Abstract
The independently folded appendages of the large α and β2 subunits of the endocytic adaptor protein (AP)-2 complex coordinate proper assembly and operation of endocytic components during clathrin-mediated endocytosis. The β2 subunit appendage contains a common binding site for β-arrestin or the autosomal recessive hypercholesterolemia (ARH) protein. To determine the importance of this interaction surface in living cells, we used small interfering RNA-based gene silencing. The effect of extinguishing β2 subunit expression on the internalization of transferrin is considerably weaker than an AP-2 α subunit knockdown. We show the mild sorting defect is due to fortuitous substitution of the β2 chain with the closely related endogenous β1 subunit of the AP-1 adaptor complex. Simultaneous silencing of both β1 and β2 subunit transcripts recapitulates the strong α subunit RNA interference (RNAi) phenotype and results in loss of ARH from endocytic clathrin coats. An RNAi-insensitive β2-yellow fluorescent protein (YFP) expressed in the β1 + β2-silenced background restores cellular AP-2 levels, robust transferrin internalization, and ARH colocalization with cell surface clathrin. The importance of the β appendage platform subdomain over clathrin for precise deposition of ARH at clathrin assembly zones is revealed by a β2-YFP with a disrupted ARH binding interface, which does not restore ARH colocalization with clathrin. We also show a β-arrestin 1 mutant, which engages coated structures in the absence of any G protein-coupled receptor stimulation, colocalizes with β2-YFP and clathrin even in the absence of an operational clathrin binding sequence. These findings argue against ARH and β-arrestin binding to a site upon the β2 appendage platform that is later obstructed by polymerized clathrin. We conclude that ARH and β-arrestin depend on a privileged β2 appendage site for proper cargo recruitment to clathrin bud sites.
INTRODUCTION
Clathrin-mediated endocytosis is a process through which many nutrient and signaling receptors, ion channels, and even pathogens, are internalized from the cell surface and delivered to endosomes. The process requires the fine coordination of cargo selection, membrane invagination, and actin dynamics (Conner and Schmid, 2003; Robinson, 2004; Edeling et al., 2006b; Kaksonen et al., 2006). At the plasma membrane, these diverse processes are managed, in part, by the adaptor protein (AP)-2 heterotetramer. The brick-like AP-2 core is composed of a small σ2 subunit, the cargo-selective μ2 subunit, along with the N-terminal trunks of two large chains, the α and β2 subunits (Collins et al., 2002; see Figure 4A). Projecting off both α and β2 subunit trunks, via flexible hinges, are bilobal appendages that contain binding sites for clathrin and numerous other endocytic proteins (Robinson, 2004; Edeling et al., 2006b; Schmid and McMahon, 2007).
Figure 4.
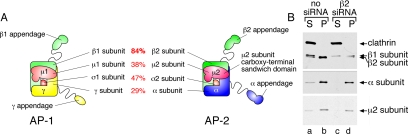
β1 subunit rescues β2 subunit knockdown of AP-2. (A) Schematic diagram of AP-1 and AP-2 complex composition and subunit homology. (B) Lysates from either untreated or β2 siRNA-treated HeLa SS6 cells were prepared and AP-2 immunoprecipitated using the anti-α subunit mAb AP.6. Aliquots of the supernatant and precipitate fractions from both control and β2 RNAi samples were resolved by SDS-PAGE and transferred to nitrocellulose. Blots were probed with the anti-AP-2 α subunit mAb clone 8, anti-β1/β2 subunit antibody mAb 100/1, anti-clathrin HC antibody mAb TD.1, or anti-AP-2 μ2 antiserum, and only the relevant portion of each blot is shown.
The four assembled subunits permit AP-2 to coordinate various aspects of clathrin coat formation. The plasma membrane-enriched lipid phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] is engaged by both the α and μ2 subunits (Gaidarov and Keen, 1999; Höning et al., 2005). YXXØ-type sorting signals (as found in the transferrin receptor) are bound directly by the μ2 subunit (Ohno et al., 1995), whereas [DE]XXXL[LIM] dileucine signals are recognized by a functional hemicomplex of the α and σ2 subunit (Janvier et al., 2003; Coleman et al., 2005; Chaudhuri et al., 2007). Various assembly protein and clathrin associations are established through the α and β2 appendages (Wang et al., 1995; McPherson and Ritter, 2005; Traub, 2005; Edeling et al., 2006b; Schmid and McMahon, 2007). The α appendage has two interaction surfaces, one each upon the platform and sandwich subdomains. The platform site can bind to DP[WF] and FXDXF interaction motifs (Brett et al., 2002; Praefcke et al., 2004), whereas the sandwich site engages the WXX[FW]Xn[DE] motif (Mishra et al., 2004; Praefcke et al., 2004; Ritter et al., 2004; Walther et al., 2004). These short interaction sequences are typically positioned within tracts of intrinsically unstructured polypeptide, and proteins bearing the former class of motifs are typically involved in cargo selection and lattice polymerization. Proteins bearing the latter set generally include regulatory proteins such as adaptor-associated kinase 1, cyclin G-dependent kinase, and the phosphoinositide polyphosphatase synaptojanin 1 (Jha et al., 2004; Praefcke et al., 2004; Walther et al., 2004). Some of the regulatory proteins, such as synaptojanin 1, also contain platform binding sites, potentially allowing them to compete previously bound clathrin-associated sorting proteins (CLASPs) off AP-2 (Mishra et al., 2004; Praefcke et al., 2004). Thus, the α appendage regulates the temporal organization of the developing clathrin lattice through binding sites that permit the recruitment of first lattice assembly and cargo selective factors, followed by regulatory proteins that control bud formation and likely promote release of proteins from AP-2 once their role in endocytosis is complete.
Similar to the α appendage, the structurally related β2 appendage also contains two interaction surfaces, one surface analogous to the α appendage-platform site, and one surface positioned on the opposite side from the cognate α appendage-sandwich site (Edeling et al., 2006a; Schmid et al., 2006). The contact sites on the β2 appendage do not seem to control accessory proteins in precisely the same manner as the hierarchical α appendage binding sites. Biochemical studies suggest the β2 appendage-platform site is largely dedicated to CLASPs, such as the β-arrestins, which concentrate G protein-coupled receptors (GPCRs) (Kim and Benovic, 2002; Laporte et al., 2002; Milano et al., 2002); the autosomal recessive hypercholesterolemia (ARH) protein, which decodes the FXNPXY-type sorting signal (He et al., 2002; Mishra et al., 2002b); and epsin 1, which recognizes poly/multiubiquitinated cargo (Barriere et al., 2006; Hawryluk et al., 2006). These discrete classes of cargo internalization signal do not bind directly to AP-2. The β2 appendage-sandwich site, along with a type I clathrin box in the β2-hinge, allows AP-2 to polymerize clathrin and regulate eps15 positioning within the assembling lattice (Cupers et al., 1998; Owen et al., 2000; Edeling et al., 2006a). Thus, the β2 subunit contains functionally distinct binding sites that could simultaneously allow privileged access of certain CLASPs to the lattice along with clathrin recruitment. Overall, AP-2 acts as a master adaptor, binding to the plasma membrane, sorting YXXØ- and [DE]XXXL[LIM]-bearing cargo, and coordinating clathrin vesicle formation while simultaneously providing access for alternative types of cargo by scaffolding CLASPs. Accordingly, targeted gene disruption or mutation of AP-2 μ2 or α subunit genes is homozygous lethal in mice (Mitsunari et al., 2005), Drosophila (Gonzalez-Gaitan and Jackle, 1997), and Caenorhabditis elegans (Shim and Lee, 2000).
In humans, inherited deficiency of one CLASP, ARH, results in a pathological hypercholesterolemia similar to, but less severe than, familial hypercholesterolemia (Eden et al., 2001; Garcia et al., 2001). Structurally, ARH contains a phosphotyrosine binding (PTB) domain that binds simultaneously to both FXNPXY peptides and PtdIns(4,5)P2 and an unstructured C terminus containing a type I clathrin box and the β2 appendage-binding sequence (Eden et al., 2002; He et al., 2002; Mishra et al., 2002b). Functionally, it may play an analogous role to the β-arrestins, which use a related, helical [DE]nX1-2FXX[FL]XXXR β2-binding sequence to usher GPCRs to preformed clathrin coats (Laporte et al., 2000; Kim and Benovic, 2002; Milano et al., 2002; Edeling et al., 2006a; Hamdan et al., 2007). ARH may likewise use primarily the β2 appendage-binding site, rather than the clathrin box, to shuttle or retain low density lipoprotein (LDL) receptors in clathrin structures.
To test ARH dependence on β2 appendage binding, we replaced endogenous AP-2 β2 subunits with various β2-yellow fluorescent protein (YFP) mutants by using RNA interference (RNAi). When characterizing the β2 subunit knockdown necessary for this approach, we found that simultaneous depletion of the β1 subunit from the related trans-Golgi network (TGN)/endosome-localized AP-1 complex is also necessary, because, otherwise, the highly related, endogenous β1 subunit compensates for β2 subunit depletion. In the context of a β1 + β2 subunit knockdown, the β2 subunit trunk is sufficient to rescue known AP-2 knockdown phenotypes (Hinrichsen et al., 2003; Motley et al., 2003), clustering transferrin in clathrin coats and restoring cellular AP-2 levels, although ARH surface localization is clearly disrupted. Rescue of the β1 + β2 subunit knockdown with a full-length β2-YFP bearing a point mutation in the β2-platform subdomain also fails to recover ARH localization to clathrin structures, whereas both wild-type β2-YFP or β2-YFP lacking clathrin binding sites restore ARH localization to clathrin puncta. This suggests that AP-2 is vital both as the YXXØ-selective CLASP and as a scaffold controlling endocytic protein recruitment to clathrin-coated pits during endocytosis.
MATERIALS AND METHODS
Plasmids, Small Interfering RNA (siRNA) and Antibodies
The siRNA duplexes used target base pairs 1384-1402 of the human β2 subunit (Huang et al., 2004) (Dharmacon RNA Technologies, Lafayette, CO), base pairs 1529-1537 of the human β1 subunit (Invitrogen, Carlsbad, CA or Dharmacon RNA Technologies), and base pairs 1052-1069 of the α subunit transcript (Dharmacon RNA Technologies) (Keyel et al., 2006). All siRNA oligonucleotides were synthesized with dTdT overhangs. The β2-YFP plasmid was a kind gift from Alexander Sorkin (University of Colorado at Denver, Aurora, CO; Sorkina et al., 2002). Generation of the β2-YFP mutants and truncation has been described previously (Keyel et al., 2006). The tandem dimer tomato (tdRFP)-β-arrestin 1 was generated by first replacing enhanced green fluorescent protein (EGFP) with tdRFP (Shaner et al., 2004) between the NheI and BspE1 restriction sites in pEGFP-C1, followed by insertion of full-length Bos taurus β-arrestin 1 between XhoI and SalI sites. The β2-YFP platform (Y888V) and sandwich (Y815A) subdomain mutants, the clathrin-box deleted (ΔLLDLD) mutant, the tdRFP-β-arrestin 1 paired I386A and V387A (IV→AA) constitutively active mutant, and the clathrin box 376LIEL→AAEA mutation or clathrin-box-deleted ΔLIELD were all generated using QuikChange mutagenesis (Stratagene, La Jolla, CA).
The anti-clathrin heavy chain (HC) monoclonal antibody (mAb) TD.1 (Nathke et al., 1992) and X22 (Brodsky, 1985), the AP-1/2 β1/β2 subunit mAb 100/1 (Ahle et al., 1988) and affinity-purified antibody GD/1 (Traub et al., 1999), the AP-2 α subunit mAb AP.6 (Chin et al., 1989), the anti-AP-1 γ subunit antibody AE/1 (Traub et al., 1995), the anti-AP-1 μ1 subunit antibody RY/1 (Traub et al., 1995), the anti-ARH antibody (Mishra et al., 2002b), the anti-cation–independent mannose 6-phosphate receptor (MPR) antibody (Zhu et al., 1999), the anti-epsin 1 polyclonal antibody (Drake et al., 2000), and the anti-disabled-2 (Dab2) polyclonal antibody (Mishra et al., 2002a) have been described previously. Affinity-purified anti-eps15 polyclonal was kindly provided by Ernst Ungewickell (Medizinische Hochschule Hannover, Hannover, Germany; Hinrichsen et al., 2003). The anti-transferrin receptor mAb H68.4 was a gift from Ian Trowbridge (The Salk Institute, La Jolla, CA; White et al., 1992), rabbit R11-29 anti-AP-2 μ2 subunit serum was kindly provided by Juan Bonifacino (National Institutes of Health, Bethesda, MD; Aguilar et al., 1997), the anti-lysosome–associated membrane protein (LAMP)-1 mAb G1/139 was kindly provided by Jack Rohrer (Zürcher Hochschule für Angewandte Wissenschaften, Wadenswil, Switzerland; Rohrer et al., 1996), and anti-green fluorescent protein (GFP) polyclonal antibody B5 was generously provided by Phyllis Hanson (Washington University School of Medicine, St. Louis, MO; Dalal et al., 2004). Anti-AP-2 α subunit mAb clone 8 and the anti-AP-1 γ subunit mAb clone 88 were both from BD Biosciences Transduction Laboratories (Lexington, KY). The anti-β-arrestin polyclonal antibody was purchased from Sigma-Aldrich (St. Louis, MO), the anti-tubulin mAb E7 was from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA), and a hybridoma secreting anti-LDL receptor mAb IgG-C7 was from the American Type Culture Collection (Manassas, VA). Horseradish peroxidase-conjugated donkey anti-mouse and anti-rabbit, Cy5-conjugated goat anti-mouse, and 15-nm gold-conjugated anti-mouse secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA), whereas Alexa Fluor 488 angiotensin II, Alexa Fluor 488-, and Alexa Fluor 568-conjugated goat anti-mouse and anti-rabbit secondary antibodies were obtained from Invitrogen.
Cell Culture, RNAi, and Reconstitution
HeLa SS6 cells (Elbashir et al., 2001) were cultured at 37°C humidified with 5% CO2 in DMEM supplemented with 10% fetal calf serum and 2 mM l-glutamine (Invitrogen). A line of HeLa SS6 cells stably expressing β2-YFP was selected in the same medium supplemented with 0.5 mg/ml G-418 (Invitrogen). Analysis of these cells shows that <10% of cellular AP-2 contains the YFP-tagged β2 subunit. Wild-type (+/+) and AP-2 β2 subunit homozygous mutant (β2 −/−) mouse embryonic fibroblasts were cultured in DMEM supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 1× antibiotic/antimycotic mixture (Sigma-Aldrich). β-Arrestin nullizygous mouse embryonic fibroblasts were kindly provided by Robert Lefkowitz (Kohout et al., 2001). Cells were maintained in DMEM supplemented with 10% fetal calf serum and 2 mM l-glutamine. Human embryonic kidney (HEK) 293 cells stably expressing a FLAG-tagged type I angiotensin II receptor were kindly provided by Stéphane Laporte (McGill University, Montreal, Quebec, Canada), and they were cultured in Earle's minimal essential medium. RNAi and reconstitution were performed as described previously (Keyel et al., 2006). For rescue experiments, 100 ng of rescue DNA was included along with the siRNA duplexes at the time of transfection and did not block RNAi-mediated knockdown of the targeted proteins (Supplemental Figure S2).
Immunofluorescence
HeLa SS6 cells were prepared for immunofluorescence as described previously (Mishra et al., 2005). To visualize only the plasma membrane associated clathrin, or only AP-2 with the anti-β1/β2 antibody GD/1, cells were treated with 10 μg/ml brefeldin A (Epicenter Technologies, Madison, WI) for 15 min, followed by permeabilization in 0.3% saponin, 25 mM HEPES-KOH, pH 7.2, 125 mM potassium acetate, and 5 mM magnesium acetate for 1 min on ice before fixation to remove cytosolic and ARF-dependent clathrin and AP-1. For experiments involving LDL receptors, the cells were grown in DMEM supplemented with 10% lipoprotein-deficient serum (Cocalico Laboratories, Reamstown, PA), 2 mM l-glutamine for 24–48 h to up-regulate LDL receptors, whereas for experiments involving transferrin, cells were placed in DMEM supplemented with 25 mM HEPES-KOH, pH 7.2, and 0.5% bovine serum albumin (starvation medium) for 1 h before experiments to unload the receptors. To measure surface levels of receptors, cells were incubated with either the appropriate antibody or 25 μg/ml transferrin conjugated to Alexa Fluor 568 (Tf568) (Invitrogen) in starvation medium for 1 h on ice at 4°C and fixed for immunofluorescence. To measure the amount of internalized receptors, cells were either incubated on ice as described above and then washed and warmed to 37°C for 15 min before fixation (see Figures 1 and 7); or after the 1 h in starvation medium, maintained in the continuous presence of 25 μg/ml Tf568 or 50 μg/ml Tf633 (Invitrogen) for 15 min at 37°C before fixation (see Figures 6 and 7). Alexa 488-labeled angiotensin II (100 nM) was added at 37°C for 5 or 20 min. For imaging of surface transferrin receptor-labeled fibroblasts, 25 μg/ml Tf488 was bound at 4°C for 1 h in starvation media. Cells were washed once and imaged at 4°C in starvation media using a temperature-controlled stage mount (Harvard Apparatus, Holliston, MA).
Figure 1.
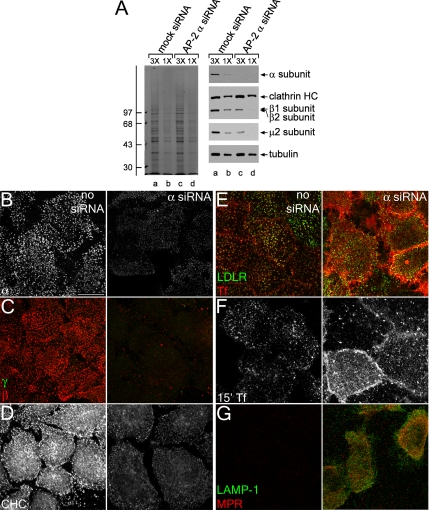
siRNA-mediated gene silencing of the AP-2 α subunit. (A) Lysates of HeLa SS6 cells untreated (lanes a and b) or treated with AP-2 α subunit siRNA (lanes c and d) were resolved by SDS-PAGE and either Coomassie stained or transferred to nitrocellulose. Sections of the blots were probed with anti-AP-2 α subunit mAb clone 8, anti-clathrin heavy chain (HC) mAb TD.1, anti-β1/β2 subunit mAb 100/1, anti-AP-2 μ2 antiserum, or anti-tubulin mAb E7, and only the relevant portion of each blot shown. The position of molecular mass standards (in kilodaltons) is indicated on the left. (B–D) HeLa SS6 cells untreated (left) or treated with AP-2 α subunit siRNA (right) were treated with 10 μg/ml brefeldin A for 15 min, permeabilized on ice for 1 min before fixation, and prepared for immunofluorescence using the anti-AP-2 α subunit mAb AP.6 (B), anti-AP-1 γ subunit mAb (C; green), anti-β1/β2 subunit antibody GD/2 (C; red), or anti-clathrin heavy chain mAb X22 (D). (E–G) HeLa SS6 cells untreated (left) or treated with AP-2 α subunit siRNA (right) were incubated on ice for 1 h with either anti-LDL receptor mAb C7 (E; green), Tf568 (E, red and F), anti-LAMP-1 mAb (G; green), or anti-MPR antibody (G; red) and fixed (E and G) or washed and warmed to 37°C for 15 min and fixed (F) followed by indirect immunofluorescence. Bar, 10 μm.
Figure 7.
Rescue of AP-2 knockdown with β2-YFP. (A) Lysates of HeLa SS6 cells untreated (lane a) or treated with either AP-2 α subunit (lane b), both AP-1 β1 subunit and AP-2 β2 subunit siRNA (lane c) along with full-length β2-YFP (lane d), or β2-YFPTRNK (lane e) were resolved by SDS-PAGE and transferred to nitrocellulose. Sections of the blots were probed with the anti-clathrin HC mAb TD.1 and anti-β1/β2 subunit mAb 100/1, anti-GFP antibody, anti-AP-2 α subunit mAb clone 8, anti-AP-2 μ2 subunit antiserum, or anti-AP-1 γ subunit antibody AE/1 and only the relevant portions show. (B–F) Mock-transfected (B and E) or β1 + β2 subunit siRNA-transfected (C, D, and F) HeLa SS6 cells were transfected with either full-length β2-YFP (B–D) or β2-YFPTRNK (E and F) (green in merged images), and either surface labeled with Tf568 (B, C, E, and F) (red in merged images) and fixed, or incubated with Tf568 continuously for 15 min at 37°C, fixed, and probed with anti-AP-2 α subunit mAb AP.6 (D) (blue in merged image). Note that β2-YFP–expressing cells cluster transferrin at the surface (C) and do not have the typical circumferential band of transferrin at 15 min (D; arrowheads) and that the β2-YFPTRNK restores clustering of surface transferrin receptors (F) as well. Bar, 10 μm.
Figure 6.
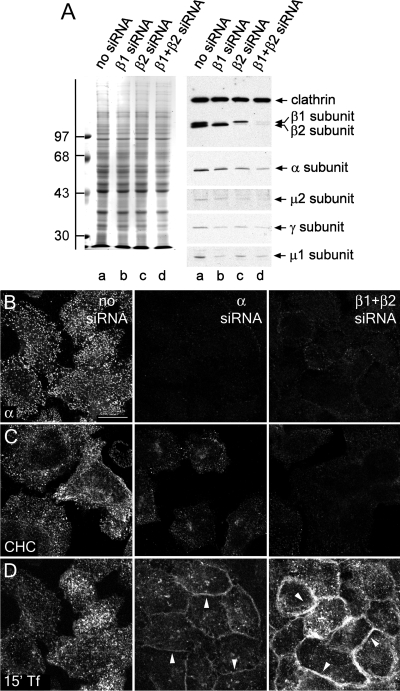
β1 + β2 subunit RNAi recapitulates α subunit RNAi phenotype. (A) Lysates of HeLa SS6 cells untreated (lane a) or treated with either AP-1 β1 (lane b), AP-2 β2 (lane c), or β1 + β2 subunit siRNAs (lane d) were resolved by SDS-PAGE and either Coomassie stained or transferred to nitrocellulose. Sections of the blots were probed with the anti-clathrin HC mAb TD.1 and anti-β1/β2 subunit mAb 100/1, anti-AP-2 α subunit mAb clone 8, anti-AP-2 μ2 subunit antiserum, anti-AP-1 γ subunit antibody AE/1, or anti-AP-1 μ1 subunit antibody RY/1 polyclonal antibodies, and only the relevant portions are shown. The position of molecular mass standards (in kilodaltons) is indicated on the left. (B–D) HeLa SS6 cells were either untreated (left), treated with either AP-2 α subunit siRNA (middle), or both AP-1 β1 and AP-2 β2 subunit siRNA (right). Next, they were either fixed and probed with anti-AP-2 α subunit mAb AP.6 (B), brefeldin treated and permeabilized on ice before fixation and probed with anti-clathrin HC mAb X22 (C), or serum starved for 1 h, given 25 μg/ml Tf568 continuously for 15 min at 37°C, and fixed (D). Arrowheads indicate accumulation of transferrin on the cell surface in contrast to the control cells. Bar, 10 μm.
Immunoprecipitation
HeLa SS6 cells grown in six-well plates, treated or untreated with siRNA against β2 subunit as described above, were trypsinized, washed in phosphate-buffered saline (PBS), and lysed in 10 mM HEPES-KOH, pH 7.2, 0.3 M sucrose, 1% Triton X-100, and Complete mini-protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) for 30 min on ice. Cell debris was removed by centrifugation at 18,000 × gmax for 10 min at 4°C, and the supernatants frozen on dry ice and stored at −80°C until use. The samples were thawed and incubated with 2 μg of anti-α subunit mAb AP.6 for 1 h at 4°C with gentle shaking, followed by the addition of protein G-coupled beads (Sigma-Aldrich), an additional hour at 4°C with gentle shaking, and then centrifugation to separate supernatant from pellet. The pellets were washed extensively in PBS and 1% Triton X-100, and then they were mixed with SDS sample buffer such that one-eighth of the pellet and ∼1/25 of the supernatant were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). AP-2 was similarly immunoprecipitated from lysates of embryonic fibroblasts prepared in 10 mM HEPES-KOH, pH 7.2, 0.3 M sucrose, 500 mM Tris-HCl, 1% Triton X-100, Complete mini-protease inhibitor cocktail, and 1 mM phenylmethylsulfonyl fluoride. After centrifugation to remove insoluble material, lysates were diluted fourfold before addition of 5 μg of mAb AP.6. Electrophoresis and immunoblotting was as described previously (Keyel et al., 2006). Blots were quantitated by measuring the integrated intensity of a fixed region containing the band in MetaMorph (MDS Analytical Technologies, Sunnyvale, CA), subtracting that intensity from the background in a similarly sized region, and normalizing expression to clathrin expression. Normalized expression levels were compared with those of mock-transfected cells to determine up- or down-regulation of AP-1 and AP-2 subunits under the various RNAi conditions.
Microscopy
Images were acquired on a FluoView FV500 or FV1000 confocal microscope (Olympus, Tokyo, Japan) as described previously (Keyel et al., 2006). Quantitation of ARH clathrin coat localization was performed using MetaMorph software (MDS Analytical Technologies) by measuring the integrated intensity of ARH staining in cell surface confocal sections that were either control (normal transferrin internalization), knocked down (blocked transferrin internalization), or rescued (YFP expression) and subtracting the intensity of knockdown cells from that of either mock transfected or neighboring rescued cells. The number of clathrin and AP-2 (α subunit) puncta were determined by manually counting all puncta within a 10 × 10 μm (clathrin) or 6 × 6 μm (AP-2) portion of cells from multiple fields and independent experiments expressing very low levels of clathrin/AP-2 (AP-2 knocked down cells) or cells expressing wild-type levels of the protein (mock). Statistical significance was determined using Student's t test. Cells with surface transferrin, transferrin uptake or AP-2 (α subunit) intensity phenotypes were manually counted in MetaMorph (MDS Analytical Technologies) as either normal or impaired, and the total percentage of cells either expressing or not expressing β2-YFP determined. Impaired phenotypes consisted of loss of transferrin localization to coated pits, very low levels of internalized transferrin, or AP-2 α subunit intensities on a par with those found with AP-2 α subunit siRNA. These percentages were averaged for two to three independent experiments and significance determined using a one-tailed Student's t test.
Total internal reflection fluorescence microscopy (TIR-FM) was performed as described previously (Keyel et al., 2004), with some modifications. Images were collected with a thermoelectrically cooled Cascade II 512 camera (Photometrics, Tucson, AZ). Data sets were collected with MetaMorph software at ∼1 frame/5 s. For quantification of colocalization of tdRFP-β-arrestin 1 with AP-2 in β2-YFP–expressing cells, an average of 55 spots/cell in 11-15 individual cells were analyzed.
Adherent plasma membrane from cells treated with α subunit siRNA as described above were prepared for rapid-freeze, deep-etch electron microscopy as described previously (Heuser, 2000). Briefly, cells grown on small oriented pieces of glass coverslip were disrupted by sonication, fixed in paraformaldehyde, and labeled with anti-transferrin receptor mAb H68.4 and then 15-nm gold-conjugated anti-mouse antibody before flash freezing in liquid helium (Keyel et al., 2006).
RESULTS
Gene Silencing of the AP-2 α Subunit
In HeLa SS6 cells, we find transfection of siRNA duplexes targeting the AP-2 α subunit mRNA reduces the protein level of all four adaptor subunits >75% within 48 h (Figure 1A and Supplemental Figure 1A), in very good agreement with previous results (Hinrichsen et al., 2003; Motley et al., 2003). Depletion of AP-2 is also seen morphologically, as a sharp decrease in the intensity of surface AP-2–positive puncta compared with the control cells (Figure 1B). It is known that AP-2 knockdown reduces the amount of clathrin positioned at the plasma membrane but not that recruited to other structures or total cytoplasmic amounts (Hinrichsen et al., 2003; Motley et al., 2003). To selectively visualize clathrin at the plasma membrane, we briefly treated cells with brefeldin A to dissociate clathrin from internal membrane structures, and then we rapidly permeabilized the cells before fixation (Figure 1, C and D). In mock-transfected cells, this treatment effectively eliminates TGN, endosomal and cytosolic clathrin, without perturbing the plasma membrane associated clathrin pool. No AP-1 γ subunit staining is observed, and the remaining β subunit signal is fully sensitive to α subunit RNAi (Figure 1C). Although the total amount of clathrin recruited to the plasma membrane is greatly diminished upon AP-2 α subunit RNAi (Figure 1D), we do not observe that the total number of surface puncta decreases >10-fold as reported originally (Hinrichsen et al., 2003; Motley et al., 2003). Rather, in α subunit–silenced cells, although very dim, the number of discrete clathrin- and AP-2–containing surface structures is 73.2 and 70.7% that of control, respectively. The discrepancy is probably because AP-2 complexes are not completely eliminated under our single transfection RNAi conditions (Figure 1B).
Extinguishing AP-2 expression also abrogates the clustering of surface receptors bearing a YXXØ internalization signal, exemplified by the transferrin receptor, at clathrin-positive puncta. Unlike the mock-treated cells, transferrin marks a population of diffuse receptors on the surface of AP-2–deficient cells (Figure 1E). Other receptors, such as the LDL receptor, that bear the FXNPXY-type internalization signal decoded by the CLASPs ARH and disabled-2 (Dab2), can internalize in an AP-2–independent manner (Eden et al., 2002; Garuti et al., 2005; Keyel et al., 2006; Maurer and Cooper, 2006; Eden et al., 2007). Nonetheless, because Dab2 and ARH each bind physically to both AP-2 and clathrin, in mock-treated cells, the LDL receptor and transferrin congregate in common surface structures (Figure 1E), which contain clathrin and AP-2 (Keyel et al., 2006). Yet, when AP-2 complexes are extinguished, only the surface distribution of the transferrin receptor, and not the LDL receptor, alters dramatically (Figure 1E). A consequence of the dispersion of the transferrin receptor is that transferrin is very poorly internalized (Figure 1F). To verify that the internalization defect is general to proteins bearing the YXXØ signal, we examined the surface distributions of the MPR and LAMP-1. The trafficking trajectory of these transmembrane proteins takes them through the plasma membrane relatively infrequently (Rohrer et al., 1996) and normally, the proteins are concentrated, at steady state, in endosomes/TGN and lysosomes, respectively. If delivered to the cell surface, both are retrieved via clathrin-mediated endocytosis (Ghosh et al., 2003). In the context of α subunit knockdown, and consistent with both the absolute dependence of the YXXØ signal on AP-2 for sorting into clathrin-coated vesicles and previous observations (Harasaki et al., 2005; Janvier and Bonifacino, 2005), a surface population of both the MPR and LAMP-1 becomes readily detectable as they stagnate at the surface (Figure 1G).
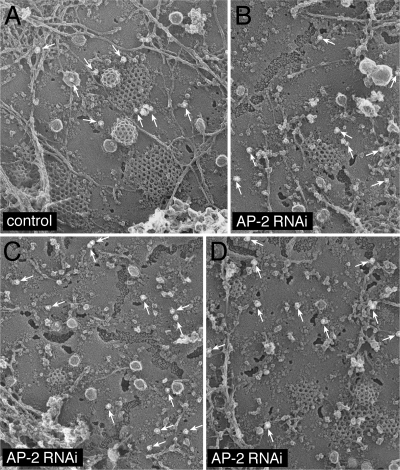
To better understand at the ultrastructural level how the AP-2 knockdown affects clathrin lattice assembly at the cell surface, adherent plasma membranes of mock- and α subunit siRNA-treated cells were analyzed by immunogold freeze-etch electron microscopy. AP-2 knocked down cells can be unambiguously identified with mAb H68.4. This antibody detects an epitope located near the amino terminus of the human transferrin receptor that overlaps with the YTRF internalization signal (White et al., 1992). Transferrin receptors positioned within clathrin lattices by engagement of the AP-2 μ2 subunit are not recognized by this mAb; only the extra-lattice population of the receptor is detected (White et al., 1992). In our images, compared with mock-transfected cells (Figure 2A), the frequency of gold labeling in the AP-2 gene-silenced cells is increased, especially the population >100 nm from assembled clathrin lattices (Figure 2, B–D). In the siRNA-transfected cells, the discernible clathrin coats are substantially smaller than normal (Motley et al., 2003), concordant with the immunofluorescence data (Figure 1) and the capability of CLASPs like Dab2 and epsin to promote clathrin lattice assembly (Drake et al., 2000; Morris and Cooper, 2001; Kalthoff et al., 2002; Mishra et al., 2002a). Despite the limited dimensions, both flattened lattices and invaginating buds are visible. Overall, we conclude that the knockdown of the α subunit of AP-2 ablates AP-2 sorting activity with similar functional consequences as reported initially (Hinrichsen et al., 2003; Motley et al., 2003), except that our knockdown procedure does not decrease as dramatically the total number of surface clathrin-containing structures.
Figure 2.
AP-2 knocked down cell ultrastructure. HeLa SS6 cells either mock transfected (A) or transfected with α subunit siRNA oligonucleotides (B–D) were briefly sonicated to prepare cell cortices, fixed, labeled with anti-transferrin receptor mAb H68.4, and then 15-nm gold-conjugated anti-mouse secondary antibodies to visualize extra-lattice receptors. Arrows indicate gold-labeled receptors.
Gene Silencing of the AP-2 β2 Subunit Does Not Inactivate AP-2
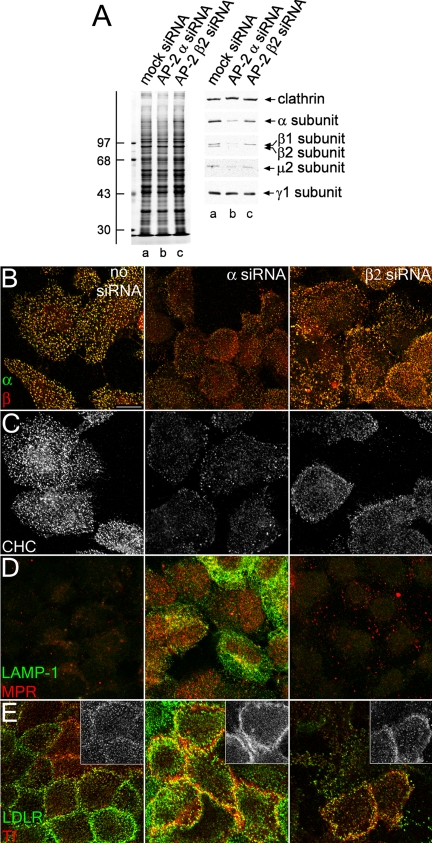
Next, as a prelude to evaluating the function of the β2 subunit hinge and appendage by reconstitution after AP-2 RNAi, we selectively knocked down the AP-2 β2 subunit in HeLa SS6 cells. Although the β2 subunit polypeptide is very effectively diminished (Huang et al., 2004), in marked contrast to the α subunit RNAi, the abundance of other AP-2 subunits is only mildly affected (Figure 3A and Supplemental Figure S1A). A comparatively weak effect on AP-2 levels is also apparent by immunofluorescence. The levels of the α, and, surprisingly, β subunits of AP-2 in the context of a β2 subunit knockdown are reduced from normal levels, but clearly not to the extent observed in an α subunit knockdown (Figure 3B). Likewise, the other phenotypes characteristic of α subunit knockdown are not as penetrant when using β2 subunit siRNA duplexes (Figure 3, C–E). The intensity of clathrin within punctate structures is intermediate between control and α subunit knockdown, which is likely due to the presence of functional AP-2 complexes in the cell (Figure 3C). Also, AP-2–dependent cargo, the MPR and LAMP-1, do not accumulate appreciably at the cell surface (Figure 3D), and although the transferrin distribution is more diffuse over the surface than in mock-transfected cells, it is also present in numerous puncta on the plasma membrane (Figure 3E). We conclude that AP-2 β2 subunit knockdown produces a milder variant of the α or μ2 subunit RNAi phenotype, due to incomplete depletion of AP-2.
Figure 3.
AP-2 β2 subunit gene silencing. (A) Lysates of HeLa SS6 cells untreated (lane a) or treated with either AP-2 α or β2 subunit siRNA (lanes b and c) were resolved by SDS-PAGE and either Coomassie stained or transferred to nitrocellulose. Sections of the blots were probed with anti-clathrin HC mAb TD.1, anti-AP-2 α subunit mAb clone 8, anti-β1/β2 subunit mAb 100/1, anti-AP-2 μ2 antiserum, or anti-AP-1 γ subunit antibody AE/1, and only the relevant portion of the blots is shown. The position of molecular mass standards (in kilodaltons) is indicated on the left. (B and C) HeLa SS6 cells untreated (left) or treated with AP-2 α- (middle), or β2 subunit siRNA (right) were treated with 10 μg/ml brefeldin A for 15 min, permeabilized on ice for 1 min before fixation, and prepared for immunofluorescence using the anti-AP-2 α subunit mAb AP.6 (B, green), anti-β1-/β2 subunit antibody GD/2 (B; red), or anti-clathrin HC mAb X22 (C). (D and E) HeLa SS6 cells untreated (left) or treated with AP-2 α (middle) or β2 subunit (right) siRNA were incubated on ice for 1 h with either anti-LAMP-1 mAb (D; green) and anti-MPR antibody (D; red), or anti-LDL receptor mAb C7 (E; green) and Tf568 (E; red), fixed, and prepared for indirect immunofluorescence. The inset in E shows Tf568 immunofluorescence from each sample.
The AP-1 β1 Subunit Is Incorporated into AP-2 in the Absence of a β2 Subunit
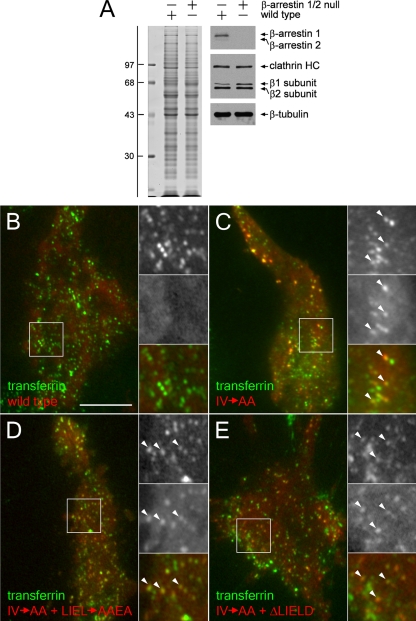
A clue to the biochemical basis for the milder β2 RNAi phenotype comes from the surface β subunit signal in cells lacking the β2 subunit (Figure 3B). The β1 and β2 subunits of AP-1 and AP-2 are ∼85% identical, in contrast to the other three subunits of the heterotetramers, which display <50% homology (Figure 4A). The appendages of the β1 and β2 subunits are structurally homologous and share many binding partners (Owen et al., 2000; Lundmark and Carlsson, 2002; Edeling et al., 2006a; Knuehl et al., 2006; Schmid et al., 2006). In fact, the Drosophila melanogaster genome encodes only a single β subunit that is incorporated into both AP-1 and AP-2 (Camidge and Pearse, 1994). Almost all available antibodies raised against the vertebrate β subunits recognize both β1 and β2 subunits, although these subunits can be resolved by SDS-PAGE (Page and Robinson, 1995; Sorkin et al., 1995; Traub et al., 1996). Thus, the fluorescent signal observed in β2 subunit-knockdown cells may be due to β1 subunit incorporated into AP-2. To test this hypothesis, we treated HeLa SS6 cells with or without β2 subunit siRNA, and then we immunoprecipitated AP-2 with an α subunit-specific mAb. In mock-transfected cells, the majority of AP-2 is recovered in the immunoprecipitate, and the principal β subunit incorporated into AP-2 is the faster migrating β2 chain (Figure 4B). However, when the β2 subunit is depleted, this smaller chain almost disappears, and the majority of the β subunit now assembled into AP-2 is the larger β1 subunit (Figure 4B).
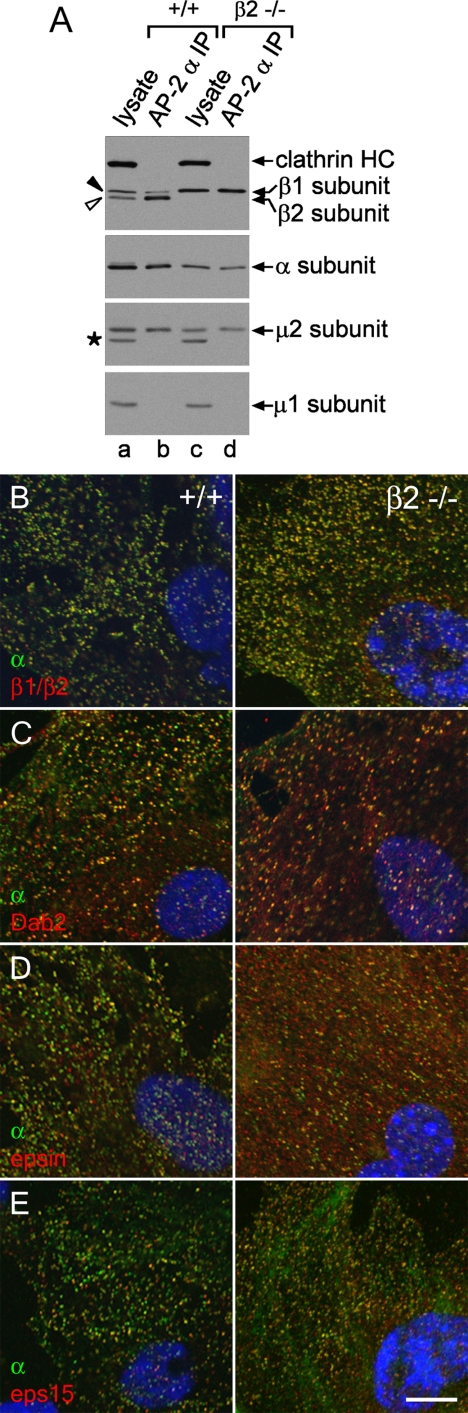
Independent verification of the incorporation of the AP-1 β1 subunit into AP-2 comes from mouse embryonic fibroblasts homozygous for a disrupted β2 subunit gene. Like HeLa SS6 cells, +/+ fibroblasts from normal littermate controls display both the β1 subunit and the faster migrating β2 polypeptide (Figure 5A). The β2-null −/− fibroblasts clearly express only the larger β1 polypeptide, and the overall abundance of the AP-2 α and μ2 subunits, but not of the AP-1 μ1 or σ1 subunits, is diminished compared with the control lysates. AP-2 immunoprecipitated from −/− cells with an α subunit-specific mAb contains only the β1 subunit (Figure 5A), similar to the β2 gene-silenced HeLa SS6 cells. Likewise, the surface distribution of AP-2, as judged by the intracellular positioning of the α subunit, is very similar to the +/+ fibroblasts (Figure 5, B–E), despite the reduced levels of the α and μ2 chains in the −/− fibroblasts. The surface distribution of CLASPs such as Dab2, epsin, and eps15 is also highly similar to the normal fibroblast controls (Figure 5, C–E). Therefore, we conclude that because of the high degree of sequence homology, the β1 subunit is promiscuously incorporated into AP-2 when cellular β2 subunit levels are depressed, and that this misassembly largely preserves the functional capabilities of the AP-2 heterotetramer. Interestingly, this substitution phenomenon does occur naturally to a limited extent, as incorporation of endogenous β2 into AP-1 in brain and β1 into AP-2 in liver has been documented (Page and Robinson, 1995; Sorkin et al., 1995; Traub et al., 1996), and some β1 subunit is present in AP-2 immunoprecipitated from +/+ fibroblasts (Figure 5A).
Figure 5.
Stable incorporation of the β1 subunit into AP-2 in β2 subunit-null mouse fibroblasts. (A) Aliquots of lysates from either control +/+ (lane a) or β2-null −/− (lane c) mouse embryonic fibroblasts and of mAb AP.6 immunoprecipitates from the +/+ (lane b) or −/− (lane d) lysates were resolved by SDS-PAGE and transferred to nitrocellulose. Blots were probed with anti-clathrin HC mAb TD.1 and affinity purified anti-β1/β2 subunit polyclonal GD/1, anti-AP-2 α subunit mAb clone 8, anti-AP-2 μ2 subunit antiserum, or affinity-purified anti-μ1 subunit polyclonal RY/1, and only the relevant portion of each blot is shown. The asterisk indicates a nonspecific band. Loss of β2-chain expression results in a compensatory increase in the steady-state level of the β1 subunit in the cultured fibroblasts, although the level of AP-2 is nevertheless reduced compared with wild-type fibroblasts. (B–E) Wild-type (+/+; left column) or β2-null (−/−; right column) mouse embryonic fibroblasts were fixed and labeled with Hoechst 33258, anti-α subunit mAb AP.6 and either anti-β1/β2 subunit GD/1 (B), anti-Dab2 (C), anti-epsin (D), or anti-eps15 (E) polyclonal antibodies. Representative confocal sections of the ventral plasma membrane are shown. In all cases, absence of β2 does not obviously perturb the punctate patterning of the α subunit or CLASPs. Bar, 10 μm.
Simultaneous Knockdown of β1 and β2 Subunits Functionally Ablates AP-2
Because gene silencing of the β2 subunit alone is insufficient to reduce AP-2 levels and disrupt sorting, we designed an siRNA duplex to extinguish the β1 subunit. Transfection of this duplex selectively targets the β1 subunit, and together the β1 + β2 subunit siRNAs silence both AP-1 and AP-2 (Figure 6A). Furthermore, the combined β1 + β2 RNAi reproduces the α subunit knockdown phenotype (Figure 6, B–D). Morphologically, the level of the AP-2 α subunit is decreased by β1 + β2 subunit RNAi similar to that observed with an α subunit knockdown (Figure 6B), and the amount of clathrin deposited on the plasma membrane is reduced to that observed with the α subunit RNAi (Figure 6C). Most importantly, transferrin receptor internalization is strongly blocked and fluorescent transferrin accumulates in a diffuse pattern over the cell surface (Figure 6D). Thus, simultaneous knockdown of both β1 and β2 subunits functionally incapacitates AP-2 and phenocopies AP-2 α or μ2 subunit ablation.
Functional Reconstitution of AP-2 with β2-YFP
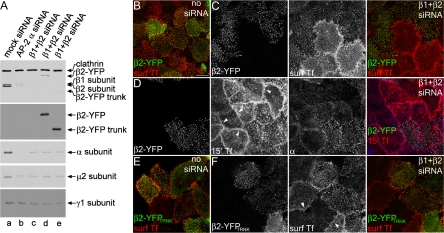
To begin to probe the function of the β2 subunit in vivo then, an siRNA-insensitive β2 subunit fused at the carboxy terminus to YFP (β2-YFP) (Sorkina et al., 2002; Hamdan et al., 2007) was ectopically expressed in HeLa SS6 cells also transfected with the β1 + β2 subunit-silencing duplexes. The transiently expressed β2 construct (Figure 7A) clearly reconstitutes AP-2 function successfully; the ectopic β2-YFP targets to α subunit puncta in control cells, and restores normal concentration of surface transferrin at clathrin-coated structures in β1 + β2 subunit knockdown cells (Figure 7, B and C). Approximately 95% of β2-YFP–expressing cells display clustered transferrin, whereas <30% of the non-GFP–producing cells have similar patches of the ligand (Supplemental Figure S1B). Expression of the plasmid-borne YFP-tagged β2 subunit in some siRNA-treated cells is confirmed by immunoblot (Figure 7A). The relatively low level of β2-YFP expression (reflecting the transfection efficiency) concurs with another recent study dealing with re-expression of a GFP-tagged AP-2 α subunit after RNAi (Rappoport and Simon, 2008). Transfected cells have apparently normal AP-2 levels, as seen by immunofluorescence, and permit efficient transferrin internalization (Figure 7D); only ∼20% of β1 + β2 RNAi-treated cells not expressing GFP show bright AP-2–positive puncta compared with ∼70% of cells synthesizing β2-YFP (Supplemental Figure S1B). Importantly, transferrin uptake is not the result of reduced siRNA transfection efficiency due to the simultaneous addition of β2-YFP plasmid DNA during RNAi treatment. Adding similar amounts of control GFP plasmid DNA restores neither AP-2 levels nor the punctate surface transferrin localization (Supplemental Figure S2). Therefore, the β2-YFP seems to functionally reconstitute AP-2 in β1 + β2 subunit knocked down cells. Fused to YFP, a β2 subunit trunk that lacks the C-terminal hinge and appendage, also targets to transferrin-positive puncta in control cells (Figure 7E), and it promotes the clustering of surface transferrin at YFP-positive patches, as opposed to the diffuse distribution of transferrin in neighboring β1- + β2-extinguished cells (Figure 7F).
ARH Requires the β2 Appendage for Clathrin Coat Localization
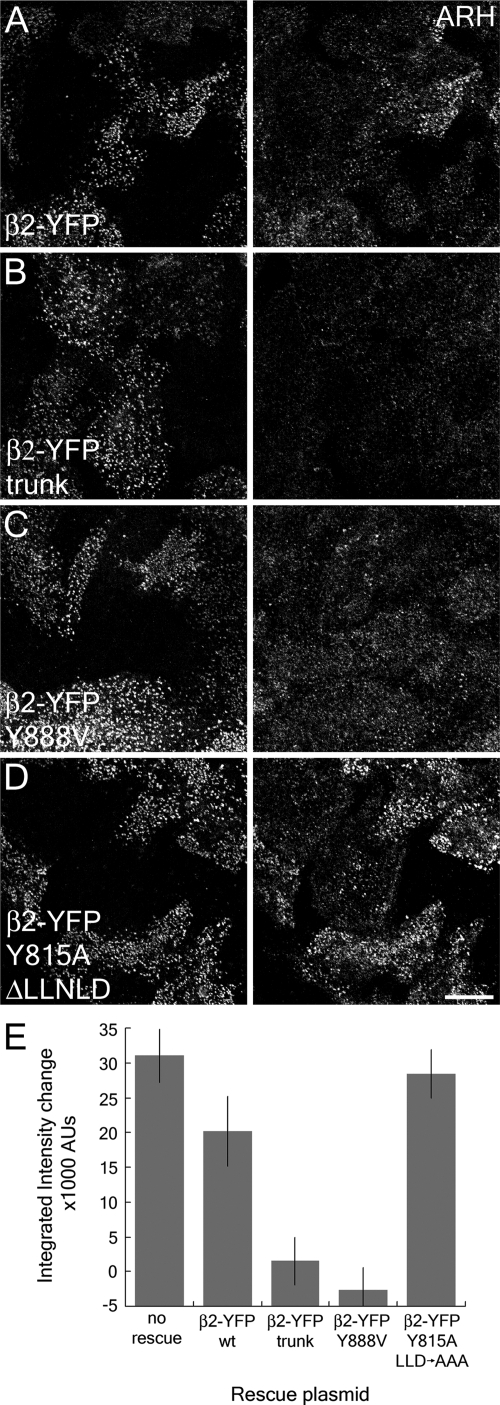
Next, we examined the effects the β1 + β2 subunit knockdown has on the distribution of the β2 subunit binding partner ARH. ARH uses a helically structured [DE]nX1-2FXX[FL]XXXR motif to engage the platform site on the β2 appendage (Edeling et al., 2006a; Schmid et al., 2006). Knockdown of the β1 + β2 subunits reduces the amount of ARH localized to clathrin-coated structures (Figure 8). This can be reversed by expression of the β2-YFP, but not β2-YFPTRNK lacking both the unstructured hinge and appendage domains (Figure 8, A and B). This failure to rescue is due solely to lack of a functional platform binding site, as expression of β2-YFP bearing the platform site-disrupting Y888V mutation fails to restore ARH localization, but simultaneous deletion of the clathrin box and introduction of the sandwich site disrupting Y815A mutation into β2-YFP reconstitutes ARH positioning in clathrin coats (Figure 8, C and D). Measurement of the difference in fluorescence intensity between transfected cells and neighboring knockdown cells demonstrates that the AP-2 dependence of ARH coated-structure localization is wholly dependent on the β2 appendage platform site (Figure 8E). We conclude that in vivo ARH requires the β2 appendage binding sequence to efficiently target to clathrin coats to promote efficient sorting of LDL receptors.
Figure 8.
ARH requires the β2 appendage platform for plasma membrane localization. HeLa SS6 cells transfected with β1 + β2 subunit siRNAs and either full-length β2-YFP (A), β2-YFP trunk (B), or full-length β2-YFP bearing Y888V (C), or dual Y815A and ΔLLNLD (D) mutations (left column) were incubated with 50 μg/ml Tf633 for 15 min at 37°C, permeabilized, fixed and stained with polyclonal anti-ARH antibodies (right column). (E) A comparison of the fluorescence intensity between β2-YFP–transfected cells and knockdown cells (as determined by lack of Tf633 internalization) shows the differences between β2-YFP fusions that can recruit ARH to the membrane and those that cannot. No rescue indicates the difference in ARH intensity between wild-type and β1 + β2 knocked down cells.
Clathrin Binding Surface on the β2 Subunit Appendage
In addition to engaging ARH and β-arrestins, the β2 subunit appendage also binds physically to clathrin (Owen et al., 2000; Lundmark and Carlsson, 2002; Edeling et al., 2006a; Knuehl et al., 2006; Schmid et al., 2006). Yet, the precise molecular basis for this association is currently controversial. There is general agreement that by binding bivalently to the clathrin heavy chain, some β2 subunit partners could be controlled spatiotemporally as the extent of polymerized clathrin lattice increases (Edeling et al., 2006a; Knuehl et al., 2006; Schmid et al., 2006; Schmid and McMahon, 2007; Ungewickell and Hinrichsen, 2007). We recently mapped the clathrin binding surface to a site, including Tyr815, on the N-terminal sandwich subdomain (Edeling et al., 2006a), and our prior results indicate that clathrin binding to the β2 appendage sandwich can displace interaction partners such as eps15 and AP180, which bind to the same site. Because clathrin trimers stoichiometrically outnumber AP-1/2 in preparations of biochemically purified clathrin-coated vesicles (Blondeau et al., 2004; Girard et al., 2005), the excess of clathrin heavy chain terminal domains, which project into the interior of the assembled coat (Musacchio et al., 1999; Edeling et al., 2006b), can bind avidly to AP-2 through the β2 subunit. Indeed, we (Edeling et al., 2006a) and others (Tebar et al., 1996) localize eps15 to only the periphery of clathrin-coated structures. Similar conclusions have been drawn for the mode of engagement of the monomeric GGA adaptor proteins and clathrin, where the C-terminal GAE domain of the GGAs only contains a sandwich subdomain (Knuehl et al., 2006). Because activation of GPCRs leads to the rapid translocation of an activated GPCR·β-arrestin complex to pre-existing clathrin-coated structures at the cell surface (Laporte et al., 2000; Santini et al., 2002; Scott et al., 2002), and because ARH (Figure 8; He et al., 2002; Mishra et al., 2002b) and β-arrestins (Kim and Benovic, 2002; Laporte et al., 2002) specifically engage the platform subdomain of the β2 appendage, we proposed that the β2 platform interaction surface represents a relatively privileged binding site (Mishra et al., 2005; Brett and Traub, 2006; Edeling et al., 2006a). Challenging these findings and calling our conclusions “misleading,” McMahon and colleagues assert that it is the platform subdomain, and not the sandwich site, that is the location of the second point of contact between the clathrin heavy chain and the β2 (and β1) hinge + appendage (Schmid et al., 2006; Schmid and McMahon, 2007). In this model, clathrin interference dislodges CLASPs such as β-arrestin and ARH from the β2 appendage, but because both these proteins also have an adjacent but separate clathrin binding sequence, the proteins would remain associated with assembling clathrin coats despite occupancy of the β2 platform with a distal leg segment of the heavy chain (Schmid et al., 2006; Schmid and McMahon, 2007). This is a chief feature of the changing hubs model formulated to account for the vectorial nature of the clathrin coat assembly process (Schmid and McMahon, 2007). Clearly then, these opposing models have importantly different implications for the molecular basis of ARH- and β-arrestin–mediated cargo capture within forming clathrin coats.
To attempt to distinguish between the two models, we used a mutant form of β-arrestin 1. Wild-type β-arrestins remain diffusely cytosolic in the absence of GPCR stimulation (Laporte et al., 2000; Santini et al., 2002; Scott et al., 2002) (Figure 9A) by maintaining a basal, inactive conformation. Several closely spaced residues within the C-terminal [DE]nX1-2FXX[FL]XXXR motif interact with the major folded N-terminal region of β-arrestin, rigidifying the protein and attenuating the CLASPs ability to engage the assembling clathrin machinery (Kim and Benovic, 2002; Gurevich and Gurevich, 2004; Edeling et al., 2006a; Burtey et al., 2007). In β-arrestin 1, immediately preceding the proximal Phe388 of the β2 platform binding motif are Ile386 and Val387. Double substitution of these residues to Ala (IV→AA) results in constitutive association of β-arrestin 1 with clathrin-coated structures in the absence of any GPCR activation (Burtey et al., 2007). If β-arrestins only remain associated with surface coated structures by switching to clathrin when assembling clathrin binds the AP-2 β2 subunit appendage, then deletion or mutation of the β-arrestin 1 clathrin box sequence 376LIELD should prevent the IV→AA mutant from colocalizing extensively with clathrin-coated structures at the plasma membrane.
Figure 9.
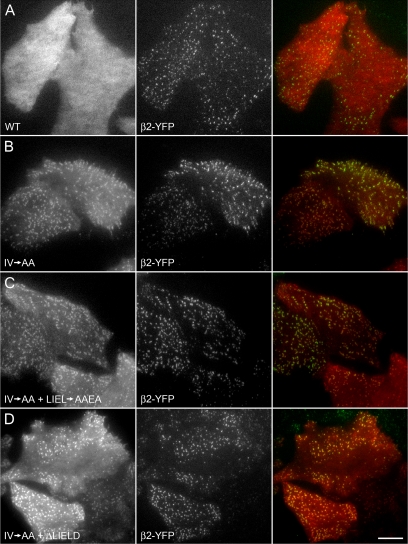
Clathrin-binding deficient β-arrestin 1 associates with AP-2. HeLa SS6 cells stably expressing β2-YFP were transiently transfected with wild-type (WT) tdRFP-β-arrestin 1 (A), tdRFP-β-arrestin 1 with the IV→AA activating mutation (B), or tdRFP-β-arrestin 1 (IV→AA) + clathrin box mutation LIEL→AAEA (C) or + clathrin box deletion ΔLIELD (D). Approximately 18 h after transfection, cells were imaged by TIR-FM to selectively observe the ventral surface. Representative images show tdRFP-β-arrestin 1 (left column), β2-YFP (middle column), and merged (right column) signals. Note that despite a considerable cytosolic pool, there is near complete colocalization of β2-YFP puncta with the tdRFP-β-arrestin 1 (IV→AA) alone or with either clathrin box disruption. Bar, 10 μm.
Using TIR-FM to inspect the ventral surface of HeLa SS6 cells stably expressing β2-YFP, we confirm that tdRFP-β-arrestin 1 is soluble while a portion of the IV→AA mutant concentrates at pre-existing, β2 subunit positive clathrin-coated structures (Figure 9, A and B) (Burtey et al., 2007). In the IV→AA background, the colocalization of neither a LIEL→AAEA substitution nor a ΔLIELD mutant with coated structures at the plasma membrane marked by β2-YFP is obviously different from the protein containing an intact clathrin box (Figure 9, C and D). Although 0.6 ± 0.3% of AP-2 structures colocalize with tdRFP-β-arrestin 1, 99.1 ± 0.6% of AP-2–positive spots contain tdRFP-β-arrestin (IV→AA). For the tdRFP-β-arrestin (IV→AA+LIEL→AAEA) and tdRFP-β-arrestin (IV→AA+ΔLIELD) mutants, 95 ± 2% and 89 ± 3% of AP-2 structures contain β-arrestin, respectively.
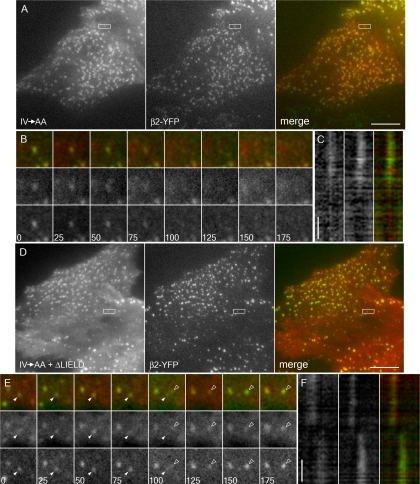
There are several distinct classes of clathrin-coated structures on the ventral surface of HeLa SS6 cells (Keyel et al., 2004), and the IV→AA β-arrestin 1 mutants localize to all of these. Analysis of the dynamics of de novo-forming puncta reveals that, like the tdRFP-β-arrestin 1 (IV→AA) (Figure 10, A–C), the IV→AA+ΔLIELD mutant concentrates at, and then disappears with, β2-YFP–tagged AP-2 structures (Figure 10 D–F). We do not observe earlier loss of the IV→AA+ΔLIELD tdRFP-β-arrestin 1 mutant from these patches that would indicate failure to switch to a strictly clathrin-dependent mode of association. Very similar results are obtained using BS-C-1 cells stably expressing GFP-tagged clathrin light chain (data not shown), a cell line that exhibits de novo coat formation almost exclusively (Ehrlich et al., 2004).
Figure 10.
Clathrin-binding deficient β-arrestin 1 dynamically associates with AP-2. HeLa SS6 cells stably expressing β2-YFP were transiently transfected with tdRFP-β-arrestin 1 (IV→AA) (A–C) or tdRFP-β-arrestin 1 (IV→AA+ΔLIELD) (D–F). Approximately 18 h after transfection, cells were imaged by TIR-FM at one frame every 5 s. (A) Representative frames from the tdRFP-β-arrestin 1 (IV→AA)–expressing cells. β-arrestin 1 (left frame) and β2-YFP (middle frame) show extensive colocalization (right frame). (B) A sequence of frames at 25-s intervals shows the simultaneous disappearance of tdRFP-β-arrestin 1 (IV→AA) (middle row) and β2-YFP (bottom row) from the evanescent field. The spot is the same as that boxed in A. (C) A line drawn along the axis of the box in A was used to create a kymograph. tdRFP-β-arrestin 1 IV→AA (left column) and β2-YFP (middle column) leave the evanescent field together (right column). (D) tdRFP-β-arrestin 1 (IV→AA+ΔLIELD)–expressing cells likewise show significant overlap with β2-YFP as in depicted in A. (E) The boxed spot in D containing tdRFP-β-arrestin 1 (IV→AA+ΔLIELD) (middle row) and β2-YFP (bottom row) disappears from the field (solid arrowhead) at 100 s as a second spot containing both proteins appears (open arrowhead). (F) A line drawn along the axis of the box in D was used to create a kymograph. tdRFP-β-arrestin 1 (IV→AA+ΔLIELD) (left column) and β2-YFP (middle column) disappear together, whereas the appearance of arrestin in the second spot is slightly preceded by β2-YFP. All horizontal bars, 10 μm (A and D); vertical time bars, 60 s (C and F).
It is important to note that in these experiments, the activation of the β-arrestin 1 (IV→AA) mutant occurs in the absence of any GPCR ligand addition, but HeLa SS6 cells also do express endogenous β-arrestins. Although there is currently some doubt that nonvisual arrestins oligomerize at physiological concentrations (Hanson et al., 2008b), overexpressed β-arrestins can form multimers (Storez et al., 2005; Milano et al., 2006). Oligomerization requires inositol hexakisphosphate binding and pertains to the soluble form of β-arrestin, because the contact sites for dimer and tetramer assembly are located on the concave surface that engages an activated receptor (Milano et al., 2006; Hanson et al., 2008a,b). Solution oligomers therefore do not bind to GPCRs, and exposure of the C-terminal region, which couples β-arrestins to the clathrin machinery, destabilizes oligomers (Hanson et al., 2008a). We thus believe it unlikely that hetero-oligomerization with endogenous β-arrestins accounts for the association of the IV→AA + clathrin box mutants with surface clathrin-coated structures. Supporting this view is that transfection of the tdRFP-β-arrestin 1 (IV→AA) clathrin-binding mutants into β-arrestin 1/2 nullizygous fibroblasts (Kohout et al., 2001) still results in constitutive accumulation of the tagged β-arrestin at membrane puncta that are also positive for surface transferrin (Figure 11).
Figure 11.
Clathrin-binding deficient β-arrestin 1 associates with clathrin-coated structures in the absence of endogenous β-arrestins. Wild-type and β-arrestin 1/2 null mouse embryonic fibroblasts were lysed and resolved by SDS-PAGE (A; left) and transferred to nitrocellulose (A; right). Portions of the blots were probed with an anti-β-arrestin polyclonal antibody, anti-clathrin HC mAb TD.1, and anti-β1/β2 subunit mAb 100/1, or with anti-tubulin mAb E7. The position of the molecular mass standards (in kilodaltons) is noted on the left. Alternatively, β-arrestin 1/2 null mouse embryonic fibroblasts were transiently transfected with tdRFP-β-arrestin 1 (red) wild type (B), the tdRFP-β-arrestin 1 (IV→AA)-activating mutation (C), IV→AA + clathrin box mutation LIEL→AAEA (D), or IV→AA + clathrin box deletion ΔLIELD (E). At 18 h after transfection, cells were incubated with 25 μg/ml Tf488 (green) on 4°C on ice for 1 h to label transferrin receptor in clathrin-coated structures, and then they were rapidly imaged at 17°C by TIR-FM. Under these conditions, transferrin is found both at clathrin coated structures on the cell surface and already in peripheral endosomes. Magnified insets are denoted by white boxes; the IV→AA and IV→AA + clathrin box disruption β-arrestin mutants (middle inset) both colocalize with Tf488 (top inset), as denoted by arrowheads. Bar, 10 μm.
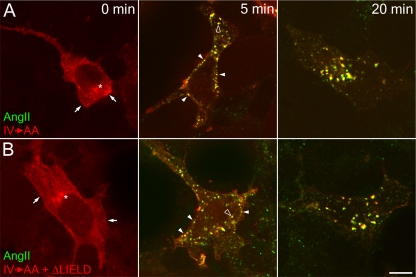
Finally, like the β-arrestin 1 (IV→AA) mutant, the compound (IV→AA+ΔLIELD) mutant translocates rapidly to the surface of angiotensin II-stimulated HEK cells stably expressing the type I angiotensin II receptor (Figure 12). At 5 min, both the β-arrestin mutants colocalize with fluorescent angiotensin II at cell surface patches and peripheral endosomes, whereas, after 20 min, both angiotensin II and β-arrestin 1 (IV→AA+ΔLIELD) are found in larger, juxtanuclear endosomes, typical of a class B GPCR (Hamdan et al., 2007). These results show that deletion of the β-arrestin clathrin box does not have a major negative affect on GPCR down-regulation and are fully consistent with a doubly mutated IV→AA+LIEF→AAEA β-arrestin 2 mutant clustering activated thyrotropin-releasing hormone receptors at AP-2–positive clathrin-coated structures and orchestrating endocytic uptake roughly equivalent to wild-type β-arrestin 2 at 10 min (Burtey et al., 2007). We conclude that β-arrestin switching to a clathrin-dependent mode of association is not an obligate step during the formation of clathrin-coated vesicles and that the [DE]nX1-2FXX[FL]XXXR binding surface upon the platform subdomain of the β2 appendage does represent a privileged interaction site, because it can be rapidly accessed by ARH or β-arrestin in the face of ongoing endocytic activity.
Figure 12.
Down-regulation of the type 1 angiotensin II receptor by both tdRFP-β-arrestin 1 (IV→AA) and β-arrestin (IV→AA+ΔLIELD). HEK293 cells stably expressing FLAG-tagged type I angiotensin II receptor were transiently transfected with tdRFP-β-arrestin 1 (IV→AA) (A), or tdRFP-β-arrestin 1 (IV→AA+ΔLIELD) (B). Cells were starved for 1 h in starvation medium and then stimulated with 100 nM angiotensin II conjugated to Alexa488 (AngII; green) for 0, 5, or 20 min. Cells were washed, fixed, and examined by confocal microscopy. Representative images of medial focal planes are shown. Before stimulation, β-arrestin is largely diffuse in the cytosol but also present at the plasma membrane (arrows) or at a juxtanuclear location (asterisks). After 5 min of stimulation, β-arrestin clusters with angiotensin II at the plasma membrane (closed arrowheads), and on endosomes (open arrowheads). At 20 min after stimulation, nearly all β-arrestin and angiotensin II colocalize on larger endosomes. Bar. 10 μm.
DISCUSSION
AP-2 ablation has a major inhibitory effect on the surface clustering and internalization of YXXØ-bearing proteins and also [DE]XXXL[LIM] (McCormick et al., 2005)-bearing proteins. This confirms the chief sorting activity that this adaptor complex confers. Yet, at least in cultured cells, AP-2 silencing is still compatible with internalization of a subset of clathrin-dependent cargo; several groups have now shown this phenomenon independently (Hinrichsen et al., 2003; Motley et al., 2003; Barriere et al., 2006; Keyel et al., 2006; Maurer and Cooper, 2006; Eden et al., 2007). The same is not true if clathrin levels are decreased by RNAi (Hinrichsen et al., 2003; Motley et al., 2003; Moskowitz et al., 2005; Barriere et al., 2006; Keyel et al., 2006; Maurer and Cooper, 2006). Careful single cell analysis of clathrin knocked down populations reveals that rates of endocytosis become severely slowed when clathrin triskelia begin to be limiting (Moskowitz et al., 2005). This difference indicates to us that although clathrin is essential for the fabrication of budding vesicles, AP-2 can be replaced to some extent by other clathrin-binding CLASPs such as Dab2, epsin, eps15, ARH, stonin 2, HIP1/Hip1R, and CALM. Correct surface deposition of Dab2 still permits clustering of LDL receptors when AP-2 is silenced; even though ARH depends upon AP-2 for proper placement in surface clathrin structures, ARH and Dab2 are functionally redundant and robust LDL uptake persists in either Dab2- or ARH-lacking HeLa cells (Keyel et al., 2006; Maurer and Cooper, 2006; Eden et al., 2007). The clathrin-, PtdIns(4,5)P2- and cargo-binding qualities of Dab2 and other CLASPs likely accounts for the preservation of clathrin-coated vesicle formation in an AP-2 compromised background. Still, our ultrastructural analysis of cell membranes under these conditions shows that the clathrin lattices are not of normal dimensions, although invaginating buds are evident. The two AP-2 appendages operate as interaction hubs that can engage >20 CLASPs and accessory factors (Wang et al., 1995; Traub, 2005; Edeling et al., 2006b; Maldonado-Baez and Wendland, 2006; Schmid and McMahon, 2007), including type I phosphatidylinositol 4-phosphate 5-kinases (Bairstow et al., 2006; Krauss et al., 2006; Nakano-Kobayashi et al., 2007) and the PtdIns(4,5)P2 phosphatases synaptojanin 1 (Haffner et al., 2000; Jha et al., 2004) and OCRL (Ungewickell et al., 2004). Given the vital role that PtdIns(4,5)P2 plays in clathrin-coated vesicle assembly and budding at the plasma membrane, we suspect the residual AP-2 we see in cells subject to siRNA knockdown probably contributes to LDL uptake. Our overall interpretation of the available data is that clathrin-coated structures never evolved to operate in the absence of AP-2, and is fully consistent with the lethal consequence of interfering with expression of certain AP-2 adaptor subunits in C. elegans (Grant and Hirsh, 1999; Shim and Lee, 2000; Kamikura and Cooper, 2003), Drosophila (Gonzalez-Gaitan and Jackle, 1997), and mice (Mitsunari et al., 2005). Harder to explain is the completely normal internalization rate of a CD8-LDL receptor chimera in HeLa cells containing <10% of the surface clathrin structures observed in AP-2 replete cells (Motley et al., 2003).
Because of the high degree of sequence identity between the adaptor β1 and β2 subunits, gene silencing or targeted gene disruption of the β2 subunit results in only a moderate endocytic phenotype. β2 is probably the only subunit of AP-2 that can be replaced by an AP-1 counterpart, because the σ1 subunit does not interact directly with the α subunit (Page and Robinson, 1995), and μ2 subunit RNAi phenocopies the α subunit RNAi (Motley et al., 2003; Huang et al., 2004). We suspect that the opposite is also true, because it is known that β2 subunits can incorporate into AP-1 in vivo (Page and Robinson, 1995; Sorkin et al., 1995; Traub et al., 1996). Also, at moderately high levels, ectopically expressed β2-YFP can incorporate into AP-1 (Huang et al., 2003; Keyel et al., 2004), and the C-terminal region of ARH interacts similarly with AP-1 and AP-2 but not with AP-3 (He et al., 2002; Mishra et al., 2002b). All of the key specificity-determining residues at the platform and sandwich subdomain interaction interfaces are conserved between the β1 and β2 polypeptides. This must account for the functional activity of a hybrid AP-2 incorporating a β1 chain. The apparent interchangeability of the β1 and β2 subunits, the localization of some eps15 at a juxtanuclear, TGN-like location (Tebar et al., 1996; Kent et al., 2002), and our detection of the β-arrestin 1 (IV→AA) mutant at the juxtanuclear/TGN region (Figure 12) raises the question of whether known AP-2 β2 binding partners also function normally together with AP-1. A very recent study in fact mapped an apparent AP-1–specific binding site on eps15 to the sequence 720ESFDGDFADFSTLS (Chi et al., 2008). Remarkably, this region is identical to a peptide segment of eps15 cocrystallized at the sandwich site of the AP-2 β2 appendage (Schmid et al., 2006). The reason why deletion of this amino acid tract from eps15 perturbs only AP-1 binding (Chi et al., 2008) is because the proximal tract of >10 DPF triplets facilitates AP-2 engagement through interaction with the platform subdomain of the α subunit appendage (Owen et al., 1999; Brett et al., 2002); the appendage of the analogous subunit in AP-1, the γ subunit, lacks a platform subdomain, and binds eps15 only very weakly (Kent et al., 2002; Nogi et al., 2002). The fact that overexpression of an eps15 mutant lacking the above-mentioned 14-amino acid β-binding tract sharply slows exit of the MPR from the TGN (Chi et al., 2008) argues that the β1 and β2 subunits do perform some similar functions at the TGN/endosome and plasma membrane, respectively.
Because eps15 contacts the β2 appendage through the sandwich subdomain, a mutation that disrupts this interaction surface interferes with eps15 binding (Edeling et al., 2006a; Schmid et al., 2006). We find that this same mutation (Y815A) abolishes clathrin binding, indicating that eps15 and clathrin compete for a common site on the β2 appendage sandwich subdomain (Edeling et al., 2006a). Biochemical experiments show plainly that eps15 preloaded onto AP-2 is expelled upon clathrin assembly and eps15 fails to be incorporated along with AP-2 into assembled clathrin coats (Cupers et al., 1998). By contrast, ARH and the β-arrestins differ significantly from eps15 and several other CLASPs and accessory factors in that they both display only a single AP-2 binding determinant with an absolute selectivity for the platform subdomain of the β2 appendage (Laporte et al., 2000; He et al., 2002; Mishra et al., 2002b). Substantial data attest to the critical and selective importance of the β2 appendage in the initial recruitment of GPCR·β-arrestin complexes to pre-existing sites of clathrin assembly. The clathrin box is located within a flexible, unstructured C-terminal loop (Milano et al., 2002), even in the basal β-arrestin conformation. Yet β-arrestins are cytosolic in the absence of GPCR activation (Hamdan et al., 2007), so, although the clathrin box is potentially accessible to the clathrin terminal domain, if the distal [DE]nX1-2FXX[FL]XXXR sequence is conformationally restrained, β-arrestins fail to associate appreciably with clathrin-coated structures. Similarly, when fused to GFP, the C-terminal region of β-arrestin 2 populates AP-2–positive clathrin structures at the cell surface but this colocalization is completely lost if the [DE]nX1-2FXX[FL]XXXR sequence is disrupted, even with an intact adjacent clathrin box (Schmid et al., 2006). Also, the β-arrestin 1 IVF→AAA triple mutant is not colocalized with AP-2 at steady state, despite the conformation relaxation affected by the IV to AA substitution (Burtey et al., 2007). Mutagenic inactivation of the AP-2 binding sequence in β-arrestin 2 still allows translocation of this CLASP to agonist-stimulated GPCRs on the cell surface, but clustering at AP-2–positive puncta is lost, again despite an intact clathrin box (Laporte et al., 2000). Indeed, bioluminescence resonance energy transfer studies show that β-arrestin binding to liganded GPCRs temporally precedes an AP-2 β2 appendage interaction (Hamdan et al., 2007) and that mutation of the interaction surface on the β2 platform subdomain prevents energy transfer to AP-2 (Hamdan et al., 2007). By contrast, even with an inactivating mutation or complete deletion of the clathrin box, β-arrestin 1 still targets to clathrin-coated structures (Figures 9–12). Altogether, this argues strongly that the [DE]nX1-2FXX[FL]XXXR sequence is the principal determinant governing initial linkage of GPCRs with the endocytic machinery (Laporte et al., 2000).
Our siRNA/reconstitution experiments reveal that ARH likewise uses the analogous interaction motif as the primary mode of clathrin-coat engagement. In fact, simultaneous gene silencing of AP-2 and Dab2 leads to stalling of the LDL receptor at the cell surface despite normal ARH levels and the clear presence of surface clathrin coats (Keyel et al., 2006; Maurer and Cooper, 2006). The binding affinity of ARH (and β-arrestin) for the β2 appendage is greater than its affinity for any other binding partner. ARH binds to the β2 appendage, with a KD value of ∼1 μM (Mishra et al., 2005), compared with 22 μM for a type I clathrin box LLDLD binding to clathrin (Miele et al., 2004), 10–100 μM for the Shc PTB domain binding to PtdIns(4,5)P2 (Zhou et al., 1995), and 2–5 μM for various PTB domains binding to FXNPXY peptides (Li et al., 1998; Howell et al., 1999; Stolt et al., 2003; Stolt et al., 2005). In a sequential hub model for clathrin-coated vesicle assembly, it is proposed that AP-2–dependent interactions are diminished as clathrin buds progress toward late-stage events (Schmid et al., 2006; Schmid and McMahon, 2007). In fact, it is conjectured that ARH and β-arrestins only remain associated with deeply invaginated buds by interacting directly with clathrin heavy chains (Schmid et al., 2006). Given the 10-fold difference in binding affinity for the β2 appendage and the clathrin terminal domain, which is also subject to direct competition by numerous other CLASPs and accessory proteins that have tandemly repeated clathrin boxes and are massed at the bud site (Robinson, 2004; Sorkin, 2004; Maldonado-Baez and Wendland, 2006), the biological advantage of moving to an apparently lower affinity association (at the clathrin hub) is not obvious.
The changing hub model was formulated, in part, to account for the inherent directionality of the clathrin-coat assembly process (Schmid et al., 2006; Schmid and McMahon, 2007). Although our view is that CLASPs like ARH and β-arrestin, which associate physically with AP-2 through the β2 appendage, do not necessarily switch to a clathrin-dependent interaction mode in a temporally-defined manner, our observations certainly do not generally invalidate the model, which invokes changing degrees of freedom as a function of ongoing lattice assembly events. Nor do our findings indicate that the clathrin box in either ARH or β-arrestin is functionally insignificant (Krupnick et al., 1997; Laporte et al., 2000; Kim and Benovic, 2002; Santini et al., 2002; Garuti et al., 2005). Rather, our data agree with several independent investigations showing the β-arrestin·AP-2 interaction typically initiates clustering of GPCRs into clathrin-coated structures and simply preclude ARH and β-arrestins from the set of potential endocytic factors subject to displacement by assembling clathrin triskelia. Intriguingly, because of the clear functional redundancy between the adaptor β1 and β2 subunits, and because only a single pool of cytosolic clathrin is used to construct all clathrin-coats within the cell, use of neither the [DE]nX1-2FXX[FL]XXXR motif nor the clathrin box will ensure translocation of β-arrestin or ARH to only those AP-2 and clathrin-containing structures positioned at the cell surface. However, both the β-arrestins (Gaidarov et al., 1999; Milano et al., 2002) and ARH (Mishra et al., 2002b) bind to PtdIns(4,5)P2, a lipid concentrated in the inner leaflet of the plasma membrane. The phenomenon of coincidence detection, involving simultaneous contacts with transmembrane cargo receptors, lipids, AP-2, and then clathrin, likely provides the necessary selectivity. The striking translocation of the cytosolic pool of β-arrestin 1 upon angiotensin II application (Figure 12) is a graphic demonstration of this avidity-based phenomenon. Overall then, the β2 appendage of AP-2 represents one hub within an intricate web of protein–protein interactions that promotes selective cargo endocytosis by facilitating initial CLASP recruitment.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sasha Sorkin for generously sharing the β2-YFP plasmid, Robert Lefkowitz for providing the arrestin-null mouse fibroblasts, Stéphane Laporte for the angiotensin II receptor-expressing HEK cells, and members of the Traub laboratory for critical reading of the manuscript. This work was funded by National Institutes of Health grants R01 DK53249 (to L.M.T.) and DE015180 (to E.T.E.), American Heart Association (AHA) Established Investigator Award 0540007N (to L.M.T.), and AHA predoctoral fellowship 0415428U (to P.A.K.).
Abbreviations used:
- ARH
autosomal recessive hypercholesterolemia protein
- CLASP
clathrin-associated sorting protein
- Dab2
disabled-2
- EGFP
enhanced green fluorescent protein
- GPCR
G protein-coupled receptor
- HC
heavy chain
- LDL
low-density lipoprotein
- mAb
monoclonal antibody
- MPR
cation-independent mannose 6-phosphate receptor
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PTB
phosphotyrosine binding
- tdRFP
tandem dimer tomato
- Tf488
transferrin conjugated to Alexa Fluor 488
- Tf568
transferrin conjugated to Alexa Fluor 568
- Tf633
transferrin conjugated to Alexa Fluor 633
- YFP
yellow fluorescent protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0712) on October 8, 2008.
REFERENCES
- Aguilar R. C., Ohno H., Roche K. W., Bonifacino J. S. Functional domain mapping of the clathrin-associated adaptor medium chains μ1 and μ2. J. Biol. Chem. 1997;272:27160–27166. doi: 10.1074/jbc.272.43.27160. [DOI] [PubMed] [Google Scholar]
- Ahle S., Mann A., Eichelsbacher U., Ungewickell U. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairstow S. F., Ling K., Su X., Firestone A. J., Carbonara C., Anderson R. A. Type Iγ 661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J. Biol. Chem. 2006;281:20632–20642. doi: 10.1074/jbc.M601465200. [DOI] [PubMed] [Google Scholar]
- Barriere H., Nemes C., Lechardeur D., Khan-Mohammad M., Fruh K., Lukacs G. L. Molecular basis of Ub-dependent internalization of membrane proteins in mammalian cells. Traffic. 2006;7:282–297. doi: 10.1111/j.1600-0854.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- Blondeau F., et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl. Acad. Sci. USA. 2004;101:3833–3838. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett T. J., Traub L. M. Molecular structures of coat and coat-associated proteins: function follows form. Curr. Opin. Cell Biol. 2006;18:395–406. doi: 10.1016/j.ceb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Brett T. J., Traub L. M., Fremont D. H. Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure. 2002;10:797–809. doi: 10.1016/s0969-2126(02)00784-0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M. Clathrin structure characterized with monoclonal antibodies. II. Identification of in vivo forms of clathrin. J. Cell Biol. 1985;101:2055–2062. doi: 10.1083/jcb.101.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtey A., Schmid E. M., Ford M. G., Rappoport J. Z., Scott M. G., Marullo S., Simon S. M., McMahon H. T., Benmerah A. The conserved isoleucine-valine-phenylalanine motif couples activation state and endocytic functions of β-arrestins. Traffic. 2007;8:914–931. doi: 10.1111/j.1600-0854.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- Camidge D. R., Pearse B.M.F. Cloning of the Drosophila β-adaptin and its localization on expression in mammalian cells. J. Cell Sci. 1994;107:709–718. [PubMed] [Google Scholar]
- Chaudhuri R., Lindwasser O. W., Smith W. J., Hurley J. H., Bonifacino J. S. CD4 downregulation by HIV-1 Nef is dependent on clathrin and involves a direct interaction of Nef with the AP2 clathrin adaptor. J. Virol. 2007;81:3877–3890. doi: 10.1128/JVI.02725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S., Cao H., Chen J., McNiven M. Eps15 mediates vesicle trafficking from the trans-Golgi network via an interaction with clathrin adaptor AP-1. Mol. Biol. Cell. 2008;19:3564–3575. doi: 10.1091/mbc.E07-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Straubinger R. M., Acton S., Nathke I., Brodsky F. M. 100-kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 1989;86:9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S. H., Van Damme N., Day J. R., Noviello C. M., Hitchin D., Madrid R., Benichou S., Guatelli J. C. Leucine-specific, functional interactions between human immunodeficiency virus type 1 Nef and adaptor protein complexes. J. Virol. 2005;79:2066–2078. doi: 10.1128/JVI.79.4.2066-2078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. M., McCoy A. J., Kent H. M., Evans P. R., Owen D. J. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Cupers P., Jadhav A. P., Kirchhausen T. Assembly of clathrin coats disrupts the association between Eps15 and AP-2 adaptors. J. Biol. Chem. 1998;273:1847–1850. doi: 10.1074/jbc.273.4.1847. [DOI] [PubMed] [Google Scholar]
- Dalal S., Rosser M. F., Cyr D. M., Hanson P. I. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol. Biol. Cell. 2004;15:637–648. doi: 10.1091/mbc.E03-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M. T., Downs M. A., Traub L. M. Epsin binds to clathrin by associating directly with the clathrin-terminal domain: evidence for cooperative binding through two discrete sites. J. Biol. Chem. 2000;275:6479–6489. doi: 10.1074/jbc.275.9.6479. [DOI] [PubMed] [Google Scholar]
- Edeling M. A., Mishra S. K., Keyel P. A., Steinhauser A. L., Collins B. M., Roth R., Heuser J. E., Owen D. J., Traub L. M. Molecular switches involving the AP-2 β2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev. Cell. 2006a;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Edeling M. A., Smith C., Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat. Rev. Mol. Cell Biol. 2006b;7:32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- Eden E. R., Naoumova R. P., Burden J. J., McCarthy M. I., Soutar A. K. Use of homozygosity mapping to identify a region on chromosome 1 bearing a defective gene that causes autosomal recessive homozygous hypercholesterolemia in two unrelated families. Am. J. Hum. Genet. 2001;68:653–660. doi: 10.1086/318795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E. R., Patel D. D., Sun X., Burden J. J., Themis M., Edwards M., Lee P., Neuwirth C., Naoumova R. P., Soutar A. K. Restoration of LDL-receptor function in cells from patients with autosomal recessive hypercholesterolemia by retroviral expression of ARH1. J. Clin. Invest. 2002;110:1695–1702. doi: 10.1172/JCI16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E. R., Sun X. M., Patel D. D., Soutar A. K. Adaptor protein Disabled-2 modulates low density lipoprotein (LDL) receptor synthesis in fibroblasts from patients with autosomal recessive hypercholesterolemia. Hum. Mol. Genet. 2007;16:2751–2759. doi: 10.1093/hmg/ddm232. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M. L., Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Gaidarov I., Keen J. H. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I., Krupnick J. G., Falck J. R., Benovic J. L., Keen J. H. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 1999;18:871–881. doi: 10.1093/emboj/18.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C. K., et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science. 2001;292:1394–1398. doi: 10.1126/science.1060458. [DOI] [PubMed] [Google Scholar]
- Garuti R., Jones C., Li W. P., Michaely P., Herz J., Gerard R. D., Cohen J. C., Hobbs H. H. The modular adaptor protein autosomal recessive hypercholesterolemia (ARH) promotes low density lipoprotein receptor clustering into clathrin-coated pits. J. Biol. Chem. 2005;280:40996–41004. doi: 10.1074/jbc.M509394200. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Dahms N. M., Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell Biol. 2003;4:202–213. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- Girard M., Allaire P. D., McPherson P. S., Blondeau F. Non-stoichiometric relationship between clathrin heavy and light chains revealed by quantitative comparative proteomics of clathrin-coated vesicles from brain and liver. Mol. Cell. Proteomics. 2005;4:1145–1154. doi: 10.1074/mcp.M500043-MCP200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M., Jackle H. Role of Drosophila α-adaptin in presynaptic vesicle recycling. Cell. 1997;88:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- Grant B., Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich V. V., Gurevich E. V. The molecular acrobatics of arrestin activation. Trends Pharmacol. Sci. 2004;25:105–111. doi: 10.1016/j.tips.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Haffner C., Paolo G. D., Rosenthal J. A., de Camilli P. Direct interaction of the 170 kDa isoform of synaptojanin 1 with clathrin and with the clathrin adaptor AP-2. Curr. Biol. 2000;10:471–474. doi: 10.1016/s0960-9822(00)00446-2. [DOI] [PubMed] [Google Scholar]
- Hamdan F. F., Rochdi M. D., Breton B., Fessart D., Michaud D. E., Charest P. G., Laporte S. A., Bouvier M. Unraveling G protein-coupled receptor endocytosis pathways using real-time monitoring of agonist-promoted interaction between β-arrestins and AP-2. J. Biol. Chem. 2007;282:29089–29100. doi: 10.1074/jbc.M700577200. [DOI] [PubMed] [Google Scholar]
- Hanson S. M., Dawson E. S., Francis D. J., Van Eps N., Klug C. S., Hubbell W. L., Meiler J., Gurevich V. V. A model for the solution structure of the rod arrestin tetramer. Structure. 2008a;16:924–934. doi: 10.1016/j.str.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S. M., Vishnivetskiy S. A., Hubbell W. L., Gurevich V. V. Opposing effects of inositol hexakisphosphate on rod arrestin and arrestin2 self-association. Biochemistry. 2008b;47:1070–1075. doi: 10.1021/bi7021359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasaki K., Lubben N. B., Harbour M., Taylor M. J., Robinson M. S. Sorting of major cargo glycoproteins into clathrin-coated vesicles. Traffic. 2005;6:1014–1026. doi: 10.1111/j.1600-0854.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- Hawryluk M. J., Keyel P. A., Mishra S. K., Watkins S. C., Heuser J. E., Traub L. M. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- He G., Gupta S., Yi M., Michaely P., Hobbs H. H., Cohen J. C. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin and AP-2. J. Biol. Chem. 2002;277:44044–44049. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- Heuser J. The production of ‘cell cortices’ for light and electron microscopy. Traffic. 2000;1:545–552. doi: 10.1034/j.1600-0854.2000.010704.x. [DOI] [PubMed] [Google Scholar]
- Hinrichsen L., Harborth J., Andrees L., Weber K., Ungewickell E. J. Effect of clathrin heavy chain- and α-adaptin specific small interfering RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 2003;278:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- Höning S., Ricotta D., Krauss M., Spate K., Spolaore B., Motley A., Robinson M., Robinson C., Haucke V., Owen D. J. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol. Cell. 2005;18:519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Howell B. W., Lanier L. M., Frank R., Gertler F. B., Cooper J. A. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol. 1999;19:5179–5188. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Jiang X., Sorkin A. Tyrosine phosphorylation of the β2 subunit of clathrin adaptor complex AP-2 reveals the role of a di-leucine motif in the epidermal growth factor receptor trafficking. J. Biol. Chem. 2003;278:43411–43417. doi: 10.1074/jbc.M306072200. [DOI] [PubMed] [Google Scholar]
- Huang F., Khvorova A., Marshall W., Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- Janvier K., Bonifacino J. S. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol. Biol. Cell. 2005;16:4231–4242. doi: 10.1091/mbc.E05-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K., Kato Y., Boehm M., Rose J. R., Martina J. A., Kim B. Y., Venkatesan S., Bonifacino J. S. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 γ-σ1 and AP-3 δ-σ3 hemicomplexes. J. Cell Biol. 2003;163:1281–1290. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A., Agostinelli N. R., Mishra S. K., Keyel P. A., Hawryluk M. J., Traub L. M. A novel AP-2 adaptor interaction motif initially identified in the long-splice isoform of synaptojanin 1, SJ170. J. Biol. Chem. 2004;279:2281–2290. doi: 10.1074/jbc.M305644200. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kalthoff C., Alves J., Urbanke C., Knorr R., Ungewickell E. J. Unusual structural organization of the endocytic proteins AP180 and epsin 1. J. Biol. Chem. 2002;277:8209–8216. doi: 10.1074/jbc.M111587200. [DOI] [PubMed] [Google Scholar]
- Kamikura D. M., Cooper J. A. Lipoprotein receptors and a disabled family cytoplasmic adaptor protein regulate EGL-17/FGF export in. C. elegans. Genes Dev. 2003;17:2798–2811. doi: 10.1101/gad.1136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent H., McMahon H., Evans P., Benmerah A., Owen D. γ-adaptin appendage domain. Structure and binding site for Eps15 and γ-synergin. Structure. 2002;10:1139–1148. doi: 10.1016/s0969-2126(02)00801-8. [DOI] [PubMed] [Google Scholar]
- Keyel P. A., Mishra S. K., Roth R., Heuser J. E., Watkins S. C., Traub L. M. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol. Biol. Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyel P. A., Watkins S. C., Traub L. M. Endocytic adaptor molecules reveal an endosomal population of clathrin by total internal reflection fluorescence microscopy. J. Biol. Chem. 2004;279:13190–13204. doi: 10.1074/jbc.M312717200. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Benovic J. L. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J. Biol. Chem. 2002;277:30760–30768. doi: 10.1074/jbc.M204528200. [DOI] [PubMed] [Google Scholar]
- Knuehl C., Chen C. Y., Manalo V., Hwang P. K., Ota N., Brodsky F. M. Novel binding sites on clathrin and adaptors regulate distinct aspects of coat assembly. Traffic. 2006;7:1688–1700. doi: 10.1111/j.1600-0854.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- Kohout T. A., Lin F. S., Perry S. J., Conner D. A., Lefkowitz R. J. β-arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc. Natl. Acad. Sci. USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M., Kukhtina V., Pechstein A., Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2μ-cargo complexes. Proc. Natl. Acad. Sci. USA. 2006;103:11934–11939. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick J. G., Santini F., Gagnon A. W., Keen J. H., Benovic J. L. Modulation of the arrestin-clathrin interaction in cells. Characterization of beta-arrestin dominant-negative mutants. J. Biol. Chem. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- Laporte S. A., Miller W. E., Kim K. M., Caron M. G. β-arrestin/AP-2 interaction in G protein-coupled receptor internalization. Identification of a β-arrestin binding site in β2-adaptin. J. Biol. Chem. 2002;277:9247–9254. doi: 10.1074/jbc.M108490200. [DOI] [PubMed] [Google Scholar]
- Laporte S. A., Oakley R. H., Holt J. A., Barak L. S., Caron M. G. The interaction of β-arrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits. J. Biol. Chem. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- Li S. C., Zwahlen C., Vincent S. J., McGlade C. J., Kay L. E., Pawson T., Forman-Kay J. D. Structure of a Numb PTB domain-peptide complex suggests a basis for diverse binding specificity. Nat. Struct. Biol. 1998;5:1075–1083. doi: 10.1038/4185. [DOI] [PubMed] [Google Scholar]
- Lundmark R., Carlsson S. R. The β-appendages of the four adaptor-protein (AP) complexes: structure and binding properties, and identification of sorting nexin 9 as an accessory protein to AP-2. Biochem. J. 2002;362:597–607. doi: 10.1042/0264-6021:3620597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Baez L., Wendland B. Endocytic adaptors: recruiters, coordinators and regulators. Trends Cell Biol. 2006;16:505–513. doi: 10.1016/j.tcb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Maurer M. E., Cooper J. A. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J. Cell Sci. 2006;119:4235–4246. doi: 10.1242/jcs.03217. [DOI] [PubMed] [Google Scholar]
- McCormick P. J., Martina J. A., Bonifacino J. S. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc. Natl. Acad. Sci. USA. 2005;102:7910–7915. doi: 10.1073/pnas.0502206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson P. S., Ritter B. Peptide motifs: building the clathrin machinery. Mol. Neurobiol. 2005;32:73–87. doi: 10.1385/MN:32:1:073. [DOI] [PubMed] [Google Scholar]
- Miele A. E., Watson P. J., Evans P. R., Traub L. M., Owen D. J. Two distinct interaction motifs in amphiphysin bind two independent sites on the clathrin terminal domain β-propeller. Nat. Struct. Mol. Biol. 2004;11:242–248. doi: 10.1038/nsmb736. [DOI] [PubMed] [Google Scholar]
- Milano S. K., Kim Y. M., Stefano F. P., Benovic J. L., Brenner C. Nonvisual arrestin oligomerization and cellular localization are regulated by inositol hexakisphosphate binding. J. Biol. Chem. 2006;281:9812–9823. doi: 10.1074/jbc.M512703200. [DOI] [PubMed] [Google Scholar]
- Milano S. K., Pace H. C., Kim Y. M., Brenner C., Benovic J. L. Scaffolding functions of arrestin-2 revealed by crystal structure and mutagenesis. Biochemistry. 2002;41:3321–3328. doi: 10.1021/bi015905j. [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Hawryluk M. J., Brett T. J., Keyel P. A., Dupin A. L., Jha A., Heuser J. E., Fremont D. H., Traub L. M. Dual-engagement regulation of protein interactions with the AP-2 adaptor α appendage. J. Biol. Chem. 2004;279:46191–46203. doi: 10.1074/jbc.M408095200. [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Keyel P. A., Edeling M. A., Owen D. J., Traub L. M. Functional dissection of an AP-2 β2 appendage-binding sequence within the autosomal recessive hypercholesterolemia (ARH) protein. J. Biol. Chem. 2005;280:19270–19280. doi: 10.1074/jbc.M501029200. [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Keyel P. A., Hawryluk M. J., Agostinelli N. R., Watkins S. C., Traub L. M. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002a;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S. K., Watkins S. C., Traub L. M. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc. Natl. Acad. Sci. USA. 2002b;99:16099–16104. doi: 10.1073/pnas.252630799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunari T., Nakatsu F., Shioda N., Love P. E., Grinberg A., Bonifacino J. S., Ohno H. Clathrin adaptor AP-2 is essential for early embryonal development. Mol. Cell. Biol. 2005;25:9318–9323. doi: 10.1128/MCB.25.21.9318-9323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. M., Cooper J. A. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- Moskowitz H. S., Yokoyama C. T., Ryan T. A. Highly cooperative control of endocytosis by clathrin. Mol. Biol. Cell. 2005;16:1769–1776. doi: 10.1091/mbc.E04-08-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A., Bright N. A., Seaman M. N., Robinson M. S. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Smith C. J., Roseman A. M., Harrison S. C., Kirchhausen T., Pearse B. M. Functional organization of clathrin in coats: combining electron cryomicroscopy and X-ray crystallography. Mol. Cell. 1999;3:761–770. doi: 10.1016/s1097-2765(01)80008-3. [DOI] [PubMed] [Google Scholar]
- Nakano-Kobayashi A., Yamazaki M., Unoki T., Hongu T., Murata C., Taguchi R., Katada T., Frohman M. A., Yokozeki T., Kanaho Y. Role of activation of PIP5Kγ661 by AP-2 complex in synaptic vesicle endocytosis. EMBO J. 2007;26:1105–1116. doi: 10.1038/sj.emboj.7601573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke I. S., Heuser J., Lupas A., Stock J., Turck C. W., Brodsky F. M. Folding and trimerization of clathrin subunits at the triskelion hub. Cell. 1992;68:899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Nogi T., et al. Structural basis for the accessory protein recruitment by the γ-adaptin ear domain. Nat. Struct. Biol. 2002;9:527–531. doi: 10.1038/nsb808. [DOI] [PubMed] [Google Scholar]
- Ohno H., Stewart J., Fournier M.-C., Bosshart H., Rhee I., Miyatake S., Saito T., Galluser A., Kirchhausen T., Bonifacino J. S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Vallis Y., Noble M. E., Hunter J. B., Dafforn T. R., Evans P. R., McMahon H. T. A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell. 1999;97:805–815. doi: 10.1016/s0092-8674(00)80791-6. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Vallis Y., Pearse B. M., McMahon H. T., Evans P. R. The structure and function of the β2-adaptin appendage domain. EMBO J. 2000;19:4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page L., Robinson M. Targeting signals and subunit interactions in coated vesicle adaptor complexes. J. Cell Biol. 1995;131:619–630. doi: 10.1083/jcb.131.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke G. J., Ford M. G., Schmid E. M., Olesen L. E., Gallop J. L., Peak-Chew S. Y., Vallis Y., Babu M. M., Mills I. G., McMahon H. T. Evolving nature of the AP2 α-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 2004;23:4371–4383. doi: 10.1038/sj.emboj.7600445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappoport J. Z., Simon S. M. A functional GFP-fusion for imaging clathrin-mediated endocytosis. Traffic. 2008 doi: 10.1111/j.1600-0854.2008.00770.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B., Denisov A. Y., Philie J., Deprez C., Tung E. C., Gehring K., McPherson P. S. Two WXXF-based motifs in NECAPs define the specificity of accessory protein binding to AP-1 and AP-2. EMBO J. 2004;23:3701–3710. doi: 10.1038/sj.emboj.7600378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Rohrer J., Schweizer A., Russell D., Kornfeld S. The targeting of lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J. Cell Biol. 1996;132:565–576. doi: 10.1083/jcb.132.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F., Gaidarov I., Keen J. H. G protein-coupled receptor/arrestin3 modulation of the endocytic machinery. J. Cell Biol. 2002;156:665–676. doi: 10.1083/jcb.200110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E. M., Ford M. G., Burtey A., Praefcke G. J., Peak Chew S. Y., Mills I. G., Benmerah A., McMahon H. T. Role of the AP2 β-appendage hub in recruiting partners for clathrin coated vesicle assembly. PLoS Biol. 2006;4:e262. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E. M., McMahon H. T. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- Scott M. G., Benmerah A., Muntaner O., Marullo S. Recruitment of activated G protein-coupled receptors to pre-existing clathrin-coated pits in living cells. J. Biol. Chem. 2002;277:3552–3559. doi: 10.1074/jbc.M106586200. [DOI] [PubMed] [Google Scholar]
- Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Shim J., Lee J. Molecular genetic analysis of apm-2 and aps-2, genes encoding the medium and small chains of the AP-2 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol. Cells. 2000;10:309–316. [PubMed] [Google Scholar]
- Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr. Opin. Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Sorkin A., McKinsey T., Shih W., Kirchhausen T., Carpenter G. Stoichiometric interaction of the epidermal growth factor receptor with the clathrin-associated protein complex AP-2. J. Biol. Chem. 1995;270:619–625. doi: 10.1074/jbc.270.2.619. [DOI] [PubMed] [Google Scholar]
- Sorkina T., Huang F., Beguinot L., Sorkin A. Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of epidermal growth factor receptor. J. Biol. Chem. 2002;277:27433–27441. doi: 10.1074/jbc.M201595200. [DOI] [PubMed] [Google Scholar]
- Stolt P. C., Chen Y., Liu P., Bock H. H., Blacklow S. C., Herz J. Phosphoinositide binding by the disabled-1 PTB domain is necessary for membrane localization and reelin signal transduction. J. Biol. Chem. 2005;280:9671–9677. doi: 10.1074/jbc.M413356200. [DOI] [PubMed] [Google Scholar]
- Stolt P. C., Jeon H., Song H. K., Herz J., Eck M. J., Blacklow S. C. Origins of peptide selectivity and phosphoinositide binding revealed by structures of Disabled-1 PTB domain complexes. Structure. 2003;11:569–579. doi: 10.1016/s0969-2126(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Storez H., et al. Homo- and hetero-oligomerization of β-arrestins in living cells. J. Biol. Chem. 2005;280:40210–40215. doi: 10.1074/jbc.M508001200. [DOI] [PubMed] [Google Scholar]
- Tebar F., Sorkina T., Sorkin A., Ericsson M., Kirchhausen T. eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of clathrin-coated pits. J. Biol. Chem. 1996;271:28727–28730. doi: 10.1074/jbc.271.46.28727. [DOI] [PubMed] [Google Scholar]
- Traub L. M. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta. 2005;1744:415–437. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Traub L. M., Bannykh S. I., Rodel J. E., Aridor M., Balch W. E., Kornfeld S. AP-2–containing clathrin coats assemble on mature lysosomes. J. Cell Biol. 1996;135:1801–1804. doi: 10.1083/jcb.135.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M., Downs M. A., Westrich J. L., Fremont D. H. Crystal structure of the α appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc. Natl. Acad. Sci. USA. 1999;96:8907–8912. doi: 10.1073/pnas.96.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M., Kornfeld S., Ungewickell E. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J. Biol. Chem. 1995;270:4933–4942. doi: 10.1074/jbc.270.9.4933. [DOI] [PubMed] [Google Scholar]
- Ungewickell A., Ward M. E., Ungewickell E., Majerus P. W. The inositol polyphosphate 5-phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc. Natl. Acad. Sci. USA. 2004;101:13501–13506. doi: 10.1073/pnas.0405664101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E. J., Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr. Opin. Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Walther K., Diril M. K., Jung N., Haucke V. Functional dissection of the interactions of stonin 2 with the adaptor complex AP-2 and synaptotagmin. Proc. Natl. Acad. Sci. USA. 2004;101:964–969. doi: 10.1073/pnas.0307862100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Sudhof T. C., Anderson R. G. The appendage domain of α-adaptin is a high affinity binding site for dynamin. J. Biol. Chem. 1995;270:10079–10083. doi: 10.1074/jbc.270.17.10079. [DOI] [PubMed] [Google Scholar]
- White S., Miller K., Hopkins C., Trowbridge I. S. Monoclonal antibodies against defined epitopes of the human transferrin receptor cytoplasmic tail. Biochim. Biophys. Acta. 1992;1136:28–34. doi: 10.1016/0167-4889(92)90081-l. [DOI] [PubMed] [Google Scholar]
- Zhou M. M., Ravichandran K. S., Olejniczak E. F., Petros A. M., Meadows R. P., Sattler M., Harlan J. E., Wade W. S., Burakoff S. J., Fesik S. W. Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature. 1995;378:584–592. doi: 10.1038/378584a0. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Traub L. M., Kornfeld S. High-affinity binding of the AP-1 adaptor complex to trans-Golgi network membranes devoid of mannose 6-phosphate receptors. Mol. Biol. Cell. 1999;10:537–549. doi: 10.1091/mbc.10.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.