Abstract
Botulinum neurotoxin E (BoNT/E) can cause paralysis in humans and animals by blocking neurotransmitter release from presynaptic nerve terminals. How this toxin targets and enters neurons is not known. Here we identified two isoforms of the synaptic vesicle protein SV2, SV2A and SV2B, as the protein receptors for BoNT/E. BoNT/E failed to enter neurons cultured from SV2A/B knockout mice; entry was restored by expressing SV2A or SV2B, but not SV2C. Mice lacking SV2B displayed reduced sensitivity to BoNT/E. The fourth luminal domain of SV2A or SV2B alone, expressed in chimeric receptors by replacing the extracellular domain of the low-density lipoprotein receptor, can restore the binding and entry of BoNT/E into neurons lacking SV2A/B. Furthermore, we found disruption of a N-glycosylation site (N573Q) within the fourth luminal domain of SV2A rendered the mutant unable to mediate the entry of BoNT/E and also reduced the entry of BoNT/A. Finally, we demonstrate that BoNT/E failed to bind and enter ganglioside-deficient neurons; entry was rescued by loading exogenous gangliosides into neuronal membranes. Together, the data reported here demonstrate that glycosylated SV2A and SV2B act in conjunction with gangliosides to mediate the entry of BoNT/E into neurons.
INTRODUCTION
Botulinum neurotoxins (BoNTs), produced by the anaerobic bacterium Clostridium botulinum, are the most potent toxins known (Schiavo et al., 2000). These toxins cause botulism, a severe disease in humans and animals. The toxins target and enter motor nerve terminals and block the release of acetylcholine at neuromuscular junctions (NMJs), causing flaccid paralysis and may lead to death due to respiratory failure (Schiavo et al., 2000; Simpson, 2004). Botulism is a rare disease in humans and thus the general population has not been immunized against these toxins; this is one of the reasons that BoNTs are among the most dangerous potential bioterrorism threats (Arnon et al., 2001).
There are seven serotypes of BoNTs (BoNT/A to G; Schiavo et al., 2000; Simpson, 2004). Each toxin is composed of a light chain (∼50 kDa) and a heavy chain (∼100 kDa), connected through a disulfide bond (Schiavo et al., 2000). The heavy chain mediates cell entry, via receptor-mediated endocytosis, and translocation of the light chain across the endosomal membrane into the cytosol (Hoch et al., 1985; Schiavo et al., 2000; Koriazova and Montal, 2003; Fischer and Montal, 2007). The light chain is a protease that cleaves target proteins in cells (Schiavo et al., 2000). BoNT/A and E cleave the peripheral membrane protein SNAP-25 (synaptosomal-associated protein of 25 kDa); BoNT/B, D, F, and G cleave the vesicle membrane protein synaptobrevin (Syb); BoNT/C cleaves both SNAP-25 and the plasma membrane protein syntaxin (Schiavo et al., 1992; Blasi et al., 1993 a,b; Schiavo et al., 1993 a,b, 1994). SNAP-25, syntaxin, and Syb are collectively referred to as SNARE (soluble N-ethylmaleimide–sensitive factor attachment receptor) proteins. These three SNAREs assemble into a complex that mediates the fusion of synaptic vesicles with the plasma membrane (Rothman and Warren, 1994; Jahn and Sudhof, 1999; Jahn and Scheller, 2006); cleavage of these proteins thus inhibits synaptic vesicle exocytosis and blocks the release of neurotransmitters. Because of their ability to inhibit synaptic transmission, BoNTs are used to treat a wide spectrum of medical conditions ranging from overactive muscle disorders to chronic pain (Johnson, 1999; Aoki, 2004; Dodick et al., 2004).
The extremely high efficacy of these toxins is not only due to their enzymatic activity, but also involves their ability to recognize and enter presynaptic nerve terminals with high affinity and specificity. Thus, a major focus has been to identify the neuronal receptors for BoNTs. A “double-receptor” hypothesis has been proposed, in which BoNTs recognize nerve terminals by binding to two components: a group of membrane glycosphingolipids called gangliosides and specific protein receptors (Montecucco, 1986).
Complex forms of gangliosides, called polysialiogangliosides (PSGs), have been shown to bind BoNT/A, B, and E with low affinity (Kitamura et al., 1980; Kamata et al., 1986; Kozaki et al., 1998; Rummel et al., 2004b; Yowler and Schengrund, 2004). Cells lacking gangliosides are resistant to the binding and entry of BoNT/A, B, and G; entry can be rescued by loading cell membranes with exogenous gangliosides (Yowler et al., 2002; Chai et al., 2006; Dong et al., 2007). Furthermore, mice lacking PSG showed decreased sensitivities to BoNT/A, B, C, and G (Kitamura et al., 1999; Bullens et al., 2002; Tsukamoto et al., 2005; Dong et al., 2007; Rummel et al., 2007). Interestingly, it was recently reported that BoNT/D does not interact with gangliosides, and loss of PSG does not diminish the entry of BoNT/D into neurons (Tsukamoto et al., 2005). Furthermore, mice lacking PSG exhibit the same sensitivity to BoNT/D as wild-type (WT) mice, indicating that not all BoNTs utilize gangliosides as coreceptors (Tsukamoto et al., 2005). It has not been reported whether gangliosides are essential for the entry of BoNT/E or BoNT/F into neurons.
Among the seven BoNTs, the protein receptors for BoNT/A, B, and G have been identified. Two homologous synaptic vesicle membrane proteins, synaptotagmins I and II (Syts I/II), were first found to bind BoNT/B (Nishiki et al., 1994, 1996) and were subsequently shown to function as the protein receptors that mediate entry of BoNT/B into cells (Dong et al., 2003, 2007). The toxin-binding site lies in a short intravesicular region that is conserved between Syt I and II (Dong et al., 2003). In addition, BoNT/G was also found to utilize Syt I/II as its receptor by recognizing the same toxin-binding site on Syt I/II as BoNT/B (Rummel et al., 2004a, 2007; Dong et al., 2007). The cocrystal structure of BoNT/B bound to the toxin-binding domain of Syt II was recently reported. This structure revealed that the toxin binds Syt II through a hydrophobic groove within the C-terminal region of BoNT/B (Chai et al., 2006; Jin et al., 2006; Rummel et al., 2007). This hydrophobic groove is conserved in all subtypes of BoNT/B, as well as in BoNT/G (Chai et al., 2006; Jin et al., 2006; Rummel et al., 2007).
The receptor for BoNT/A was recently identified as another synaptic vesicle membrane protein, SV2 (Dong et al., 2006; Mahrhold et al., 2006). All three isoforms of SV2 in mammals (SV2A, B, and C) can bind BoNT/A and mediate its entry into cells (Dong et al., 2006). SV2 contains 12 transmembrane domains with one large luminal domain (the fourth luminal domain, L4) between the seventh and eighth transmembrane domains (Bajjalieh et al., 1992; Feany et al., 1992; Bajjalieh et al., 1993; Janz and Sudhof, 1999). SV2 is a proteoglycan on synaptic vesicles and is heavily glycosylated, possibly through three putative N-glycosylation sites within the L4 luminal domain (Buckley and Kelly, 1985; Bajjalieh et al., 1992; Feany et al., 1992; Scranton et al., 1993; Janz and Sudhof, 1999). Interestingly, the BoNT/A-binding site was mapped to a region within the SV2-L4 domain that contains two putative glycosylation sites (Dong et al., 2006). It is not clear whether glycosylation of SV2 affects the binding of BoNT/A.
BoNT/E is one of four BoNTs (BoNT/A, B, E, and rarely F) that are associated with human botulism (Sobel, 2005; Yule et al., 2006). It is also one of the leading causes of botulism outbreaks among wild fish and birds (Sobel, 2005; Yule et al., 2006). The protein receptor for BoNT/E has not yet been identified. The goal of this study is to identify the protein receptor for BoNT/E and to determine whether gangliosides serve as coreceptors for this toxin. Previous studies revealed that neuronal activity facilitated paralysis in diaphragm muscle preparations exposed to BoNT/E and increased the cleavage of the substrate protein SNAP-25 in cultured hippocampal neurons (Keller et al., 2004; Lawrence et al., 2007), providing indirect evidence that synaptic vesicle recycling may enhance the entry of BoNT/E. However, it was reported that BoNT/E does not bind to the recombinant luminal domains of Syt I/II or SV2 purified from Escherichia coli (Dong et al., 2003, 2006; Rummel et al., 2004a; Mahrhold et al., 2006).
Here we report the surprising finding that two isoforms of SV2—SV2A and SV2B—mediate the entry of BoNT/E into neurons. We found that glycosylation at the third N-glycosylation site within the SV2-L4 domain is essential for binding BoNT/E and also plays a role in the entry of BoNT/A into neurons. We also found that the L4 domain alone, engineered to replace the extracellular domain of low-density lipoprotein receptor (LDLR), is sufficient to mediate the entry of BoNT/A and E. Finally, we report that gangliosides are essential for binding and entry of BoNT/E into neurons, thus extending the “double-receptor” model to BoNT/E.
MATERIALS AND METHODS
Antibodies, Materials, and Mouse Lines
Monoclonal antibodies directed against Syb II (Cl 69.1), Syt I (Syt IN Ab: Cl 604.4; anti-Syt I cytoplasmic domain: Cl 41.1), SV2 (pan-SV2), synaptophysin (Cl 7.2), and SNAP-25 (Cl 71.2) were generously provided by R. Jahn (Max-Planck-Institute for Biophysical Chemistry, Goettingen, Germany). A human anti-BoNT/B was generously provided by J. Lou and J. Marks (University of California, San Francisco, CA). Rabbit polyclonal anti-BoNT/A, B, and E antibodies and anti-SV2C antibodies were described previously (Janz and Sudhof, 1999; Dong et al., 2003). Guinea pig anti-vesicular glutamate transporter I (vGlut) was purchased from Chemicon (Temecula, CA). Chicken polyclonal anti-green fluorescent protein (GFP), rabbit polyclonal anti-GFP, mouse monoclonal anti-GFP, and rabbit monoclonal anti-LDLR were all purchased from Abcam (Cambridge, MA).
Bovine brain gangliosides were obtained from Matreya (State College, PA). Tetanus neurotoxin was purchased from List Biological Laboratories (Campbell, CA).
A Syt I knockout mouse line was obtained from Jackson Laboratory (Bar Harbor, ME; Geppert et al., 1994). Ganglioside knockout mice lack the gene encoding GM2/GD2 synthase (gene symbols: Galgt1; Liu et al., 1999) and were obtained from the Consortium for Functional Glycomics (Grant GM62116). The SV2A, SV2B, and SV2A/B knockout mouse lines were described previously (Janz et al., 1999).
cDNA and Constructs
Rat SV2A, B, and C cDNAs were described previously (Bajjalieh et al., 1992, 1993; Feany et al., 1992; Janz and Sudhof, 1999). Human low-density lipoprotein receptor (LDLR-2) cDNA was generously provided by S. Blacklow (Harvard Medical School, Boston, MA).
Full-length SV2A, B, and C were subcloned into the Lox-Syn-Syn lentivirus vector (Gascon et al., 2008). This vector contains two separate neuronal-specific promoters (synapsin promotor). One promoter controls the expression of SV2 isoforms and the other controls expression of EGFP. Point mutations at N-glycosylation sites of SV2A were generated with a QuickChange mutagenesis kit (Stratagene, La Jolla, CA).
Chimeric receptors were generated by fusing the fourth luminal domain of each SV2 isoform (residues 468-595 in SV2A, 410-539 in SV2B, and 453-580 in SV2C) to the N-terminus of a fragment encoding the transmembrane and cytosolic domain of human LDLR-2 (residues 788-860). In addition, a preprolactin signal sequence was fused to the N-terminus of the chimeric receptors (Miesenbock and Rothman, 1997). The cDNAs encoding these chimeric receptors were subcloned into the pEGFP-N1 vector to generate GFP-tagged receptors, which were used in the experiments described in Figure 4, B and C. These cDNAs were also subcloned into the Lox-Syn-Syn lentivirus vector to generate untagged receptors and to produce lentiviruses. Deletion mutations of the chimeric receptors described in Figure 5A were generated by PCR with addition of a tag derived from the first 11 amino acids of rat Syt I (Chapman and Jahn, 1994).
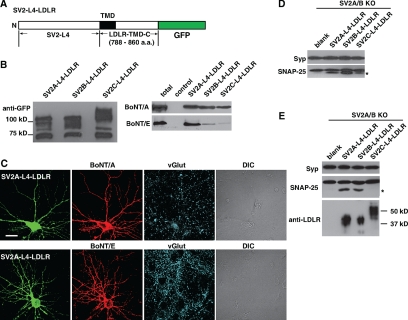
Figure 4.
The luminal domains of SV2 A and B mediate the binding and entry of BoNT/E into neurons. (A) Schematic drawings of SV2 and the chimeric receptors. The chimeric receptors are composed of the L4 domain of SV2A/B/C, and the transmembrane domain (TMD) and the cytosolic domain of the LDL-receptor (LDLR-TMD-C). The constructs used in B and C also contain a GFP tag that was fused to the C-terminus of the chimeric receptor. (B) Coimmunoprecipitation of BoNT/A (100 nM) or BoNT/E (250 nM) with SV2-L4-LDLR chimeric receptors, expressed in HEK293 cells, was carried out using a mAb against GFP, in the presence of exogenous gangliosides (0.6 mg/ml). Left panel, immunoprecipitated chimeric receptors were subjected to SDS-PAGE and immunoblot analysis using a polyclonal GFP antibody. Right panel, BoNT/A coimmunoprecipitated with all three chimeric receptors; BoNT/E coimmunoprecipitated with SV2A-L4-LDLR, and to a much lesser degree with SV2B-L4-LDLR. (C) SV2 A/B KO neurons were transfected with SV2A-L4-LDLR and exposed to BoNT/A (20 nM) or BoNT/E (30 nM) in normal culture media for 10 min. Cells were fixed for immunocytochemistry. vGlut was labeled as a marker for synapses. Expression of SV2A-L4-LDLR restored the binding of BoNT/A (top panel) or BoNT/E (bottom panel). (D) SV2A/B KO neurons were infected with lentiviruses that express chimeric receptors containing the L4 domains of SV2A, B, or C, respectively. Neurons were exposed to BoNT/A (10 nM) in culture media for 10 min and were harvested 12 h later. Cleavage of SNAP-25 was observed for neurons that were infected with SV2A, B, or C chimeric receptors. (E) Experiments were carried out as described in D, except that neurons were exposed to BoNT/E (2 nM). The cleavage of SNAP-25 was observed for neurons infected with lentiviruses that express SV2A-L4 or SV2B-L4 chimeric receptors, but not with viruses that express the SV2C-L4 chimeric receptor. Expression of chimeric receptors was determined using an antibody that recognizes the C-terminal region of the LDLR (bottom panel).
Figure 5.
Binding of BoNT/E to SV2A requires the middle portion of the SV2A-L4 domain. (A) Schematic drawing of the chimeric receptors containing a series of truncations within the SV2A-L4 domain. A small tag derived from the first 11 amino acids of rat Syt I was fused to the N-terminus. (B) SV2A/B KO neurons were transfected with the truncation mutants D1, D7, and D8 described in A. Transfected neurons were identified by GFP expression. Neurons were exposed to BoNT/E (30 nM) and Syt IN Ab (1:200) in media for 10 min. D1, D7, and D8 mutants all mediated the binding of Syt IN Ab, indicating that their L4 domains are exposed at the cell surface. D1 and D8 both restored the binding of BoNT/E or BoNT/A (20 nM, right panel) to neurons. D7 failed to restore binding of BoNT/E or BoNT/A. (C) Mouse neurons were transfected with the D2 and D6 mutants; representative examples are shown. Left panel, permeabilized neurons were positive for immunostaining with Syt IN Ab, indicating that these mutants were expressed in transfected neurons. Right panel, Syt IN Ab uptake experiments were carried out as described in Figure 1A. The L4 domains of D2 and D6 mutants failed to take-up Syt IN Ab.
Neuronal Cell Cultures, Transfection, Viral Infection, and Loading Gangliosides
Cultured rat hippocampal neurons were prepared from embryonic day (E)18–19 rats. Cultured SV2 knockout (KO), Syt I KO, and ganglioside-deficient hippocampal neurons were prepared from P1 mice. Neurons were plated on poly-d-lysine–coated glass coverslips (12 mm) at a density of 50,000/cm2 and cultured in Neurobasal medium supplemented with B-27 (2%) and Glutamax (2 mM). Neurons were generally analyzed at 12–14 days in vitro (DIV).
Transient transfection of neurons was performed at 5 DIV using Lipofectamine 2000 (Invitrogen, Carlsbad. CA). Transient transfection of HEK cells was also performed using Lipofectamine 2000. Lentiviral particles were generated as described previously (Dong et al., 2006). Viruses were added to neurons at 5 DIV.
To load cells with exogenous gangliosides, ganglioside-deficient neurons were incubated in media plus a 250 μg/ml gangliosides mixture for 12 h at 13 DIV.
Immunocytochemistry and Analysis of Neuronal Lysates
The buffers used in Figure 1A were: control buffer (mM: NaCl 140, KCl 3, KH2PO4 1.5, Na2HPO4 8, and MgCl2 0.5), high K+ (same as control buffer but adjusted to 56 mM KCl and 87 mM NaCl, and containing 1 mM CaCl2). Unless specified in the text, hippocampal neurons were generally exposed to toxins in high K+ buffer for 5 min. Neurons were subjected to immunocytochemistry analysis as described previously (Dong et al., 2006). All images were collected using a confocal microscope (Olympus FV1000,Melville, NY; 60× objective). Scale bars represent 20 μm in all images.
Figure 1.
BoNT/E enters neurons via recycling synaptic vesicles and coimmunoprecipitates with the synaptic vesicle membrane protein SV2. (A) Cultured rat hippocampal neurons were exposed to BoNT/E (30 nM) and an antibody against the luminal domain of Syt I (Syt IN Ab, 1:200) for 5 min in either resting conditions (control buffer: PBS) or stimulated conditions (high K+ buffer: PBS with 56 mM KCl and 1 mM Ca2+). Cells were washed and fixed for immunocytochemistry. Binding of BoNT/E was detected using a rabbit anti-BoNT/E antibody. Depolarization-induced synaptic vesicle recycling increased the binding of BoNT/E and the Syt IN Ab to neurons. High K+ buffer was used in all following experiments unless otherwise indicated in the figure legends. Scale bars in all figures, 20 μm. (B) Rat hippocampal neurons were first incubated with tetanus neurotoxin (TeNT, 15 nM) for 24 h. Binding of BoNT/E to these neurons was tested under stimulated conditions (30 nM, 5 min in high K+ buffer). Control cells were not treated with TeNT. Pretreatment with TeNT resulted in the cleavage of Syb and diminished the binding of BoNT/E. Vesicular glutamate transporter I (vGlut) was also labeled as a marker for synapses. (C) Monoclonal antibodies were used to immunoprecipitate synaptic vesicle membrane proteins SV2 (pan-SV2), synaptophysin (Syp, Cl 7.2), and Syt I (Cl 41.1) from rat brain detergent extracts in the presence of BoNT/E (250 nM), with (+) or without (−) the addition of exogenous gangliosides (0.6 mg/ml). Immunoprecipitated vesicle proteins and BoNT/E were detected by SDS-PAGE and immunoblot analysis. BoNT/E coimmunoprecipitated with SV2. Addition of exogenous gangliosides enhanced BoNT/E · SV2 interactions.
To monitor the entry of BoNTs into neurons, neurons were briefly exposed to toxins in high K+ buffer (5 min) or in normal culture media (10 min, see Figures 4 and 5). Neurons were then washed and further incubated in toxin-free media. Neuronal lysates were collected using 100 μl per well (24-well plate) of the lysate buffer (PBS with 1% Triton X-100, 0.05% SDS, and protease inhibitor cocktail; Roche Diagnostics, Indianapolis, IN). Lysates were centrifuged for 10 min at maximum speed using a microcentrifuge at 4°C, and the supernatants were subjected to SDS-PAG and immunoblot analysis.
Diaphragm Preparation, Extracellular Field Potential Measurements, and Rapid BoNT Toxicity Assays in Mice
The extracellular field potential (EFP) recording on mouse diaphragm preparations was performed as described previously (Zhou et al., 1998). Briefly, diaphragms, with the phrenic nerve attached, were excised from mice (postnatal day: P21–P28) and placed immediately into oxygenated Ringer's solution. Diaphragms were pinned down in a recording chamber. The nerve was stimulated with brief stimuli (15–20V/1 ms) every 2 min with a bipolar electrode connected to the voltage output of a Grass Stimulator (SD9). The EFP was recorded using an EPC-10/2 amplifier (HEKA Electronics, Lambrecht, Germany) with PATCHMASTER software (HEKA), filtered at 2.9 kHz, and digitized at 5 kHz. The recording electrode was pressed gently against the diaphragm surface (see Figure 3A). The bath was continuously perfused with oxygenated Ringer's solution at a rate of 2–3 ml/min. BoNT/E was added to the bath at a final concentration of 10 nM after the fifth stimulus, while the perfusion was stopped for 5 min to let the neurons to take up the toxin. The normal Ringer's solution, bathing the nerve-muscle preparation, contained (in mM): NaCl (129), KCl (3.0), CaCl2 (2.4), MgSO4 (1.3), NaHCO3 (20), glucose (20), and HEPES (3). The solution was vigorously bubbled with 95% O2/5% CO2 to a pH of 7.4. Data were analyzed using Igor (WaveMetrics, Lake Oswego, OR). All experiments were carried out at room temperature.
Figure 3.
SV2B KO mice are less sensitive to BoNT/E than WT mice. (A) Schematic drawing of the phrenic nerve-diaphragm preparation. (B) Diaphragms, dissected from SV2B KO and WT mice as described in A, were exposed to BoNT/E briefly (10 nM, 5 min, at 0 min). Stimulation of the phrenic nerve with a patch pipette triggers muscle contraction. Muscle action potentials were recorded as a readout for contraction (extracellular field potential, EFP) every 2 min until they became undetectable. Time-to-paralysis is the time it takes for the EFP signal to disappear. Representative EFP traces from WT and SV2B KO mice are shown. (C) The time-to-paralysis of three WT samples and five SV2B KO samples were determined as described in B. Diaphragms from SV2B KO displayed significantly longer (62.4 ± 5.6 min) time-to-paralysis than diaphragms from WT(36.7 ± 2.7 min). (D) The susceptibility of SV2B(−/−) mice and their WT littermates to BoNT/E was determined using a rapid time-to-death assay. The same amount of BoNT/E was injected into each mouse, and their survival time (time-to-death) was monitored. SV2B(−/−) mice live significantly longer on average than WT mice. The effective toxicity of BoNT/E in WT mice is about threefold greater than in SV2B KO mice.
The effective toxicity of BoNT/E in mice was estimated using the intravenous method described previously (Dong et al., 2003).
Coimmunoprecipitation
Coimmunoprecipitation experiments were carried out as described previously (Dong et al., 2003). Briefly, BoNT/E (250 nM) was mixed with either rat brain detergent extracts (400 μl, 3 mg/ml, with and without exogenous gangliosides, 0.6 mg/ml; see Figure 1C) or cell lysates from HEK cells that express SV2-L4-LDLR receptors (with 0.6 mg/ml exogenous gangliosides, see Figure 4B), for 1 h at 4°C, and then antibodies were added and were further incubated for 1 h. Protein G Fast Flow beads (50 μl, Amersham Biosciences, Piscataway, NJ) were added last and incubated for additional 1 h. Beads were washed three times in PBS plus 0.5% Triton X-100. Bound material (25%) was subjected to SDS-PAGE and immunoblot analysis.
RESULTS
BoNT/E Enters Neurons via Recycling Synaptic Vesicles and Coimmunoprecipitates with SV2
In the first series of experiments, we determined whether BoNT/E enters neurons via synaptic vesicle recycling. Exocytosis of synaptic vesicles can be triggered by depolarizing neurons with buffers containing a high concentration of K+, and can be blocked by treating neurons with tetanus neurotoxin (TeNT), which cleaves Syb (Schiavo et al., 1992). Depolarization of neurons with high K+ increased the binding of an antibody that recognizes the luminal domain of Syt I (Syt IN Ab), which serves as an internal control to monitor the exposure of luminal domains of synaptic vesicle proteins (Figure 1A). This treatment also resulted in increased binding of BoNT/E (Figure 1A). We also found that pretreatment of neurons with TeNT diminished the binding of BoNT/E (Figure 1B). These data indicate that the binding site for BoNT/E is likely to be localized to synaptic vesicles.
The major synaptic vesicle membrane proteins were then screened for their abilities to bind BoNT/E in coimmunoprecipitation experiments. An mAb that recognizes all isoforms of SV2 (pan-SV2) was able to coimmunoprecipitate BoNT/E (250 nM) from rat brain detergent (Triton X-100) extracts (Figure 1C). Addition of exogenous gangliosides to the brain detergent extract significantly increased the degree of coimmunoprecipitation, suggesting that gangliosides enhance BoNT/E · SV2 interactions. Antibodies against synaptophysin (Syp) and Syt I failed to pull down BoNT/E, indicating BoNT/E specifically interacts with SV2.
SV2A or SV2B Is Required for the Binding and Entry of BoNT/E into Neurons
Next, we determined whether BoNT/E · SV2 interactions play functional roles in the binding and entry of BoNT/E into neurons. Among the three SV2 isoforms, KO mice have been generated for SV2A and B, but not C (Crowder et al., 1999; Janz et al., 1999). Because hippocampal neurons express mainly SV2A and B, neurons from SV2A/B double KO mice serve as a useful loss-of-function model in which we could examine whether the binding and entry of BoNT/E depends on the expression of SV2 (Bajjalieh et al., 1994; Janz and Sudhof, 1999; Dong et al., 2006).
SV2A/B double KO mice were generated by breeding SV2A(+/−)SV2B(−/−) mice with each other. Thus, all of the newborn mice were SV2B(−/−), with varying levels of SV2A: SV2A(+/+), SV2A(+/−), and SV2A(−/−). Neurons cultured from these littermates were exposed to BoNT/B and E simultaneously, and toxin-binding was assayed via immunocytochemistry. We found that binding of BoNT/E to SV2A(+/−) neurons was reduced (Figure 2A, middle panel) compared with SV2A(+/+) neurons (Figure 2A, top panel). Binding to SV2A/B double KO neurons was completely abolished (Figure 2A, bottom panel). The binding of BoNT/B, which uses Syt I/II as its protein receptor, to neurons with each genotype remained the same, thus serving as an internal control; neurons lacking SV2 are capable of taking up BoNTs through synaptic vesicle recycling.
Figure 2.
Expression of SV2A or SV2B in neurons is essential for the binding and entry of BoNT/E. (A) Hippocampal neurons from littermates with the following genotypes: SV2A(+/+)SV2B(−/−), SV2A(+/−)SV2B(−/−), and SV2A(−/−)SV2B(−/−), were exposed to BoNT/E (30 nM) and BoNT/B (10 nM) simultaneously for 5 min. Triple immunostaining was performed to detect BoNT/B, BoNT/E, and SV2. Representative images are shown. BoNT/E failed to bind SV2A/B double KO neurons. (B) SV2A(+/+)SV2B(−/−) neurons, SV2A/B KO neurons, and neurons infected with lentiviruses expressing SV2A, B, or C, were briefly exposed to BoNT/E (200 pM, 5 min) and then incubated for 4 h in media. Cells were harvested, and cell lysates were subjected to SDS-PAGE and immunoblot analysis using antibodies against SV2, Syp, and SNAP-25. Cleavage of SNAP-25 was detected using an antibody that recognizes both intact SNAP-25 and the cleavage product (indicated by an asterisk). Syp was assayed as an internal control for loading of cell lysates. BoNT/E failed to enter SV2A/B KO neurons, and entry was rescued by expressing SV2A or SV2B, but not SV2C in neurons. (C) Experiments were carried out as described in B, except that neurons were exposed to BoNT/A (10 nM, 5-min exposure, 12-h incubation). The BoNT/A cleavage product of SNAP-25 is indicated by an asterisk. BoNT/A failed to enter SV2A/B KO neurons, and entry was rescued by expressing SV2A, B, or C. (D) Hippocampal neurons from Syt I KO mice were exposed to BoNT/E (50 pM) as described in B. The degree of cleavage of SNAP-25 by BoNT/E was similar in WT neurons and Syt I KO neurons. (E) SV2A(+/+)SV2B(−/−) neurons and SV2A/B KO neurons were assayed for the entry of BoNT/B (10 nM, 5-min exposure, 24-h incubation), as described in B. The cleavage of Syb by BoNT/B resulted in loss of Syb signals detected using an anti-Syb antibody. Syb, in both SV2A(+/+)SV2B(−/−) and SV2A/B KO neurons, was cleaved by BoNT/B.
It was previously reported that a subpopulation of GABAergic nerve terminals of cultured hippocampal neurons may also express SV2C (Verderio et al., 2007). We also observed that a small fraction of synapses in SV2A/B KO neurons were recognized by the pan-SV2 antibody as well as an SV2C-specific polyconal antibody (Figure 2A, bottom panel, Supplemental Figure 1). BoNT/A, which can use all three SV2 isoforms as its receptor, bound to synapses that were stained by the SV2C antibody (Supplemental Figure 1A). Interestingly, we did not detect binding of BoNT/E to SV2C-positive synapses (Supplemental Figure 1B), suggesting that BoNT/E may not exploit SV2C to enter neurons.
We next examined whether neurons lacking SV2 are resistant to the entry of BoNT/E, and if they are resistant, whether toxin entry can be restored by expressing SV2A, B, or C. Functional entry of BoNT/E can be assayed by monitoring the cleavage of its substrate protein SNAP-25. BoNT/E cleaves 26 amino acids from the C-terminus of SNAP-25, and the remaining fragment of SNAP-25 can be detected by immunoblotting with SNAP-25 antibodies. SV2A(+/+)SV2B(−/−) neurons from littermates of double KO mice served as controls. Neurons were briefly exposed to BoNT/E and further incubated for 4 h in normal culture media; neuronal lysates were then subjected to SDS-PAGE and immunoblot analysis. SNAP-25 was cleaved by BoNT/E in SV2A(+/+)SV2B(−/−) neurons, whereas SNAP-25 in SV2A/B double KO neurons was protected from BoNT/E (Figure 2B).
We then carried out rescue experiments by infecting SV2A/B KO neurons with lentiviruses that express SV2A, B, or C. The infection efficiency is >90%, so the expression of SV2A, B, or C can be restored in the majority of neurons. As shown in Figure 2B, expression of SV2A or SV2B, but not SV2C, rescued the entry of BoNT/E as evidenced by the cleavage of SNAP-25. We carried out parallel experiments with BoNT/A (10 nM, 5 min in high K+ buffer, 12-h incubation). BoNT/A cleaves nine amino acids from the C-terminus of SNAP-25. We found that SV2A, B, and C were all able to restore the entry of BoNT/A into SV2A/B KO neurons, as shown by the cleavage of SNAP-25 (Figure 2C). These findings are consistent with our previous report that SV2A, B and C all can function as receptors for BoNT/A in cells (Dong et al., 2006).
The inability of BoNT/E to enter neurons lacking SV2 is specific, because BoNT/E can readily enter neurons lacking Syt I (Figure 2D). In addition, BoNT/B, which cleaves Syb, can enter SV2A/B KO neurons (Figure 2E), further demonstrating that neurons lacking SV2 are able to take-up BoNTs via recycling synaptic vesicles.
SV2B KO Mice Display Reduced Sensitivity to BoNT/E
BoNTs cause death in humans and animals by blocking the release of neurotransmitters from motor nerve terminals at the diaphragm (Dolly et al., 1984). These motor nerve terminals express all three isoforms of SV2 (Dong et al., 2006). Because SV2C KO mice have not been generated and SV2A KO mice do not survive to adulthood (Crowder et al., 1999; Janz et al., 1999), we determined whether motor nerve terminals from SV2B KO mice display decreased susceptibility to BoNT/E compared with motor nerve terminals from WT mice. To test this, we used a phrenic nerve and diaphragm preparation (Figure 3A; Zhou et al., 1998). Stimulation of the phrenic nerve triggers the contraction of the diaphragm muscle, which can be recorded as an extracellular field potential (EFP; Figure 3, A and B). The EFPs in both WT and SV2B KO last more than 3 h in the absence of BoNT/E (data not shown), consistent with a previous report (Zhou et al., 1998). The EFPs are similar between SV2B KO and WT before adding BoNT/E (Figure 3B, 0 min). After a brief exposure to BoNT/E (10 nM, 5 min), the EFP decreases over time and eventually disappears, indicating that neurotransmitter release from motor nerve terminals has been blocked by the toxin (Figure 3B). We define the time it takes for EFPs to fall below the detection threshold as the time-to-paralysis. The average time-to-paralysis in SV2B KO (62.4 ± 5.6 min) was significantly longer than that in WT (36.7 ± 2.7 min; Figure 3C).
We then carried out whole-animal studies to determine the physiological significance of SV2 expression on the action of BoNT/E in vivo. Sensitivity to BoNT/E was assessed with an established rapid assay, in which large doses of toxin are injected intravenously and the survival time (time-to-death) is monitored on a time scale of minutes. This survival time can be converted to intraperitoneal toxicity by using a standard curve (Dong et al., 2003). Identical amounts of BoNT/E were injected into SV2B KO and WT littermate control mice. The survival times are shown in Figure 3D. SV2B KO mice survived significantly longer than WT littermates (42 ± 3 vs. 33 ± 3 min in average). The toxin was threefold more effective in WT mice than in SV2B KO mice (apparent LD50, Figure 3D), indicating that mice lacking SV2B display reduced susceptibility to BoNT/E. The remaining toxicity of BoNT/E in SV2B KO mice was presumably mediated by SV2A and SV2C, which are still expressed in motor nerve terminals (Dong et al., 2006).
The Fourth Luminal Domain of SV2A and SV2B Mediates the Binding and Entry of BoNT/E into Neurons
Because the luminal domains of SV2 are the only regions that are exposed to the outside of cells, we next determined whether BoNT/E enters cells by binding to the luminal domains of SV2A and B. SV2 has only one luminal domain of significant length (SV2-L4; Figure 4A). It has been reported that the L4 domains of SV2A, B, and C, purified as GST fusion proteins in E. coli, directly bind BoNT/A, but not BoNT/E (Dong et al., 2006). However, GST-L4 fragments may lack critical posttranslational modifications, such as glycosylation within the L4 domain. Thus, it is necessary to test the binding of BoNT/E to the L4 domain that has been expressed in mammalian cells.
To exclude other regions of SV2, and also to present the L4 domain on the cell surface, we constructed three chimeric receptors by replacing the extracellular domain of the LDLR with the L4 domains of SV2A, B, or C (Figure 4A). When expressed in HEK cells, these chimeric receptors displayed higher apparent molecular weight than the putative size of the chimeras calculated from the primary protein sequences (∼55 kDa, including a GFP tag at the C-terminus, Figure 4B, left panel). Because there are three putative N-linked glycosylation sites within the L4 domain and it has been demonstrated that native SV2 is glycosylated (Buckley and Kelly, 1985; Bajjalieh et al., 1992; Feany et al., 1992; Scranton et al., 1993; Janz and Sudhof, 1999), it is likely that these chimeric receptors are glycosylated within their L4 domains in HEK cells. Interestingly, SV2C displayed a higher molecular weight than SV2A and B, despite the fact that their L4 domains have similar amino acid sequence lengths.
We first carried out coimmunoprecipitation experiments using a GFP antibody to pull down chimeric receptors from HEK cell lysates, in the presence of BoNT/A and exogenous gangliosides. As expected, we found that all three chimeric receptors coimmunoprecipitated with BoNT/A (Figure 4B, right panel). Parallel experiments were carried out using BoNT/E, and we found that BoNT/E was coimmunoprecipitated with the chimeric receptor containing SV2A-L4 (Figure 4B, right panel). The levels of coimmunoprecipitation of BoNT/E with SV2B-L4 and SV2C-L4 were much less than with SV2A-L4, but were still slightly higher than the control that did not contain SV2-L4 (Figure 4B, right panel).
We next assessed whether the L4 domain alone was sufficient to mediate the binding of BoNT/A and BoNT/E to neurons. The SV2A-L4-LDLR chimeric receptor was expressed in SV2A/B KO neurons. These neurons were exposed to BoNT/A or BoNT/E under resting conditions (10 min in culture media). Binding of BoNT/A and BoNT/E was observed for neurons that expressed the SV2A-L4-LDLR receptor (Figure 4C).
We then determined whether the chimeric receptors can mediate functional entry of BoNT/A and BoNT/E into neurons. SV2A/B KO neurons were infected with lentiviruses that express chimeric receptors and exposed to BoNT/A (10 nM, Figure 4D) or BoNT/E (2 nM, Figure 4E) under resting conditions (10 min in culture media), followed by further incubation for 12 h. Cells were harvested, and cell lysates were subjected to SDS-PAGE and immunoblot analysis. Cleavage of SNAP-25 by BoNT/A was observed in SV2A/B KO neurons that had been infected with chimeric receptors containing the L4 domains of SV2A, B, or C (Figure 4D), indicating that the L4 domain alone can mediate the entry of toxins into neurons under resting conditions. The entry of BoNT/E into SV2A/B KO neurons was also restored by the expression of the SV2A-L4 or SV2B-L4 receptors, but not by the SV2C-L4 receptor (Figure 4E), further indicating that BoNT/E can enter neurons via binding to the luminal domain of SV2A or SV2B, but not SV2C.
Consistent with what we have observed for chimeric receptors expressed in HEK cells (Figure 4B, left panel), the SV2C-L4 chimeric receptor displayed a significantly higher molecular weight than the SV2A-L4 or SV2B-L4 receptors (Figure 4E, please note that the chimeric receptors expressed in neurons are not fused with GFP tags), further suggesting that glycosylation of SV2C-L4 is somehow distinct from SV2A-L4 and SV2B-L4.
Binding of BoNT/E to SV2A Requires the Middle Portion of the SV2A-L4 Domain
We next attempted to determine the minimal protein sequence within the SV2A-L4 domain that mediates binding of BoNT/E. Because BoNT/E does not bind the recombinant SV2A-L4 domain in vitro, we approached this question by testing binding of BoNT/E to a series LDLR-based chimeric receptors that contain various truncations and deletions of the SV2A-L4 domain (Figure 5A). Because the truncations of the L4 domain may change the membrane targeting/topology of the chimeric receptors, a tag derived from the first 11 amino acids of rat Syt I was fused to the N-terminus of all the constructs (Figure 5A). This tag contains the epitope for the Syt IN antibody and can be used for antibody uptake experiments (Figure 1A; Chapman and Jahn, 1994). Interestingly, the mouse version of Syt I cannot take up the Syt IN antibody, possibly because of sequence differences between rat and mouse Syt I (data not shown). These features enabled us to monitor the surface exposure of the chimeric receptors expressed in mouse neurons by testing whether they can take up the Syt IN antibody.
Among the eight mutants tested, mutants D1, D7, and D8 were able to take-up the Syt IN antibody when expressed in SV2A/B KO mouse neurons, indicating that they are targeted correctly to the cell surface (Figure 5B). When exposed to BoNT/E, mutants D1 and D8 mediated the binding of BoNT/E, whereas D7 failed to restore binding of BoNT/E to neurons (Figure 5B). These results indicate that the N-terminal (amino acids 468–505) and the C-terminal portion (583–590) of the SV2A-L4 domain are not required for binding BoNT/E. Similar results were obtained for BoNT/A, which is consistent with our previous findings that the binding site of BoNT/A lies in the middle of the SV2 luminal domain (amino acids 529–566 in SV2C, corresponding to 543–580 in SV2A; Dong et al., 2006).
The expression of the D2 and D6 mutants in transfected neurons was detected by immunostaining permeabilized cells with the Syt IN antibody (Figure 5C, left panel). However, both mutants failed to take up the Syt IN antibody in the live-cell up take experiments, indicating that their L4 domains were not exposed to the cell surface (Figure 5C, right panel). Similar results were observed for other mutants (D3, D4, and D5; data not shown). The mistargeting of these mutants prevented us from further mapping the binding site for BoNT/E within the luminal domain of SV2A.
Glycosylation at the Third N-linked Glycosylation Site within the SV2A-L4 Domain is Required for the Entry of BoNT/E and Enhances the Entry of BoNT/A, into Neurons
SV2 is a major synaptic vesicle proteoglycan and N-linked glycosylation was shown to be the predominant form of modification (Buckley and Kelly, 1985; Bajjalieh et al., 1992; Feany et al., 1992; Scranton et al., 1993; Janz and Sudhof, 1999). SV2 has only three putative N-linked glycosylation sites (N-X-S/T consensus sequence, where X can be any amino acid except proline), all of which are localized within the L4 domain (Figures 4A and 6A).
Figure 6.
Glycosylation of the third glycosylation site within the SV2A-L4 domain is essential for entry of BoNT/E and affects the sensitivity of neurons to BoNT/A. (A) Partial amino acid sequence of the SV2A-L4 domain, with putative N-glycosylation sites and point mutation sites, described in the following panels, indicated. The amino acid sequences of the corresponding regions of SV2B and SV2C are also shown. (B) Three putative glycosylation sites within the SV2A-L4 domain were abolished by site-directed mutagenesis (N to Q), respectively. These mutants were expressed in SV2 A/B KO neurons via lentiviral infection. Neurons were exposed to BoNT/E (200 pM) and were assayed as described in Figure 2B. Substitution of the third glycosylation site (N573Q) abolished the entry of BoNT/E into neurons. (C) Experiments were carried out as described in B, except that neurons were exposed to a higher concentration of BoNT/E (1 nM). SV2A(+/+)SV2B(−/−) neurons were also tested in parallel as a control. (D) SV2 A/B KO neurons were infected with WT, or the N498/548Q double mutant form of SV2A, using lentiviruses. The SV2A(N498/548Q) double mutant mediated entry of BoNT/E. (E) A new N-linked glycosylation site was created by exchanging R570 for T in the SV2A(N573Q) mutant. When expressed in SV2A/B KO neurons, this mutant displayed a similar molecular weight to WT SV2; however, this mutant failed to mediate the entry of BoNT/E. (F) Experiments were carried out as described in B, except that cells were exposed to BoNT/A (7 nM, 5-min exposure, incubated for 12 h). The N573Q mutation reduced the entry of BoNT/A into neurons, reflected by the partial cleavage of SNAP-25. (G) Experiments were carried out as described in F, but using a range of BoNT/A concentrations. When exposed to 1 nM BoNT/A, more extensive cleavage was observed in neurons expressing WT SV2, compared with neurons expressing the N573Q mutant.
Attempts to remove all of the N-glycans in SV2 with PNGase F, under nondenaturing conditions, were unsuccessful, possibly because the N-glycosylation sites are not fully accessible to this enzyme (data not shown; Scranton et al., 1993). This prevented us from testing the effect of de-glycosylation on binding of BoNT/E in vitro. Thus, we relied on testing whether disruption of the glycosylation of SV2, through site-directed mutagenesis, affects the binding of BoNT/E to neurons.
Each of three N-linked glycosylation sites in SV2A-L4 was disrupted by a point mutation (N to Q). These mutants were expressed in SV2A/B KO neurons using lentiviruses, and the entry of BoNT/E into these neurons was detected by assaying for the cleavage of SNAP-25. As shown in Figure 6B, all three SV2 mutants ran at a lower apparent molecular weight compared with WT SV2 on SDS-PAGE gels, indicating that all three putative N-linked glycosylation sites are glycosylated in neurons. Mutations at the first or the second glycosylation sites (N498Q, N548Q) did not affect the entry of BoNT/E, whereas mutation at the third glycosylation site (N573Q) completely abolished the entry of BoNT/E as evidenced by the lack of cleavage of SNAP-25 (Figure 6B). Even when the toxin concentration was increased fivefold (1 nM, Figure 6C), cleavage of SNAP-25 was not observed in neurons expressing the N573Q mutant form of SV2A.
We next assayed whether glycosylation at the third site alone is sufficient to mediate the entry of BoNT/E. SV2A, harboring mutations at both the first and the second glycosylation sites (N498,548Q), was expressed in SV2A/B KO neurons. As shown in Figure 6D, the N498,548Q mutant was capable of mediating the entry of BoNT/E as monitored by the cleavage of SNAP-25. This finding demonstrated that glycosylation at the N573 position alone, among three glycosylation sites, is sufficient for SV2 to mediate the entry of BoNT/E.
We also created an SV2A mutant in which we generated a new N-linked glycosylation site in the SV2A(N573Q) mutant through a point mutation at a nearby site (R570T); in effect this shifted the N-linked glycosylation site from N573 to N568. Once expressed in neurons, this mutant (R570T, N573Q) had a molecular weight similar to WT SV2A (Figure 6E), indicating that the new glycosylation site is glycosylated. However, this mutant failed to mediate the entry of BoNT/E (Figure 6E), suggesting that the loss of entry of BoNT/E, due to loss of glycosylation at N573 site, cannot be rescued by compensatory glycosylation at a nearby site.
Because the BoNT/A-binding site also includes the second and the third glycosylation sites of SV2, we assayed whether abolishing these glycosylation sites affects the entry of BoNT/A into neurons. As shown in Figure 6F, entry of BoNT/A was not affected by mutations at the first and the second glycosylation sites, whereas cleavage of SNAP-25 was reduced, but not completely blocked, by mutating the third glycosylation site. To confirm these findings, we titrated the concentration of BoNT/A and compared the cleavage of SNAP-25 in neurons expressing WT SV2 and the SV2A(N573Q) mutant (Figure 6G). At low concentrations of BoNT/A (1–3 nM), less cleavage of SNAP-25 was observed in neurons that expressed the N573Q mutant compared with neurons that expressed WT SV2A; at higher [BoNT/A] (10 nM), this difference became negligible. These results indicate that neurons expressing WT SV2A have a higher sensitivity to BoNT/A than neurons that express the SV2A(N573Q) mutant, suggesting that glycosylation of the N573 site is not essential for, but may enhance, the entry of BoNT/A mediated by SV2A.
Importantly, the finding that SV2A(N573Q) can mediate the entry of BoNT/A into neurons demonstrated that loss of glycosylation at the third N-linked glycosylation site does not alter the expression or targeting of SV2A in neurons. This finding is further supported by our observation that SV2A(N573Q) targeted to synapses in neurons, as evidenced by the high degree of colocalization with the synaptic marker Syb II (Supplemental Figure S2).
Gangliosides Are Essential for the Binding and Entry of BoNT/E into Neurons
Finally, we sought to address whether gangliosides are essential coreceptors for the binding and entry of BoNT/E into neurons. We found BoNT/E failed to bind hippocampal neurons cultured from mice lacking gangliosides (Liu et al., 1999); binding was restored by loading exogenous gangliosides into neuronal membranes (Figure 7A). Furthermore, BoNT/E failed to enter ganglioside-deficient neurons as demonstrated by the lack of cleavage of SNAP-25 (Figure 7B). Loading ganglioside-deficient neurons with exogenous gangliosides rescued the entry of BoNT/E, resulting in the cleavage of SNAP-25 (Figure 7B). Together, these data demonstrate that gangliosides are essential for the binding and entry of BoNT/E into neurons.
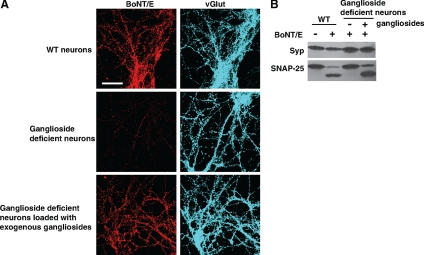
Figure 7.
Gangliosides are essential for the binding and entry of BoNT/E into neurons. (A) Cultured WT and ganglioside-deficient neurons were exposed to BoNT/E (30 nM) as described in Figure 1A. Ganglioside deficient neurons, preloaded with exogenous gangliosides (250 μg/ml ganglioside mixture, 12 h), were assayed in parallel. Binding of BoNT/E to ganglioside-deficient neurons was abolished but was rescued by loading neurons with exogenous gangliosides. (B) Cultured WT and ganglioside-deficient neurons were exposed to BoNT/E (200 pM) as described in Figure 2B. Neurons were harvested and subjected to immunoblot analysis. In ganglioside-deficient neurons, SNAP-25 was protected from BoNT/E, whereas loading these neurons with exogenous gangliosides resulted in entry of BoNT/E, as monitored by the cleavage of SNAP-25.
DISCUSSION
Using cultured hippocampal neurons from SV2A/B KO mice, we found that two of three isoforms of SV2, SV2A and SV2B, mediate the binding and entry of BoNT/E into neurons. We also demonstrated that mice lacking SV2B are less sensitive to BoNT/E. The entry of BoNT/E is mediated by the major luminal domain (the L4 domain) in SV2A and SV2B because 1) BoNT/E coimmunoprecipitates with the L4 domains of SV2A and SV2B expressed in HEK cells, and 2) SV2A-L4 or SV2B-L4 luminal domains alone, expressed on the cell surface through fusion with the transmembrane and cytosolic domain of the LDLR, can mediate activity-independent entry of BoNT/E into SV2A/B KO neurons. Finally, we found that glycosylation of the third glycosylation site of SV2A (N573) is essential for entry of BoNT/E. Together, our data establish that glycosylated SV2A and B are functional protein receptors for BoNT/E in neurons.
In contrast to SV2A and SV2B, SV2C (expressed through lentiviral infection) failed to rescue the entry of BoNT/E into SV2A/B KO hippocampal neurons (Figure 2B). We noticed that the expression levels of SV2C were consistently lower than SV2A and SV2B (Figure 2B). However, the expression levels of SV2C, achieved in our experiments, were sufficient to mediate the entry of BoNT/A (Figure 2C). It remains to be determined whether SV2C, when expressed at high levels in hippocampal neurons, can mediate the entry of BoNT/E.
Among three SV2-L4 chimeric receptors expressed in HEK cells, SV2A-L4 coimmunoprecipitated much higher levels of BoNT/E than SV2B-L4 and SV2C-L4 (Figure 4B, right panel), suggesting that SV2A might be the preferred binding partner for BoNT/E. Both SV2B-L4 and SV2C-L4 immunoprecipitated only minimal levels of BoNT/E (Figure 4B, right panel), indicating their weak association with BoNT/E in vitro. However, when expressed in neurons, it is clear that SV2B-L4, but not SV2C-L4, can mediate the entry of BoNT/E (Figure 4E). One possible explanation for this apparent discrepancy is that the neuronal surface might provide an optimal environment for SV2B-BoNT/E interactions, as opposed to the artificial conditions that occur in the immunoprecipitates (e.g., the presence of detergents).
The observation that the SV2C-L4 chimeric receptor cannot mediate the entry of BoNT/E (Figure 4E) is consistent with the inability of SV2C to rescue entry of BoNT/E in cultured neurons (Figure 2B). Interestingly, the chimeric receptor containing SV2C-L4 domain displayed a higher apparent molecular weight than receptors containing SV2A-L4 or SV2B-L4, suggesting that the glycosylation pattern might differ between SV2C and SV2A/B, because their primary protein sequences are of similar lengths. Further studies are needed to assess whether the amino acid sequence differences between these SV2 isoforms determine their differential glycosylation patterns.
We found all three putative N-linked glycosylation sites in the L4 domain of SV2 are glycosylated in neurons (Figure 6B). A key finding was that a point mutation, which abolishes the third glycosylation site (N573Q) in the SV2A-L4 domain, rendered SV2A unable to mediate the entry of BoNT/E into neurons (Figure 6B). This defect is not due to mis-sorting/mis-targeting of the mutant SV2A because it can still mediate the entry of BoNT/A into neurons (Figure 6, F and G). The finding that glycosylation at N573 site is essential for SV2A-mediated entry of BoNT/E provides a possible explanation for previous reports that BoNT/E does not bind to recombinant SV2-L4 fragments purified from E. coli (Dong et al., 2006; Mahrhold et al., 2006).
In contrast to BoNT/E, BoNT/A can bind to recombinant SV2-L4 fragments directly (Dong et al., 2006; Mahrhold et al., 2006), indicating that L4 · BoNT/A interactions in vitro do not completely rely on glycosylation of SV2. However, neurons expressing the glycosylation-mutant SV2A(N573Q) displayed reduced sensitivity to BoNT/A compared with neurons expressing WT SV2A (Figure 6G). Together, these findings suggest that the glycosylation of SV2A at N573 is not essential, but may facilitate, binding of BoNT/A. We also found that all three chimeric receptors, containing the L4 domains of SV2A, B, or C, can bind BoNT/A and mediate its entry into neurons (Figure 4D), which confirmed our previous finding that all three SV2 isoforms can function as receptors for BoNT/A (Dong et al., 2006).
The nature and extent of the glycosylation of SV2 is not currently understood. Cleavage of N-glycan from denatured SV2 using PNGaseF or blocking N-glycosylation with tunicamycin in PC12 cells yielded SV2 protein band that runs close to estimated molecular weight based on primary protein sequence (Buckley and Kelly, 1985; Janz and Sudhof, 1999), suggesting that N-linked glycosylation is the dominant form of modification of SV2.
There are two possiblities for the importance of N-linked glycosylation at position N573 of SV2A. First, glycosylation might be critical for helping the SV2-L4 domain fold into a certain structure that is essential for BoNT/E recognition and that also enhances the binding of BoNT/A. Interestingly, the L4 domain has an unusually high percentage of hydrophobic amino acids, particularly phenylalanine (Bajjalieh et al., 1993; Janz and Sudhof, 1999). This feature will probably require the L4 domain to fold in a manner that minimizes the exposure of hydrophobic surfaces. Alternatively, the N-glycan at N573 might contain specific structural groups that can bind directly to BoNT/E and BoNT/A. Analysis of the structure of this N-glycan is needed in order to determine whether there is a specific binding site for BoNT/E and A within the N-glycan itself.
We also addressed the role of gangliosides in the binding and entry of BoNT/E, using cultured hippocampal neurons from ganglioside-deficient mice as a model system. We found that BoNT/E failed to bind and enter neurons lacking gangliosides and that this defect can be rescued by loading neurons with exogenous gangliosides. These data support a “double-receptor” model for BoNT/E in which functional receptors are composed of both protein receptor SV2A/B and gangliosides in neurons.
Although it remains to be determined whether there are other proteins that serve as coreceptors by providing additional toxin-binding sites or by enhancing the affinity of toxin · SV2 complexes (Baldwin and Barbieri, 2007), the finding that chimeric receptors, SV2-L4-LDLR, can mediate the binding and entry of BoNT/A and E into neurons demonstrated that the L4 domain is sufficient to act as the toxin-binding site on the surface of neurons. These findings also revealed that entry pathways other than synaptic vesicle recycling can result in the functional entry of BoNT/A and E, thus opening the possibility for targeting toxins to specific neurons or even nonneuronal cells through the recycling endosomal pathway, to block SNARE-mediated exocytosis or protein delivery to target membranes.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Consortium for Functional Glycomics, Grant GM62116, for providing ganglioside-deficient mice. We thank members of the Chapman lab for discussions. We thank S. Y. Chiu for help with the diaphragm experiments. This work was supported by grants from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases, Grant R01 AI057744 to E.R.C. and grant EY016452 from the National Eye Institute to R.J. E.R.C. and E.A.J. acknowledge membership and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases (GLRCE; NIH award 1-U54-AI-057153). E.R.C. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0765) on September 24, 2008.
REFERENCES
- Aoki K. R. Botulinum toxin: a successful therapeutic protein. Curr. Med. Chem. 2004;11:3085–3092. doi: 10.2174/0929867043363802. [DOI] [PubMed] [Google Scholar]
- Arnon S. S., et al. Botulinum toxin as a biological weapon: medical and public health management. J. Am. Med. Assoc. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- Bajjalieh S. M., Frantz G. D., Weimann J. M., McConnell S. K., Scheller R. H. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J. Neurosci. 1994;14:5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajjalieh S. M., Peterson K., Linial M., Scheller R. H. Brain contains two forms of synaptic vesicle protein 2. Proc. Natl. Acad. Sci. USA. 1993;90:2150–2154. doi: 10.1073/pnas.90.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajjalieh S. M., Peterson K., Shinghal R., Scheller R. H. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257:1271–1273. doi: 10.1126/science.1519064. [DOI] [PubMed] [Google Scholar]
- Baldwin M. R., Barbieri J. T. Association of botulinum neurotoxin serotypes a and B with synaptic vesicle protein complexes. Biochemistry. 2007;46:3200–3210. doi: 10.1021/bi602396x. [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Sudhof T. C., Niemann H., Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993a;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Yamasaki S., Binz T., Niemann H., Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993b;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K., Kelly R. B. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J. Cell Biol. 1985;100:1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullens R. W., O'Hanlon G. M., Wagner E., Molenaar P. C., Furukawa K., Plomp J. J., Willison H. J. Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J. Neurosci. 2002;22:6876–6884. doi: 10.1523/JNEUROSCI.22-16-06876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Q., Arndt J. W., Dong M., Tepp W. H., Johnson E. A., Chapman E. R., Stevens R. C. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature. 2006;444:1096–1100. doi: 10.1038/nature05411. [DOI] [PubMed] [Google Scholar]
- Chapman E. R., Jahn R. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J. Biol. Chem. 1994;269:5735–5741. [PubMed] [Google Scholar]
- Crowder K. M., Gunther J. M., Jones T. A., Hale B. D., Zhang H. Z., Peterson M. R., Scheller R. H., Chavkin C., Bajjalieh S. M. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc. Natl. Acad. Sci. USA. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick D., Blumenfeld A., Silberstein S. D. Botulinum neurotoxin for the treatment of migraine and other primary headache disorders. Clin. Dermatol. 2004;22:76–81. doi: 10.1016/j.clindermatol.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Dolly J. O., Black J., Williams R. S., Melling J. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature. 1984;307:457–460. doi: 10.1038/307457a0. [DOI] [PubMed] [Google Scholar]
- Dong M., Richards D. A., Goodnough M. C., Tepp W. H., Johnson E. A., Chapman E. R. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell Biol. 2003;162:1293–1303. doi: 10.1083/jcb.200305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M., Tepp W. H., Liu H., Johnson E. A., Chapman E. R. Mechanism of botulinum neurotoxin B and G entry into hippocampal neurons. J. Cell Biol. 2007;179:1511–1522. doi: 10.1083/jcb.200707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M., Yeh F., Tepp W. H., Dean C., Johnson E. A., Janz R., Chapman E. R. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- Feany M. B., Lee S., Edwards R. H., Buckley K. M. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70:861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- Fischer A., Montal M. Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc. Natl. Acad. Sci. USA. 2007;104:10447–10452. doi: 10.1073/pnas.0700046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon S., Paez-Gomez J. A., Diaz-Guerra M., Scheiffele P., Scholl F. G. Dual-promoter lentiviral vectors for constitutive and regulated gene expression in neurons. J. Neurosci. Methods. 2008;168:104–112. doi: 10.1016/j.jneumeth.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Sudhof T. C. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R., Simpson L. L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc. Natl. Acad. Sci. USA. 1985;82:1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Scheller R. H. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jahn R., Sudhof T. C. Membrane fusion and exocytosis. Annu. Rev. Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Janz R., Goda Y., Geppert M., Missler M., Sudhof T. C. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24:1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Janz R., Sudhof T. C. SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience. 1999;94:1279–1290. doi: 10.1016/s0306-4522(99)00370-x. [DOI] [PubMed] [Google Scholar]
- Jin R., Rummel A., Binz T., Brunger A. T. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature. 2006;444:1092–1095. doi: 10.1038/nature05387. [DOI] [PubMed] [Google Scholar]
- Johnson E. A. Clostridial toxins as therapeutic agents: benefits of nature's most toxic proteins. Annu. Rev. Microbiol. 1999;53:551–575. doi: 10.1146/annurev.micro.53.1.551. [DOI] [PubMed] [Google Scholar]
- Kamata Y., Kozaki S., Sakaguchi G., Iwamori M., Nagai Y. Evidence for direct binding of Clostridium botulinum type E derivative toxin and its fragments to gangliosides and free fatty acids. Biochemical and biophysical research communications. 1986;140:1015–1019. doi: 10.1016/0006-291x(86)90736-9. [DOI] [PubMed] [Google Scholar]
- Keller J. E., Cai F., Neale E. A. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry. 2004;43:526–532. doi: 10.1021/bi0356698. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Iwamori M., Nagai Y. Interaction between Clostridium botulinum neurotoxin and gangliosides. Biochim. Biophys. Acta. 1980;628:328–335. doi: 10.1016/0304-4165(80)90382-7. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Takamiya K., Aizawa S., Furukawa K. Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim. Biophys. Acta. 1999;1441:1–3. doi: 10.1016/s1388-1981(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Koriazova L. K., Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 2003;10:13–18. doi: 10.1038/nsb879. [DOI] [PubMed] [Google Scholar]
- Kozaki S., Kamata Y., Watarai S., Nishiki T., Mochida S. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb. Pathog. 1998;25:91–99. doi: 10.1006/mpat.1998.0214. [DOI] [PubMed] [Google Scholar]
- Lawrence G., Wang J., Chion C. K., Aoki K. R., Dolly J. O. Two protein trafficking processes at motor nerve endings unveiled by botulinum neurotoxin E. J. Pharmacol. Exp. Therapeut. 2007;320:410–418. doi: 10.1124/jpet.106.108829. [DOI] [PubMed] [Google Scholar]
- Liu Y., et al. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J. Clin. Invest. 1999;103:497–505. doi: 10.1172/JCI5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrhold S., Rummel A., Bigalke H., Davletov B., Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580:2011–2014. doi: 10.1016/j.febslet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Miesenbock G., Rothman J. E. Patterns of synaptic activity in neural networks recorded by light emission from synaptolucins. Proc. Natl. Acad. Sci. USA. 1997;94:3402–3407. doi: 10.1073/pnas.94.7.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco C. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem. Sci. 1986:314–317. [Google Scholar]
- Nishiki T., Kamata Y., Nemoto Y., Omori A., Ito T., Takahashi M., Kozaki S. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J. Biol. Chem. 1994;269:10498–10503. [PubMed] [Google Scholar]
- Nishiki T., Tokuyama Y., Kamata Y., Nemoto Y., Yoshida A., Sato K., Sekiguchi M., Takahashi M., Kozaki S. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 1996;378:253–257. doi: 10.1016/0014-5793(95)01471-3. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Rummel A., et al. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc. Natl. Acad. Sci. USA. 2007;104:359–364. doi: 10.1073/pnas.0609713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel A., Karnath T., Henke T., Bigalke H., Binz T. Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J. Biol. Chem. 2004a;279:30865–30870. doi: 10.1074/jbc.M403945200. [DOI] [PubMed] [Google Scholar]
- Rummel A., Mahrhold S., Bigalke H., Binz T. The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol. Microbiol. 2004b;51:631–643. doi: 10.1046/j.1365-2958.2003.03872.x. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Malizio C., Trimble W. S., Polverino de Laureto P., Milan G., Sugiyama H., Johnson E. A., Montecucco C. Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala-Ala peptide bond. J. Biol. Chem. 1994;269:20213–20216. [PubMed] [Google Scholar]
- Schiavo G., Matteoli M., Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Rossetto O., Catsicas S., Polverino de Laureto P., DasGupta B. R., Benfenati F., Montecucco C. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J. Biol. Chem. 1993a;268:23784–23787. [PubMed] [Google Scholar]
- Schiavo G., Shone C. C., Rossetto O., Alexander F. C., Montecucco C. Botulinum neurotoxin serotype F is a zinc endopeptidase specific for VAMP/synaptobrevin. The J. Biol. Chem. 1993b;268:11516–11519. [PubMed] [Google Scholar]
- Scranton T. W., Iwata M., Carlson S. S. The SV2 protein of synaptic vesicles is a keratan sulfate proteoglycan. J. Neurochem. 1993;61:29–44. doi: 10.1111/j.1471-4159.1993.tb03535.x. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. Identification of the major steps in botulinum toxin action. Annu. Rev. Pharmacol. Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- Sobel J. Botulism. Clin. Infect. Dis. 2005;41:1167–1173. doi: 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K., Kohda T., Mukamoto M., Takeuchi K., Ihara H., Saito M., Kozaki S. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 2005;280:35164–35171. doi: 10.1074/jbc.M507596200. [DOI] [PubMed] [Google Scholar]
- Verderio C., Grumelli C., Raiteri L., Coco S., Paluzzi S., Caccin P., Rossetto O., Bonanno G., Montecucco C., Matteoli M. Traffic of botulinum toxins A and E in excitatory and inhibitory neurons. Traffic. 2007;8:142–153. doi: 10.1111/j.1600-0854.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- Yowler B. C., Kensinger R. D., Schengrund C. L. Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J. Biol. Chem. 2002;277:32815–32819. doi: 10.1074/jbc.M205258200. [DOI] [PubMed] [Google Scholar]
- Yowler B. C., Schengrund C. L. Botulinum neurotoxin A changes conformation upon binding to ganglioside GT1b. Biochemistry. 2004;43:9725–9731. doi: 10.1021/bi0494673. [DOI] [PubMed] [Google Scholar]
- Yule A. M., Austin J. W., Barker I. K., Cadieux B., Moccia R. D. Persistence of Clostridium botulinum neurotoxin type E in tissues from selected freshwater fish species: implications to public health. J. Food Protect. 2006;69:1164–1167. doi: 10.4315/0362-028x-69.5.1164. [DOI] [PubMed] [Google Scholar]
- Zhou L., Zhang C. L., Messing A., Chiu S. Y. Temperature-sensitive neuromuscular transmission in Kv1.1 null mice: role of potassium channels under the myelin sheath in young nerves. J. Neurosci. 1998;18:7200–7215. doi: 10.1523/JNEUROSCI.18-18-07200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.