Abstract
Ydj1 of Saccharomyces cerevisiae is an abundant cytosolic Hsp40, or J-type, molecular chaperone. Ydj1 cooperates with Hsp70 of the Ssa family in the translocation of preproteins to the ER and mitochondria and in the maturation of Hsp90 client proteins. The substrate-binding domain of Ydj1 directly interacts with steroid receptors and is required for the activity of diverse Hsp90-dependent client proteins. However, the effect of Ydj1 alteration on client interaction was unknown. We analyzed the in vivo interaction of Ydj1 with the protein kinase Ste11 and the glucocorticoid receptor. Amino acid alterations in the proposed client-binding domain or zinc-binding domain had minor effects on the physical interaction of Ydj1 with both clients. However, alteration of the carboxy-terminal farnesylation signal disrupted the functional and physical interaction of Ydj1 and Hsp90 with both clients. Similar effects were observed upon deletion of RAM1, which encodes one of the subunits of yeast farnesyltransferase. Our results indicate that farnesylation is a major factor contributing to the specific requirement for Ydj1 in promoting proper regulation and activation of diverse Hsp90 clients.
INTRODUCTION
Molecular chaperones are a diverse group of highly conserved proteins that transiently interact with partially folded polypeptide chains during normal cellular processes such as protein translation and disassembly of protein complexes. Heat-shock protein 70 (Hsp70) and Hsp40 chaperones are among the most conserved, being present in nearly all organisms. Hsp70 and Hsp40 cooperate in the refolding of denatured proteins in vitro and in vivo. Hsp70s promote folding of misfolded proteins through cycles of ATP-regulated binding and release, and Hsp40s regulate the ATPase activity of Hsp70. Hsp70s bind polypeptides with exposed hydrophobic stretches that are prone to aggregation or misfolding. Some Hsp40s also bind unfolded polypeptide substrates and are able to prevent their aggregation independently of Hsp70 action. According to a current model of the cycle of Hsp70 and Hsp40 action, Hsp40 binds unfolded protein substrate first. The ATPase activity of Hsp70 is stimulated as Hsp40 transfers the bound polypeptide to Hsp70, resulting in stable interaction of Hsp70 with the unfolded polypeptide (Mayer and Bukau, 2005).
Hsp40s are a group of diverse proteins defined by the J domain, which directly interacts with and stimulates the ATPase activity of Hsp70. Hsp40s are further classified according to the domains they share in common with DnaJ of Escherichia coli. Ydj1 shares the general domain structure of DnaJ, in which the J domain is followed by a region rich in glycine-phenylalanine (G/F) residues, and a substrate-binding domain (SBD; Lu and Cyr, 1998b), which is further subdivided into domains I, II, and III. Domain I cocrystallized with a substrate peptide GWLYEIS and thus contains the presumed substrate-binding site; domain II contains a zinc-binding domain; and domain III contains a dimerization site (Li et al., 2003; Wu et al., 2005). In contrast to DnaJ, Ydj1 possesses a carboxy-terminal CAAX box, where A is an aliphatic amino acid and X is any amino acid (Wright and Philips, 2006). This sequence specifies the addition of a farnesyl group to the carboxy-terminus of Ydj1. Ydj1 is primarily cytosolic, but this modification facilitates attachment of a portion of Ydj1 to ER and perinuclear membranes (Caplan et al., 1992b).
Ydj1 cooperates with Hsp70s of the Ssa class to refold denatured luciferase in vitro (Lu and Cyr, 1998b) and facilitate transport of preproteins to mitochondrial and ER membranes (Caplan and Douglas, 1991; Atencio and Yaffe, 1992; Becker et al., 1996). Ydj1 is also required for the maturation of heterologous Hsp90-client proteins such as steroid receptors, as well as endogenous client proteins such as the heme-regulated transcription factor Hap1 and the Ste1l kinase (Kimura et al., 1995; Fliss et al., 1999; Johnson and Craig, 2000; Hon et al., 2001; Lee et al., 2004). However, little is known about how Ydj1 interacts with Hsp90 client proteins in vivo. We compared the ability of Ydj1 containing alterations in the zinc-binding domain, the presumed client-binding domain and the farnesylation signal to interact with members of two main classes of Hsp90 client proteins, transcription factors, and protein kinases. Specifically, we monitored the effects of YDJ1 mutation on the in vivo interaction of Ydj1 with the glucocorticoid receptor (GR) and the Ste11 kinase, the equivalent of mammalian Raf (Pratt and Toft, 2003). Our results indicate that the farnesylation modification of Ydj1 is critical for its interaction with these diverse Hsp90 client proteins.
MATERIALS AND METHODS
Media, Chemicals, Antibodies, and Plasmids
5-fluoroorotic acid (5-FOA) was obtained from Toronto Research Chemicals (Downsview, ON, Canada). Deoxycorticosterone (DOC) was obtained from Sigma (St. Louis, MO). Polyclonal antibodies against the carboxy-terminus of Hsc82/Hsp82 have been described (Flom et al., 2006). Polyclonal antibodies against Ydj1, Sis1, and Ssa were generous gifts from Elizabeth Craig (University of Wisconsin, Madison, WI). S. cerevisiae strain JJ160 (MATa trp1-2 ura3-1 leu2-3112 his3-11,15 ade2-1 can1-100 met2-Δ1 lys2-Δ2 ydj1::HIS3/pRS316-YDJ1) is isogenic to W303 and has been described (Johnson and Craig, 2000). Strain JJ264 (ram1:kanr, ydj1::HIS3/pRS316-YDJ1) is isogenic to W303. Briefly, a homozygous ydj1::HIS3 diploid strain containing the pRS316-YDJ1 plasmid was transformed with an amplified ram1::kanr cassette derived from the genome knockout collection (Open Biosystems, Huntsville, AL). Haploid strain JJ264 (ram1 ydj1::HIS3/pRS316-HIS3) was obtained after dissection of resultant spores and was confirmed by PCR to contain ram1::kanr. RAM1 deletion strains C1B (JJ217) and C5A (JJ218) were similarly obtained after transformation of the isogenic diploid strain PJ53 (Yan and Craig, 1999) with ram1::kanr. The pheromone-response element (PRE)-lacZ reporter construct was a gift from Dr. Kevin Morano (University of Texas Medical School, Houston, TX; Morano and Thiele, 1999). A plasmid expressing the GR and the corresponding glucocorticoid-response element (GRE)-lacZ reporter plasmid were generous gifts from Didier Picard (University of Geneva).
Ydj1 Plasmids
WT or mutant YDJ1 expressed from the plasmid pRS317 was transformed into strain JJ160 (ydj1::HIS3/pRS316-YDJ1). Resulting colonies were grown in the presence of 5-FOA to counterselect for the presence of the pRS316-YDJ1 plasmid. The truncation mutants and ydj1-C406S have been described (Johnson and Craig, 2000). Additional point mutations (H34Q, L137HF249S, C159T, and C143SC162S were constructed using site-directed mutagenesis (QuikChange, Stratagene, La Jolla, CA) or other PCR based-methods. All mutant constructs were sequenced completely using automated DNA sequencing. Sequences of mutagenic primers are available on request.
Ste11 Plasmids
A construct expressing full-length WT Ste11 or the isolated coding sequence was isolated from strain BY4171 (Open Biosystems, Huntsville, AL) using standard PCR methods. To construct His-Ste11, the coding sequence for full-length Ste11 or the isolated kinase domain (amino acids 364-717, Ste11ΔN) was cloned into pRSETA (Invitrogen, Carlsbad, CA) to insert an amino terminal 6XHis tag plus an Xpress epitope in frame with the Ste11 coding sequence. The 6X-His-Ste11 coding sequence was subsequently cloned into p414GPD (Mumberg et al., 1995) to generate constitutively expressed p414GPD-His-Ste11 or p414GPD-HisSte11ΔN. To place WT His-tagged full-length Ste11 or Ste11ΔN under the GAL1 promoter, SacI and NdeI restriction sites flanking the promoter were introduced into the pESC-TRP plasmid (Stratagene). Standard cloning methods were used to swap the GPD and GAL1 promoters, resulting in p414GAL-His-Ste11 and p414GAL-HisSte11ΔN. Full-length His-Ste11 WT and His-Ste11-K444R were expressed at similar levels in cells expressing WT YDJ1 (not shown). Constitutive expression of His-Ste11ΔN-K444R did not significantly affect cell growth (not shown), and GAL-Ste11ΔN-K444R exhibited markedly reduced activation of the PRE-lacZ reporter plasmid (not shown), indicating that the K444R mutation disrupts Ste11 activity.
Isolation of His-Ste11ΔN-K444R Complexes
Cells expressing WT or mutant YDJ1 were transformed with the plasmid expressing p414GPDHis-Ste11, p414GPDHis-Ste11ΔN-K444R, or p414GPD as a control. Transformants were grown overnight in selective media to an OD600 of 2.5–3.0. Cultures were grown at 30°C except for ram1, ydj1, or ydj1-H34Q strains, which were grown at 25°C. Twenty-five OD600 units of cells were harvested, washed with water, and resuspended in 650 μl lysis buffer (20 mM Tris, pH 7.5, 100 mM KCl, 5 mM MgCl2, 10 mM sodium molybdate, and 5 mM imidazole containing a dissolved mini-protease inhibitor tablet; Roche Applied Science, Indianapolis, IN). Cells were disrupted in the presence of glass beads with eight 30-s pulses. After centrifugation, His-Ste11ΔN-K444R complexes were isolated by incubation with 35 μl of a 1:1 slurry of nickel resin (1 h with rocking, 4°C) followed by four washes with lysis buffer plus 0.1% Tween-20 and 35 mM imidazole. Nickel resin was boiled in SDS-PAGE sample buffer, and protein complexes were separated by gel electrophoresis followed by Coomassie Blue staining or immunoblot analysis. As needed to reduce background binding of mutant Ydj1 to the resin (specifically Ydj1-C159T and -C143SC162S), His-Ste11ΔN-K444R complexes were eluted from the nickel resin by incubation with lysis buffer containing 200 mM imidazole (three times, 2-min washes) and then concentrated by trichloroacetic acid (TCA) precipitation before SDS-PAGE and immunoblot analysis. Typically we used gels containing 7.5% acrylamide except when analyzing Ydj1 truncation mutants, in which case we used gels containing 15% acrylamide. Gels were transferred to nitrocellulose, and immunoblots were performed using Super Signal West Pico Chemiluminescent reagents (Pierce, Rockford, IL). Quantification of immunoblots was performed using ImageJ (http://rsbweb.nih.gov/ij/).
Ste11 Activity
Cells expressing WT or mutant YDJ1 were transformed with a plasmid expressing GAL-Ste11ΔN (TRP+) along with the corresponding reporter plasmid PRE-lacZ (URA+). Unless noted otherwise, cultures were grown at 30°C in raffinose selective media to midlog phase. To induce Ste11ΔN, galactose was added to a final concentration of 2%. After 6 h of induction, cells were harvested for β-galactosidase assays. β-Galactosidase units were calculated as 103× OD420 divided by the OD600× elapsed time (in minutes; Johnson and Craig, 2000). Each experiment contained triplicate samples, and the activity of each mutant was assayed at least two independent times. To assay activity of endogenous Ste11, cells expressing WT or mutant YDJ1 along with the corresponding reporter plasmid PRE-lacZ were grown overnight in selective media. Yeast alpha factor (Zymo Research, Orange, CA) was added to a final concentration of 5 μM and cells were grown for an additional 2 or 3 h as indicated. β-Galactosidase units were calculated as described above.
GR Assay
The rat GR expressed from a multicopy plasmid under control of the constitutive GPD promoter was coexpressed with a GRE-lacZ reporter plasmid. Briefly, cultures were grown at 30° in selective media to midlog phase (OD600 0.4–0.8). To activate the GR, ethanol (as a control) or 1 μM deoxycorticosterone (final concentration) was added to the culture for a 1-h incubation. β-Galactosidase assays were performed as described (Johnson and Craig, 2000).
In Vivo Interaction of Ydj1 with the GR
A plasmid constitutively expressing rat GR containing an amino-terminal Flag-tag was a gift from Susan Lindquist (Whitehead Institute for Biomedical Research, Cambridge, MA; Kimura et al., 1995). Cells expressing various ydj1 mutants along with Flag-GR were harvested and lysed in buffer (20 mM Tris, pH 7.5, 100 mM KCl, 5 mM MgCl2, and 10 mM sodium molybdate) as described above for cells expressing His-Ste11ΔN-K444R. Cell lysate was incubated for 1.5 h (with rocking) with 25 μl of a 1:1 slurry of anti-Flag M2 affinity resin (Sigma; cat. no. F2426). After incubation, resin was washed with lysis buffer plus 0.1% Tween-20 and then incubated with Flag peptide (Sigma; 100 μg/ml final concentration) for 30 min, 4°C with rocking, to elute GR and associated proteins from the antibody resin. The eluate was concentrated by TCA precipitation before SDS-PAGE and immunoblot analysis. Samples were analyzed as described above, except that GR was detected using an antibody specific for the GR (Affinity Bioreagents, Golden, CO; cat. no. MA1-510).
RESULTS
Direct interaction between the substrate binding domain (SBD) of Ydj1 and a steroid receptor appears to be the first step in the assembly of steroid-receptor Hsp90 complexes (Hernandez et al., 2002; Cintron and Toft, 2006), and deletion of the SBD of Ydj1 disrupts the activity of diverse Hsp90 clients in vivo (Johnson and Craig, 2000). However, the mechanism by which Ydj1 binds steroid receptors and subsequently delivers them to Hsp70 and Hsp90 is unclear (Wegele et al., 2006). Also unclear is whether kinases and steroid receptors have similar requirements for Ydj1 interaction, particularly because many kinases are also dependent on the cochaperone Cdc37 for both stability and activity (Caplan et al., 2007). The goal of this study was to determine whether the Ste11 kinase and the glucocorticoid receptor have similar requirements for Ydj1 interaction in vivo.
Ste11 Accumulation Is Disrupted by YDJ1 Mutation
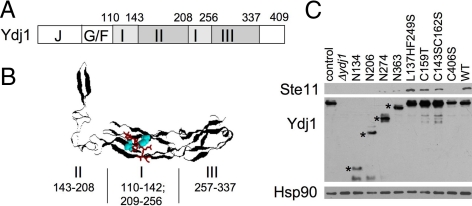
Ste11 is a mitogen-activated kinase kinase kinase that has critical roles in the cellular response to pheromone and nutrient depletion. Ste11 and the mammalian equivalent, Raf, formed complexes with Hsp90, and decreased accumulation of Ste11 was observed in cells expressing mutant forms of Hsp90 or reduced Hsp90 levels (Louvion et al., 1998; Pratt and Toft, 2003). We tested the effect of YDJ1 mutation on the accumulation of Ste11 protein. The J+G/F domains of Ydj1 are required to stimulate the ATPase activity of the partner Hsp70 Ssa1. The SBD of Ydj1 contains multiple distinct features (Figure 1, A and B): domain I (residues 110-142 and 209-256) cocrystallized with the peptide GWLYEIS and thus contains a presumed client-binding site; domain II (residues 143-208, the zinc-binding domain) is postulated to function in substrate binding and/or the transfer of substrates to Hsp70; and domain III (residues 257-337) contains both a region structurally similar to domain I and sequences required for dimerization of Ydj1. The four terminal amino acids (CASQ), which are not included in the determined structure, contain the farnesylation signal that dictates attachment of a 15-carbon isoprenyl lipid to the cysteine residue (C406 in Ydj1; Caplan et al., 1992b; Lu and Cyr, 1998b; Li et al., 2003; Fan et al., 2005; Wu et al., 2005).
Figure 1.
Effect of Ydj1 alteration on Ste11 accumulation. (A) Schematic of the domain structure of Ydj1. The amino acid sequence encoding domain I is split by the sequences encoding domain II (the zinc-binding domain). (B) A ribbon diagram of the x-ray crystal structure of the SBD of Ydj1 (amino acids 110-337) cocrystallized with the GWLYEIS peptide (Li et al., 2003). The residues comprising each domain are shown below the structure. The GWLYEIS peptide is depicted in red stick mode and the L137 and F249 residues are aqua in space filling mode. The figure was generated with RasMol (PDB file 1NLT). (C) Full-length WT Ste11 was constitutively expressed in a ydj1::HIS3 strain expressing the indicated ydj1 mutation. His-Ste11 was isolated by incubation of cell lysate with nickel resin. Control sample expressed WT YDJ1 but lacked plasmid expressing His-Ste11. Top, His-Ste11was eluted from nickel resin in SDS-sample buffer, separated by SDS-PAGE and immunoblotted with anti-Xpress (His-Ste11). Middle and bottom, whole cell lysate was immunoblotted with antibodies specific for Ydj1 or Hsp90. The truncated forms of Ydj1 are indicated with an asterisk (*).
We examined the stability of full-length WT Ste11 in cells expressing truncated forms of Ydj1 or containing amino acid alterations in distinct subdomains of the SBD (Figure 1C). Because Ste11 is normally expressed at low levels, we expressed His-Ste11 under a strong constitutive promoter. His-Ste11 accumulated in cells expressing WT YDJ1, but YDJ1 deletion or truncation of Ydj1 (Ydj1-N134, N206, N274, or N363), or alteration of the carboxy-terminal farnesylation signal (C406S), resulted in a dramatic loss in accumulation of WT His-Ste11. In contrast, mutation of domain I did not affect Ste11 accumulation (L137HF249S, 110% WT Ydj1 level), whereas mutations in domain II resulted in partially reduced Ste11 levels C159T and C143SC162S (78 and 58% of WT Ste11 levels, respectively). Although the level of Hsp90 in cell extracts was unchanged by YDJ1 mutation, the expression of Ydj1-L137HF249S, -C159T, and -C143SC162S was slightly higher than that of WT Ydj1.
Impact of YDJ1 Mutation on Ste11-Ydj1 Interaction
We detected interaction of WT Ydj1 with full-length His-Ste11 (not shown), but because multiple ydj1 mutations resulted in dramatically reduced accumulation of His-Ste11, we were unable to examine the impact of ydj1 mutation on Ydj1-Ste11 interaction. Prior studies established that a truncated form of Ste11 consisting of only the kinase domain (Ste11ΔN) stably bound Hsp90, Cdc37, Sti1, and Hsp70 (Louvion et al., 1998; Abbas-Terki et al., 2000; Lee et al., 2004). The accumulation pattern of His-Ste11ΔN expressed from an inducible GAL1 promoter in ydj1 mutant cells was similar to that shown in Figure 1C, and constitutive expression of His-Ste11ΔN was toxic to cells (not shown). However, constitutively expressed His-Ste11ΔN-K444R, which contains an alteration of a conserved lysine (residue K444) found in the vast majority of serine/threonine kinases (Hanks and Hunter, 1995), was not toxic to yeast (not shown). WT Ste11 and Ste11-K444R exhibited similar interactions in two-hybrid assays, suggesting that this mutation does not cause global misfolding of Ste11 in vivo (Printen and Sprague, 1994). In the following experiments we used His-Ste11ΔN-K444R to circumvent problems that arose because of Ste11 instability and Ste11ΔN toxicity.
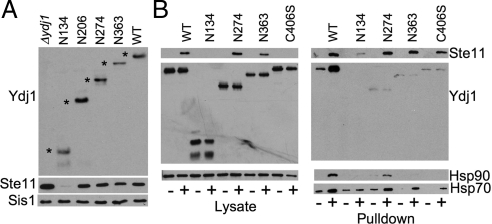
Figure 2A shows the accumulation of His-Ste11ΔN-K444R in cells lacking YDJ1 or expressing ydj1 truncation mutants. Cells lacking YDJ1 accumulate His-Ste11ΔN-K444R to a high level (∼190% of WT levels), but cells expressing ydj1-N134 failed to accumulate significant levels of His-Ste11ΔN-K444R (>5% of WT levels). The reason for this difference is unknown. His-Ste11ΔN-K444R accumulation was not affected in cells expressing ydj1-N206 or N274 (110 and 100% WT levels, respectively). However, cells expressing ydj1-N363 consistently expressed reduced levels of His-Ste11ΔN-K444R (∼75% WT levels).
Figure 2.
Effect of Ydj1 truncation on Ste11ΔN accumulation and Ydj1-Ste11ΔN interaction. Strain JJ160 expressing indicated ydj1 mutant was transformed with a plasmid constitutively expressing His-Ste11ΔN-K444R or a control plasmid. (A) Cells expressing His-Ste11ΔN-K444R were disrupted with glass beads in the presence of protease inhibitors and centrifuged to remove cellular debris. Equal amounts of cell lysates were separated by SDS-PAGE (15% acrylamide to detect Ydj1 truncations using Ydj1-specific antisera, 10% acrylamide in other instances) followed by immunoblot analysis. Top panel, the truncated forms of Ydj1 are indicated with an asterisk (*). Bottom panels, immunoblots using antibodies specific for the Xpress epitope present in His-Ste11ΔN-K444R or an antibody that recognizes the Hsp40 Sis1 (as a loading control). (B) Left, extracts were prepared from cells that lacked a His-tagged protein (−) or from those expressing His-Ste11ΔN-K444R (+). Right, cell extracts were incubated with nickel resin for 1 h at 4°C. After washing nickel resin with lysis buffer containing 35 mM imidazole, His-Ste11ΔN-K444R complexes were eluted into SDS sample buffer, separated by SDS-PAGE, and subjected to immunoblot analysis using antibodies specific for the Xpress epitope (Ste11ΔN), Ydj1, Hsp90, or Hsp70 of the Ssa family.
We examined the ability of truncated forms of Ydj1 to copurify with His-Ste11ΔN-K444R. For each mutant we compared the binding of Ydj1 to nickel resin incubated with lysates from cells that did not express any His-tagged proteins (−) to that from cells expressing His-Ste11ΔN-K444R (+). Cells expressing full-length Ydj1 or truncation mutants were lysed in a nondenaturing buffer and incubated with nickel resin. Resin was washed with lysis buffer containing low levels of imidazole and analyzed by SDS-PAGE and immunoblot analysis. As expected, significant levels of His-Ste11ΔN-K444R failed to bind nickel resin in cells expressing Ydj1-N134 (>10% WT levels), but near WT levels of His-Ste11ΔN-K444R were recovered in cells expressing N274 or N363 (99 and 91% WT levels, respectively). Ydj1-C406S resulted in a reduced level of His-Ste11ΔN-K444R in the cell lysate and a slight reduction in the level of His-Ste11ΔN-K444R bound to nickel resin (73% WT level). Although WT Ydj1 specifically bound resin in the presence of His-Ste11ΔN-K444R (Figure 2B right), no specific binding of the Ydj1 truncation mutants or Ydj1-C406S to resin was observed. We also examined whether binding of Hsp90 and Hsp70 to His-Ste11ΔN-K444R was disrupted by Ydj1 mutation. In most cases, loss of stable Ydj1 interaction resulted in loss of stable Hsp90 binding. However, some Hsp90 binding to His-Ste11ΔN-K444R was observed in the presence of Ydj1-N274 (∼38% WT level). In contrast, the level of Hsp70 (Ssa family) interaction with His-Ste11ΔN-K444R was not as sensitive to ydj1 mutation, although slightly reduced levels of Hsp70 bound His-Ste11ΔN-K444R in the presence of Ydj1-C406S.
Figure 3 shows the impact of amino acid alterations in Ydj1 on His-Ste11ΔN-K444R interaction. C143, C159, and C162 are cysteine residues that contact bound zinc, and alterations of these residues led to various degrees of growth defects (Atencio and Yaffe, 1992; Li et al., 2003; Fan et al., 2005). ydj1-L137HF249S is a novel mutation that alters two hydrophobic residues that contacted bound GWLYEIS peptide (Li et al., 2003). Ydj1-C159T did not result in reduced Ste11ΔN accumulation, but Ydj1-C143SC162 exhibited a reduced level of His-Ste11ΔN-K444R in cell lysate (Figure 3A, 35% WT level) or bound to nickel resin (Figure 3B, 60% WT levels). Ydj1-C159T did not affect Ydj1, Hsp90, or Hsp70 interaction with His-Ste11ΔN-K444R. Ydj1-C143SC162S appeared to have reduced interaction with both Ydj1 and Hsp90 (Figure 3B). Ydj1-L137HF249S did not affect the level of His-Ste11ΔN-K444R bound to nickel resin (100% WT level) or interaction of Ydj1, Hsp70, or Hsp90 with His-Ste11ΔN-K444R.
Figure 3.

Effect of Ydj1 point mutations on His-Ste11ΔN-K444R interaction. Strain JJ160 expressing indicated ydj1 mutant was transformed with a plasmid constitutively expressing His-Ste11ΔN-K444R or a control plasmid. (A) Extracts were prepared from cells that lacked a His-tagged protein (−) or from those expressing His-Ste11ΔN-K444R (+). Equal amounts of cell lysates were separated by SDS-PAGE followed by immunoblot analysis to detect His-Ste11ΔN-K444R and Ydj1. (B) Cell extracts were incubated with nickel resin for 1 h at 4°C. In general, His-Ste11ΔN-K444R complexes were isolated as described for Figure 2. The exception was that in the experiment with C159T and C143SC162S, complexes bound to nickel resin were eluted with 200 mM imidazole (three times, 2-min washes) and then precipitated with TCA to reduce background binding to the nickel resin. Samples were analyzed by immunoblot as described in Figure 2.
To determine whether the SBD of Ydj1 interacts with His-Ste11ΔN-K444R in the absence of functional Ydj1-Hsp70 interaction, we examined the interaction of His-Ste11ΔN-K444R with Ydj1 containing a mutation in the conserved histidine residue (H34) of the HPD motif of the J domain. Mutations in the HPD motif disrupt Hsp40-Hsp70 interaction, and purified Ydj1-H34Q was unable to stimulate the ATPase activity of the Hsp70 Ssa1 (Lu and Cyr, 1998b). Yeast expressing ydj1-H34Q exhibit a null phenotype with regard to cell growth and Ste11ΔN activity (not shown). However, Ydj1-H34Q did not result in a dramatic reduction in either the accumulation of His-Ste11ΔN-K444R or interaction with Ydj1, Hsp70 or Hsp90. This result suggests that the SBD of Ydj1 directly interacts with His-Ste11ΔN-K444R and is consistent with a prior study that demonstrated that purified Dja1/Hdj2 containing an alteration in the HPD motif maintained the ability to interact with purified progesterone receptor (Cintron and Toft, 2006).
Next we examined the effect of YDJ1 mutation on growth and Ste11 activity. Deletion of YDJ1 causes very slow growth at 30°C and inviability at higher temperatures (Caplan and Douglas, 1991; Atencio and Yaffe, 1992). The effect of ydj1 mutations that eliminate various domains or point mutations within subdomains of the SBD on the ability to support growth of a ydj1 strain is shown in Figure 4A. Alteration (C406S) or truncation of the farnesylation signal disrupted the ability of Ydj1 to support growth at 37°C and caused altered activity of Hsp90 clients, including steroid receptors and the v-src kinase (Caplan et al., 1992a; Johnson and Craig, 2000). Alteration of residue C159 caused defects in import into mitochondria and a temperature-sensitive phenotype (Atencio and Yaffe, 1992). In a prior study, ydj1-C162S was unable to support growth at 37°C, and ydj1-C143S exhibited slight growth defects at 37°C. As expected, the ydj1-C143SC162S double mutant also exhibited growth defects at 37°C. A prior study demonstrated that the L137A or F249A alterations did not affect growth at 37°C (Li and Sha, 2005). The combination of alterations in these residues had only slight effects on the ability of Ydj1 to support growth at 37°C.
Figure 4.
Effect of YDJ1 mutation on growth and Ste11 activity. (A) Strain JJ160 (ydj1::HIS3) was transformed with a plasmid expressing WT YDJ1, indicated mutant or pRS317 vector (as a control). Growth was assessed after 2 d on selective media at the indicated temperature. To measure Ste11 activity, strains were transformed with plasmids expressing Ste11ΔN under the GAL1 promoter along with the PRE-lacZ reporter plasmid. β-Galactosidase activity was assessed after a 6-h induction with galactose. Activity of each mutant was assayed in triplicate at least twice. The value shown is the average activity (relative to WT Ydj1) of independent assays. (B) To measure the effect of YDJ1 mutation on activity of endogenous WT Ste11, ydj1::HIS3 cells expressing indicated mutant along with the PRE-lacZ reporter plasmid were exposed to 5 μM α-factor for 0, 2, or 3 h. Ste11 activation was monitored using β-galactosidase assays.
Full-length Ste11 phosphorylates target proteins such as the transcription factor Ste12 in response to pheromone, whereas Ste11ΔN exhibits constitutive kinase activity. In prior studies, in vivo activity of Ste11 was dependent on Ydj1, Hsp90, and the Hsp90 cochaperones Cdc37, Cpr7, and Sti1, and deletion of Hsp90 cochaperones had similar effects on the activity of full-length Ste11 or Ste11ΔN (Louvion et al., 1998; Abbas-Terki et al., 2000; Lee et al., 2004). We compared the ability of strains overexpressing GAL1-Ste11ΔN to activate a reporter construct driven by a PRE-lacZ (Morano and Thiele, 1999). Relative to a ydj1 strain expressing WT YDJ1, Ste11ΔN activity was greatly reduced in the ydj1 null strain or in the presence of ydj1-N134 (∼3.5% activity; Figure 4B). We monitored Ste11ΔN activity in a ydj1 strain expressing additional mutations within the SBD. Our results demonstrate that the ydj1 mutant strains with the greatest growth defects exhibit the strongest defects in Ste11ΔN activity. Similarly, a ydj1 mutant strain that exhibited near WT growth (L137HF249S) had less dramatic effects on Ste11ΔN activity.
To further examine the impact of loss of the SBD or farnesylation signal on the activity of full-length WT Ste11, we examined the ability of Ste11 to activate transcription of Ste12 in a pheromone-responsive manner (Figure 4B). As observed with Ste11ΔN, loss of the entire SBD (Ydj1-N134) dramatically reduced Ste11 function. Although the effects are not as dramatic as for Ydj1-N134, alteration of the farnesylation signal of Ydj1 caused defects in the activity of both Ste11ΔN and full-length Ste11.
Deletion of a Gene Encoding a Subunit of the Yeast Farnesyltransferase, Ram1, Disrupts Ydj1-Ste11 Interaction.
The results presented above indicate that alteration or truncation of the farnesylation signal disrupts the interaction of Ydj1 and Hsp90 with Ste11, and results in decreased Ste11 accumulation and activity. A role of Ydj1 farnesylation in client interaction was unexpected because Ydj1 purified from E. coli, which lacks enzymes required for the farnesylation modification, was able to bind unfolded polypeptides and cooperate with Hsp70 in refolding of denatured luciferase and assembly of Hsp90 complexes in vitro (Lu and Cyr, 1998b; Hernandez et al., 2002). To confirm the importance of the farnesylation modification in Ydj1-Ste11 interaction, we established that deletion of a subunit of the enzyme required for farnesylation also results in loss of the Ydj1-Ste11 interaction.
The first step in the farnesylation modification is carried out by farnesyltransferase, which transfers a farnesyl group from farnesyldiphosphate to the thiol group of the cysteine in the CAAX motif (C406 of Ydj1). A prior study demonstrated that WT Ydj1, but not Ydj1-C406S could be labeled with [3H]farnesyl diphosphate in vitro. Ydj1-C406S also migrated more slowly than WT Ydj1 by SDS-PAGE (Caplan et al., 1992b). In S. cerevisiae, farnesyltransferase is comprised of two subunits, Ram1 and Ram2. RAM2 is essential, but RAM1 is not (Wright and Philips, 2006). Figure 5A shows that all detectable WT Ydj1 is shifted to a form that migrates more slowly in a strain lacking Ram1 (ram1) and that the mobility of WT Ydj1 in the ram1 strain is indistinguishable from the mobility of Ydj1-C406S expressed in the presence or absence of RAM1. This result indicates that all detectable Ydj1 is likely farnesylated in vivo.
Figure 5.
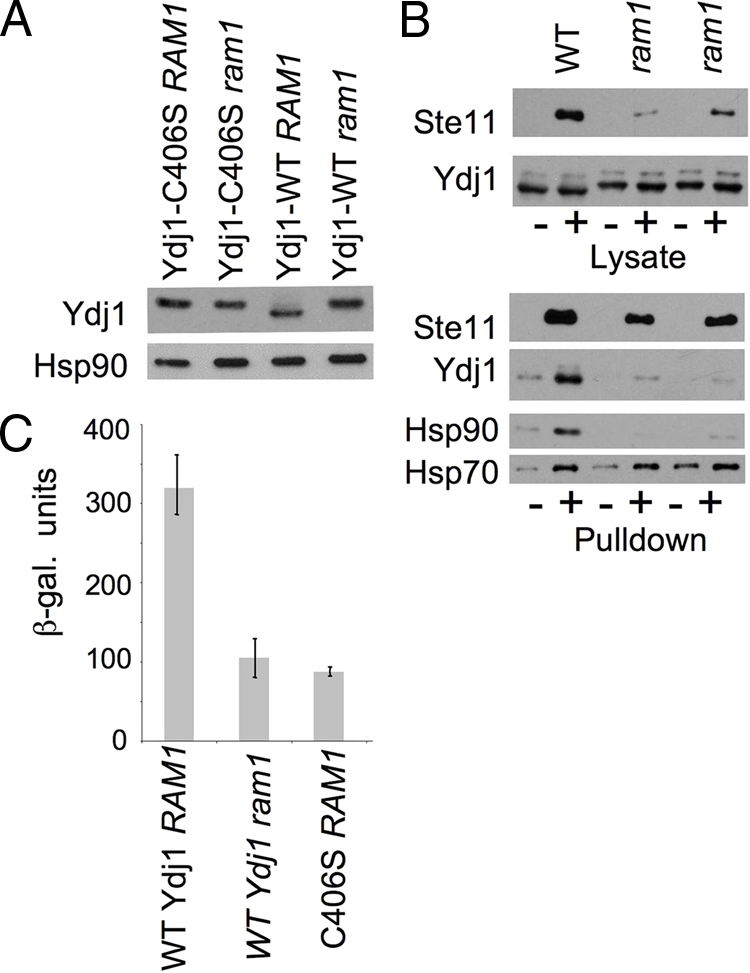
Effect of RAM1 deletion on Ste11ΔN activity and interaction with Ydj1. (A) Mobility of WT Ydj1 or Ydj1-C406S in isogenic strains JJ160 (ydj1 RAM1) and strain JJ264 (ydj1 ram1). (B) As described for Figure 2: (+) His-Ste11ΔN-K444R complexes were isolated out WT strain PJ43-2B (Yan and Craig, 1999) and two isogenic independent ram1 strains (JJ217 and JJ218) grown overnight at 25°C; (−) control, lysates from cells lacking His-tagged protein were isolated in parallel. Cell lysates were immunoblotted with antibodies specific for Xpress or Ydj1. Nickel-bound proteins were eluted into sample buffer and analyzed by SDS-PAGE following by immunoblot analysis using indicated antibodies. (C) Strain JJ160 (ydj1 RAM1) expressing WT YDJ1 or ydj1-C406S and strain JJ264 (ydj1 ram1) expressing WT YDJ1 were transformed with plasmids expressing GAL1-Ste11ΔN and the PRE-lacZ reporter plasmid. β-Galactosidase activity was assessed after a 6-h induction with galactose at 25°C. Error bars, activity of triplicate samples.
We determined how RAM1 deletion affected the interaction of WT Ydj1 with His-Ste11ΔN-K444R. The level of Ydj1 was not significantly affected by RAM1 deletion, but, as expected, WT Ydj1 migrated more slowly in the ram1 strains than in the WT strain (Figure 5B, top). The level of accumulated His-Ste11ΔN-K444R was reduced in the ram1 lysates compared with the isogenic WT strain (10 and 36% WT levels) and reduced amounts (58 and 65% WT levels) of His-Ste11ΔN-K444R bound nickel resin (Figure 5B, bottom). As observed with cells expressing ydj1-C406S, little or no Ydj1 or Hsp90 bound His-Ste11ΔN-K444R in the absence of RAM1, but the level of Hsp70 bound to His-Ste11ΔN-K444R was not dramatically affected. Next we compared Ste11 activity in a ydj1 strain expressing WT YDJ1 or ydj1-C406S with an isogenic ram ydj1 strain expressing WT YDJ1. As shown in Figure 5C, yeast strains lacking RAM1 or expressing ydj1-C406S exhibited <30% WT Ste11ΔN activity.
Effect of YDJ1 Mutation on the Physical and Functional Interaction with the Glucocorticoid Receptor.
Ydj1 and Hsp90 interact with diverse clients that have different sequences, structures and cellular localizations (Pratt and Toft, 2003; Wegele et al., 2004). To determine if Ydj1 farnesylation is required for interaction with additional Hsp90 clients, we examined the interaction of Ydj1 with the GR. Although yeast do not express endogenous steroid receptors, expression of rat GR in yeast results in hormone- and Hsp90-dependent activation of a GR-dependent reporter plasmid (Picard et al., 1990). In addition, isolation of Flag-GR out of yeast lysates resulted in the copurification of Hsp90, Ydj1, and Hsp70 (Kimura et al., 1995). Cell lysates from strains expressing the indicated Ydj1 mutant with (+) or without (−) Flag-GR were harvested and incubated with anti-Flag antibody resin. GR and associated proteins were eluted from the resin with Flag peptide and then analyzed by SDS-PAGE and immunoblot analysis. Similar levels of Flag-GR were recovered after elution with Flag peptide, except in the case of Ydj1-N134 and Ydj1-N206, which exhibited slightly lower levels of Flag-GR (Figure 6). Although WT Ydj1 coeluted with Flag-GR, none of the truncated forms of Ydj1 nor Ydj1-C406S stably bound Flag-GR. Thus the farnesylation signal of Ydj1 appears critical for in vivo interaction with both Ste11 and GR. Similar to the results with His-Ste11ΔN-K444R, the interaction of Flag-GR with Hsp70 was not dramatically affected by Ydj1 mutation. Although Flag-GR bound near WT levels of Hsp90 in the presence of Ydj1-N206 or N274, reduced Flag-GR-Hsp90 interaction was observed in the presence of Ydj1-N134, N363, and C406S.
Figure 6.

Effect of Ydj1 truncation on Flag-GR interaction. Strain JJ160 expressing indicated ydj1 mutant was transformed with a plasmid constitutively expressing Flag-GR or a control plasmid. Cells expressing Flag-GR were disrupted with glass beads in the presence of protease inhibitors and centrifuged to remove cellular debris. Equal amounts of cell lysates were separated by SDS-PAGE (15% acrylamide to detect Ydj1 truncations, 10% acrylamide in other instances) followed by immunoblot analysis. Top panel, immunoblot of yeast lysate using antibody specific for Ydj1. Bottom panels, Extracts were prepared from cells that lacked a Flag-tagged protein (−) or from those expressing Flag-GR (+). Cell extracts were incubated with anti-Flag resin for 1.5 h at 4°C. After washing resin with lysis buffer, Flag-GR complexes were eluted from resin with Flag peptide, precipitated with TCA, separated by SDS-PAGE, and subjected to immunoblot analysis using antibodies specific for GR, Ydj1, Hsp90, or Hsp70.
The impact of amino acid alterations within domains I and II on Flag-GR interaction is shown in Figure 7. Ydj1- L137HF249S and Ydj1-C159T exhibited near-WT binding to Ydj1, Hsp70, and Hsp90. Ydj1-C143SC162S did not affect Flag-GR interaction with Hsp70 and Hsp90, but exhibited increased interaction with Flag-GR. Both Ydj1-C143S and Ydj1-C162S were previously shown to exhibit enhanced interaction with the androgen receptor (Fan et al., 2005).
Figure 7.
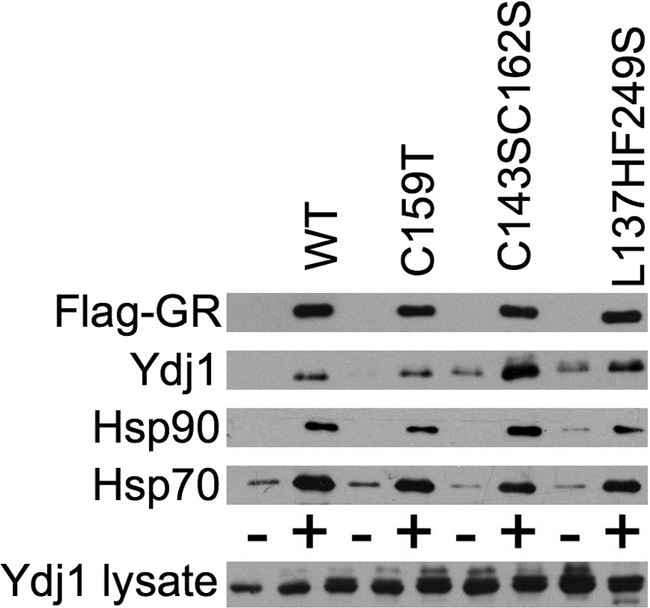
Effect of Ydj1 point mutations on Flag-GR interaction. Strain JJ160 expressing indicated ydj1 mutant was transformed with a plasmid constitutively expressing Flag-GR (+) or a control plasmid (−). Flag-GR complexes were isolated and analyzed as described for Figure 6.
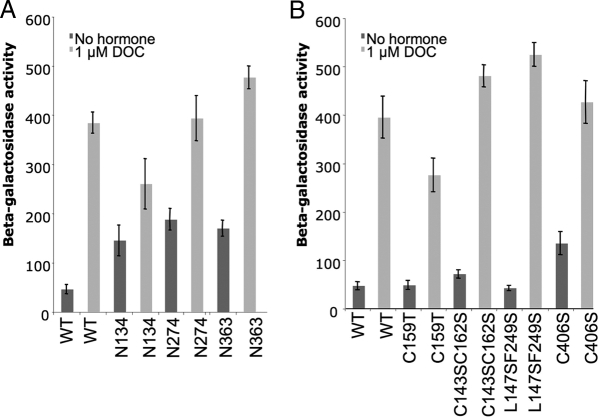
Mutations in Hsp90 and cochaperones result in reduced hormone-dependent activation of the GR and other steroid receptors (Pratt and Toft, 2003). In contrast, multiple mutations in YDJ1 resulted in increased constitutive activity of the GR in the absence of hormone (Kimura et al., 1995; Johnson and Craig, 2000). An example of the effect of ydj1 mutation on the ability of GR to activate a GR-driven lacZ reporter gene is shown in Figure 8. Yeast cells expressing WT or mutant Ydj1 along with the GR and a corresponding GRE-lacZ reporter construct were grown to midlog phase at 30°C. After a 1-h incubation with 1 μM DOC or ethanol (as a control), cells were harvested and β-galactosidase activity was assessed. The basal activity of cells expressing Ydj1 mutants that delete or alter the farnesylation signal (N134, N274, N363, and C406S) exhibit the highest level of activity in the absence of hormone. In addition, two of the mutants, Ydj1-N134 and Ydj1-C159T, exhibited defects in activation of GR in the presence of hormone. This result supports the conclusion that Ydj1 farnesylation is required for Ydj1 to suppress GR activity in the absence of hormone but is not required for hormone-dependent activation of the GR.
Figure 8.
Effect of ydj1 mutation on in vivo activity of the GR. (A) Strain JJ160 expressing indicated ydj1 truncation mutant was transformed with plasmids expressing GR and corresponding GRE-lacZ reporter construct. Cells were grown overnight in selective media to midlog phase. GR was activated by the addition of 1 μM DOC or ethanol (as a control). β-Galactosidase activity was assessed after 1 h. (B) As in A, except with Ydj1 point mutants. Error bars, activity of triplicate samples. Each experiment contained triplicate samples and was conducted at least two times. A representative experiment is shown.
Effect of RAM1 Deletion on Ydj1 Interaction with Flag-GR and GR Activity.
Figure 9 shows the effect of RAM1 deletion on the interaction of WT Ydj1 with Flag-GR and GR activity. Deletion of RAM1 did not significantly reduce the level of Flag-GR isolated out of cell lysates. However, the level of Ydj1 and Hsp90 bound to Flag-GR was dramatically reduced in cell lysates lacking RAM1, whereas the level of Hsp70 interaction with Flag-GR was only slightly reduced (Figure 9A). In addition, deletion of RAM1 or the ydj1-C406S mutation resulted in similar increases in constitutive activity of the GR, but did not disrupt the activation of GR in the presence of hormone (Figure 9B), supporting a role for Ydj1 farnesylation in suppression of GR activity in the absence of hormone.
Figure 9.
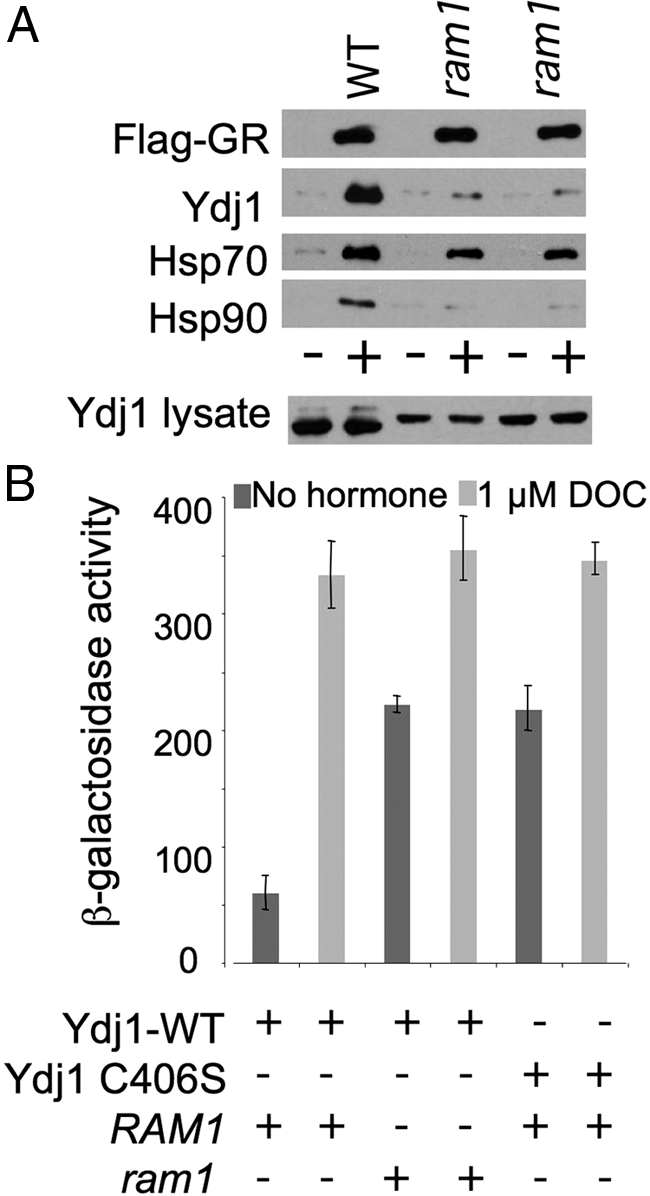
Effect of ram1 mutation on Ydj1-GR interaction and GR activity. (A) Isogenic WT (PJ43-2B) and ram1 strains (JJ217 and JJ218) were transformed with Flag-GR or a control plasmid. Flag-GR complexes were isolated and analyzed as described for Figure 6 except that cells were grown at 25°C. (B) Strain JJ160 (ydj1 RAM1) expressing WT YDJ1 or ydj1-C406S and strain JJ264 (ydj1 ram1) expressing WT were transformed with plasmids expressing GR and corresponding GRE-lacZ reporter construct. β-Galactosidase activity was assessed as in Figure 8, except that cells were grown at 25°C.
DISCUSSION
A role for Hsp40 in maturation of Hsp90 clients was first identified in yeast (Kimura et al., 1995; Dey et al., 1996). Subsequent studies established that direct interaction of Ydj1 or the human homolog DjA1/Hdj2 with full-length progesterone receptor was required for receptor interaction with both Hsp70 and Hsp90 (Hernandez et al., 2002; Cintron and Toft, 2006). This study is the first to compare the impact of YDJ1 mutation on the in vivo interaction with two established Hsp90 client proteins. Our results indicate that even though the Ste11 protein kinase and the GR have very different sequences and structures, they have similar requirements for Ydj1 interaction.
Ydj1 Farnesylation Is Critical for the Physical and Functional Interaction with Client Proteins
Ydj1 farnesylation was required for interaction of Ydj1 with both Ste11 and GR, although the modification had differing effects on Ste11 and GR accumulation and activity. Loss of Ydj1 farnesylation resulted in reduced interaction of Ste11 with Ydj1 and Hsp90, as well as reduced Ste11 accumulation and activity. We have not yet established whether Ydj1 farnesylation directly impacts Ste11 kinase activity independent of its effects on Ste11 accumulation. Hsp90 and associated chaperones have dual roles in steroid receptor function: repressing DNA binding and transcriptional activity in the absence of hormone and promoting hormone binding (Pratt and Toft, 2003). Ydj1 farnesylation was required for GR interaction with Ydj1 and Hsp90 and repression of GR activity in the absence of hormone but not activation of transcription in the presence of hormone or GR accumulation. This result is consistent with a prior study that demonstrated that cells expressing ydj1-C406S do not have defects in the ability of the androgen receptor to bind hormone (Fliss et al., 1999). It is not known whether alteration of the human homolog of Ydj1, DjA1/Hdj2, results in constitutive GR activity in mammalian cells. However, overexpression of DjA1/Hdj2 rescues subnuclear targeting, transactivation and transrepression defects caused by a GR mutation (Tang et al., 1997), suggesting a role for DjA1/Hdj2 in regulating GR-DNA interactions in mammalian cells.
In a current model of Hsp40 interaction with client proteins (Pratt and Toft, 2003; Mayer and Bukau, 2005), Hsp40 interaction precedes both Hsp70 and Hsp90. However, none of the ydj1 mutations used in our study dramatically disrupted Hsp70-client interaction. We do not know whether this difference reflects client-specific requirements for Hsp90 complex assembly or differences that arise between whole cell lysate and purified systems. Our results are consistent with the model that Ydj1 interaction precedes and is required for Hsp90 interaction in vivo, except that Hsp90 binding to both GR and Ste11 was observed in cells expressing ydj1-N274, which did not stably bound Ydj1. Further studies will be required to determine whether Ydj1-N274 transiently interacts with clients in vivo or whether another protein facilitates or stabilizes Hsp90-client interaction in cells expressing ydj1-N274.
How Does Ydj1 Farnesylation Impact Client Interaction?
A prior study demonstrated that Ydj1-C406S could not be farnesylated in vitro and that cells expressing ydj1-C406S were unable to grow at 37°C (Caplan et al., 1992b). Additional studies established that ydj1-C406S caused defects in translocation of preproteins across membranes and regulation of Hsp90 client activity (Caplan et al., 1992a; Johnson and Craig, 2000). The majority of Ydj1 (>80%) is cytosolic, and Ydj1-C406S displayed increased cytosolic localization compared with WT Ydj1 (Caplan et al., 1992b). Because virtually all Ydj1 in the cell appears to be farnesylated, yet only a portion is membrane associated, it is possible that the membrane-associated forms of Ydj1 contain another unidentified modification that promotes stable membrane binding. Alternatively, farnesylated Ydj1 may cycle between cytosolic and membrane environments as it performs its chaperoning duties. To further examine the importance of Ydj1 farnesylation, we constructed pairs of Ydj1 mutants (Ydj1-N137, Ydj1-N209, and Ydj1-N310) that contained either a functional (CASQ) or nonfunctional farnesylation signal (SASQ). When expressed in a ydj1::HIS3 strain, each of these pairs exhibited similar growth defects at 37°C, as well as similar defects in GR, v-src, and Ste11ΔN activity (not shown and Johnson and Craig, 2000). This result indicates that the farnesylation signal of Ydj1 must be in the proper context to function properly.
Our results suggest that Ydj1 farnesylation plays an important role in facilitating transfer of client proteins from Hsp70 to Hsp90, but we are unsure whether Ydj1 localization plays a role in this process. An alternate hypothesis is that addition of the hydrophobic farnesyl group enhances or creates a client-binding site within the SBD or creates a binding surface that facilitates interaction with a cochaperone involved in transfer of clients from Hsp70 to Hsp90. Within other proteins, the farnesylation modification has been shown to have specific roles in protein–protein interactions, particularly those that facilitate protein trafficking and subcellular localization (Sinensky, 2000). Because the ydj1-C406S and ram1 strains exhibit similar behavior with regard to Ste11 and GR interactions and activity, we conclude that loss of Ydj1 farnesylation is the primary cause of ram1-mediated defects. However, other proteins within the cell are also farnesylated, and we cannot rule out pleiotropic effects caused by ram1 deletion.
Studies that established the molecular chaperone functions of Ydj1 used bacterially expressed Ydj1 (Lu and Cyr, 1998b; Fan et al., 2005; Li and Sha, 2005), and thus used the nonfarnesylated form. Because a fragment of Ydj1 containing only the J + G/F regions was sufficient to stimulate the ATPase activity of Hsp70, we do not expect that Ydj1 farnesylation will affect that function. Additional studies will be required to determine whether WT Ydj1 and Ydj1-C406S purified out of bacterial and yeast lysates exhibit similar activities in established in vitro assays of chaperone function, including steroid receptor complex assembly and refolding of denatured luciferase (Lu and Cyr, 1998b; Hernandez et al., 2002; Cintron and Toft, 2006).
Role of Domains I, II, and III in Ydj1–Client Interaction
Within the cocrystal structure of the SBD of Ydj1 (amino acids 102-350) and the peptide substrate GWLYEIS (Li et al., 2003), the peptide bound a hydrophobic pocket in domain I consisting of residues I116, L135, L137, L216, and F249. Purified Ydj1 containing alterations in these residues exhibited defects in binding denatured polypeptides and cooperating with Ssa1 to refold denatured luciferase (Li and Sha, 2005). In our assays, the Ydj1-L137HF249S alteration caused only slight defects in growth and client activity, and did not disrupt the interaction of Ste11 and GR with Ydj1, Hsp70, or Hsp90. Thus it appears unlikely that the peptide-binding site in domain I binds Hsp90 client proteins. However, it is possible that domain III, which has a structure similar to that of domain I, may have overlapping roles with domain I in client interaction. In support of that conclusion, Ydj1-G315D, which contains an alteration in a conserved residue of domain III, caused a severe temperature-sensitive growth defect at 37°C, reduced interaction with Flag-GR and denatured luciferase and other defects in molecular chaperone activity (Kimura et al., 1995; Lu and Cyr, 1998b).
Previous studies have variously implicated the zinc-binding domain of DnaJ or Ydj1 in substrate binding (Szabo et al., 1996) or transfer of bound substrates to Hsp70 (Banecki et al., 1996; Linke et al., 2003; Fan et al., 2005). Purified Ydj1-C159T, C143S, or C162S was able to stimulate the ATPase activity of the Hsp70 Ssa1 and bind to unfolded proteins (Lu and Cyr, 1998b; Fan et al., 2005), but each of the mutant proteins was defective in cooperating with Ssa1 to refold denatured luciferase (Lu and Cyr, 1998b; Fan et al., 2005). We observed strong defects in Ste11ΔN activity in the presence of either Ydj1-C159T or Ydj1-C143SC162S, but only Ydj1-C143SC162S exhibited reduced Ste11ΔN interaction. In contrast, Ydj1-C143SC162S exhibited enhanced interaction with Flag-GR, and Ydj1-C159T caused a slight decrease in GR activity in the presence of hormone. Thus, our results concur that the zinc-binding domain is important for Ydj1 function, but suggest that it may have client-specific functions. In particular, since Ydj1-N206, which contains an intact zinc-binding domain (domain II) but not an intact domain I, promoted accumulation of WT levels of Ste11ΔN-K444R protein, whereas little or no Ste11ΔN-K444R accumulated in cells expressing Ydj1-N134, it seems possible that the zinc-binding domain of Ydj1 has a critical role in promoting the accumulation and activity of Ste11 protein. A recent study identified a panel of kinases that accumulate in a Ydj1-dependent manner (Mandal et al., 2008), and it will be interesting to determine whether accumulation and activity of those kinases is dependent on the zinc-binding domain of Ydj1.
Specificity of Hsp40 Functions
Two Hsp40s of S. cerevisiae, Ydj1 and Sis1, function with Hsp70s of the Ssa family, share significant structural homolog within their SBDs, and have some overlapping functions (Lu and Cyr, 1998a; Johnson and Craig, 2001; Li et al., 2003). However, Ydj1 has specific functions in maturation of Hsp90 clients (Johnson and Craig, 2000; Mandal et al., 2008). Unique sequences within the G/F and G/M regions enable Sis1, but not Ydj1, to function in maintenance of the yeast prion, [Rnq+] (Yan and Craig, 1999; Lopez et al., 2003). Similarly, our studies suggest that the farnesylation signal and zinc-binding domains of Ydj1, which are not present in Sis1, enable Ydj1 to function in the maturation of Hsp90 clients. Thus, differences in domain structure are a major factor contributing to the specificity of Hsp40 functions.
ACKNOWLEDGMENTS
We thank Elizabeth Craig, Susan Lindquist, Avrom Caplan, Didier Picard (University of Geneva), and Kevin Morano for reagents. We also thank Avrom Caplan (City College of New York) for helpful advice regarding the Ste11 kinase. This publication was made possible by Grant P20 RR15587 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and a grant from the National Science Foundation (NSF, MCB-0744522). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR, NIH, or NSF. This project was also funded in part by the U.S. Department of Agriculture HATCH/CREES Grant IDA01266 and additional funds from the University of Idaho Research Council.
Abbreviations used:
- Hsp
heat-shock protein
- GR
glucocorticoid receptor
- SBD
substrate-binding domain.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0435) on October 1, 2008.
REFERENCES
- Abbas-Terki T., Donze O., Picard D. The molecular chaperone Cdc37 is required for Ste11 function and pheromone-induced cell cycle arrest. FEBS Lett. 2000;467:111–116. doi: 10.1016/s0014-5793(00)01134-0. [DOI] [PubMed] [Google Scholar]
- Atencio D. P., Yaffe M. P. MAS5, a yeast homolog of DnaJ involved in mitochondrial protein import. Mol. Cell Biol. 1992;12:283–291. doi: 10.1128/mcb.12.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banecki B., Liberek K., Wall D., Wawrzynow A., Georgopoulos C., Bertoli E., Tanfani F., Zylicz M. Structure-function analysis of the zinc finger region of the DnaJ molecular chaperone. J. Biol. Chem. 1996;271:14840–14848. doi: 10.1074/jbc.271.25.14840. [DOI] [PubMed] [Google Scholar]
- Becker J., Walter W., Yan W., Craig E. A. Functional interaction of cytosolic hsp70 and a DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol. Cell. Biol. 1996;16:4378–4386. doi: 10.1128/mcb.16.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. J., Cyr D. M., Douglas M. G. YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell. 1992a;71:1143–1155. doi: 10.1016/s0092-8674(05)80063-7. [DOI] [PubMed] [Google Scholar]
- Caplan A. J., Douglas M. G. Characterization of YDJ1, a yeast homologue of the bacterial dnaJ protein. J. Cell Biol. 1991;114:609–621. doi: 10.1083/jcb.114.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. J., Mandal A. K., Theodoraki M. A. Molecular chaperones and protein kinase quality control. Trends Cell Biol. 2007;17:87–92. doi: 10.1016/j.tcb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Caplan A. J., Tsai J., Casey P. J., Douglas M. G. Farnesylation of YDJ1p is required for function at elevated growth temperatures in Saccharomyces cerevisiae. J. Biol. Chem. 1992b;267:18890–18895. [PubMed] [Google Scholar]
- Cintron N. S., Toft D. Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J. Biol. Chem. 2006;281:26235–26244. doi: 10.1074/jbc.M605417200. [DOI] [PubMed] [Google Scholar]
- Dey B., Caplan A. J., Boschelli F. The Ydj1 molecular chaperone facilitates formation of active p60v-src in yeast. Mol. Biol. Cell. 1996;7:91–100. doi: 10.1091/mbc.7.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. Y., Ren H. Y., Lee P., Caplan A. J., Cyr D. M. The type I Hsp40 zinc finger-like region is required for Hsp70 to capture non-native polypeptides from Ydj1. J. Biol. Chem. 2005;280:695–702. doi: 10.1074/jbc.M410645200. [DOI] [PubMed] [Google Scholar]
- Fliss A. E., Rao J., Melville M. W., Cheetham M. E., Caplan A. J. Domain requirements of DnaJ-like (Hsp40) molecular chaperones in the activation of a steroid hormone receptor. J. Biol. Chem. 1999;274:34045–34052. doi: 10.1074/jbc.274.48.34045. [DOI] [PubMed] [Google Scholar]
- Flom G., Weekes J., Williams J. J., Johnson J. L. Effect of mutation of the tetratricopeptide repeat and aspartate-proline 2 domains of Sti1 on Hsp90 signaling and interaction in Saccharomyces cerevisiae. Genetics. 2006;172:41–51. doi: 10.1534/genetics.105.045815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- Hernandez M. P., Chadli A., Toft D. O. HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J. Biol. Chem. 2002;277:11873–11881. doi: 10.1074/jbc.M111445200. [DOI] [PubMed] [Google Scholar]
- Hon T., Lee H. C., Hach A., Johnson J. L., Craig E. A., Erdjument-Bromage H., Tempst P., Zhang L. The Hsp70-Ydj1 molecular chaperone represses the activity of the heme activator protein Hap1 in the absence of heme. Mol. Cell. Biol. 2001;21:7923–7932. doi: 10.1128/MCB.21.23.7923-7932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Craig E. A. A role for the Hsp40 Ydj1 in repression of basal steroid receptor activity in yeast. Mol. Cell. Biol. 2000;20:3027–3036. doi: 10.1128/mcb.20.9.3027-3036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Craig E. A. An essential role for the substrate-binding region of Hsp40s in Saccharomyces cerevisiae. J. Cell Biol. 2001;152:851–856. doi: 10.1083/jcb.152.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Yahara I., Lindquist S. Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science. 1995;268:1362–1365. doi: 10.1126/science.7761857. [DOI] [PubMed] [Google Scholar]
- Lee P., Shabbir A., Cardozo C., Caplan A. J. Sti1 and Cdc37 can stabilize Hsp90 in chaperone complexes with a protein kinase. Mol. Biol. Cell. 2004;15:1785–1792. doi: 10.1091/mbc.E03-07-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Qian X., Sha B. The crystal structure of the yeast Hsp40 Ydj1 complexed with its peptide substrate. Structure. 2003;11((12)):1475–1483. doi: 10.1016/j.str.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Li J., Sha B. Structure-based mutagenesis studies of the peptide substrate binding fragment of type I heat-shock protein 40. Biochem. J. 2005;386:453–460. doi: 10.1042/BJ20041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke K., Wolfram T., Bussemer J., Jakob U. The roles of the two zinc binding sites in DnaJ. J. Biol. Chem. 2003;278:44457–44466. doi: 10.1074/jbc.M307491200. [DOI] [PubMed] [Google Scholar]
- Lopez N., Aron R., Craig E. A. Specificity of class II Hsp40 Sis1 in maintenance of yeast prion [RNQ+] Mol. Biol. Cell. 2003;14((3)):1172–1181. doi: 10.1091/mbc.E02-09-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvion J. F., Abbas-Terki T., Picard D. Hsp90 is required for pheromone signaling in yeast. Mol. Biol. Cell. 1998;9:3071–3083. doi: 10.1091/mbc.9.11.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Cyr D. M. Protein folding activity of Hsp70 is modified differentially by the hsp40 co-chaperones Sis1 and Ydj1. J. Biol. Chem. 1998a;273:27824–27830. doi: 10.1074/jbc.273.43.27824. [DOI] [PubMed] [Google Scholar]
- Lu Z., Cyr D. M. The conserved carboxyl terminus and zinc finger-like domain of the co-chaperone Ydj1 assist Hsp70 in protein folding. J. Biol. Chem. 1998b;273:5970–5978. doi: 10.1074/jbc.273.10.5970. [DOI] [PubMed] [Google Scholar]
- Mandal A. K., Nillegoda N., Chen J. A., Caplan A. J. Ydj1 protects nascent protein kinases from degradation and controls the rate of their maturation. Mol. Cell. Biol. 2008;28:4434–4444. doi: 10.1128/MCB.00543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. P., Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol. Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano K. A., Thiele D. J. The Sch9 protein kinase regulates Hsp90 chaperone complex signal transduction activity in vivo. EMBO J. 1999;18:5953–5962. doi: 10.1093/emboj/18.21.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D., Muller R., Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M., Fortin M., Lindquist S., Yamamoto K. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Toft D. O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Printen J. A., Sprague G. F., Jr. Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics. 1994;138:609–619. doi: 10.1093/genetics/138.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Functional aspects of polyisoprenoid protein substituents: roles in protein-protein interaction and trafficking. Biochim. Biophys. Acta. 2000;1529:203–209. doi: 10.1016/s1388-1981(00)00149-9. [DOI] [PubMed] [Google Scholar]
- Szabo A., Korszun R., Hartl F. U., Flanagan J. A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. EMBO J. 1996;15:408–417. [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Ramakrishnan C., Thomas J., DeFranco D. B. A role for HDJ-2/HSDJ in correcting subnuclear trafficking, transactivation, and transrepression defects of a glucocorticoid receptor zinc finger mutant. Mol. Biol. Cell. 1997;8:795–809. doi: 10.1091/mbc.8.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegele H., Muller L., Buchner J. Hsp70 and Hsp90–a relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- Wegele H., Wandinger S. K., Schmid A. B., Reinstein J., Buchner J. Substrate transfer from the chaperone Hsp70 to Hsp90. J. Mol. Biol. 2006;356:802–811. doi: 10.1016/j.jmb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Wright L. P., Philips M. R. Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J. Lipid Res. 2006;47:883–891. doi: 10.1194/jlr.R600004-JLR200. [DOI] [PubMed] [Google Scholar]
- Wu Y., Li J., Jin Z., Fu Z., Sha B. The crystal structure of the C-terminal fragment of yeast Hsp40 Ydj1 reveals novel dimerization motif for Hsp40. J. Mol. Biol. 2005;346:1005–1011. doi: 10.1016/j.jmb.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Yan W., Craig E. A. The glycine-phenylalanine-rich region determines the specificity of the yeast Hsp40 Sis1. Mol. Cell Biol. 1999;19:7751–7758. doi: 10.1128/mcb.19.11.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]