1RK–expressing cells may also be due to decreases in the movement of vesicles toward the plasma membrane that would accompany fuller fusion pore dilation. More direct evidence for reduced fusion pore expansion was provided by the observed reduced vesicle capture of ∼6-nm dextran (Figure 5) by cells expressing Syt-1RK. These observations directly implicate Ca2+-dependent binding of Syt-1 to SNAREs as essential for the postfusion dilation of the fusion pore. Evidently Syt-1 interactions with membrane are inadequate to compensate for a loss in SNARE interactions. Previous studies showed that the overexpression of an inter-C2 domain linker Syt-1 mutant, which also exhibits reduced Ca2+-dependent SNARE binding, failed to extend the lifetime of PSFs compared with overexpressed wild-type Syt-1 (Bai et al., 2004). Syt-1RK replacement would likely extend the lifetime of PSFs because of its inability to promote fusion pore expansion but amperometry could not be utilized in the current work because of the loss in catecholamine loading into vesicles that occurs in Syt-1–deficient PC12 cells (Fukuda et al., 2002; Lynch and Martin, 2007).
We utilized Syt-14W and -14A mutants to selectively alter Ca2+-dependent membrane interactions. Previous studies showed that Syt-14A retained Ca2+-dependent liposome binding to a large extent but failed to induce positive membrane curvature (Martens et al., 2007). In the current work, Syt-14A exhibited reduced Ca2+-dependent liposome binding (Figure 1B), but the nonequilibrium binding assay used may have emphasized the increased membrane dissociation rates that would be expected of a Syt mutant that was loosely anchored to the membrane. In any case, the reduced or enhanced activity in bilayer penetration and membrane curvature for Syt-14A and -14W, respectively, likely represent the key properties of these mutants in functional studies. The role of Syt-1 membrane interactions in determining Ca2+-dependent fusion pore opening probabilities has previously been characterized. Mutation of hydrophobic residues in the Ca2+-binding C2 loops 1 and 3 to tryptophan increase the Ca2+-dependent probability of synaptic vesicle exocytosis (Rhee et al., 2005). The importance of Syt-1 membrane interactions for determining fusion pore opening probabilities was confirmed in the current work showing that cells expressing Syt-14A exhibited release probabilities near zero, whereas Syt-14W exhibited normal probabilities (Figure 2B). It is likely that cells expressing Syt-14W exhibited wild-type fusion probabilities, rather than enhanced probabilities reported for an Syt-16W mutant (Rhee et al., 2005), because maximal rather than graded stimulus strength was used to trigger exocytosis.
The role of Syt-1 membrane interactions in regulating fusion pore dynamics had not been previously determined. We found that the replacement of vesicle Syts with the gain-of-function Syt-14W mutant had a dramatic effect in promoting extensive fusion pore dilation. Cells with Syt-14W exhibited much more release of ANF-EGFP (∼6 nm; Figure 3) and, unlike wild-type cells, also released BDNF-EGFP (Figure 4). Whereas the majority of fusion pores in wild-type PC12 cells reach a diameter of 6 nm with very few dilating to 12 nm, many fusion pores in cells expressing Syt-14W routinely dilated to or beyond 12 nm based on dextran-capture studies (Figure 5). In addition, there may be increased proton flux through the fusion pore in Syt-14W–expressing cells compared with wild-type events based on the increased brightening of BDNF-EGFP–containing vesicles (Figure 4A). Taken together, these results indicate that the fusion pore expands to a greater diameter in cells expressing Syt-14W, and this is likely achieved by expansion at a greater rate. Even though full release of ANF-EGFP occurred for many vesicles in Syt-14W–expressing cells (Figure 3E), full fusion was not achieved as indicated by the limited release of BDNF-EGFP (Figure 4A). Thus, it was apparent that exocytosis with extensive fusion pore dilation in Syt-14W–expressing cells still terminated in cavicapture (Figure 5).
Our results with loss-of-function Syt-1RK and gain-of-function Syt-14W mutant proteins imply important roles for both Ca2+-dependent SNARE binding and Ca2+-triggered bilayer insertion by Syt-1 in regulating fusion pore expansion. The cluster of basic residues in C2A that mediates Ca2+-dependent SNARE interactions is orthogonal to the membrane-penetrating loops (Lynch et al., 2007), which would enable simultaneous interactions of Syt-1 with SNAREs and the target membrane, which has been shown in studies in vitro (Davis et al., 1999; Dai et al., 2007). Both interactions appear to function to promote fusion pore expansion but the exact mechanisms utilized are uncertain.
The composition of the fusion pore, whether lipidic or protein-lined or both, remains to be determined (Chernomordik et al., 2006). Recent studies suggested that transmembrane segments of syntaxin-1 line the fusion pore on the plasma membrane (Han et al., 2004). Continued Syt-1 interactions with SNAREs beyond initial fusion pore formation might drive the lateral separation of SNARE complexes to expand the pore. Syt-1 penetration of the plasma membrane by the tandem C2 domains would induce positive membrane curvature, possibly within a ring of SNARE complexes, to lower the activation barrier for bilayer fusion in opening of the fusion pore (Martens et al., 2007). The lateral tension of the curved plasma membrane bilayer would be expected to drive further expansion of the fusion pore (Chernomordik and Kozlov, 2005). Although higher resolution studies will be required to define the underlying mechanisms, the current work indicates that both SNAREs as well as membrane tension participate in pore expansion and its regulation by Syt-1.
Supplementary Material
ACKNOWLEDGMENTS
The authors appreciate the advice on statistical analysis provided by Dr. K. B. Strier. This work was supported by US Public Health Service Grant DK25861 to T.F.J.M. and by a Ruth L. Kirschstein predoctoral fellowship to K.L.L. H.T.M. and S.M. were supported by the Medical Research Council (UK) and by a long-term fellowship from the European Molecular Biology Organization (ALTF 21-2006) to S.M.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-03-0235) on September 17, 2008.
REFERENCES
- Albillos A., Dernick G., Horstmann H., Almers W., Alvarez de Toledo G., Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernandez-Chacon R., Fernandez J. M. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Bai J., Chapman E. R. The C2 domains of synaptotagmin—partners in exocytosis. Trends Biochem. Sci. 2004;29:143–151. doi: 10.1016/j.tibs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Bai J., Wang C. T., Richards D. A., Jackson M. B., Chapman E. R. Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron. 2004;41:929–942. doi: 10.1016/s0896-6273(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Bai J., Wang P., Chapman E. R. C2A activates a cryptic Ca2+-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA. 2002;99:1665–1670. doi: 10.1073/pnas.032541099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg S., Olofsson C. S., Schreiver-Abeln J., Wendt A., Gebre-Medhin S., Renstrom E., Rorsman P. Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron. 2002;33:287–299. doi: 10.1016/s0896-6273(02)00563-9. [DOI] [PubMed] [Google Scholar]
- Bhalla A., Chicka M. C., Tucker W. C., Chapman E. R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 2006;13:323–330. doi: 10.1038/nsmb1076. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature. 1987;328:814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Brigadski T., Hartmann M., Lessmann V. Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J. Neurosci. 2005;25:7601–7614. doi: 10.1523/JNEUROSCI.1776-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. R. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Kozlov M. M. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123:375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Zimmerberg J., Kozlov M. M. Membranes of the world unite! J. Cell Biol. 2006;175:201–207. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R. H., von Ruden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Cohen F. S., Melikyan G. B. The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement. J. Membr. Biol. 2004;199:1–14. doi: 10.1007/s00232-004-0669-8. [DOI] [PubMed] [Google Scholar]
- Craxton M. Evolutionary genomics of plant genes encoding N-terminal-TM-C2 domain proteins and the similar FAM62 genes and synaptotagmin genes of metazoans. BMC Genomics. 2007;8:259. doi: 10.1186/1471-2164-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Shen N., Arac D., Rizo J. A quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. J. Mol. Biol. 2007;367:848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. F., Bai J., Fasshauer D., Wolowick M. J., Lewis J. L., Chapman E. R. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- Earles C. A., Bai J., Wang P., Chapman E. R. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J. Cell Biol. 2001;154:1117–1123. doi: 10.1083/jcb.200105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Alfonso T., Ryan T. A. The efficiency of the synaptic vesicle cycle at central nervous system synapses. Trends Cell Biol. 2006;16:413–420. doi: 10.1016/j.tcb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R., Alvarez de Toledo G. Cytosolic calcium facilitates release of secretory products after exocytotic vesicle fusion. FEBS Lett. 1995;363:221–225. doi: 10.1016/0014-5793(95)00319-5. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kanno E., Satoh M., Saegusa C., Yamamoto A. Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+-dependent exocytosis in PC12 cells. J. Biol. Chem. 2004;279:52677–52684. doi: 10.1074/jbc.M409241200. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kowalchyk J. A., Zhang X., Martin T. F., Mikoshiba K. Synaptotagmin IX regulates Ca2+-dependent secretion in PC12 cells. J. Biol. Chem. 2002;277:4601–4604. doi: 10.1074/jbc.C100588200. [DOI] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Sudhof T. C. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Gerona R. R., Larsen E. C., Kowalchyk J. A., Martin T. F. The C terminus of SNAP25 is essential for Ca2+-dependent binding of synaptotagmin to SNARE complexes. J. Biol. Chem. 2000;275:6328–6336. doi: 10.1074/jbc.275.9.6328. [DOI] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J. Chromatograph. 1967;28:69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- Gustavsson N., Lao Y., Maximov A., Chuang J. C., Kostromina E., Repa J. J., Li C., Radda G. K., Sudhof T. C., Han W. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc. Natl. Acad. Sci. USA. 2008;105:3992–3997. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Wang C. T., Bai J., Chapman E. R., Jackson M. B. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science. 2004;304:289–292. doi: 10.1126/science.1095801. [DOI] [PubMed] [Google Scholar]
- Hartmann J., Lindau M. A novel Ca2+-dependent step in exocytosis subsequent to vesicle fusion. FEBS Lett. 1995;363:217–220. doi: 10.1016/0014-5793(95)00318-4. [DOI] [PubMed] [Google Scholar]
- Henkel A. W., Almers W. Fast steps in exocytosis and endocytosis studied by capacitance measurements in endocrine cells. Curr. Opin. Neurobiol. 1996;6:350–357. doi: 10.1016/s0959-4388(96)80119-x. [DOI] [PubMed] [Google Scholar]
- Herrick D. Z., Sterbling S., Rasch K. A., Hinderliter A., Cafiso D. S. Position of synaptotagmin I at the membrane interface: cooperative interactions of tandem C2 domains. Biochemistry. 2006;45:9668–9674. doi: 10.1021/bi060874j. [DOI] [PubMed] [Google Scholar]
- Iezzi M., Eliasson L., Fukuda M., Wollheim C. B. Adenovirus-mediated silencing of synaptotagmin 9 inhibits Ca2+-dependent insulin secretion in islets. FEBS Lett. 2005;579:5241–5246. doi: 10.1016/j.febslet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Chapman E. R. Fusion pores and fusion machines in Ca2+-triggered exocytosis. Annu. Rev. Biophys. Biomol. Struct. 2006;35:135–160. doi: 10.1146/annurev.biophys.35.040405.101958. [DOI] [PubMed] [Google Scholar]
- Lindau M., Alvarez de Toledo G. The fusion pore. Biochim. Biophys. Acta. 2003;1641:167–173. doi: 10.1016/s0167-4889(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Lynch K. L., Gerona R. R., Larsen E. C., Marcia R. F., Mitchell J. C., Martin T. F. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol. Biol. Cell. 2007;18:4957–4968. doi: 10.1091/mbc.E07-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K. L., Martin T. F. Synaptotagmins I and IX function redundantly in regulated exocytosis but not endocytosis in PC12 cells. J. Cell Sci. 2007;120:617–627. doi: 10.1242/jcs.03375. [DOI] [PubMed] [Google Scholar]
- Martens S., Kozlov M. M., McMahon H. T. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- Obermuller S., Lindqvist A., Karanauskaite J., Galvanovskis J., Rorsman P., Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J. Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- Paddison P. J., Caudy A. A., Bernstein E., Hannon G. J., Conklin D. S. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z. P., Shin O. H., Meyer A. C., Rosenmund C., Sudhof T. C. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D., Kleppe I. C., Taraska J. W., Almers W. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J. Physiol. 2004;560:413–428. doi: 10.1113/jphysiol.2004.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J. S., Li L. Y., Shin O. H., Rah J. C., Rizo J., Sudhof T. C., Rosenmund C. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. USA. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J., Chen X., Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Rizo J., Sudhof T. C. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- Sawano A., Miyawaki A. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res. 2000:28–e78. doi: 10.1093/nar/28.16.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers T. J., Visser G. J., Flik G., Theuvenet A. P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- Schonn J. S., Maximov A., Lao Y., Sudhof T. C., Sorensen J. B. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof T. C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sun J., Pang Z. P., Qin D., Fahim A. T., Adachi R., Sudhof T. C. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Kishimoto T., Nemoto T., Kadowaki T., Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297:1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- Taraska J. W., Perrais D., Ohara-Imaizumi M., Nagamatsu S., Almers W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc. Natl. Acad. Sci. USA. 2003;100:2070–2075. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker W. C., Edwardson J. M., Bai J., Kim H. J., Martin T. F., Chapman E. R. Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J. Cell Biol. 2003;162:199–209. doi: 10.1083/jcb.200302060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. T., Bai J., Chang P. Y., Chapman E. R., Jackson M. B. Synaptotagmin-Ca2+ triggers two sequential steps in regulated exocytosis in rat PC12 cells: fusion pore opening and fusion pore dilation. J. Physiol. 2006;570:295–307. doi: 10.1113/jphysiol.2005.097378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. T., Grishanin R., Earles C. A., Chang P. Y., Martin T. F., Chapman E. R., Jackson M. B. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- Wang C. T., Lu J. C., Bai J., Chang P. Y., Martin T. F., Chapman E. R., Jackson M. B. Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature. 2003;424:943–947. doi: 10.1038/nature01857. [DOI] [PubMed] [Google Scholar]
- Wang P., Chicka M. C., Bhalla A., Richards D. A., Chapman E. R. Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol. Cell. Biol. 2005;25:8693–8702. doi: 10.1128/MCB.25.19.8693-8702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Sollner T. H., Rothman J. E. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wu M. M., Grabe M., Adams S., Tsien R. Y., Moore H. P., Machen T. E. Mechanisms of pH regulation in the regulated secretory pathway. J. Biol. Chem. 2001;276:33027–33035. doi: 10.1074/jbc.M103917200. [DOI] [PubMed] [Google Scholar]
- Xiong X., Zhou K. M., Wu Z. X., Xu T. Silence of synaptotagmin I in INS-1 cells inhibits fast exocytosis and fast endocytosis. Biochem. Biophys. Res. Commun. 2006;347:76–82. doi: 10.1016/j.bbrc.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Xu J., Mashimo T., Sudhof T. C. Synaptotagmin-1, -2, and-9, Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang X., Kim-Miller M. J., Fukuda M., Kowalchyk J. A., Martin T. F. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34:599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]
- Zhang X., Rizo J., Sudhof T. C. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry. 1998;37:12395–12403. doi: 10.1021/bi9807512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.