Abstract
Saccharomyces cerevisiae Cbk1 is a LATS/Ndr protein kinase and a downstream component of the regulation of Ace2 and morphogenesis (RAM) signaling network. Cbk1 and the RAM network are required for cellular morphogenesis, cell separation, and maintenance of cell integrity. Here, we examine the phenotypes of conditional cbk1 mutants to determine the essential function of Cbk1. Cbk1 inhibition severely disrupts growth and protein secretion, and triggers the Swe1-dependent morphogenesis checkpoint. Cbk1 inhibition also delays the polarity establishment of the exocytosis regulators Rab-GTPase Sec4 and its exchange factor Sec2, but it does not interfere with actin polarity establishment. Cbk1 binds to and phosphorylates Sec2, suggesting that it regulates Sec4-dependent exocytosis. Intriguingly, Cbk1 inhibition causes a >30% decrease in post-Golgi vesicle accumulation in late secretion mutants, indicating that Cbk1 also functions upstream of Sec2-Sec4, perhaps at the level of the Golgi. In agreement, conditional cbk1 mutants mislocalize the cis-Golgi mannosyltransferase Och1, are hypersensitive to the aminoglycoside hygromycin B, and exhibit diminished invertase and Sim1 glycosylation. Significantly, the conditional lethality and hygromycin B sensitivity of cbk1 mutants are suppressed by moderate overexpression of several Golgi mannosyltransferases. These data suggest that an important function for Cbk1 and the RAM signaling network is to regulate growth and secretion via Golgi and Sec2/Sec4-dependent processes.
INTRODUCTION
Cell polarity is a common cellular feature among eukaryotic cells that is characterized by asymmetry in cell shape, protein distribution, and cellular function (Nelson, 2003; Pruyne et al., 2004). The establishment and maintenance of polarized growth are critical for the development and function of specialized cells, tissues, and organs. Polarized growth is particularly important for the development and function of neuronal cells, which can reach several meters in length, and for epithelial cells, which regulate homeostasis and maintain physical barriers between biological compartments (Arimura and Kaibuchi, 2005; Dow and Humbert, 2007; Tang, 2008). Dynamic changes in cell polarity are essential for cell migration during cell and tissue development. Polarized growth and morphogenesis are also important for yeast and filamentous fungi development (Harris, 2006).
Saccharomyces cerevisiae is an excellent model organism for studying conserved mechanisms of polarized growth and cell division. Saccharomyces cerevisiae cells undergo polarized growth during cell division, mating, and pseudohyphae formation (Pruyne et al., 2004). Many of the evolutionarily conserved elements of cell growth and proliferation were first identified and characterized in yeast (Pruyne et al., 2004). Yeast polarity establishment involves the recruitment and activation of Cdc42 GTPase to a cortical landmark. Cdc42 regulates several polarity processes, including septin assembly and actin cytoskeleton polarization (Park and Bi, 2007). Polarized actin cables are necessary for delivering organelles and secretory vesicles to the cortical sites of polarized growth (for review, see Pruyne et al., 2004; Park and Bi, 2007). Defects in yeast polarity establishment, vesicle trafficking, or exocytosis can yield a variety of phenotypes ranging from minor morphological aberrations to severe growth inhibition and cellular lysis.
Intracellular trafficking pathways are important for polarized growth (Cole and Fowler, 2006). The endoplasmic reticulum (ER) and the Golgi apparatus are essential for the synthesis, posttranslational modification, and trafficking of secretory proteins and vesicles (Brennwald and Rossi, 2007). Plasma membrane and cell wall proteins are synthesized in the ER and transported to the Golgi apparatus, in which they are commonly modified by glycosylation (Jigami, 2008). Cargo-bearing vesicles from the Golgi are delivered to endosomes, vacuoles, or the plasma membrane. Vesicle sorting, trafficking, and fusion are mediated by numerous GTPases and vesicle-tethering complexes (Whyte and Munro, 2002; Lundquist, 2006; Park and Bi, 2007). Similarly, Golgi structure and function are maintained by retrograde and intra-Golgi trafficking pathways, which are also controlled by Rab family GTPases and membrane docking complexes (Oka and Krieger, 2005). Retrograde trafficking mechanisms are essential for retrieval of ER and Golgi enzymes and for maintenance of organelle function. Membrane docking complexes cooperate with soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins to mediate membrane fusion (Whyte and Munro, 2001, 2002; Bruinsma et al., 2004; Hsu et al., 2004; Oka and Krieger, 2005; Zolov and Lupashin, 2005). ER and Golgi trafficking defects often compromise protein glycosylation, cell wall biogenesis, and cell integrity.
In yeast, many post-Golgi secretory vesicles are delivered to the sites of polarized growth along actin cables in a myosin V-dependent manner (Pruyne et al., 2004; Lowe and Barr, 2007). Both vesicle delivery and docking to the plasma membrane are regulated by the Rab GTPase Sec4 and its GEF Sec2 (Nair et al., 1990; Guo et al., 1999). Sec4 and Sec2 associate with post-Golgi secretory vesicles and are required for myosin V-dependent transport along actin cables to the sites of polarized growth. In addition Sec4 stimulates the assembly of the exocyst, an eight-subunit vesicle tethering complex on the plasma membrane (Salminen and Novick, 1987; Guo et al., 1999; Hsu et al., 2004), which mediates membrane fusion and exocytosis via an interaction with SNARE proteins (Hsu et al., 2004). Sec4 function is dependent on the guanyl nucleotide exchange factor (GEF) Sec2, which converts inactive Sec4-GDP to active Sec4-GTP (Walch-Solimena et al., 1997; Elkind et al., 2000). Sec2 is regulated by phosphorylation in vivo; however, the kinase(s) responsible for Sec2 phosphorylation has not been identified (Elkind et al., 2000). Two-hybrid data suggest Sec2 might be regulated by Cbk1 kinase, a component of the conserved S. cerevisiae RAM signaling network (Racki et al., 2000).
Cbk1 kinase and the RAM signaling network are important for polarized growth, differential gene expression, and maintenance of cell integrity (Dorland et al., 2000; Racki et al., 2000; Bidlingmaier et al., 2001; Colman-Lerner et al., 2001; Weiss et al., 2002; Nelson et al., 2003; Kurischko et al., 2005). RAM is made up of two kinases, Cbk1 and Kic1, and several associated proteins, Mob2, Hym1, Tao3, and Sog2 (Nelson et al., 2003). Cbk1 is a member of the Lats/NDR/Dbf2 kinase family, which includes the mammalian and Drosophila Lats/warts tumor suppressor, Schizosaccharomyces pombe Orb6, and Caenorhabditis elegans Sax1 (Xu et al., 1995; Verde et al., 1998; Geng et al., 2000; Racki et al., 2000; Zallen et al., 2000; Hergovich et al., 2006). The other RAM components are also highly conserved among eukaryotes (Hou et al., 2003; Emoto et al., 2004; He et al., 2005; Hergovich et al., 2006). Deletion of S. cerevisiae CBK1 or other RAM genes causes cell morphology defects, cellular lysis, and death (Sullivan et al., 1998; Du and Novick, 2002; Kurischko et al., 2005). Dosage suppressors of cbk1Δ and other RAM mutants include genes expressing cell wall components, suggesting that Cbk1 and RAM maintain cell integrity by controlling cell wall biosynthesis (Du and Novick, 2002; Vink et al., 2002; Kurischko et al., 2005). The lethality of RAM gene deletions, designated ramΔ, is also suppressed by mutations in the enigmatic SSD1 gene, which encodes an RNA binding protein of unknown function (Sutton et al., 1991; Du and Novick, 2002; Jorgensen et al., 2002). In the absence of SSD1, ramΔ cells are spherical in morphology, fail to form robust mating projections and fail to degrade the septum after cytokinesis (Nelson et al., 2003). The latter phenotype is due to misregulation of Ace2 transcription factor (Racki et al., 2000; Colman-Lerner et al., 2001; Weiss et al., 2002).
Cbk1 and RAM proteins localize to sites of polarized growth (similarly to Sec2 and Sec4 proteins), which include the bud cortex during bud emergence and growth, the tips of mating projections and the bud neck region during mitotic exit (Bidlingmaier et al., 2001; Colman-Lerner et al., 2001; Du and Novick, 2002; Weiss et al., 2002; Nelson et al., 2003). In addition, a fraction of Cbk1 and Mob2, which associate as a complex, colocalizes with Ace2 transcription factor in the daughter cell nucleus during mitotic exit (Colman-Lerner et al., 2001; Weiss et al., 2002). Ace2 activity and localization are dependent on Cbk1 kinase activity; however, Ace2 is not essential for viability, cell morphogenesis, or maintenance of cell integrity (Dohrmann et al., 1992; O'Conallain et al., 1998; Bidlingmaier et al., 2001; Weiss et al., 2002; Kurischko et al., 2005). These data indicate that Cbk1 and the RAM network maintain cell integrity and control polarized growth independently of Ace2.
The mechanism for Cbk1 and the RAM network in regulating polarized growth and maintenance of cell integrity is not known. To gain insight into the essential function of the RAM network, we investigated the consequence of Cbk1 inhibition in vivo. Here, we present evidence that Cbk1 and the RAM signaling network maintain cell integrity and control polarized growth via regulating Golgi function and exocytosis. For Cbk1 and other RAM proteins among eukaryotes, these findings suggest universal mechanisms for RAM in regulating cell integrity and cell polarity.
MATERIALS AND METHODS
Yeast Strains and Plasmid Construction
The yeast strains and relevant genotypes used in this study are listed in Table 1. All strains from our laboratory and from the deletion consortium (available from Open Biosystems, Huntsville, AL) are isogenic S288c derivatives. S. cerevisiae strains were maintained and grown using standard conditions (Guthrie and Fink, 1991). The cbk1-as allele (Weiss et al., 2002) was subcloned into the integrative vector pRS403. The plasmid was linearized in the CBK1 promoter and integrated upstream of cbk1Δ in the heterozygous diploid strain FLY1509. The diploid was sporulated and dissected to obtain FLY2084 (MATa cbk1-as::HIS3::cbk1Δ::KANMX). Strain FLY2531 was constructed by transforming FLY2084 with pRC556 (SEC4-GFP) (Schott et al., 2002). The temperature sensitive allele cbk1-8 was generated by random polymerase chain reaction (PCR) mutagenesis of the DNA encoding the kinase domain (amino acids 351-672), as described previously (Luca and Winey, 1998), by using the following oligonucleotide primers: forward, agaagatttccacactgtga and reverse, ttgtctgattgtattccaat. DNA sequence analysis reveals that cbk1-8 has point mutations that cause two amino acid substitutions in the open reading frame (E430V T550A). cbk1-8 was subcloned into pRS403 and integrated into FLY1509, as described above, to yield FLY2661.
Table 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| BY4741 | MATa ura3Δ0 his3Δ0 leu2Δ0 met15Δ0 | Deletion consortium |
| FLY869 | MATa CBK1-GFP::KANMX ssd1-d | Weiss et al. (2002) |
| FLY905 | MATa CBK1-myc::KANMX ssd1-d | This study |
| FLY906 | MATa CBK1-myc::KANMX mob2Δ::HIS3 ssd1-d | Weiss et al. (2002) |
| FLY1050 | MATa SIM1-CFP::KANMX ssd1-d | This study |
| FLY1509 | MATa/α cbk1Δ::KANMX/CBK1 | Deletion consortium |
| FLY1632 | MATα ace2Δ::KANMX | Deletion consortium |
| FLY2084 | MATa cbk1-as2::HIS3::cbk1Δ::KANMX | This study |
| FLY2086 | MATα cbk1-as2::HIS3::cbk1Δ::KANMX | This study |
| FLY2184 | MATα ssd1Δ::NATMX | This study |
| FLY2204 | MATa cbk1-as2::HIS3::cbk1Δ::KANMX ssd1-d | This study |
| FLY2236 | MATa cbk1-as2::HIS3::cbk1Δ::KANMX swe1Δ::KANMX | This study |
| FLY2288 | MATa CBK1-myc::KANMX SSD1-HA::HIS3 | This study |
| FLY2293 | MATa cbk1Δ::KANMX ssd1Δ::NATMX | This study |
| FLY2339 | MATa ABP1-GFP::KANMX cbk1-as2::HIS3-cbk1Δ::KANMX | This study |
| FLY2373 | MATa/α SEC2-GFP::KANMX/SEC2-GFP::KANMX cbk1-as2::HIS3::cbk1Δ::KANMX/cbk1-as2::HIS3::cbk1Δ::KANMX | This study |
| FLY2386 | MATa/α ABP140-GFP::KANMX/ABP140-GFP::KANMX cbk1-as2::HIS3-cbk1Δ::KANMX/cbk1-as2::HIS3-cbk1Δ::KANMX | This study |
| FLY2389 | MATa sec2-41 swe1Δ::KANMX | This study |
| FLY2452 | MATα SEC2-myc::KANMX CBK1-HA::HIS3 | This study |
| FLY2485 | MATa sec2-41 CBK1-GFP::KANMX | This study |
| FLY2489 | MATa MYO2-GFP::KANMX cbk1-as2::HIS3::cbk1Δ::KANMX | This study |
| FLY2531 | MATa cbk1-as2::HIS3-cbk1Δ::KANMX [pSEC4-GFP] | This studya |
| FLY2535 | MATa CBK1-GFP::KANMX MYO2::HIS3 | This study |
| FLY2539 | MATa CBK1-GFP::KANMX myo2-66::HIS3 | This study |
| FLY2574 | MATα bgl2Δ::KANMX | Deletion consortium |
| FLY2575 | MATα vps1Δ::KANMX | Deletion consortium |
| FLY2661 | MATa cbk1-8::HIS3-cbk1Δ::KANMX | This study |
| FLY2704 | MATa/α cbk1-8::HIS3::cbk1Δ::KANMX/cbk1-8::HIS3::cbk1Δ::KANMX SEC2-GFP::KANMX/SEC2-GFP::KANMX | This study |
| FLY2764 | MATα cog1Δ::KANMX | Deletion consortium |
| FLY2765 | MATα cog8Δ::KANMX | Deletion consortium |
| FLY2766 | MATα ypt6Δ::KANMX | Deletion consortium |
| FLY2567 | MATa SUC2-Myc:KANMX | This study |
| FLY2789 | MATa cbk1-as::HIS3::cbk1Δ::KANMX sec6–4 SSD1 | This study |
| FLY2792 | MATa cbk1-as::HIS3::cbk1Δ::KANMX sec2–41 SSD1 | This study |
| FLY2802 | MATα SUC2-Myc:KANMX cog1Δ::KANMX | This study |
| FLY2847 | MATa/α SIM1-CFP::KANMX/SIM1-CFP::KANMX cbk1-8::HIS3-cbk1Δ::KANMX/cbk1-8::HIS3-cbk1Δ::KANMX | This study |
| FLY2848 | MATa/α SUC2-myc::KANMX/SUC2-myc::KANMX cbk1-8::HIS3::cbk1Δ::KANMX/cbk1-8::HIS3::cbk1Δ::KANMX | This study |
| FLY2866 | MATα dse4Δ::KANMX | Deletion consortium |
| FLY2867 | MATa DSE4-TAP::HIS6MX | Open Biosystems |
| FLY2865 | MATα SIM-CFP::KANMX cog1Δ::KANMX | This study |
| FLY2885 | MATa CTS1-TAP::HIS6MX | Open Biosystems |
| FLY2886 | MATα cts1Δ::KANMX | Deletion consortium |
| FLY2922 | MATa cbk1-8::HIS3::cbk1Δ::KANMX OCH1-GFP::URA3 | This study |
| GY2479 | MATa exo84-121 | Zhang et al. (2005) |
| JLY284 | MATα OCH1-GFP::URA3 | Chris Burd (UPenn) |
| NY416 | MATα sec16-2 | Wei Guo (UPenn) |
| NY770 | MATα sec2-41 | Nair et al. (1990) |
a Plasmid from Wei Guo, UPenn.
The plasmid pMAL-SEC2 (encoding full-length MBP-Sec2) was provided by Ruth Collins (Cornell University). Derivative plasmids encoding maltose binding protein (MBP MalE)-tagged Sec2451-759, Sec21-508, Sec21-204, and Sec2205-450 were constructed by polymerase chain reaction (PCR) amplifying and subcloning the corresponding SEC2 fragments with combinations of the following forward and reverse oligonucleotides: FLO350 (forward), ggggaattcgatgcttctgaggaagcaaa; FLO354 (forward), aaactgcagttgctgttcctgggcatcat; and FLO383 (forward), ggggaattcggaattgtgtactcgccaag; and FLO351 (reverse), aaactgcagtgaagaattgataccaagtc; FLO352 (reverse), aaactgcagtccaatttttgaagaaattg; and FLO353 (reverse), ggggaattcacaaaaaataagccaaagat.
Dosage Suppressor Screen
cbk1-8 cells were transformed with an ordered array of high copy plasmids containing overlapping fragments of yeast genomic DNA (Open Biosystems) (Jones et al., 2008) and screened for growth at 34°C. Some relevant dosage suppressors are listed in Supplemental Table S1 and were confirmed by PCR and by retransformation. The remaining suppressors from this screen will be reported in a separate manuscript.
Cbk1-as Inhibition In Vivo
Cells were synchronized in G1 by treatment with α factor or in G0, as described previously (Weiss and Winey, 1996; Ayscough et al., 1997). Cells were released from G1 or G0 block at a cell density of 5 × 106 cells/ml, and 5 μM kinase inhibitor 4-Amino-1-tert-butyl-3-(1′-naphthyl)pyrazolo[3,4-d]pyrimidine (1NA-PP1) (Bishop et al., 2000) was added at 0 or 30 min after release. At least 200 cells were counted per time point to determine the percentage of budded cells.
Electrophoresis and Immunoblot Methods
Standard SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting were conducted as described previously (Laemmli, 1970; Towbin et al., 1979). Radiolabeled gels were analyzed with a STORM PhosphorImager (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Immunoblots were probed with various primary antibodies followed by alkaline phosphatase-conjugated anti-mouse or anti-rabbit IgG secondary antibodies (Promega, Madison, WI). Immunoblots were processed for enhanced chemifluorescence (GE Healthcare) and scanned on a STORM PhosphorImager (GE Healthcare). Mouse monoclonal anti-Myc was obtained from Covance Research Products (Princeton, NJ), monoclonal anti-green fluorescent protein (GFP) was obtained from Roche Diagnostics, Indianapolis, IN); and rabbit polyclonal anti-alcohol dehydrogenase (ADH) was obtained from Abcam (Cambridge, MA).
Secretion Assays
Bgl2 secretion assays were conducted as described previously (Adamo et al., 1999; He et al., 2007). Briefly, cells were grown to mid-log phase and shifted to 22 or 37°C for 2.5 h. Cells were collected and treated with 0.2 mg/ml Zymolyase (USB, Cleveland, OH) supplemented with 2.5 U/ml chitinase (Sigma-Aldrich, St. Louis, MO) for 40–60 min to yield 100% spheroplasts, as determined by microscopy. The internal (cellular) and external (solubilized cell wall) fractions were separated by centrifugation at 2000 rpm for 5 min, and the pellet (internal fractions) and supernatant (external fractions) were subjected to SDS-PAGE and immunoblot analysis. Immunoblots were probed with anti-Bgl2 antibody (He et al., 2007), processed for ECF (GE Healthcare), and analyzed on a STORM PhosphorImager (GE Healthcare).
Invertase secretion assays were conducted as described previously (Adamo et al., 1999; He et al., 2007). Cells were grown to mid-log phase in YPD medium (2% glucose), and shifted to experimental conditions (22° or 37°C) for 2 h in low glucose medium (YPD containing 0.1% glucose). Samples were collected at t = 0 and after 2 h in low glucose medium. Invertase activity was determined using a colorimetric assay to measure glucose released from sucrose (Adamo et al., 1999; He et al., 2007). The ratio of secreted invertase/total invertase activity was calculated using the following formula: (external invertase activity at T = 2 h − external invertase activity at T = 0)/(external invertase activity at 2 h − external invertase activity at T = 0) + (internal invertase activity at 2 h − internal invertase activity at T = 0) (He et al., 2007).
The degree of invertase glycosylation was determined in strains expressing Myc-tagged invertase (Suc2-Myc) as described previously (Click et al., 2002). Briefly, invertase expression was induced by transferring to low glucose medium (0.1% glucose) for 2.5 h. Total protein was extracted by vortexing cells in protein sample buffer containing glass beads and loaded onto 7.5% polyacrylamide gels, immunoblotted, and probed with anti-Myc antibody (9E10; Covance Research Products).
Media secretion assays were performed as described previously (Gaynor and Emr, 1997). A logarithmic culture of asynchronous growing cells was incubated in methionine- and cysteine-deficient medium containing 10 μCi of [35S]methionine/cysteine (Trans label; MP Biomedicals, Irvine, CA) per 1 OD600 of cells for 30 min at 37°C. Cells were chased for 45 min with medium containing 125 mM methionine and 25 mM cysteine at 37°C, and protein synthesis/secretion was halted by adding NaF and NaN3 to a final concentration of 20 mM. Cells were pelleted and conditioned medium was collected. The secreted proteins were precipitated with 10% trichloroacetic acid (TCA). Proteins were visualized by SDS-PAGE analysis followed by autoradiography.
In vivo pulse-labeling experiments of carboxypeptidase Y (CPY) were done as described previously (Franzusoff and Schekman, 1989). Logarithmically growing cells were collected, washed once, and resuspended in 3 ml of methionine and cysteine deficient medium at a concentration of ∼5 × 107 cells/ml. The cells were preincubated for 30 min at 37°C and then labeled with 300 μCi of [35S]Met (MP Biomedicals) for 10 min at 37°C. The cells were chased for 5 and 15 min in chase solution (5% yeast extract, 125 mM Met, and 25 mM Cys) and the cells were precipitated with TCA. CPY was immunoprecipitated with a rabbit polyclonal CPY antibody (Abcam) and analyzed by 10% SDS-PAGE followed by autoradiography.
Light Microscopy and Cytology
Fluorescent and differential interference contrast microscopy was carried out as described previously (Luca et al., 2001). Fluorescence-activated cell sorting (FACS) analysis was conducted as described in Weiss and Winey (1996). Cells were treated with Alexa 594-conjugated concanavalin A (Invitrogen, Carlsbad, CA) and N-[3-triethylammoniumpropyl]-4-[p-diethylaminophenylhexatrienyl] pyridinium dibromide (FM4-64; Invitrogen) as described previously (Lew and Reed, 1993; Vida and Emr, 1995). Where designated, cells were treated with latrunculin A (Invitrogen) to disrupt the actin cytoskeleton, as described previously (Ayscough et al., 1997). FM4-64 uptake experiments were conducted as described previously (Vida and Emr, 1995; Wang et al., 1996). Vacuoles were also visualized by incubating cells with 100 μM CellTracker Blue 7-amino-4-chloromethylcoumarin (CMAC) (Invitrogen). Cells expressing Och1-GFP were methanol/acetone fixed before imaging as described previously (Guo et al., 2001).
Electron Microscopy and Quantification
Conventional transmission electron microscopy experiments were carried out as described previously (Rieder et al., 1996). For quantification of secretory vesicles, 25 images from each experimental parameter were acquired on an FEI Tecnai 12 TWIN electron microscope equipped with a SIS Megaview III wide-angle camera yielding a total n of 50 cells/parameter. The numbers of vesicles in the bud and mother were tabulated using the “touch-counting” function in iTEM version 5.0 software (Olympus Soft Imaging Solutions, Münster, Germany). Subsequently, the numbers of vesicles in the bud and mother from each sample type were entered into Excel (Microsoft, Redmond, WA). The averages and standard deviations were calculated using the function feature, and the resulting data, including averages and SD, were transferred to Graph Pad Prism 4 (GraphPad Software, San Diego, CA). The bar-graphs were created with the calculated SEM.
In Vitro Kinase Assay and Affinity Precipitation Experiments
Cbk1 kinase assays were performed as in (Weiss et al., 2002). MBP and MBP-Sec2 substrates were expressed in Escherichia coli from pMAL2 (New England Biolabs, Ipswich, MA) and pMAL2-Sec2 vectors (gifts from Dr. Ruth Collins, Cornell University) and purified according to the manufacturer's protocol (New England Biolabs). The affinity precipitation experiments with MBP and MBP-Sec2 immobilized on amylose-agarose were carried out as described previously (Rahl et al., 2005), with slight modifications. Approximately 0.2 mg of yeast cell extract containing Cbk1-Myc was incubated for 30 min with 50 μl of immobilized amylose resin containing ∼2 mg/ml MBP or MBP-Sec2. The amylose-agarose pellet was washed three times in lysis buffer containing 1% NP-40 and protease inhibitors, three times in Tris-buffered saline containing 500 mM NaCl and 1 mM EDTA, and resuspended in protein sample buffer for immunoblot analysis. Immunoprecipitation of epitope-tagged yeast proteins and immunoblots were conducted as described previously (Weiss et al., 2002; Kurischko et al., 2005) with monoclonal anti-Myc (9E10; Covance Research Products) or anti-HA antibodies (12CA5; Covance Research Products).
RESULTS
Cbk1 Is Required for Bud Emergence
To determine the essential function of Cbk1 kinase and the RAM signaling network, we investigated the phenotypes of conditional cbk1 mutants, cbk1-as and cbk1-8. The analogue-sensitive allele cbk1-as encodes a kinase (Cbk1-as) that is specifically inhibited by the drug 1NA-PP1 (Weiss et al., 2002), whereas cbk1-8 is a recessive conditional loss-of-function allele that causes lethality at restrictive temperature (34 or 37°C). In the absence of 1NA-PP1, cbk1-as cells are indistinguishable from wild-type cells. However, in the presence of 1NA-PP1, cbk1-as cells display severe growth and morphology defects (Figure 1). When Cbk1-as was inhibited in G0 or G1 synchronized cbk1-as cells, bud emergence was severely delayed in comparison with mock-treated cbk1-as cells or 1NA-PP1–treated wild-type cells (Figure 1A and Supplemental Figure S1). Conditional cbk1-8 cells displayed similar bud delays when shifted to restrictive temperature (data not shown). Typically, it took >4 h for 50% of 1NA-PP1-treated cbk1-as cells to form buds after release from G0 or G1. In contrast, 50% of 1NA-PP1–treated wild-type cells or mock-treated cbk1-as cells initiated buds within 60–90 min after G0 or G1 release. The phenotypes of Cbk1-as inhibition were similar regardless of whether cells were synchronized by nutrient deprivation (G0) or by treatment with mating pheromone, which arrests cells in G1 at Start (Figure 1A and Supplemental Figure S1).
Figure 1.
Cbk1 kinase inhibition interferes with bud emergence and induces a Swe1-dependent cell cycle delay. (A) Cbk1 inhibition delays bud emergence. cbk1-as (FLY2084) and wild-type cells (BY4741) were released from G1 arrest and placed into fresh medium with 0 μM (DMSO), 5 μM, or 10 μM 1NA-PP1. The percentage of budded cells was plotted over time (n > 200 cells). (B) Cbk1 inhibition induces a Swe1-dependent G2 arrest. cbk1-as and cbk1-as swe1Δ (FLY2236) cells were released from G0 into medium ± 5 uM 1NA-PP1, and at designated time points samples were processed for FACS to reveal the relative DNA content in the cell population. Four hours after release from G0 block, 0 and 11% of mock and drug treated cbk1-as swe1Δ cells contained two nuclei. T0 is time of 1NA-PP1 addition. Note the modest delay in S phase initiation. See Supplemental Figure S3 for data from a similar experiment. (C) Cbk1 inhibition induces a modest Ssd1-independent delay in bud emergence. cbk1-as ssd1-d cells (FLY2204) were synchronized in G1 and released into medium ± 5 μM 1NA-PP1. The percentage of buds was scored over time (n > 200). Similar phenotypes were observed with cbk1Δ ssd1Δ cells (data not shown).
On continuous exposure to 1NA-PP1, many cbk1-as cells eventually developed buds; however, they commonly exhibited morphology and lysis defects (Supplemental Figure S2). Surviving cells developed abnormally wide bud necks (mother-daughter cell junctions), were spherical in morphology, and displayed cell separation defects. The number of colony-forming units of cbk1-as cultures remained stable for up to 8 h in 1NA-PP1 (Supplement Figure S2), suggesting that the overall viability of the population remained constant. In contrast, cbk1-8 cells displayed a precipitous loss of viability when maintained at restrictive temperature for greater than 2 h (Supplemental Figure S2). Similarly, expression of catalytically inactive Cbk1 (Cbk1-KD) failed to rescue the lethality of cbk1Δ cells (data not shown). These data indicate that Cbk1 kinase activity is necessary for bud emergence and maintenance of cell integrity, and they suggest that some in vivo Cbk1-as kinase activity persists in the presence of 1NA-PP1.
Cbk1 Inhibition Triggers the G2 Morphogenesis Checkpoint
Delayed bud emergence could be caused by a defect in cell cycle progression. Thus, we monitored DNA content in 1NA-PP1–treated cbk1-as cells to determine whether Cbk1 inhibition interferes with S phase entry. Intriguingly, Cbk1 inhibition during G1 led to a G2 cell cycle arrest, as determined by FACS and microscopic analyses (Figure 1B). 1NA-PP1–treated cbk1-as cells arrested uniformly as unbudded cells with 2N DNA content and single nuclei. We observed similar results with cbk1-8 cells (data not shown), although a partial cell separation defect at permissive temperature (22°C) hampered flow cytometric analysis (Supplemental Figure S2). These data indicate that Cbk1 kinase activity is critical for bud emergence and polarized growth but that it is not essential for cell cycle entry or for G1 and S phase progression.
The G2 arrest in 1NA-PP1–treated cbk1-as cells could be the consequence of a cell cycle checkpoint or could reflect a role for Cbk1 in M phase entry. Polarized growth defects often activate the morphogenesis checkpoint, which induces G2 arrest by inhibiting mitotic Cdk1 activation (McMillan et al., 1998; Lew, 2003). To test whether Cbk1 inhibition leads to morphogenesis checkpoint activation, we assayed DNA content, chromosome segregation, and nuclear number in cbk1-as swe1Δ double mutant cells, which lack Swe1 kinase, the central component of the morphogenesis checkpoint. In the absence of 1NA-PP1, cbk1-as swe1Δ cells were indistinguishable from wild-type, swe1Δ or cbk1-as single mutant cells (data not shown). Significantly, Cbk1 inhibition failed to induce a G2 arrest in cbk1-as swe1Δ cells, but instead caused a severe loss of viability, preceded by a modest increase in binucleate cells (Figure 1B; unpublished data). These data indicate that diminished Cbk1 activity leads to morphogenesis checkpoint activation and that the morphogenesis checkpoint is essential for maintaining cell viability when RAM signaling is compromised.
Cbk1 Inhibition Causes a Modest Delay in S Phase Entry
A previous study suggested that at least one RAM component is required for G1 progression (Bogomolnaya et al., 2004). In agreement, FACS analysis of synchronized cbk1-as cells consistently revealed a modest delay in S phase initiation upon Cbk1 inhibition (Figure 1B, compare the histograms of cbk1-as cells at 1 h; and Supplemental Figure S3). The modest G1 delay was not diminished in swe1Δ cells, indicating that it was not caused by the morphogenesis checkpoint (Figure 1C). These data suggest that Cbk1 and RAM have a nonessential role in G1 progression. Nevertheless, the apparent G1 delay upon Cbk1 inhibition is not long enough to account for the >2 h delay in bud emergence.
It is well established that the lethality of cbk1Δ and other ramΔ mutations is suppressed by truncation or deletion of the poorly understood SSD1 gene (Du and Novick, 2002; Jorgensen et al., 2002; Kurischko et al., 2005). We investigated whether SSD1 mutations (ssd1Δ or ssd1-d) rescue the bud and cell cycle delays of conditional cbk1 mutants. We found that Cbk1 inhibition in ssd1-d cells caused a very brief (10–15′) delay in bud emergence and S phase initiation, but it did not trigger a G2 arrest or cause a loss of viability (Figure 1C; unpublished data). The apparent G1 delay in cbk1-as ssd1-d cells was Swe1 independent and did not occur in mock-treated cbk1-as ssd1-d cells or 1NA-PP1–treated wild-type cells (Figure 1, B and C; unpublished data). Thus, in addition to playing a major role in bud emergence (Figure 1A), Cbk1 and RAM have a nonessential role in G1 progression or S phase initiation that is independent of Ssd1 or the Swe1 morphogenesis checkpoint.
Cbk1 Is Required for Bud Growth
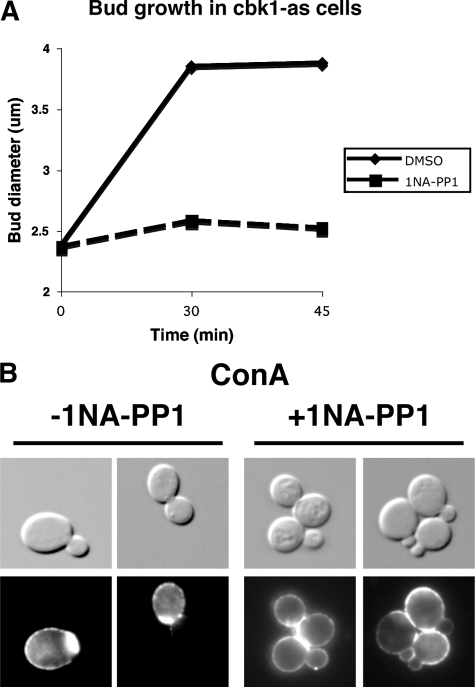
To address whether Cbk1 kinase activity is required for bud growth after bud emergence, we measured bud size in 1NA-PP1–treated cbk1-as cells at various times after release from G0 or G1 arrest (see Materials and Methods). When Cbk1-as was inhibited in late G1/early S phase (after ∼50% of the cells formed small buds), bud size remained constant compared with mock-treated cbk1-as cells (Figure 2A). Similarly, the percentage of unbudded and small budded cells remained relatively constant for over an hour (Supplemental Figure S4). The size of mother cells also remained constant upon Cbk1 inhibition (data not shown). Thus, Cbk1 kinase activity is required for both apical (bud emergence) and isotropic growth (bud and mother cell growth).
Figure 2.
Cbk1 kinase activity is required for bud growth. (A) Bud length was measured in synchronized cells upon Cbk1 inhibition. G1 synchronized cbk1-as cells (FLY2084) were incubated until ∼50% of the cells formed small buds. At that time (T = 0), 0 μm (DMSO) or 10 μM 1NA-PP1 was added, and samples were taken and fixed in 3.7% formaldehyde at designated times. (B) Cbk1 kinase inhibition prevents cell wall deposition. Asynchronously growing cbk1-as cells were treated with ConA-Alexa594 and transferred into ConA-free medium ± 5 um 1NA-PP1 for 60 min.
Cbk1 Is Required for Secretion
The significant delay in bud emergence and growth in conditional cbk1 mutants could reflect a role for the RAM network in cell wall remodeling and/or plasma membrane expansion. To test whether Cbk1 kinase inhibition interferes with cell wall deposition, we pulse-labeled cbk1-as cells with fluorescent concanavalin A (Alexa594-ConA), a lectin that binds cell wall glycoproteins (Figure 2B). After ConA washout, new cell wall deposition during polarized growth causes the development of a ConA-free zone on the cell surface (Lew and Reed, 1993; Pruyne et al., 2004). Most mock-treated cbk1-as cells displayed unlabeled buds within 30 min of ConA washout, as observed in wild-type cells. In contrast, nearly all of the buds in 1NA-PP1–treated cbk1-as cells displayed prominent fluorescent ConA-labeling, suggesting that Cbk1 kinase activity is essential for the polarized secretion of cell wall proteins.
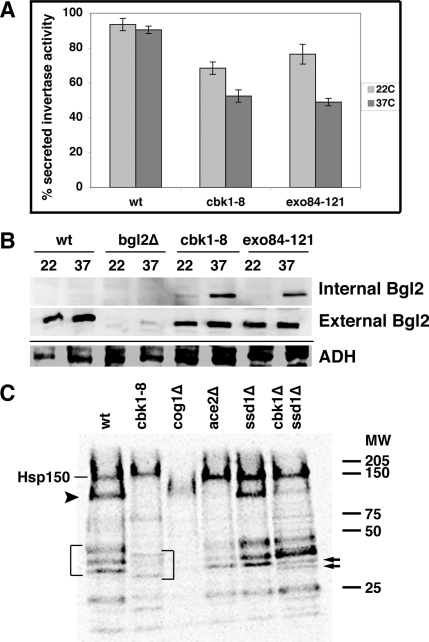
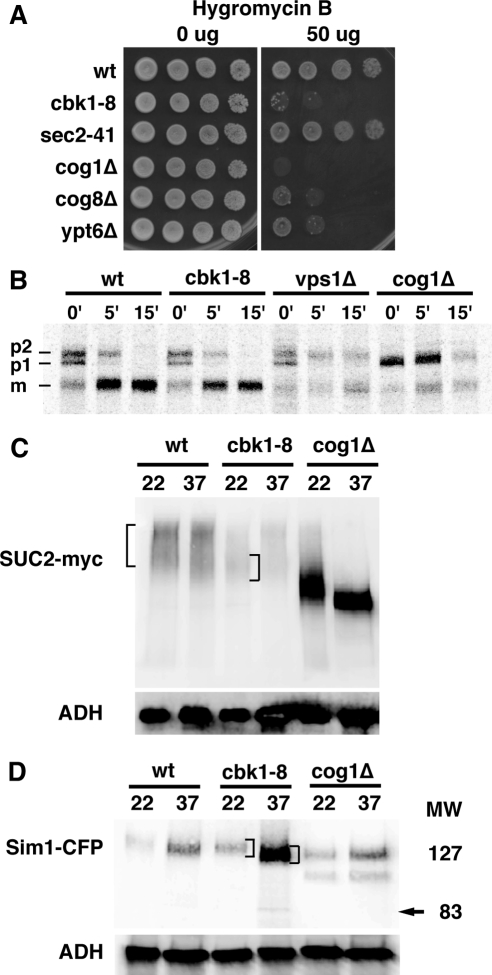
We also tested whether Cbk1 is required for the periplasmic secretion of well-characterized markers for the two major classes of post-Golgi vesicles, invertase and Bgl2 endo-β-1,3-glucanase (Harsay and Bretscher, 1995). We first measured the ratio of secreted invertase activity to total invertase activity in cbk1-8, wild-type cells and a conditional exocytosis mutant (exo84-121) at 22 and 37°C. The amount of secreted invertase activity was diminished by ∼50% in cbk1-8 cells at 37°C in comparison to similarly treated wild type cells (Figure 3A). Secreted invertase activity of cbk1-8 cells was similar to that of the exocyst mutant exo84-121. Interestingly, both cbk1-8 and exo84-121 mutants were moderately impaired for invertase secretion at 22°C. These data are consistent with a role for Cbk1 in secretion.
Figure 3.
Cbk1 is required for secretion of invertase, Bgl2 endo-β-1,3-glucanase, and other proteins. (A) Invertase secretion is diminished in conditional cbk1-8 cells. The ratio of secreted invertase/total invertase is plotted for wild-type cells (BY4741), cbk1-8 cells (FLY2661), and the conditional exocyst mutant exo84-121 (GY2479). See Materials and Methods for details. (B) The cell wall protein Bgl2 accumulates inside cbk1-8 and exo84-121 cells at 37°C. Top, immunoblot of Bgl2 in the internal fraction (cytoplasmic) of wild type, cbk1-8, and exo84-121 cells shifted to restrictive temperature. Middle, immunoblot of Bgl2 from the external (solubilized cell wall) fraction of cells. Bottom, immunoblot of the internal fractions was stripped and reprobed with anti-ADH (Abcam) as a protein loading. bgl2Δ cells (FLY2574) were used as a negative control. (C) Autoradiogram of media secretion assays for wild-type cells (BY4741), cbk1-8 cells (FLY2704), cog1Δ cells (FLY2764), ace2Δ cells (FLY1632), ssd1Δ cells (FLY2184), and cbk1Δ ssd1Δ cells (FLY2293). Proteins secreted into the media of [35S]methionine-labeled cells were TCA precipitated, separated on protein gels, and processed for autoradiography. The brackets denote several proteins that seem to be hypoglycosylated in cbk1-8 cells. Hsp150 is noted and the arrowhead below Hsp150 points to a protein whose expression and/or secretion is dependent on Cbk1 and Ace2. The two arrows on the right point to a protein that seems to split into two bands in cbk1Δ ssd1Δ double mutant cells.
We monitored Bgl2 secretion in conditional cbk1 mutants by analyzing the relative amounts of Bgl2 in internal (cytoplasmic) and external (periplasmic space/cell wall) fractions of yeast cells. Bgl2 is rapidly secreted in wild type cells, so it is almost exclusively present in the external fraction at 22 and 37°C (Figure 3B; Adamo et al., 1999; He et al., 2007). When cbk1-8 and exo84-121 cells were grown at 22°C, most Bgl2 was present in the external fraction of cells (the removed cell wall fraction). In contrast, when cbk1-8 and exo84-121 cells were shifted to 37°C, a significant amount of Bgl2 remained in the internal fraction of cells. Immunoblots of total cell extracts indicate that total Bgl2 level remain the same in all experimental conditions (data not shown). These data indicate that Cbk1 is required for efficient secretion of Bgl2 and invertase.
In wild-type yeast, most secreted proteins remain in the periplasmic space and contribute to cell wall biogenesis; however, pulse-labeling experiments revealed that some glycosylated proteins are secreted beyond the cell wall and into the medium (Adamo et al., 1999). To determine whether Cbk1 is required for protein secretion into the medium, we conducted media secretion assays with [35S]methionine-labeled cells. Wild-type cells secreted a reproducible pattern of 35S-labeled proteins into the medium (Figure 3C) (Gaynor and Emr, 1997; Schmitz et al., 2008). One of the most prominent secreted proteins in the medium was identified previously as Hsp150 (Gaynor and Emr, 1997). We observed that the overall level of most 35S-labeled proteins in the medium of cbk1-8 cells was diminished at 37°C, with the exception of Hsp150 (Figure 3C). In addition, there were intriguing differences in the electrophoretic patterns of secreted proteins in cbk1-8 cells that are discussed below. Collectively, these data suggest that Cbk1 is necessary for the efficient secretion of multiple, but not all, cargos.
Cbk1 Inhibition Does Not Disrupt the Establishment of Actin Cytoskeleton Polarity
Polarized secretion and bud emergence are mediated by the actin cytoskeleton, which is organized in cables and cortical actin patches (Pruyne et al., 2004). Actin cables polarize at the incipient bud site and serve as tracks for the myosin-dependent transport of secretory vesicles to the plasma membrane, whereas cortical actin patches polarize to the incipient bud site and mediate endocytosis. Establishment and maintenance of actin cable and patch polarity are dependent on the rho-like GTPase Cdc42 and septins, the latter of which assemble into an hourglass-shaped ring at the mother-daughter cell junction and serve as scaffolds for the actin cytoskeleton and other proteins (Longtine and Bi, 2003; Pruyne et al., 2004; Versele and Thorner, 2005).
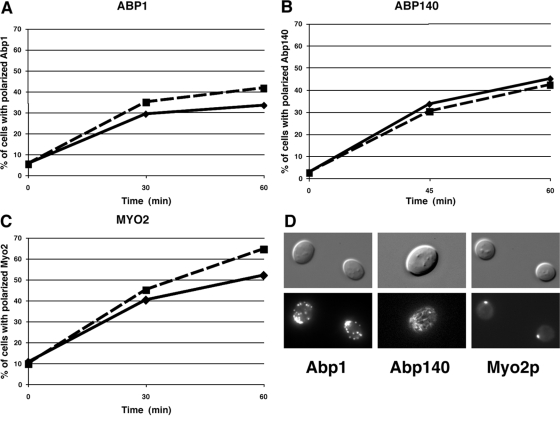
To determine whether Cbk1 regulates the establishment or maintenance of actin cytoskeleton polarity, we monitored actin cable and patch associated proteins Abp140 and Abp1 in synchronized cbk1-as cells (Figure 4), as described in Huckaba et al. (2004). Notably, Cbk1-as inhibition did not prevent the establishment of actin cable associated Abp140-GFP or actin patch protein Abp1-GFP polarity to the incipient bud site (Figure 4, A, B, and D). Polarization of both proteins occurred with similar kinetics in 1NA-PP1 and mock-treated cbk1-as cells. Cbk1 inhibition also did not interfere with Cdc42 GTPase or septin (Cdc3) localization (data not shown). These data indicate that the severe delay in bud emergence in drug-treated cbk1-as cells is not caused by gross disruption of the actin cytoskeleton organization.
Figure 4.
Cbk1 kinase inhibition does not interfere with actin cytoskeleton polarization. G0-synchronized cbk1-as cells expressing the actin patch-associated Abp1-GFP (FLY2339) (A), the actin cable-associated Abp140-GFP (FLY2386) (B), or type V myosin Myo2-GFP (FLY2489) (C) were released into medium ± 5 μm 1NA-PP1. The percentage of budded cells with polarized GFP was scored over time (—, 0 μm 1NA-PP1; - - -, 5 μM 1NA-PP1). 1NA-PP1 was added 30 min after G0 release. T0 is time of 1NA-PP1 addition. (D) Representative images of 1NA-PP1–treated cells from A-C at T = 60 min.
Delayed bud emergence and growth might reflect a problem with the delivery of secretory vesicles and other cargo to the incipient bud site. Myosin V, encoded by MYO2, is the principle motor for actin-dependent organelle and post-Golgi vesicle transport (Pruyne et al., 2004). In wild-type cells, myosin V accumulates at the incipient bud site before bud emergence (Lillie and Brown, 1994). We monitored Myo2-GFP in synchronized cbk1-as cells to determine whether Cbk1 is necessary for myosin V function or localization. We observed no detectable delay in the polarized localization of myosin V to the incipient bud site upon Cbk1 inhibition (Figure 4, C and D). Thus, the delayed bud emergence in cbk1 mutants is not caused by gross defects in actin polarity or myosin V activity.
Cbk1 Regulates Sec2 and Sec4 Localization
Polarized growth and secretion are dependent on the Rab GTPase Sec4 and its guanyl nucleotide exchange factor Sec2 (Walworth et al., 1989; Nair et al., 1990). GTP-bound Sec4 stimulates the polarized delivery of post-Golgi secretory vesicles to the plasma membrane and associates with Sec15, a component of the exocyst vesicle-tethering complex (Salminen and Novick, 1989; Walch-Solimena et al., 1997; Guo et al., 1999). GTP-bound Sec4 also contributes to the assembly of the exocyst complex, which tethers secretory vesicles to the plasma membrane and facilitates membrane fusion via interactions with SNARE proteins (Guo et al., 1999; Hsu et al., 2004). In wild-type cells, Sec2 and Sec4 associate with vesicles and concentrate at the bud cortex via myosin V-dependent transport (Walch-Solimena et al., 1997). In the absence of Sec2 or Sec4-GTP activity, many post-Golgi secretory vesicles accumulate in the cytoplasm because they are not delivered to the sites of polarized growth and are not tethered to the plasma membrane by the exocyst complex (Walworth et al., 1989; Walch-Solimena et al., 1997; Elkind et al., 2000).
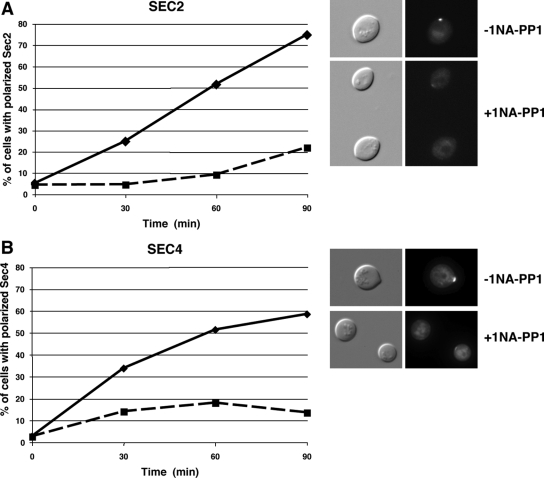
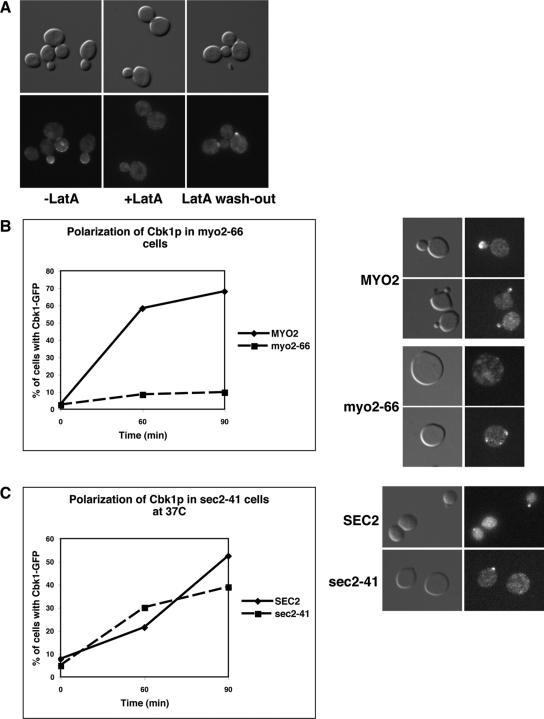
We monitored Sec2-GFP and Sec4-GFP localization in G0 block and released cbk1-as cells to determine whether Cbk1 controls Sec2 or Sec4 function during polarized secretion. When Cbk1 was inhibited upon G0 release, Sec2 and Sec4 recruitment to the incipient bud site was severely delayed (Figure 5). In contrast, Cbk1 localization was not impaired in conditional sec2-41 mutants at restrictive temperature, indicating that Cbk1 influences Sec2-Sec4 localization but not vice versa (Figure 6C). Intriguingly, polarized Cbk1 localization is abolished by disruption of the actin cytoskeleton with latrunculin A or by disruption of myosin V, indicating that the Cbk1 polarity establishment requires actin and myosin V, but is independent of Sec2-associated secretory vesicles (Figure 6, A and B). These data support the hypothesis that Cbk1 regulates polarized secretion, at least in part, via Sec2 and Sec4. Alternatively, Cbk1 might influence Sec2 and Sec4 localization by regulating an upstream function, such as ER or Golgi trafficking.
Figure 5.
Cbk1 kinase inhibition delays Sec2 and Sec4 localization to the incipient bud site. cbk1-as cells expressing Sec2-GFP and GFP-Sec4 (FLY2373 and FLY2531) were released from G0. After 30 min of release, 0 or 5 μM 1NA-PP1 was added (T = 0), and the percentage of cells with polarized Sec2 and Sec4 was scored over time (0 μM 1NA-PP1; - - -, 5 μM 1NA-PP1). Representative images of cells are shown for the T = 60-min time points.
Figure 6.
Cbk1p localization is dependent on actin and myosin V but is Sec2 independent. (A) Cbk1 kinase localization is dependent on the actin cytoskeleton. Logarithmically growing Cbk1-GFP cells (FLY869) were treated with latrunculin A (LatA) for 30 min (middle). Almost all Cbk1-GFP disappears from the cortex of buds, bud neck, and the nucleus. On LatA washout (right), Cbk1-GFP reappears on the cell cortex and bud neck within 30 min. (B) Cbk1 polarity establishment is dependent on myosin V (Myo2). (C) Cbk1 polarity establishment is independent of Sec2. G0 synchronized CBK1-GFP myo2-66 (FLY2539), CBK1-GFP (FLY2535), and CBK1-GFP sec2-41 cells (FLY2485) were released into 36°C medium, and the percentage of cells with polarized Cbk1-GFP was scored over time. Samples were methanol/acetone fixed at designated time points and observed by fluorescence microscopy. Representative images of cells at T = 60 min are shown on the right.
Cbk1 Binds to and Phosphorylates Sec2
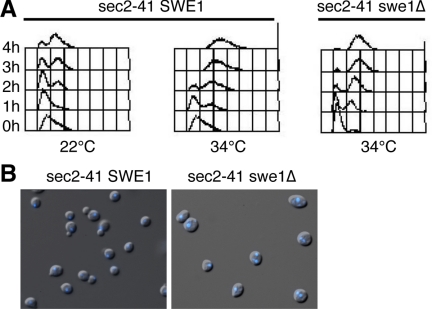
Because Cbk1 is required for the establishment of Sec2 and Sec4 localization in G0 synchronized cells, it seemed plausible that Cbk1 regulates Sec2 or Sec4 directly. In support, all three proteins localize similarly in vivo (Novick and Brennwald, 1993; Walch-Solimena et al., 1997; Weiss et al., 2002), and two-hybrid experiments suggest that Cbk1 binds Sec2 (Racki et al., 2000). Furthermore, like conditional cbk1 mutants, conditional sec2-41 cells arrest in G2 at the Swe1-dependent morphogenesis checkpoint when shifted to 34°C (Figure 7). In the absence of Swe1, sec2-41 cells do not arrest in G2 and thus accumulate as binucleate cells.
Figure 7.
Conditional sec2 mutants induce the Swe1-dependent checkpoint. (A) G0 synchronized sec2-41 (NY770) and sec2-41 swe1Δ cells (FLY2389) were transferred to fresh medium at 22 and 34°C. Samples were fixed and processed for FACS analysis. sec2-41 SWE1+ cells arrest in G2 (with post-S phase DNA content and unsegregated chromatin) at 34°C. (B) After 4h at 34°C, 0% of the sec2-41 SWE1 cells are binucleate. By 4 h at 34°C almost all sec2-41 swe1Δ cells remain unbudded and 45% are binucleate (n > 200).
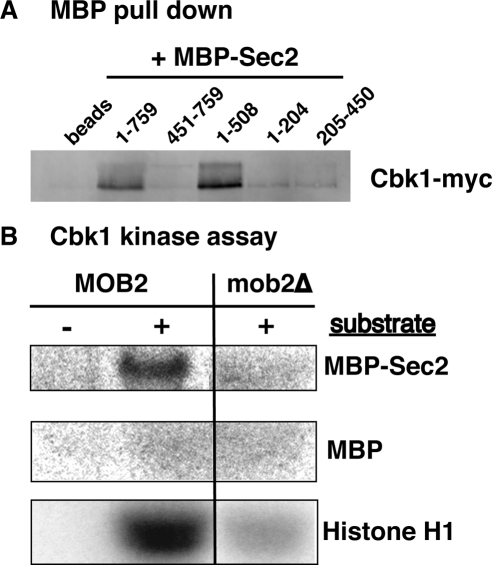
To further explore the interactions between Cbk1 and Sec2, we conducted affinity precipitation experiments with recombinant Sec2 and epitope-tagged Cbk1 from yeast cell extracts. In agreement with previous two-hybrid data, recombinant full length Sec2 specifically precipitated physiologically expressed Cbk1 and its regulatory subunit Mob2 (Figure 8A; unpublished data). We also found that Cbk1 binds the N-terminal half (amino acids 1-508) of Sec2 but that it does not efficiently bind the 1-204 amino acid region, which contains the GEF domain, or the 205-450 amino acid or C-terminal regions of Sec2 (Figure 8A). Moreover, recombinant Sec2 precipitates Cbk1 from extracts of mob2Δ cells, indicating that Sec2 can bind catalytically inactive Cbk1 (data not shown). Coprecipitation experiments with yeast extracts confirm the Cbk1 and Sec2 interaction (data not shown). These data support the model that Cbk1 regulates secretion via Sec2.
Figure 8.
Cbk1 binds and phosphorylates Sec2. (A) Immunoblot showing the results of Sec2 affinity precipitation experiments with MBP fusion proteins. Recombinant MBP-Sec21-759 and MBP-Sec21-508 precipitate Cbk1-Myc from yeast cell extracts (from FLY2288). MBP-Sec2451-759, MBP-Sec21-204, and MBP-Sec2205-450 do not efficiently precipitate Cbk1-Myc. Amylose-resin (beads) and MPB (data not shown) do not precipitate Cbk1-Myc. Similar results were obtained with extracts of mob2Δ ssd1-d cells (data not shown). (B) Autoradiograph of Cbk1 kinase assay using MBP-Sec21-759, MBP or histone H1 as substrate. The uncropped image and corresponding Coomassie-stained gel is shown in Supplemental Figure S5. Cbk1-Myc was immunoprecipitated from MOB2+ or mob2Δ cells (FLY905, FLY906).
Sec2 is phosphorylated in vivo; however, the kinase responsible for its phosphorylation has not been identified (Elkind et al., 2000). To test whether Cbk1 phosphorylates Sec2, we used in vitro Cbk1 kinase assays using recombinant MBP-Sec2 fusion proteins as substrate (Figure 8B and Supplemental Figure S5). As demonstrated previously (Weiss et al., 2002; Nelson et al., 2003), immunoprecipitated Cbk1 phosphorylates itself and the exogenous substrate histone H1 in vitro. Immunoprecipitated Cbk1 also phosphorylated MBP-Sec2 but not MBP in vitro. In the absence of the Cbk1 regulatory subunit Mob2, Cbk1 kinase activity is greatly diminished (Weiss et al., 2002). Significantly, Cbk1 from mob2Δ cells failed to phosphorylate Sec2-MBP in vitro, confirming that Sec2 phosphorylation in vitro is dependent on active Cbk1 (Figure 8B). In addition, Cbk1 did not efficiently phosphorylate N-terminal or C-terminal truncated forms of Sec2 (Sec21-508, Sec21-204, or Sec2205-450) in vitro, suggesting that native structure of Sec2 is important for Cbk1-dependent phosphorylation (data not shown). These data suggest Cbk1 and Sec2 are functionally related and support the model that Cbk1 controls Sec2 function.
Cbk1 Is Required for Post-Golgi Vesicle Formation
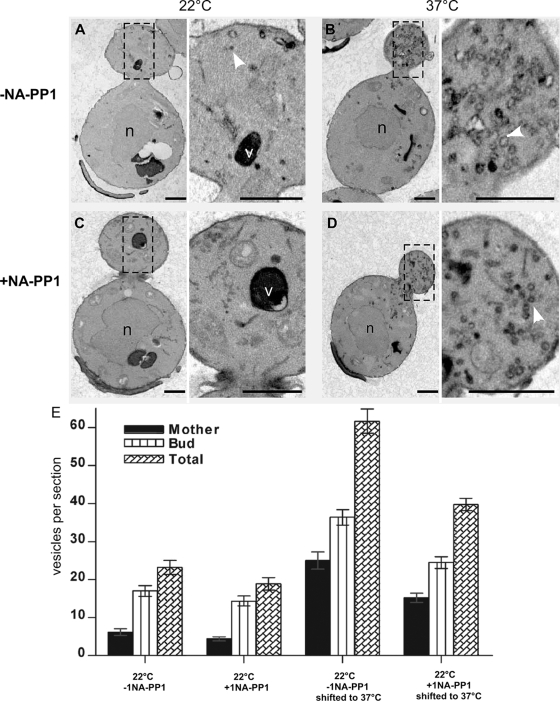
If Cbk1 regulates secretion and bud emergence exclusively via Sec2 or Sec4, then Cbk1 inhibition should cause an accumulation of post-Golgi vesicles, as shown for conditional sec2 and sec4 mutants. Alternatively, if Cbk1 functions upstream of Sec2 or Sec4, then Cbk1 inhibition might interfere with post-Golgi vesicle accumulation in conditional sec2 or sec4 mutants. Accordingly, we analyzed the phenotypes of conditional cbk1-as and cbk1-8 cells by conventional electron microscopy (EM). Significantly, Cbk1 inactivation in cbk1-as or in conditional cbk1-8 cells did not cause an accumulation of vesicles (Figure 9; data not shown). EM morphological analysis did not reveal any obvious structural cell wall defects in cbk1-as and cbk1-8 cells. However, both cbk1-as and cbk1-8 cells are sensitive to Calcofluor White, indicating that they have elevated levels of cell wall chitin. The lack of vesicle accumulation in cbk1 mutants indicates that Cbk1 does not mediate bud growth or secretion exclusively via Sec2 or Sec4 and suggests that Cbk1 has multiple roles in the secretory pathway.
Figure 9.
Cbk1 inhibition diminishes secretory vesicle accumulation in sec2-41 cells. cbk1-as sec2-41 cells (FLY2792) were synchronized in early S phase by G0 block and release. When ∼50% of the cells developed small buds, the cells were incubated at 22°C for 30′ ± 1NA-PP1. The cells were then shifted to 37°C for 15′ (to inactivate Sec2), and samples were collected for EM. (A–D) representative EM figures of cbk1-as sec2-41 cells. (n = nucleus; v = vacuole; arrows indicate vesicles.) (E) Average number of secretory vesicles per EM section of cbk1-as sec2-41 cells is plotted. The data are compiled from two independent experiments.
If Cbk1 functions before Sec2-Sec4 in the secretory pathway, then Cbk1 inhibition might prevent or diminish the accumulation of post-Golgi secretory vesicles in conditional sec2 or exocyst mutants. Accordingly, we synchronized cbk1-as sec2-41 and cbk1-as sec6-4 double mutant cells and allowed them to bud (and enter S phase) before inhibiting Cbk1 for 30 min. We then shifted the cells to 37°C for 15 min to inactivate Sec2 or Sec6 (a component of the exocyst tethering complex) and quantified the average number of secretory vesicles per EM section. Strikingly, Cbk1 inhibition diminished vesicle accumulation in sec2-41 mutants by approximately ∼35% at 37°C (Figure 9). Cbk1 inhibition also causes a ∼20% reduction in secretory vesicles in sec2-41 cells at 22°C. We observed a similar reduction in secretory vesicle accumulation in sec6-4 cells at 37°C (Supplemental Figure S8). These data indicate that Cbk1 is required for the efficient formation of post-Golgi secretory vesicles and implicate RAM signaling in regulating ER or Golgi trafficking functions.
Cbk1 Mutants Are Hypersensitive to Hygromycin B and Display Glycosylation Defects, Suggesting a Role in Golgi Trafficking
If Cbk1 and the RAM network are required for Golgi function, then conditional Cbk1 mutants should display glycosylation defects. Golgi trafficking and glycosylation mutants are often hypersensitive to the drug hygromycin B (Dean, 1995; Dean and Poster, 1996). We therefore assayed for hygromycin B sensitivity and observed that cbk1-8 cell growth was severely impaired on medium containing 50 μg/ml hygromycin B at 22°C. The cbk1-8 cells were as sensitive to hygromycin B as several severe Golgi trafficking mutants, including the conserved oligomeric Golgi (COG) complex mutants cog1Δ and cog8Δ. In contrast, wild-type and sec2-41 cells were resistant to 50 μg/ml hygromycin B (Figure 10A). Likewise, ssd1Δ and ace2Δ cells were not sensitive to hygromycin B (data not shown). The enhanced hygromycin B sensitivity of cbk1-8 cells is consistent with the model that Cbk1 function is important for glycosylation. The lack of hygromycin B sensitivity for sec2-41, ssd1Δ, and ace2Δ cells suggest that the hygromycin B-sensitive phenotype of cbk1 cells is not simply caused by misregulation of Sec2, Ssd1, or Ace2 transcription factor.
Figure 10.
Cbk1 inhibition causes glycosylation defects. (A) cbk1-8 cells are sensitive to hygromycin B. Ten-fold dilution series of cells were spotted onto plates containing 50 μg/ml hygromycin B. Strains used are BY4741, FLY2661, NY770, FLY2765, and FLY2766. (B) CPY is glycosylated and processed normally in cbk1-8 cells at 37°C. Wild type, cbk1-8, cog1Δ, and vps1Δ cells were incubated and pulse labeled with [35S]Met at 37°C. 35S-labeled CPY was immunoprecipitated and analyzed on 10% SDS-PAGE gels. The radiolabeled gels were digitized by a STORM PhosphorImager. (C) Immunoblot of Myc-tagged invertase shows that invertase is hypoglycosylated in cbk1-8 cells at 22°C. Note that invertase levels are diminished at 37°C. (D) Immunoblot of Sim1-CFP shows Sim1 hypoglycosylation in cbk1-8 cells at 37°C. Wild-type, cbk1-8, and cog1Δ cells expressing SIM1-CFP tag were incubated at 22 or 37°C for 2.5 h. Immunoblots were probed with anti-GFP antibody. Brackets denote the major range of electrophoretic mobilities for invertase and Sim1. The yeast strains used for A–C are BY4741, FLY2661, NY770, FLY2764, FLY2765, FLY2766, FLY2704, FLY2575, FLY2567, FLY2848, FLY2802, FLY1050, FLY2847, and FLY2865.
To further explore the role of Cbk1 in secretion and/or Golgi function, we assayed for glycosylation of several secreted cargos. ER or Golgi trafficking mutants often disrupt the glycosylation or processing of cargos, such as CPY. In wild-type cells, CPY is synthesized and modified in the ER as preproenzyme (P1) and is further glycosylated in the Golgi to yield a P2 form, which is subsequently transported to the vacuole where it is proteolytically processed to the mature form (M). ER-to-Golgi trafficking or glycosylation mutants cause an accumulation of the P1 or P2 forms of CPY (Gaynor and Emr, 1997; Nothwehr et al., 2000). We pulse-labeled cbk1-8 cells with [35S]Met at 37°C and analyzed CPY by autoradiography and observed no detectable defect in CPY processing (Figure 10B). In contrast, CPY processing was incomplete in vps1Δ and cog1Δ cells, which are defective in Golgi-to-vacuole trafficking and intra-Golgi trafficking, respectively. These data indicate that Cbk1 inhibition does not globally inhibit ER-to-Golgi trafficking, glycosylation, or Golgi-to-vacuole trafficking.
It is possible that Cbk1 controls the trafficking and/or glycosylation of a subset of cargos. Thus, we investigated whether Cbk1 kinase is required for invertase glycosylation. In wild-type cells, invertase is glycosylated in the Golgi, which causes it to migrate as a characteristic smear on protein gels. In Golgi trafficking mutants, such as cog1Δ, invertase is hypoglycosylated and thus migrates more rapidly on protein gels (Figure 10C; Gaynor and Emr, 1997; Whyte and Munro, 2001). Immunoblot analysis demonstrates that total invertase levels are diminished in cbk1-8 cells (Figure 10C). More significantly, the electrophoretic mobility of invertase is reproducibly aberrant in cbk1-8 cells at 22°C than in similarly prepared wild-type cells (Figure 10C, see brackets). When cbk1-8 cells were grown at 22°C, invertase migrates slightly faster on protein gels than invertase from wild-type cells, suggesting that invertase is moderately hypoglycosylated in cbk1 mutants. Curiously, the overall electrophoretic pattern of invertase from cbk1-8 cells at 37°C was not as dramatically affected. Nevertheless, these results are consistent with the hygromycin B sensitivity phenotype of cbk1-8 cells at 22°C and suggest that Cbk1 is required for the glycosylation of some proteins.
Many proteins involved in cell wall biosynthesis are glycosylated in the Golgi. The cell wall protein Sim1 was identified as a dosage suppressor of cbk1-8 mutants (see below) and ramΔ mutants (Du and Novick, 2002; Kurischko et al., 2005). Sim1 is a glycosylated protein that migrates much slower on protein gels than predicted from its amino acid composition, which is 48 kDa (Velours et al., 2002). In wild-type cells, Sim1-CFP migrates as an ∼127-kDa smear on one-dimensional protein gels (Figure 10D). Impaired Golgi function in cog1Δ cells leads to increased electrophoretic mobility of Sim1-CFP, due to impaired glycosylation (Figure 10). To determine whether Cbk1 is required for Sim1 glycosylation, we assayed the electrophoretic mobility of Sim1-CFP in cbk1-8 cells. Significantly, Sim1-CFP migrates faster in cbk1-8 cells at 37°C than in similarly treated wild-type cells or in cbk1-8 cells at 22°C (Figure 10D, see brackets). Moreover, a small amount of Sim1-CFP migrates as a discrete ∼83-kDa band in cbk1-8 cells at 37°C. There are differences in the electrophoretic patterns of Sim1-CFP from cbk1-8 and cog1Δ cells, indicating that Cbk1 does not function exclusively via Cog1. Nevertheless, these data strongly suggest that Cbk1 kinase is required for proper Sim1 and invertase glycosylation and support the model that Cbk1 and the RAM network regulate Golgi trafficking and/or sorting.
Media Secretion Assays Support a Role for Cbk1 in Glycosylation
To investigate whether Cbk1 is required for the glycosylation of other secreted proteins, we analyzed the electrophoretic mobilities of proteins that are secreted into the medium. As noted above, the overall level of protein secretion (with the exception of Hsp150) was diminished in cbk1-8 cells at 37°C (Figure 3C). In addition, at least three unidentified secreted proteins seem to migrate more rapidly on protein gels of cbk1-8 cells, suggesting that they are hypoglycoslyated (see brackets in Figure 3C). Hsp150 glycosylation seems normal in cbk1-8 cells. By comparison, the Golgi mutant cog1Δ does not efficiently secrete or glycosylate most cargos (Figure 3C). Hsp150 from cog1Δ cells is hypoglycosylated and migrates more rapidly on protein gels. These data suggest that Cbk1 is required for efficient glycosylation and secretion of a subset of cargos and support the model that Cbk1 regulates Golgi trafficking. Nevertheless, definitive proof that the relevant differences in patterns of secreted proteins are caused by aberrant Cbk1-dependent glycosylation, and not by differences in the overall content of secreted proteins, will require identification of the proteins. Thus far, the low abundance of the secreted proteins has impeded their purification and identification.
We conducted parallel media secretion assays with ace2Δ and ssd1-d cells to determine whether differences in Cbk1-dependent glycosylation or secretion require the Cbk1-regulated transcription factor Ace2 or Ssd1. The protein mobility of all but one secreted protein from ace2Δ cells and all proteins in ssd1-d cells seem normal (compare protein patterns to that of wild-type cells), suggesting that glycosylation and Golgi trafficking are not dependent on Ace2 or Ssd1. Strikingly, both cbk1-8 and ace2Δ cells failed to secrete an ∼125-kDa protein that is only second in prominence to Hsp150 (Figure 3C, arrow), suggesting that the expression or secretion of the protein is dependent on Ace2 transcription. The identity of the 125-kDa protein is unknown; however, we have established that it is not the Ace2-regulated Dse4/Eng1 gluconase or Cts1 chitinase (Supplemental Figure S6).
The overall pattern of secreted proteins from cbk1Δ ssd1-d cells was nearly identical to that of ssd1-d and wild-type cells, suggesting that, in the absence of functional Ssd1, Cbk1 is dispensable for secretion or glycosylation of most cargos. There were two notable differences in the pattern of secreted proteins from cbk1Δ ssd1-d cells and ssd1-d cells. First, the secretion (or expression) of the Ace2-dependent 125-kDa band was diminished in cbk1Δ ssd1-d cells, which is consistent with the hypothesis that the 125-kDa protein's expression is Ace2 dependent. Second, one of the glycosylated proteins from cbk1Δ ssd1-d cells seems to migrate as two bands on protein gels, suggesting that its glycosylation (or other posttranslational modification) is diminished in the absence of Cbk1 (Figure 3C, see arrowheads). This observation suggests that at least one protein is glycosylated in a Cbk1-dependent and Ssd1-independent manner. Nevertheless, in the absence of functional Ssd1, Cbk1 is dispensable for the glycosylation and secretion of other cargos.
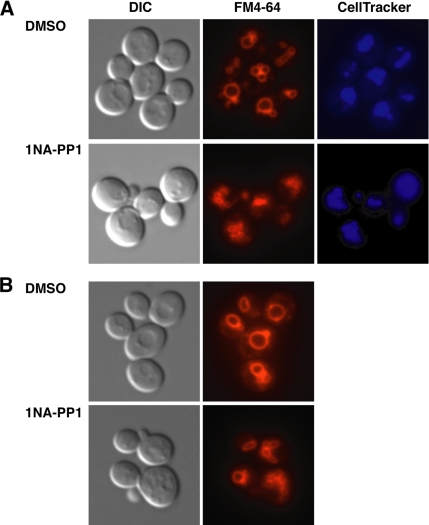
Cbk1 Is Not Required for FM4-64 Uptake
A reduction in Golgi trafficking or exocytosis could be caused by defects in endocytosis or membrane recycling. Thus, we treated cbk1-as cells with the fluorescent endocytic dye FM4-64 to determine whether Cbk1 inhibition interferes with endocytosis. Wild-type cells and untreated cbk1-as cells readily take up FM4-64, as detected by prominent labeling of endocytic vesicles and vacuoles (Figure 11). 1NA-PP1–treated cbk1-as cells also internalized FM4-64, but they displayed aberrant vacuole morphology. The majority of inhibited cbk1-as cells contained smaller vacuole structures. The FM4-64 structures colocalized with the vacuole dye CellTracker Blue CMAC (Invitrogen) in both cbk1-as and wild-type cells. The smaller vacuole structures in cbk1 cells were more definitively observed upon hypertonic expansion. These data indicate that Cbk1 kinase activity is not essential for endocytosis, but demonstrate that Cbk1 is required for normal vacuolar morphology. Early and late Golgi trafficking defects cause similar vacuole morphology defects (Lafourcade et al., 2004). Thus, these findings are consistent with a role for Cbk1 in trafficking.
Figure 11.
Cbk1 kinase inhibition causes aberrant vacuole morphology. (A) Cbk1-kinase inhibition does not prevent FM4-64 (red) uptake, but it does cause the aberrant accumulation of many small vacuoles. The cells were treated with the vacuole dye CellTracker Blue CMAC to confirm that FM4-64 is delivered to the vacuoles. (B) Cells were treated in water for 1h after FM4-64 labeling to increase vacuole size.
Genetic Interactions Support a Role for Cbk1 in Regulating Golgi Function
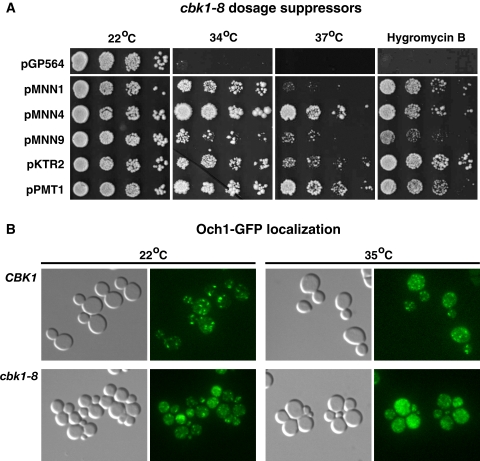
To gain insight to the essential function of Cbk1, we screened a yeast DNA library for dosage (high copy) suppressors of the temperature sensitive phenotypes of cbk1-8 mutants. The complete set of dosage suppressors will be reported in a separate manuscript. Several dosage suppressors were previously identified as suppressors of cbk1Δ lethality (Du and Novick, 2002; Kurischko et al., 2005). These include genes involved in cell wall biogenesis (CCW12, SIM1, and SRL1). Significantly, we also discovered that several cbk1-8 dosage suppressors encode Golgi-localized mannosyltransferases or regulators Mnn1, Mnn4, Mnn6, Mnn9, Ktr2, Pmt1, and Pmt3 (Figure 12 and Supplemental Table S2). Each of the mannosyltransferase plasmids also suppressed the hygromycin B sensitivity of cbk1-8 mutants. These data support a role for Cbk1 in Golgi regulation and suggest that the cell growth and cell integrity defects of cbk1-8 cells derive from Golgi misregulation. Moderate mannosyltransferase overexpression may ameliorate the effects of Golgi misregulation in cbk1-8 cells by restoring or elevating the glycosylation of Golgi-derived cargos.
Figure 12.
Cbk1 kinase and Golgi mannosyltransferases are functionally linked. (A) The conditional lethality of cbk1-8 mutants is suppressed by mannosyltransferase overexpression. Ten-fold dilution series of cbk1-8 cells containing empty vector (pGP654) or dosage suppressor plasmids were spotted onto YPD plates and incubated at the designated temperatures. Cells were also spotted onto YPD plates containing 50 μg/ml hygromycin B and incubated at 22°C. The suppressor plasmids are detailed in Supplemental Table S1. (B) Och1 mannosyltransferase localization is aberrant in cbk1-8 cells. Och1-GFP was monitored in wild type and cbk1-8 cells (JLY284, FLY2922). Cells were grown at 22°C, and, where designated, transferred to 35°C for 1 h. Each image was obtained from a single optical plane.
Mutations in functionally related genes often cause slow growth or death when combined with each other (Kaiser and Schekman, 1990; Finger and Novick, 2000; Schuldiner et al., 2005). Thus, we also investigated the genetic relationships between CBK1 and several genes involved in vesicle trafficking. We discovered that cbk1-8 is lethal in combination with sec2-41, cog1Δ, ypt6Δ, and sec16-2 (Supplemental Figure S7). Cog1 and Ypt6 mediate Golgi trafficking and Sec16 is involved in ER-to-Golgi trafficking. These data are consistent with the finding that a conditional RAM mutant (tao3) is lethal in gyp1Δ strains, which lack a nonessential cis-Golgi localized GTPase-activation protein for Rab-GTPases (Du and Novick, 2002). Intriguingly, late secretory mutants, such as sec2-41 and exocyst mutants are not commonly lethal in combination with Golgi trafficking or glycosylation mutants and vice versa (Finger and Novick, 2000). These genetic interactions support the model that Cbk1 and the RAM network are required for multiple steps in secretion, including 1) an early role in mediating Golgi trafficking and 2) a later role in Sec2-dependent trafficking and secretion.
Och1 Mannosyltransferase Mislocalizes in cbk1-8 Cells
To investigate whether Golgi trafficking is disrupted in cbk1-8 cells, we monitored the localization of Och1 mannosyltransferase, a common marker for the cis-Golgi (Gaynor et al., 1994). In wild-type cells at 22 and 35°C, Och1 localizes to cis-Golgi vesicles, which are evident as numerous cytoplasmic puncta (Figure 12). In contrast, Och1-GFP localized to smaller, more dispersed puncta in cbk1-8 cells at 35°C (Figure 12). The more dispersed Och1 distribution in cbk1-8 cells was similar to, although less pronounced than that described for cog mutants (Bruinsma et al., 2004), which are deficient in Golgi enzyme retrieval. We also observed that the Och1-GFP fluorescence is brighter in cbk1-8 cells than wild-type cells, which was expected because Och1 expression is elevated in RAM and several cell wall biosynthesis mutants (Yamamoto and Jigami, 2002; Nelson et al., 2003). Together, the hypoglycosylation and Och1 mislocalization phenotypes of cbk1 mutants may implicate Cbk1 in Golgi enzyme retrieval.
DISCUSSION
Mutations in the RAM signaling network were shown previously to cause morphogenesis and cellular lysis defects; however, the specific roles of the RAM network had not been determined (Du and Novick, 2002; Vink et al., 2002; Nelson et al., 2003; Kurischko et al., 2005). This study clearly establishes that the S. cerevisiae Lats/NDR kinase Cbk1 is required for Golgi function and secretion, thereby revealing a previously unknown function of the RAM signaling network. In support, we establish that Cbk1 kinase activity is essential for bud emergence and cell growth independently of actin polarity establishment. Cbk1 inactivation disrupts Och1 mannosyltransferase localization, invertase and Sim1 glycosylation, and efficient post-Golgi vesicle formation and secretion of several proteins. In addition to Golgi regulation, it is likely that Cbk1 kinase also regulates Sec2, because Cbk1 binds to and phosphorylates Sec2 in vitro and is necessary for Sec2 and Rab GTPase Sec4 recruitment to the incipient bud site. The sum of these data suggest that Cbk1 and the RAM network regulate growth and maintain cell integrity by controlling Golgi and Sec2-Sec4 functions.
Cbk1 and Golgi Function
Several lines of evidence implicate Cbk1 in regulating Golgi function. First, cbk1 mutants display enhanced sensitivity to hygromycin B, which is a phenotype that strongly correlates with diminished or aberrant glycosylation (Dean, 1995; Dean and Poster, 1996). Moreover, glycosylation of invertase, the cell wall protein Sim1, and several secreted proteins is significantly diminished in conditional cbk1 mutants. Significantly, Cbk1 inhibition disrupts the localization of the cis-Golgi enzyme Och1 and reduces the amount of trans-Golgi-derived vesicles in late secretory mutants by 20–35%. Genetic interactions, such as the identification of several mannosyltransferases genes as cbk1-8 dosage suppressors, also support a role for Cbk1 in regulating Golgi function. We propose that mannosyltransferase overexpression rescues the temperature sensitivity and hygromycin B sensitivity of cbk1-8 mutants by compensating for the diminished glycosylation of Golgi-derived cargos.
The Golgi-related phenotypes of conditional cbk1 mutants may reflect direct mechanisms for Cbk1 in regulating intra-Golgi trafficking, recycling, or structure. For example, Cbk1 may regulate the Golgi via direct phosphorylation of Golgi trafficking mediators, such as those that regulate the retrieval of Golgi enzymes required for glycosylation and/or secretion. Indeed, other protein kinases, such as Cdk1, were shown to directly regulate the Golgi structure (Draviam et al., 2001; Preisinger and Barr, 2005). The hypoglycosylation phenotypes of cbk1 mutants might reflect decreased Golgi enzyme activity caused by protein mislocalization, aberrant intra-Golgi trafficking or alterations in the luminal environment of the Golgi (de Graffenried and Bertozzi, 2004; Schmitz et al., 2008). In support, cbk1 mutants and the Golgi trafficking cog mutants both cause similar Och1 mislocalization phenotypes. Thus, Cbk1 might regulate Golgi enzyme retrieval mechanisms. In principle, some of the mutant cbk1 phenotypes could be caused by defective ER function or trafficking; however, unimpaired CPY processing activities in cbk1 mutants strongly argue against a role for Cbk1 in ER-mediated glycosylation, ER-to-Golgi or Golgi-to-vacuole trafficking.
It is possible that Cbk1 controls Golgi function indirectly. For example, Cbk1 might regulate the expression of glycosylation mediators and/or Golgi trafficking proteins. Indeed, several Golgi mannosyltransferases and associated cofactors are transcriptionally regulated (Igual et al., 1996; Jigami, 2008). Although this indirect mechanism is formally possible, the Cbk1-dependent Ace2 transcription factor cannot be responsible for the Golgi-related phenotypes of cbk1 mutants, because deletion of ACE2 does not cause cellular lysis, hygromycin B sensitivity, or detectable hypoglycosylation defects. Nevertheless, Cbk1 might control Golgi enzyme expression independently of Ace2. Indeed, Och1 mannosyltransferase expression is induced by HOG pathway inactivation and is enhanced in RAM deletion mutants, likely as a consequence of cell wall integrity pathway activation (Nelson et al., 2003; Narang et al., 2008). Nonetheless, Och1 induction alone cannot account for the diminished glycosylation of invertase and Sim1 in conditional cbk1 mutants.
Interactions between Cbk1 and Sec2 Suggest a Post-Golgi Function for Cbk1 in Secretion
The relationship between Cbk1 and Sec2 suggests that Cbk1 regulates bud emergence and secretion, at least in part, via the Rab GTPase Sec4 during post-Golgi trafficking and secretion. Sec2 associates with trans-Golgi–derived secretory vesicles and mediates the guanyl nucleotide exchange to yield active Sec4-GTP, which stimulates vesicle trafficking to the plasma membrane and contributes to exocyst assembly. We demonstrated that Cbk1 binds to and phosphorylates full-length Sec2 in vitro. Notably, Cbk1 binds Sec2 within the N-terminal ∼500-amino acid region (Sec21-508), but it does not efficiently bind a smaller fragment (Sec21-204) that contains the GEF domain. Currently, the Cbk1-dependent phosphorylation sites of Sec2 are unknown. In vivo labeling experiments (analyzed on one-dimensional gels) did not reveal any significant differences in Sec2 phosphorylation levels from wild-type and cbk1-8 cells (data not shown); however, modest but functionally relevant changes in phosphorylation might be obscured if Sec2 is phosphorylated by multiple kinases. The synthetic lethality of cbk1-8 and sec2-41 alleles is also consistent with a cooperative function for Cbk1 and Sec2 in growth control (Supplemental Figure S7).
The cbk1-sec2 interactions support the model that Cbk1 directly regulates the late secretory pathway. Our data indicate that Cbk1 kinase is necessary for the recruitment of Sec2 and Sec4 to the incipient bud site in synchronized cells. Although Sec2 and Sec4 are critical for exocyst assembly and protein secretion (He et al., 2007), the primary consequence of Sec2-Sec4 misregulation in cbk1 mutants is not known. The apparent Sec2-Sec4 mislocalization and secretion defects of cbk1 mutants could be caused by diminished secretory vesicle formation or by misregulation of another upstream process. For example, the consequence of Sec2-Sec4 misregulation may be masked by an earlier defect in Golgi function or secretory vesicle formation. Further insight to the purpose of the cbk1-sec2 interaction will come from the identification and functional analysis of Sec2 phosphorylations. Nevertheless, taken together with the apparent Golgi phenotypes, the cbk1-sec2 interactions suggest that Cbk1 regulates multiple steps in trafficking that are critical for cell growth and maintenance of cell integrity.
Many of the cbk1 mutant phenotypes, including Swe1 checkpoint activation, could be caused by diminished Golgi or diminished Sec2–Sec4 function. Some Golgi mutants exhibit enhanced hygromycin B sensitivity, diminish glycosylation activity and trigger the morphogenesis checkpoint, just as observed for cbk1 mutants (Mondesert and Reed, 1996; Mondesert et al., 1997). Conditional sec2 mutants also trigger the Swe1 morphogenesis checkpoint activation (Figure 7). Thus, we speculate that loss of Cbk1 or RAM activity ultimately causes aberrant cell wall biosynthesis via misregulation of both Golgi and Sec2-Sec4, thereby leading to checkpoint activation and diminished cell integrity.
Functional Relationship between RAM and Ssd1
Curiously, the lethality of CBK1 and RAM gene deletions is suppressed by loss-of-function mutations in the enigmatic SSD1 gene, which encodes a conserved RNA-binding protein of unknown function (Sutton et al., 1991; Costigan et al., 1992; Du and Novick, 2002; Jorgensen et al., 2002). Although it is probable that Cbk1 has a modest role in Golgi regulation in the absence of Ssd1 (see Figure 3C, cbk1Δ ssd1Δ lane), Cbk1 is not essential for Sec2 function, bud emergence, or maintenance of cell integrity in ssd1Δ cells (Racki et al., 2000; Bidlingmaier et al., 2001; Weiss et al., 2002; Nelson et al., 2003; Kurischko et al., 2005). Moreover, ssd1Δ cells do not exhibit secretion or hypoglycosylation defects. Nevertheless, ssd1Δ and ssd1-d loss-of-function mutations share synthetic lethal or enhanced phenotypes with mutations in a variety of genes encoding trafficking proteins, such as Rab and Ras family GTPase and COG proteins (Li and Warner, 1998; Rosenwald et al., 2002; Collins et al., 2007). We speculate that Ssd1 functions as an inhibitor of Cbk1-dependent trafficking and secretion events and that Cbk1, in turn, negatively regulates Ssd1. It is probable that Cbk1-dependent growth control also involves the regulation of Ssd1-dependent RNA functions. In support, Cbk1 binds Ssd1 by two-hybrid (Racki et al., 2000). Moreover, the RAM network protein Tao3 was recently shown to be involved in small nucleolar RNA maturation and localization (Qiu et al., 2008). Further work is necessary to elucidate the mechanism of Ssd1 function with regard to Cbk1 and RAM network function.
Given the conservation of RAM network proteins and vesicle trafficking regulators, our data suggest a conserved role for Cbk1-related kinases in regulating Golgi trafficking and secretion. In agreement, mutations in S. pombe orb6 (CBK1) and other RAM orthologues halt cellular growth and cause a dramatic loss of polarized growth (Verde et al., 1998; Hirata et al., 2002; Hou et al., 2003; Huang et al., 2005; Kanai et al., 2005). Moreover, Drosophila and C. elegans NDR mutations cause morphogenic and developmental defects in a variety of cell types, including neuronal tissues where NDR mutations cause neuronal tiling defects (Geng et al., 2000; Zallen et al., 2000; Emoto et al., 2004; Gallegos and Bargmann, 2004). These phenotypes could be caused by compromised Golgi, Rab GTPase, or exocyst function. In support, Rab GTPases regulate vesicle trafficking and influence polarized morphogenesis and development of a variety of cell types (Stenmark and Olkkonen, 2001). Furthermore, the evolutionary conserved eight-subunit exocyst complex was shown to regulate post-Golgi vesicle transport and to localize to regions of membrane expansion, including growth cones and the tips of growing developing neurons (Hsu et al., 2004). Thus, we suggest that a conserved function for Cbk1-related kinases among eukaryotic cells is to regulate cell growth and development via modulating Golgi function and secretion.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michelle Ottey, Beomhee Hong, and Zachary Kern for technical assistance. We are indebted to Erfei Bi, Chris Burd, Wei Guo, Aaron Gitler, Charlie Boone, Tony Bretscher, Liza Pon, Ruth Collins, and Eric Weiss for yeast strains, plasmids, and helpful discussions. We also thank Erfei Bi, Chris Burd, and Wei Guo for critically reading this manuscript. This work was supported by American Cancer Society grant RSG0508401 and National Institutes of Health grants GM-60575 (to F.C.L.) and AI44009 (to K.M.S.).
Abbreviations used:
- COG
conserved oligomeric Golgi complex
- EM
electron microscopy
- ER
endoplasmic reticulum
- GEF
guanine nucleotide exchange factor
- RAM
regulation of Ace2 and morphogenesis
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-05-0455) on October 8, 2008.
REFERENCES
- Adamo J. E., Rossi G., Brennwald P. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell. 1999;10:4121–4133. doi: 10.1091/mbc.10.12.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura N., Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Ayscough K. R., Stryker J., Pokala N., Sanders M., Crews P., Drubin D. G. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmaier S., Weiss E. L., Seidel C., Drubin D. G., Snyder M. The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:2449–2462. doi: 10.1128/MCB.21.7.2449-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A. C., et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- Bogomolnaya L. M., Pathak R., Guo J., Cham R., Aramayo R., Polymenis M. Hym1p affects cell cycle progression in Saccharomyces cerevisiae. Curr. Genet. 2004;46:183–192. doi: 10.1007/s00294-004-0527-3. [DOI] [PubMed] [Google Scholar]
- Brennwald P., Rossi G. Spatial regulation of exocytosis and cell polarity: yeast as a model for animal cells. FEBS Lett. 2007;581:2119–2124. doi: 10.1016/j.febslet.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma P., Spelbrink R. G., Nothwehr S. F. Retrograde transport of the mannosyltransferase Och1p to the early Golgi requires a component of the COG transport complex. J. Biol. Chem. 2004;279:39814–39823. doi: 10.1074/jbc.M405500200. [DOI] [PubMed] [Google Scholar]
- Click E. S., Stearns T., Botstein D. Systematic structure-function analysis of the small GTPase Arf1 in yeast. Mol. Biol. Cell. 2002;13:1652–1664. doi: 10.1091/mbc.02-01-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. A., Fowler J. E. Polarized growth: maintaining focus on the tip. Current opinion in plant biology. 2006;9:579–588. doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Collins S. R., et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- Colman-Lerner A., Chin T. E., Brent R. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell. 2001;107:739–750. doi: 10.1016/s0092-8674(01)00596-7. [DOI] [PubMed] [Google Scholar]
- Costigan C., Gehrung S., Snyder M. A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol. Cell. Biol. 1992;12:1162–1178. doi: 10.1128/mcb.12.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graffenried C. L., Bertozzi C. R. The roles of enzyme localisation and complex formation in glycan assembly within the Golgi apparatus. Curr. Opin. Cell Biol. 2004;16:356–363. doi: 10.1016/j.ceb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Dean N. Yeast glycosylation mutants are sensitive to aminoglycosides. Proc. Natl. Acad. Sci. USA. 1995;92:1287–1291. doi: 10.1073/pnas.92.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N., Poster J. B. Molecular and phenotypic analysis of the S. cerevisiae MNN10 gene identifies a family of related glycosyltransferases. Glycobiology. 1996;6:73–81. doi: 10.1093/glycob/6.1.73. [DOI] [PubMed] [Google Scholar]
- Dohrmann P. R., Butler G., Tamai K., Dorland S., Greene J. R., Thiele D. J., Stillman D. J. Parallel pathways of gene regulation: homologous regulators SWI5 and ACE2 differentially control transcription of HO and chitinase. Genes Dev. 1992;6:93–104. doi: 10.1101/gad.6.1.93. [DOI] [PubMed] [Google Scholar]
- Dorland S., Deegenaars M. L., Stillman D. J. Roles for the Saccharomyces cerevisiae SDS3, CBK1 and HYM1 genes in transcriptional repression by SIN3. Genetics. 2000;154:573–586. doi: 10.1093/genetics/154.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow L. E., Humbert P. O. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int. Rev. Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- Draviam V. M., Orrechia S., Lowe M., Pardi R., Pines J. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J. Cell Biol. 2001;152:945–958. doi: 10.1083/jcb.152.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L. L., Novick P. Pag1p, a novel protein associated with protein kinase Cbk1p, is required for cell morphogenesis and proliferation in Saccharomyces cerevisiae. Mol. Biol. Cell. 2002;13:503–514. doi: 10.1091/mbc.01-07-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind N. B., Walch-Solimena C., Novick P. J. The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles. J. Cell Biol. 2000;149:95–110. doi: 10.1083/jcb.149.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto K., He Y., Ye B., Grueber W. B., Adler P. N., Jan L. Y., Jan Y. N. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245–256. doi: 10.1016/j.cell.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Finger F. P., Novick P. Synthetic interactions of the post-Golgi sec mutations of Saccharomyces cerevisiae. Genetics. 2000;156:943–951. doi: 10.1093/genetics/156.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A., Schekman R. Functional compartments of the yeast Golgi apparatus are defined by the sec7 mutation. EMBO J. 1989;8:2695–2702. doi: 10.1002/j.1460-2075.1989.tb08410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos M. E., Bargmann C. I. Mechanosensory neurite termination and tiling depend on SAX-2 and the SAX-1 kinase. Neuron. 2004;44:239–249. doi: 10.1016/j.neuron.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gaynor E. C., Emr S. D. COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J. Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor E. C., te Heesen S., Graham T. R., Aebi M., Emr S. D. Signal-mediated retrieval of a membrane protein from the Golgi to the ER in yeast. J. Cell Biol. 1994;127:653–665. doi: 10.1083/jcb.127.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng W., He B., Wang M., Adler P. N. The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics. 2000;156:1817–1828. doi: 10.1093/genetics/156.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Tamanoi F., Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Fink G. R. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:1–933. [PubMed] [Google Scholar]
- Harris S. D. Cell polarity in filamentous fungi: shaping the mold. Int. Rev. Cytol. 2006;251:41–77. doi: 10.1016/S0074-7696(06)51002-2. [DOI] [PubMed] [Google Scholar]
- Harsay E., Bretscher A. Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Xi F., Zhang J., TerBush D., Zhang X., Guo W. Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth. J. Cell Biol. 2007;176:771–777. doi: 10.1083/jcb.200606134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Emoto K., Fang X., Ren N., Tian X., Jan Y. N., Adler P. N. Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol. Biol. Cell. 2005;16:4139–4152. doi: 10.1091/mbc.E05-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A., Stegert M. R., Schmitz D., Hemmings B. A. NDR kinases regulate essential cell processes from yeast to humans. Nat. Rev. Mol. Cell Biol. 2006;7:253–264. doi: 10.1038/nrm1891. [DOI] [PubMed] [Google Scholar]
- Hirata D., et al. Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis and its mutation induces Wee1-dependent G(2) delay. EMBO J. 2002;21:4863–4874. doi: 10.1093/emboj/cdf495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M. C., Wiley D. J., Verde F., McCollum D. Mob2p interacts with the protein kinase Orb6p to promote coordination of cell polarity with cell cycle progression. J. Cell Sci. 2003;116:125–135. doi: 10.1242/jcs.00206. [DOI] [PubMed] [Google Scholar]
- Hsu S. C., TerBush D., Abraham M., Guo W. The exocyst complex in polarized exocytosis. Int. Rev. Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- Huang T. Y., Renaud-Young M., Young D. Nak1 interacts with Hob1 and Wsp1 to regulate cell growth and polarity in Schizosaccharomyces pombe. J. Cell Sci. 2005;118:199–210. doi: 10.1242/jcs.01608. [DOI] [PubMed] [Google Scholar]
- Huckaba T. M., Gay A. C., Pantalena L. F., Yang H. C., Pon L. A. Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J. Cell Biol. 2004;167:519–530. doi: 10.1083/jcb.200404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igual J. C., Johnson A. L., Johnston L. H. Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 1996;15:5001–5013. [PMC free article] [PubMed] [Google Scholar]
- Jigami Y. Yeast glycobiology and its application. Biosci. Biotechnol. Biochem. 2008;72:637–648. doi: 10.1271/bbb.70725. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Stalker J., Humphray S., West A., Cox T., Rogers J., Dunham I., Prelich G. A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat. Methods. 2008;5:239–241. doi: 10.1038/nmeth.1181. [DOI] [PubMed] [Google Scholar]
- Jorgensen P., Nelson B., Robinson M. D., Chen Y., Andrews B., Tyers M., Boone C. High-resolution genetic mapping with ordered arrays of Saccharomyces cerevisiae deletion mutants. Genetics. 2002;162:1091–1099. doi: 10.1093/genetics/162.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kanai M., Kume K., Miyahara K., Sakai K., Nakamura K., Leonhard K., Wiley D. J., Verde F., Toda T., Hirata D. Fission yeast MO25 protein is localized at SPB and septum and is essential for cell morphogenesis. EMBO J. 2005;24:3012–3025. doi: 10.1038/sj.emboj.7600782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurischko C., Weiss G., Ottey M., Luca F. C. A role for the Saccharomyces cerevisiae regulation of Ace2 and polarized morphogenesis signaling network in cell integrity. Genetics. 2005;171:443–455. doi: 10.1534/genetics.105.042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafourcade C., Galan J. M., Gloor Y., Haguenauer-Tsapis R., Peter M. The GTPase-activating enzyme Gyp1p is required for recycling of internalized membrane material by inactivation of the Rab/Ypt GTPase Ypt1p. Mol. Cell. Biol. 2004;24:3815–3826. doi: 10.1128/MCB.24.9.3815-3826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J. The morphogenesis checkpoint: how yeast cells watch their figures. Curr. Opin. Cell Biol. 2003;15:648–653. doi: 10.1016/j.ceb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Reed S. I. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Warner J. R. Genetic interaction between YPT6 and YPT1 in Saccharomyces cerevisiae. Yeast. 1998;14:915–922. doi: 10.1002/(SICI)1097-0061(199807)14:10<915::AID-YEA291>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Lillie S. H., Brown S. S. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol. 2003;13:403–409. doi: 10.1016/s0962-8924(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Lowe M., Barr F. A. Inheritance and biogenesis of organelles in the secretory pathway. Nat. Rev. Mol. Cell Biol. 2007;8:429–439. doi: 10.1038/nrm2179. [DOI] [PubMed] [Google Scholar]
- Luca F. C., Mody M., Kurischko C., Roof D. M., Giddings T. H., Winey M. Saccharomyces cerevisiae Mob1p is required for cytokinesis and mitotic exit. Mol. Cell. Biol. 2001;21:6972–6983. doi: 10.1128/MCB.21.20.6972-6983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca F. C., Winey M. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol. Biol. Cell. 1998;9:29–46. doi: 10.1091/mbc.9.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist E. A. Small GTPases. WormBook. 2006:1–18. doi: 10.1895/wormbook.1.67.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J. N., Sia R. A., Lew D. J. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J. Cell Biol. 1998;142:1487–1499. doi: 10.1083/jcb.142.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesert G., Clarke D. J., Reed S. I. Identification of genes controlling growth polarity in the budding yeast Saccharomyces cerevisiae: a possible role of N-glycosylation and involvement of the exocyst complex. Genetics. 1997;147:421–434. doi: 10.1093/genetics/147.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesert G., Reed S. I. BED1, a gene encoding a galactosyltransferase homologue, is required for polarized growth and efficient bud emergence in Saccharomyces cerevisiae. J. Cell Biol. 1996;132:137–151. doi: 10.1083/jcb.132.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J., Muller H., Peterson M., Novick P. Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. J. Cell Biol. 1990;110:1897–1909. doi: 10.1083/jcb.110.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang S. S., Malone C. L., Deschenes R. J., Fassler J. S. Modulation of yeast Sln1 kinase activity by the CCW12 cell wall protein. J. Biol. Chem. 2008;283:1962–1973. doi: 10.1074/jbc.M706877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B., et al. RAM: A Conserved Signaling Network That Regulates Ace2p Transcriptional Activity and Polarized Morphogenesis. Mol. Biol. Cell. 2003;14:3782–3803. doi: 10.1091/mbc.E03-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr S. F., Ha S. A., Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 2000;151:297–310. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Brennwald P. Friends and family: the role of the Rab GTPases in vesicular traffic. Cell. 1993;75:597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- O'Conallain C., Doolin T., Butler G. Inappropriate expression of the yeast transcription factor Ace2p affects cell growth. Biochem. Soc. Trans. 1998;26:S78. doi: 10.1042/bst026s078. [DOI] [PubMed] [Google Scholar]
- Oka T., Krieger M. Multi-component protein complexes and Golgi membrane trafficking. J. Biochem. 2005;137:109–114. doi: 10.1093/jb/mvi024. [DOI] [PubMed] [Google Scholar]
- Park H. O., Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 2007;71:48–96. doi: 10.1128/MMBR.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger C., Barr F. A. Kinases regulating Golgi apparatus structure and function. Biochem. Soc. Symp. 2005;72:15–30. doi: 10.1042/bss0720015. [DOI] [PubMed] [Google Scholar]
- Pruyne D., Legesse-Miller A., Gao L., Dong Y., Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- Qiu H., et al. Identification of Genes that Function in the Biogenesis and Localization of Small Nucleolar RNAs in Saccharomyces cerevisiae. Mol. Cell. Biol. 2008 doi: 10.1128/MCB.01115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racki W. J., Becam A. M., Nasr F., Herbert C. J. Cbk1p, a protein similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in Saccharomyces cerevisiae. EMBO J. 2000;19:4524–4532. doi: 10.1093/emboj/19.17.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahl P. B., Chen C. Z., Collins R. N. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol. Cell. 2005;17:841–853. doi: 10.1016/j.molcel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Rieder S. E., Banta L. M., Kohrer K., McCaffery J. M., Emr S. D. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol. Biol. Cell. 1996;7:985–999. doi: 10.1091/mbc.7.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A. G., Rhodes M. A., Van Valkenburgh H., Palanivel V., Chapman G., Boman A., Zhang C. J., Kahn R. A. ARL1 and membrane traffic in Saccharomyces cerevisiae. Yeast. 2002;19:1039–1056. doi: 10.1002/yea.897. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J. Cell Biol. 1989;109:1023–1036. doi: 10.1083/jcb.109.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz K. R., Liu J., Li S., Setty T. G., Wood C. S., Burd C. G., Ferguson K. M. Golgi localization of glycosyltransferases requires a Vps74p oligomer. Dev. Cell. 2008;14:523–534. doi: 10.1016/j.devcel.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D. H., Collins R. N., Bretscher A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J. Cell Biol. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner M., et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Olkkonen V. M. The Rab GTPase family. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-reviews3007. REVIEWS3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. S., Biggins S., Rose M. D. The yeast centrin, cdc31p, and the interacting protein kinase, Kic1p, are required for cell integrity. J. Cell Biol. 1998;143:751–765. doi: 10.1083/jcb.143.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Immanuel D., Arndt K. T. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol. Cell. Biol. 1991;11:2133–2148. doi: 10.1128/mcb.11.4.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B. L. Emerging aspects of membrane traffic in neuronal dendrite growth. Biochim. Biophys. Acta. 2008;1783:169–176. doi: 10.1016/j.bbamcr.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velours G., Boucheron C., Manon S., Camougrand N. Dual cell wall/mitochondria localization of the ‘SUN′ family proteins. FEMS Microbiol. Lett. 2002;207:165–172. doi: 10.1111/j.1574-6968.2002.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Verde F., Wiley D. J., Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc. Natl. Acad. Sci. USA. 1998;95:7526–7531. doi: 10.1073/pnas.95.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versele M., Thorner J. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 2005;15:414–424. doi: 10.1016/j.tcb.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T. A., Emr S. D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink E., Vossen J. H., Ram A. F., Van Den Ende H., Brekelmans S., De Nobel H., Klis F. M. The protein kinase Kic1 affects 1,6-beta-glucan levels in the cell wall of Saccharomyces cerevisiae. Microbiology. 2002;148:4035–4048. doi: 10.1099/00221287-148-12-4035. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C., Collins R. N., Novick P. J. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth N. C., Goud B., Kabcenell A. K., Novick P. J. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 1989;8:1685–1693. doi: 10.1002/j.1460-2075.1989.tb03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. X., Zhao H., Harding T. M., Gomes de Mesquita D. S., Woldringh C. L., Klionsky D. J., Munn A. L., Weisman L. S. Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Mol. Biol. Cell. 1996;7:1375–1389. doi: 10.1091/mbc.7.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. L., Kurischko C., Zhang C., Shokat K., Drubin D. G., Luca F. C. The Saccharomyces cerevisiae Mob2p-Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to facilitate daughter cell-specific localization of Ace2p transcription factor. J. Cell Biol. 2002;158:885–900. doi: 10.1083/jcb.200203094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J. R., Munro S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell. 2001;1:527–537. doi: 10.1016/s1534-5807(01)00063-6. [DOI] [PubMed] [Google Scholar]
- Whyte J. R., Munro S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- Xu T., Wang W., Zhang S., Stewart R. A., Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Jigami Y. Mutation of TRS130, which encodes a component of the TRAPP II complex, activates transcription of OCH1 in Saccharomyces cerevisiae. Curr. Genet. 2002;42:85–93. doi: 10.1007/s00294-002-0336-5. [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Peckol E. L., Tobin D. M., Bargmann C. I. Neuronal cell shape and neurite initiation are regulated by the Ndr kinase SAX-1, a member of the Orb6/COT-1/warts serine/threonine kinase family. Mol. Biol. Cell. 2000;11:3177–3190. doi: 10.1091/mbc.11.9.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolov S. N., Lupashin V. V. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J. Cell Biol. 2005;168:747–759. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.