Abstract
It is known that nuclear lipids play a role in proliferation, differentiation, and apoptotic process. Cellular nuclei contain high levels of phosphatidylcholine and sphingomyelin, which are partially linked with cholesterol and proteins to form lipid–protein complexes. These lipids are also associated with transcription factors and newly synthesized RNA but, up to date, their organization is still unknown. The aim of the present work was to study if these specific lipid–protein interactions could be nuclear membrane microdomains and to evaluate their possible role. The results obtained demonstrate for the first time the existence of nuclear microdomains characterized by a specific lipid composition similar to that of intranuclear lipid–protein complexes previously described. Nuclear microdomain lipid composition changes during cell proliferation when the content of newly synthesized RNA increases. Because previous data show a correlation between nuclear lipids and transcription process, the role of nuclear microdomains in cellular functions is discussed.
INTRODUCTION
Extensive research on biological membranes has led to the modification of the Singer-Nicolson fluid-mosaic model and has indicated that certain classes of lipids and proteins are not randomly distributed over the membrane but form distinct microdomains (Lichtenberg et al., 2005) that can exist in equilibrium (Brown, 2006). The most studied class of microdomain are the cholesterol (CHO) and sphingomyelin (SM)-enriched lipid rafts (Edidin, 2003). However, the distinct concepts of lipid rafts, detergent-resistant membranes and liquid-ordered lipid phases are often confused in the current literature (Lichtenberg et al., 2005). Rafts are described as transient detergent-resistant membrane microdomains enriched in sphingolipids and CHO, whereas “detergent-resistant membranes” are formed after detergent treatment and do not correspond to any membrane structure. In contrast, “liquid-ordered lipid phase domains” are the result of interactions between high levels of CHO and phospholipid fatty acyl chains (Lichtenberg et al., 2005). On the other hand, several lines of investigation have supported the idea that detergent-resistant membranes are not detergent-induced artifacts, but do exist as domains in cellular membranes (Brown and London, 1997). As visualization of rafts in living cells is difficult, their existence and function rely on indirect methods such as detergent extraction (Munro, 2003). However, Schuck et al. (2003) have found that various detergents differ considerably in their ability to selectively solubilize membrane proteins and enrich the content of sphingolipids and CHO. Insolubility in detergents like Triton X-100 was observed in lipid bilayers, which exist in physical states where lipid packing is tight (London and Brown, 2000). However, Braccia et al. (2003) have demonstrated that isolation of lipid rafts from microvillar membrane with “conventional” Triton X-100 at low temperature and with Brij 98 at 37°C have essentially similar profiles of protein and lipid components, suggesting that they are not “low temperature artifacts” but do exist at physiological temperature.
Membrane microdomains are thought to act as platforms for specific proteins (Edidin, 2003; Kenworthy et al., 2004) and to have different functions for protein and lipid sorting (Ikoen, 2001) in the regulation of cell signaling (Simons and Toomre, 2000). Today, it is widely recognized that lipids are new intranuclear components (Tamiya-Koizumi et al., 1989; Martelli et al., 2004; Albi and Viola Magni, 2004; Ledeen and Wu, 2004, 2006). However, these molecules are quickly metabolized in relation to the cellular function due to the activity of neutral-sphingomyelinase (N-SMase; Tamiya-Koizumi et al., 1989; Alessenko and Chatterjee, 1995; Romanenko et al., 1998; Tsugane et al., 1999; Neitcheva and Peeva, 2001; Mizutani et al., 2001; Albi and Viola Magni, 2004; Watanabe et al., 2004), sphingomyelin-synthase (SM-synthase; Kavok et al., 2003; Albi and Viola Magni, 2004), phosphatidylcholine-specific phospholipase C (PC-PLC; Albi and Viola Magni, 2004; Ramoni et al., 2004) and reverse sphingomyelin-synthase (RSM-synthase; Albi and Viola Magni, 2003a), enzymes responsible for SM and phosphatidylcholine (PC) metabolism. Recently, it has been demonstrated that SM/PC metabolism cross-talk occurs in the nucleus, regulating the intranuclear pool of ceramide/diacylglycerol (Albi et al., 2008). Nevertheless, these nuclear lipids affect cellular functions not only as intranuclear signaling molecules but also, by modifying the subnuclear structures (Albi and Viola Magni, 2006a). In fact, during cellular proliferation induced by partial hepatectomy, the activation of SMase changes SM and CHO levels, therefore modifying the fluidity of the nuclear membrane and consequently regulating the nucleus-cytoplasm efflux of mRNA (Albi and Viola Magni, 2006a). Because nuclear-cytoplasmic exchange is a critical cellular process for eukaryotes, this highlights the importance of these lipids (Terry et al., 2007). Moreover, in the same experimental conditions, modification of the CHO-SM/PC ratio during the S phase of the cell cycle causes decreased fluidity of the nuclear matrix, suggesting that it could represent a structure involved in DNA duplication (Albi et al., 2003b). In chromatin, two pools of CHO exist: an “SM-free CHO” pool that does not change during cellular proliferation and another “SM-linked CHO” pool that can change during the S-phase of the cell cycle in relation to N-SMase activation (Albi and Viola Magni, 2002).
Recently, the association between PC-SM-CHO and transcription process was demonstrated (Rossi et al., 2007a,b). During liver regeneration, an intranuclear complex was isolated in which N-SMase activity was responsible for STAT3 activation (Rossi et al., 2007b). Moreover, newly synthesized RNA was protected from RNase digestion through acquiring a double-strand structure that was stabilized by SM and CHO (Rossi et al., 2007a). As this intranuclear complex contains lamin B, it was suggested to correspond to transcription sites associated with the inner nuclear membrane (Rossi et al., 2007b), recently described as a transcription factor resting place (Heessen and Fornerod, 2007). Together, these observations led to the hypothesis that the nuclear lipids SM-CHO and PC are organized in microdomains that could regulate nuclear function.
MATERIALS AND METHODS
Materials
Chemicals, bovine serum albumin, CHO, PC, phenylmethylsulfonylfluoride (PMSF), SM, SMase, polyclonal anti-Signal transducer and activator of transcription-3 (STAT3), IgG peroxidase coniugate were obtained from Sigma Chemical Co. (St. Louis, MO). TLC plates (silica Gel G60) were from Merck (Darmstadt, Germany). Radioactive [Me-14C] SM (54.5 Ci/mol, 2.04 GBq/mmol), [Me-3H] (L-3-phosphatidyl [N-Me-3H] choline 1,2 dipalmitoyl, 81.0 Ci/mmol, 3.03 TBq/mmol), and [3H] UTP (41 Ci/mmol, 1.52 TBq/mmol) were from Amersham Pharmacia Biotech (Rainham, Essex, United Kingdom), Ecoscint A from National Diagnostic (Atlanta, GA). Monoclonal anti-lamin B was from Oncogene (Boston, MA), polyclonal anti-giantin from Covance (Berkeley, CA), and SDS-PAGE Molecular Weight Standard (Bio-Rad Laboratories, Hercules, CA).
Animals and Treatments
Sixty-day-old Sprague Dawley female rats (Harlan Nossan, Milan, Italy), kept at normal light-dark periods, were used. They had free access to pelleted food and water. [3H]uridine incorporation was studied in liver regeneration induced by partial hepatectomy corresponding to 75% of total liver, which stimulates liver cells to proliferate. Hepatectomy was performed between 8 and 10 a.m. as previously reported (Albi et al., 2002). Sham-operated animals were used as controls. Animals were injected intraperitoneally with 100 μCi [3H]uridine 23 h after operation, for 1 h and then killed. All the treatments were performed according to the international regulation of National Institutes of Health.
Preparation of Homogenate and Isolation of Nuclei and Subnuclear Fractions
The liver was homogenized in 10 mM Tris-HCl buffer, pH 7.4, containing 0.25 M sucrose, 1 mM EDTA, 0.1% ethanol, 0.1 M PMSF, and 0.2 M dithiothreitol using the Thomas homogenizer; the homogenate was filtered through two layers of surgical gauze. The hepatocyte nuclei, chromatin, nuclear membranes, and nuclear matrix were prepared as previously described (Albi et al., 2003b).
Purification of Triton X-100–insoluble Microdomains from Isolated Hepatocyte Nuclei
For the isolation of nuclear microdomains, hepatocyte nuclei were washed twice with Barnes solution (Barnes et al. 1957; 0.085 M KCl, 0.0085 M NaCl, 0.0025 M MgCl2, 0.005 M trichloroacetic acid-HCl, 0.1 M PMSF, pH 7.2) as previously reported (Albi et al., 2003b). This treatment, during which the nuclei are collected at 2000 × g avoids the presence of mitocondria and microsomes, which require a higher gravity value for sedimentation. The absence of cytoplasmic contamination was shown by electrophoretic analysis of RNA extracted from this preparation as previously reported (Albi et al., 2006a). The microdomains were prepared from normal and hepatectomized rats according to Braccia et al. (2003) with the following modifications: the extraction was carried out with Triton X-100 dissolved in distilled water (10% vol/vol), on ice. This solution was added to the purified nuclei to a final detergent concentration of 1% (vol/vol). The extract was placed in a cushion of 80% sucrose with a gradient of 15–40% sucrose on top. After centrifugation overnight, the gradients were collected in 12 1-ml fractions for subsequent analysis by SDS-PAGE and Western blotting. In other experiments floating fractions, corresponding to 3 ml, were carefully collected with a pipette, diluted five times with 25 mM HEPES-HCl, 150 mM NaCl, pH 7.1, and centrifuged at 100,000 × g for 120 min to obtain a pellet of microdomains for biochemical and electron microscopy analysis.
To test the purity of nuclear microdomain preparations, the activity of microsomal and/or mitocondria markers such as G6P and NADH-cytochrome C-reductase, respectively, was performed as previously reported (Albi et al., 2006b). Moreover, the presence of giantin as marker protein for Golgi membrane was evaluated by immunoblotting analysis to probe for contamination of Golgi apparatus (Satoh et al., 2005).
Electron Microscopy
Pellets of rat hepatocyte nuclei and nuclear microdomains were fixed in 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.2, for 2 h at 4°C according to Braccia et al. (2003). After washing in 0.1 M sodium phosphate buffer, the samples were treated with osmium tetroxide in 0.1 M sodium phosphate buffer for 1 h at 4°C, dehydrated in graded concentration of acetone, and finally embedded in Epon. Ultrathin sections were cut on a Reichert-Jung Ultracut E (ultramicrotome), stained in 1% uranyl acetate in water and lead citrate, and finally examined in Philips EM208 electron microscope (Electronic Instruments, Mahwah, NJ) equipped with a camera system at constant temperature of 18°C and 60 KW high tension.
Biochemical Analysis
Protein and RNA content were determined as previously described (Rossi et al., 2007a). [3H]uridine incorporation in homogenate, nuclei, and nuclear microdomains isolated from either regenerating liver of hepatectomized animals or normal liver of sham-operated animals was evaluated in counting vials containing 2 ml of Ecoscint A and 0.2 ml distilled water. Radioactivity was measured with a Packard liquid scintillation analyzer. Phospholipids (PLs) and CHO content, N-SMase, SM-synthase, PC-PLC (Albi et al., 2003) and RSM-synthase (Albi et al., 2003a) activity were evaluated as previously described.
Electrophoresis and Western Blot Analysis
Thirty micrograms of protein from homogenate, nuclei, Barnes washing nuclei, and microdomains were submitted to SDS-PAGE electrophoresis in 8% polyacrylamide slab gel according to Laemmli (1970). For the electrophoresis image analysis, the gel was stained by Coomassie blue. The transfer of protein was carried out into nitrocellulose for 90 min according to Towbin et al. (1979). The membranes were blocked for 30 min with 5% nonfat dry milk in PBS, pH 7.5, and incubated over night at 4°C with mAb anti-lamin B (diluted 1:1000), polyclonal antibody anti-STAT3 (diluted 1:1000), or polyclonal antibody anti-giantin (diluted 1: 5000). The blots were treated with horseradish-conjugated secondary antibodies for 90 min (diluted 1:100,000). Visualization was performed with the enhanced chemiluminescence kit from Amersham.
RESULTS
Lipid Microdomains from Purified Nuclei
Highly purified hepatocyte nuclei were used to study the nuclear existence of microdomains enriched in SM, PC, and CHO content. To isolate the microdomains, flotation in a density sucrose gradient after Triton X-100 extraction on ice was used. The activity of both glucose-6-phosphates (G6P) and NADH cytocrome C reductase in the nuclei was ∼16% of that present in homogenate (Table 1). After Barnes treatment, the activity of G6P decreased to 4%, whereas NADH cytocrome C reductase was undetectable. No activity of either enzyme was detected in the nuclear microdomains (Table 1), indicating the absence of cytoplasmic contamination. These data are strongly supported by immunoblot analysis with giantin antibodies, a marker protein for Golgi membrane. The results showed that the band for giantin corresponding to apparent molecular weight of 367 kDa was very high in homogenate, but was reduced in the nuclei and absent in nuclei after Barnes treatment and in the microdomains (Figure 1).
Table 1.
Cytoplasm markers in the homogenate, nuclei, and nuclear microdomains
| H | N | Na | NMd | |
|---|---|---|---|---|
| G6P | 193 ± 32 | 32 ± 7 | 8 ± 1 | Undetectable |
| NADH cytocrome C reductase | 18 ± 2 | 3 ± 1 | Undetectable | Undetectable |
The data are expressed as nmol/mg protein/min and represent the mean ± SD of three experiments performed in duplicate. H, homogenate; N, nuclei; Na,nuclei after Barnes treatment; NM, nuclear membrane; NMd, nuclear microdomains.
Figure 1.

Presence of giantin in the homogenate, nuclei, Barnes washing nuclei and nuclear microdomains. The different fractions were prepared as reported in Materials and Methods. The amount correspondent to 30 μg proteins were loaded onto SDS-PAGE electrophoresis in 8% polyacrylamide slab gel. Immunoblot of proteins were probed with anti-giantin (apparent molecular weight 367 kDa) antibodies and visualized by ECL. C, positive control, H35 hepatoma cells; H, homogenate; N, nuclei; Na, nuclei after Barnes treatment; NMd, nuclear microdomains.
Protein and Lipid Composition
The protein content was ∼17.00 mg/g liver and 3.0 mg/g liver in both the homogenate and the nuclei (Table 2). Further analysis in different subnuclear fractions showed that ∼27% of the total nuclear proteins was localized in the nuclear membrane, 44% in the chromatin, 22% in the nuclear matrix, and only 0.09% in the nuclear microdomains. The PL content was ∼0.45 mg/g liver in the homogenate and 0.12 mg/g liver in the nuclei, of which ∼79% belongs to nuclear membranes, 8% to chromatin, 8% to nuclear matrix, and 2.5% to nuclear microdomains. The ratio PL to protein of the nuclear microdomains was very similar to that of nuclear membranes, suggesting that it could correspond to a specific section. To verify this, analysis of lipid composition was carried out by TLC. Nuclear microdomains had a specific ratio among CHO, PC, and SM that equals to ∼1:1:1 (Figure 2), characteristic of the lipid microdomains enriched in CHO and SM content (Kenworthy et al., 2004). Therefore, the lipid composition of the microdomains was different from that of the nuclear membranes where the CHO, PC, and SM ratio was 1:1.5:0.6. Similar values for SM and CHO but higher for PC were present in either chromatin or the nuclear matrix (Figure 2).
Table 2.
Protein and phospholipid content in the homogenate, nuclei, and subnuclear fractions
| H | N | NM | C | NMx | NMd | |
|---|---|---|---|---|---|---|
| Protein | 16877 ± 198 | 3097 ± 88 | 833 ± 51 | 1375 ± 44 | 666 ± 30 | 27 ± 5 |
| PL | 447 ± 50 | 120 ± 11 | 95 ± 9 | 10 ± 2 | 10 ± 2 | 3 ± 1 |
| μPL/mg protein | 26.48 | 38.75 | 114.05 | 7.27 | 15.01 | 111.11 |
The data are expressed as μg protein or phospholipid/g liver and represent the mean ± SD of five experiments performed in duplicate. H, homogenate; N, nuclei; NM, nuclear membrane; C, chromatin; NMx, nuclear matrix; NMd, nuclear microdomains.
Figure 2.
Cholesterol, phosphatidylcholine, and sphingomyelin content in the homogenate, nuclei, and subnuclear fractions. The data are expressed as μg/mg protein and represent the average ± SD of five experiments performed in duplicate. In the nuclear rafts the value of the three lipids is very similar and it increases significantly with respect to that present in other subnuclear fractions (*p < 0.01). H, homogenate; N, nuclei; NM, nuclear membrane; C, chromatin; NMx, nuclear matrix; NMd, nuclear microdomains.
The protein profiles of nuclear microdomains with those of other gradient fractions were compared by electrophoresis. Results showed that nuclear microdomain proteins range in apparent molecular weight from 40 to 100 kDa (Figure 3). Here it was not possible to use markers of microdomains isolated from cell membranes, e.g., galectin, β-galactoside–binding proteins and others (Braccia et al., 2003) as probes for nuclear microdomains as they are not present in the nuclei. Therefore, lamin B was used as specific marker of nuclear membrane and STAT3, the nuclear protein involved in the transcription process, was used to highlight a nuclear microdomain marker. The specific distribution in the density gradient of the two proteins described above was revealed by Western blotting. As shown in Figure 4, top, the band of lamin B, corresponding to 68 kDa apparent molecular weight, appeared in the fractions 1 to 7. Lamim B was also partially present in nuclear microdomains, indicating that these domains are specific parts of nuclear membrane. Differently, STAT3, corresponding to 92 kDa apparent molecular weight, was exclusively present in the raft fractions (Figure 4, bottom), confirming STAT3 being a nuclear microdomain marker.
Figure 3.
Comparison of protein profile of different gradient fractions. Lipid microdomains were isolated by sucrose gradient centrifugation as reported in Materials and Methods. After centrifugation, the gradients were fractionated, and samples of equal volume were subjected to SDS-PAGE. Proteins were visualized by staining with Coomassie brilliant blue. Molecular mass values (kDa) are indicated. The nuclear lipid microdomain protein pattern appears different with respect to other fractions; it ranges in apparent molecular weight from 40 to 100 kDa. Fractions 1–7, no-microdomain fractions; fractions 8–10, microdomain fractions.
Figure 4.
Distribution of nuclear membrane and nuclear microdomain markers. The density gradient fractions were analyzed by Western blotting. Immunoblots of proteins were probed after stripping with mAb anti-lamin B (top) and polyclonal antibody anti-STAT (bottom) and visualized by ECL. The band of lamin B corresponds to apparent molecular weight of the 68kDa; C, positive control, H35 hepatoma nuclei. The band of STAT3 corresponds to apparent molecular weight of 92 kDa; C, positive control, H35 hepatoma cells. Fractions 1–7, no-microdomain fractions; fractions 8–10, microdomain fractions.
Lipid Metabolism Enzyme Activities
The activities of enzymes responsible for PC and SM metabolism were assayed, as they were previously reported in the nucleus (Albi and Viola Magni, 2006a). Importantly, PC-PLC activity was absent (Figure 5), whereas, in addition, SM-synthase activity was 41% lower with respect to the intact nuclei. In contrast, nuclear microdomain N-SMase and RSM-synthase activities were ∼1.9 and 63 times higher of those present in the whole nuclei. N-SMase and RSM-synthase activity in nuclear microdomains was also significantly higher than that present in the chromatin and nuclear matrix. Moreover, SM-synthase activity in nuclear microdomains was lower than that present in the chromatin but higher than that present in nuclear matrix. In comparison to the whole nuclear membrane, N-SMase and SM-synthase activities were lower in nuclear microdomains, whereas RSM-synthase activity was higher.
Figure 5.

Metabolism lipid enzymes in nuclei and subnuclear fractions. Nuclear microdomains were prepared from highly purified hepatocyte nuclei and the enzymatic activities of N-sphingomyelinase (N-SMase), sphingomyelin-synthase (SM-synthase), phosphatidylcholine-dependent phospholipase C (PC-PLC), and reverse sphingomyelin-synthase (RSM-synthase) were evaluated as reported in Materials and Methods and compared with the enzymes present in other preparations. The values were expressed as pmol/mg protein/min and represent the average ± SD of four independent experiments performed in duplicate. Significance with respect to other preparations, *p < 0.01. N, nuclei; NM, nuclear membrane; C, chromatin; NMx, nuclear matrix; NMd, nuclear microdomains.
Morphology of Nuclear Lipid Microdomains
As shown in Figure 6a, after Barnes washing, nuclei were highly purified with an average diameter in the range of 6–8 μm. Nuclear microdomains appeared as a homogenous population of closed, spherical, or ovoid vesicle-like structures with an average diameter in the range of 300–600 nm (Figure 6b). Well-defined borders were more evident at 65,000× and 200,000× magnification (Figure 6, c and d, respectively). This morphology is similar to that of microdomains isolated from cellular membrane, indicating that Triton X-100 extraction on ice results in preservation of vesicle-like structures as previously reported (Braccia et al., 2003).
Figure 6.
Morphology of nuclei and nuclear microdomains. Sections of purified hepatocyte nuclei and nuclear lipid raft pellets were treated for electron microscopy analysis as reported in Materials and Methods. The nuclei have an average diameter in the range of 6–8 μm; they appear well purified with a nuclear membrane well defined and complete (12,000×, a). Nuclear microdomains appear as a population of closed, spherical or ovoid vesicle-like structures with an average diameter in the range of 300–600 nm. Magnification, (b) 48,000×; (c) 65,000×; (d) 200,000×.
Nuclear Microdomains Constitute a Platform for Transcription Process
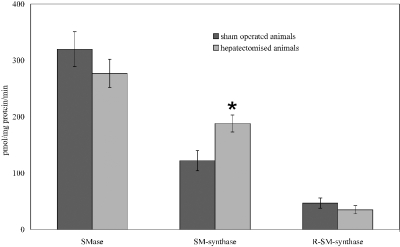
To test if transcription is localized to regions of the nuclear membrane that are enriched in specific lipids and proteins, liver regeneration was induced by partial hepatectomy. Animals were injected with [3H]uridine 23 h after operation and killed 1 h later, a time in which RNA synthesis is increased. Nuclear microdomains were purified from hepatocyte nuclei, and the level of labeled uridine was evaluated. Aliquots from homogenate, purified nuclei, and nuclear microdomains were used to evaluate the RNA specific activity. Results showed that the value of radioactivity was approximately 3000 cpm/mg RNA in the homogenate and 5390 cpm/mg RNA in the nuclei, increasing 3.5 times in the nuclear microdomains with respect to that of nuclei (Figure 7). In the sham-operated animals the radioactivity was 342 cpm/mg RNA in the homogenate and 604 cpm/mg RNA in the nuclei, increasing 1.5 times in the nuclear microdomains with respect to that of nuclei (Figure 7). To evaluate if nuclear raft composition changes during RNA synthesis, lipid analysis was carried out. It was observed that CHO and SM increased 20 and 29%, respectively, in comparison with sham-operated animals, and the CHO/SM ratio decreased from 1.25 to 1.11; however, the PC content did not change significantly (Figure 8). These variations are due mainly to increased SM-synthase activity in nuclear microdomains isolated from regenerating liver with respect to those isolated from sham-operated animals (Figure 9). These results clearly suggest that the microdomains composition changes in relationship with cellular functions implying a specific role in transcription process.
Figure 7.
Incorporation of [3H]uridine in homogenate, nuclei, and nuclear microdomains. [3H]uridine, 100 μCi, was injected intraperitoneally in rats 23 h after hepatectomy, and the animals were killed 1 h later. Sham-operated animals were used as controls. The hepatocyte nuclei were purified, and nuclear microdomains were prepared as reported in Materials and Methods. The radioactivity was evaluated in each preparation. The data were expressed as cpm/mg RNA and represent the average ± SD of three independent experiments performed in duplicate. The value of incorporation increases significantly in nuclear microdomains with respect to the homogenate and/or nuclei (*p < 0.01). H, homogenate; N, nuclei; NMd, nuclear microdomains.
Figure 8.
Lipid composition of nuclear microdomains during liver regeneration. Nuclear microdomains were prepared from hepatocyte nuclei isolated from regenerating liver 24 h after partial hepatectomy. The lipids were extracted and PC, SM, and CHO separated as reported in Materials and Methods. The values were expressed as μg/mg protein and represent the average ± SD of three independent experiments performed in duplicate. SM and CHO content increases significantly with respect to that of nuclear microdomains isolated from sham-operated animal hepatocyte nuclei (*p < 0.01). PC, phosphatidylcholine; SM, sphingomyelin; CHO, cholesterol.
Figure 9.
Lipid enzymes metabolism in nuclei and nuclear microdomains during liver regeneration. Nuclear microdomains were prepared from highly purified hepatocyte nuclei isolated from regenerating liver 24 h after partial hepatectomy and the enzymatic activities of neutral-sphingomyelinase (N-SMase), sphingomyelin-synthase (SM-synthase), phosphatidylcholine-dependent phospholipase C (PC-PLC), and reverse-sphingomyelin-synthase (RSM-synthase) were evaluated as reported in Materials and Methods. The values were expressed as pmol/mg protein/min and represent the average ± SD of four independent experiments performed in duplicate. Only the SM-synthase activity increases significantly with respect to sham-operated animals (*p < 0.01), justifying the increase of SM content.
DISCUSSION
Recent data support the hypothesis that lipid domains in the nuclear membrane perform specific functions (Graumann et al., 2007; Sun et al., 2007; Manfiolli et al., 2008). Braccia et al. (2003) showed that cold Triton X-100 extraction results in the isolation of microdomains rich in lipid content. This detergent preserves vesicle-like structures whereas extraction with Brij 98 at 37°C largely breaks up microvillar vesicles into open membrane fragments. Therefore, we have isolated the lipid microdomains from highly purified hepatocyte nuclei with Triton X-100 at low temperature.
Our results show that 1) microdomains isolated from nuclei are characterized by specific lipid composition; 2) lipid composition of nuclear microdomains changes during cellular proliferation in regenerating liver; and 3) nuclear microdomains are enriched in labeled uridine when RNA synthesis increases.
The possibility that the isolated microdomains are artifacts due to detergent treatment was reported in the literature (Munro 2003; Schuck et al., 2003). Nevertheless, we obtained by Triton X-100 treatment, a sample with properties similar to other microdomains isolated from cells (Braccia et al., 2003) but corresponding in composition to the purified intranuclear complex previously reported (Rossi et al., 2007a,b). In fact, we have previously demonstrated that it is possible to isolate from the nucleus a complex that is associated to inner nuclear membrane and constituted by proteins, PC-SM-CHO, enzymes for lipid metabolism and newly synthesized RNA (Rossi et al., 2007a,b). In the present work, we obtained through biochemical isolation of microdomains, a sample that presents similar biochemical characteristics of intranuclear complex and morphological properties of lipid microdomains.
The lipid composition is characterized by an exact ratio of PC:SM:CHO maintained by N-SMase, SM-synthase, and RSM-synthase activities: N-SMase degrades SM to form phosphocholine and ceramide; SM-synthase resynthesizes SM using ceramide and phosphocholine derived from PC freeing diacylglycerol and finally, RSM-synthase synthesizes PC using diacylglycerol and phosphocholine derived from SM producing ceramide. Therefore, these enzymes regulate the balance of PC and SM levels. This becomes very important considering the dynamic of nuclear membrane in interphase and mitosis cells (Ellenberg et al., 1997).
As a strong relationship between structure and function exists in the nuclear membrane (Jacobson et al., 1984) and as microdomains are proposed to act as platforms for protein segregation and cell signaling (Simons and Ikonen, 1997; Simons and Toomre, 2000; Hancock, 2003; Gómez-Moutón et al., 2004), we have studied nuclear lipid microdomains during cell proliferation. Our data have demonstrated that, during liver regeneration, nuclear microdomains are characterized by an increased SM content due to higher activity of SM-synthase. Because recent studies provide evidence that the presence of SM and CHO in rafts leads to strongly packed and rigid bilayers (Niemelä et al., 2007), this possibly results in a more rigid structure that could act as an anchor for RNA synthesis, as demonstrated by labeled uridine incorporation during the S phase of the cell cycle. This observation induces to think over the nuclear plasticity in relation to cell function as indicated for nuclear bodies during mitosis (Chen et al., 2008). On the other hand, electron microscopy analysis on the rat liver sections performed with a complex of N-SMase conjugated to colloidal gold particles showed that SM is present in nuclear domains active in DNA replication, transcription, and possibly in different steps of mRNA processing as well (unpublished data). Therefore, on the basis of our current data it is supposed that the nuclear lipid microdomains act as platform for the transcription process.
ACKNOWLEDGMENTS
We thank Dr. Ilaria Bernardini and Remo Lazzarini for technical assistance and Christopher J. Clarke for the manuscript revision. We thank support from Ministero dell' Università e Ricerca (PRIN project), ASI (Agenzia Spaziale Italiana), and the Fondazione Cassa di Risparmio di Perugia.
Abbreviations used:
- N-SMase
neutral-sphingomyelinase
- PC
phosphatidylcholine
- PC-PLC
phosphatidylcholine-specific phospholipase C
- PPC
phosphocholine
- SM
sphingomyelin
- SM-synthase
sphingomyelin-synthase
- RSM-synthase
reverse sphingomyelin-synthase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-05-0517) on October 15, 2008.
REFERENCES
- Albi E., Viola Magni M. P. The presence and the role of chromatin cholesterol in rat liver regeneration. J. Hepatol. 2002;36:395–400. doi: 10.1016/s0168-8278(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Albi E., Lazzarini R., Viola Magni M. Reverse sphingomyelin-synthase in rat liver chromatin. FEBS Lett. 2003a;549:152–156. doi: 10.1016/s0014-5793(03)00810-x. [DOI] [PubMed] [Google Scholar]
- Albi E., Cataldi S., Rossi G., Viola Magni M. A possible role of cholesterol-sphingomyelin/phosphatidylcholine in nuclear matrix during rat liver regeneration. J. Hepatol. 2003b;38:623–628. doi: 10.1016/s0168-8278(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Albi E., Viola Magni M. P. The role of intranuclear lipids. Biol. Cell. 2004;96:657–667. doi: 10.1016/j.biolcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Albi E., Viola Magni M. P. Sphingomyelin: a small-big molecule in the nucleus. In: Albi E., editor. Sphingolipid and Cell Function. Kerala, India: Research Signpost; 2006a. pp. 211–227. [Google Scholar]
- Albi E., Cataldi S., Bartoccini E., Viola Magni M., Marini F., Mazzoni F., Rainaldi G., Evangelisti M., Garcia-Gil M. Nuclear sphingomyelin pathway in serum deprivation-induced apoptosis of embryonic hippocampal cells. J. Cell. Physiol. 2006b;206:189–195. doi: 10.1002/jcp.20448. [DOI] [PubMed] [Google Scholar]
- Albi E., Lazzarini R, Viola Magni M. Phosphatidylcholine/sphingomyelin metabolism crosstalk inside the nucleus. Biochem. J. 2008;410:381–389. doi: 10.1042/BJ20070758. [DOI] [PubMed] [Google Scholar]
- Alessenko A., Chatterjee S. Neutral sphingomyelinase: localization in rat liver nuclei and involvement in regeneration/proliferation. Mol. Cell Biochem. 1995;143:169–174. doi: 10.1007/BF01816950. [DOI] [PubMed] [Google Scholar]
- Braccia A., Villani M., Immerdal L., Niels-Christiansen L., Nystrom B. T., Hansen G. H., Danielsen E. M. Microvillar membrane microdomains exist at physiological temperature. Role of galectin-4 as lipid raft stabilizer revealed by “superrafts.”. J. Biol. Chem. 2003;278:15679–15684. doi: 10.1074/jbc.M211228200. [DOI] [PubMed] [Google Scholar]
- Brown D. Structure and function of membrane rafts. Int. J. Med. Microbiol. 2006;291:433–437. doi: 10.1078/1438-4221-00150. [DOI] [PubMed] [Google Scholar]
- Brown D. A., London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Kappel C., Beaudouin J., Eils R., Spector D. L. Live cell dynamics of PML nuclear bodies upon entry into and exit from mitosis. Mol. Biol. Cell. 2009;19:3147–3162. doi: 10.1091/mbc.E08-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. The state of lipid rafts: from model membrane to cells. Annu. Rev. Biophys. Biomol. Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- Ellenberg J., Siggia E. D., Moreira J. E., Smith C. L., Presley J. F., Worman H. J., Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Moutón C., Lacalle R. A., Mira E., Jiménez-Baranda S., Barbe D. F., Carrera A. C., Martínez A. C., Mañes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J. Cell Biol. 2004;164:759–768. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graumann K., Irons S. L., Runions J., Evans D. E. Retention and mobility of the mammalian lamin B receptor in the plant nuclear envelope. Biol. Cell. 2007;99:553–562. doi: 10.1042/bc20070033. [DOI] [PubMed] [Google Scholar]
- Hancock J. F. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- Heessen S., Fornerod M. The inner nuclear envelope as a transcription factor resting place. EMBO Rep. 2007;8:914–919. doi: 10.1038/sj.embor.7401075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoen E. Role of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Jacobson K., O'Dell D., August J. T. Lateral diffusion of an 80,000-dalton glycoprotein in the plasma membrane of murine fibroblasts: relationships to cell structure and function. J. Cell Biol. 1984;99:1624–1633. doi: 10.1083/jcb.99.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavok N. S., Krasilnikova O. A., Babenko N. A. Increase in diacylglycerol production by liver and liver cell nuclei at old age. Exp. Gerontol. 2003;38:441–447. doi: 10.1016/s0531-5565(02)00246-2. [DOI] [PubMed] [Google Scholar]
- Kenworthy A. K., Nichols B. J., Remmert C. L., Hendrix G. M., Kumar M., Zimmemberg J., Lippincott-Schwartz J. Dynamic of putative raft-associated proteins at the cell surface. J. Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structure proteins during the assembly of bacteriophage T4. Nature. 1970;227:680–683. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Wu G. Nuclear lipids: key signalling effectors in the nervous system and other tissues. J. Lipid. Res. 2004;45:1–8. doi: 10.1194/jlr.R300015-JLR200. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Wu G. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim. Biophys. Acta. 2006;1761:588–598. doi: 10.1016/j.bbalip.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Goni F. M., Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- London E., Brown D. A. Insolubility of lipids in triton X- 100, physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim. Biophys. Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Manfiolli A. O., Maragno A. L., Baqui M. M., Yokoo S., Teixeira F. R., Oliveira E. B., Gomes M. D. FBXO25-associated nuclear domains: a novel subnuclear structure. Mol. Biol. Cell. 2008;19:1848–1861. doi: 10.1091/mbc.E07-08-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli A. M., Fala F., Faenza I., Billi A. M., Cappellini A., Manzoli F. A., Cocco L. Metabolism and signaling activities of nuclear lipids. Cell Mol. Life Sci. 2004;61:1143–1156. doi: 10.1007/s00018-004-3414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani Y., Tamiya-Koizumi K., Nakamura N., Kobayashi M., Hirabayashi Y., Yoshida S. Nuclear localization of neutral sphingomyelinase 1, biochemical and immunocytochemical analyses. J. Cell Sci. 2001;114:3727–3736. doi: 10.1242/jcs.114.20.3727. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Niemelä P. S., Ollila S., Hyvönen M. T., Karttunen M., Vattulainen I. Assessing the nature of lipid raft membranes. PLoS Comput. Biol. 2007;3(2):e34. doi: 10.1371/journal.pcbi.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitcheva T., Peeva D. Phospholipid composition, phospholipase A2 and sphingomyelinase activities in rat liver nuclear membrane and matrix. Int. J. Biochem. Cell Biol. 2001;27:995–1001. doi: 10.1016/1357-2725(95)00087-6. [DOI] [PubMed] [Google Scholar]
- Ramoni C., Spadaro F., Barletta B., Dupuis M. L., Podo F. Phosphatidylcholine-specific phospholipase C in mitogen-stimulated fibroblasts. Exp. Cell Res. 2004;299:370–382. doi: 10.1016/j.yexcr.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Romanenko E. B., Demidenko Z. N., Vanyushin B. F. RNA-polymerase, DNA-polymerase, DNA-methyltransferase and sphingomyelinase activities in liver nuclei of rats of different age. Biochemistry. 1998;63:159–163. [PubMed] [Google Scholar]
- Rossi G., Magni M., Albi E. Sphingomyelin-cholesterol and double stranded RNA relationship in the intranuclear complex. Arch. Biochem. Biophys. 2007a;459:27–32. doi: 10.1016/j.abb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Rossi G., Viola Magni M., Albi E. Signal transducer and activator of transcription 3 and sphingomyelin metabolism in intranuclear complex during cell proliferation. Arch. Biochem. Biophys. 2007b;464:138–143. doi: 10.1016/j.abb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Satoh A., Beard M., Warren G. Preparation and characterization of recombinant golgin tethers. Methods Enzymol. 2005;404:279–296. doi: 10.1016/S0076-6879(05)04026-7. [DOI] [PubMed] [Google Scholar]
- Schuck S., Honsho M., Ekroos K., Shevchenko A., Simons K. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–41. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Sun J., Xu H., Negi S., Subramony S. H., Hebert M. D. Differential effects of polyglutamine proteins on nuclear organization and artificial reporter splicing. J. Neurosci. Res. 2007;85:2306–2317. doi: 10.1002/jnr.21369. [DOI] [PubMed] [Google Scholar]
- Tamiya-Koizumi K., Umekawa H., Yoshida S., Kojima K. Existence of Mg2+-dependent, neutral sphingomyelinase in nuclei of rat ascites hepatoma cells. J. Biochem. 1989;106:593–598. doi: 10.1093/oxfordjournals.jbchem.a122901. [DOI] [PubMed] [Google Scholar]
- Terry L. J., Shows E. B., Wente S. R. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugane K., Tamiya-Koizumi K., Nagino M., Nimura Y., Yoshida S. A possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J. Hepatol. 1999;31:8–17. doi: 10.1016/s0168-8278(99)80158-5. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Kitano T., Kondo T., Yabu T., Taguchi Y., Tashima M., Umehara H., Domae N., Uchiyama T., Okazaki T. Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. Cancer Res. 2004;64:1000–1007. doi: 10.1158/0008-5472.can-03-1383. [DOI] [PubMed] [Google Scholar]