Abstract
Type I interferons (IFNs) activate Janus tyrosine kinase-signal transducer and activator of transcription pathway for exerting pleiotropic biological effects, including antiviral, antiproliferative, and immunomodulatory responses. Here, we demonstrate that filamin B functions as a scaffold that links between activated Rac1 and a c-Jun NH2-terminal kinase (JNK) cascade module for mediating type I IFN signaling. Filamin B interacted with Rac1, mitogen-activated protein kinase kinase kinase 1, mitogen-activated protein kinase kinase 4, and JNK. Filamin B markedly enhanced IFNα-dependent Rac1 activation and the sequential activation of the JNK cascade members. Complementation assays using M2 melanoma cells revealed that filamin B, but not filamin A, is required for IFNα-dependent activation of JNK. Furthermore, filamin B promoted IFNα-induced apoptosis, whereas short hairpin RNA-mediated knockdown of filamin B prevented it. These results establish a novel function of filamin B as a molecular scaffold in the JNK signaling pathway for type I IFN-induced apoptosis, thus providing the biological basis for antitumor and antiviral functions of type I IFNs.
INTRODUCTION
Interferons (IFNs) are a family of cytokines that mediate antiviral, antiproliferative, antitumor, and immunomodulatory responses (Boehm et al., 1997; Stark et al., 1998; Kalvakolanu, 2000; Sen, 2000; Taniguchi and Takaoka, 2001; Platanias, 2005). IFNs are divided into two groups: type I (including IFNα and IFNβ) and type II (IFNγ). Of these, type I IFNs mediate the pleiotropic biological effects by activating tyrosine kinase (TYK)-2 and Janus tyrosine kinase (JAK)-1 kinases, which are associated with type I interferon receptor (IFNAR) 1 and IFNAR2 subunits of type I IFN receptors, respectively. The activated JAK kinases regulate downstream engagement of multiple signal transducer and activator of transcription (STAT) proteins that translocate to the nucleus to induce gene transcription via binding to the promoters of IFN-stimulated genes.
The first signaling pathway shown to be activated by IFNs is JAK-STAT pathway, but it becomes evident that other IFN-regulated signaling cascades are required for the generation of pleiotropic responses to IFNs. Examples of the cascades that operate independently of JAK-STAT pathway include mitogen-activated protein kinase (MAPK) signaling pathways that link a variety of extracellular signals to cellular responses as diverse as proliferation, differentiation, and apoptosis (Whitmarsh and Davis, 1998; Garrington and Johnson, 1999; Schaeffer and Weber, 1999; Widmann et al., 1999). Mitogen-activated protein (MAP) kinases are classified into four main groups: p38, extracellular signal-regulated kinase (ERK)1/2, ERK5, and JNK families (Wang and Tournier, 2006). Of MAPK pathways, p38 pathway has most extensively been studied for its role in the generation of IFN-mediated responses. p38 is phosphorylated and activated in a type I IFN-dependent manner and the inhibition of p38 activity blocks IFNα-dependent transcription of genes that are regulated by IFN-stimulated response elements (ISREs) (Goh et al., 1999; Uddin et al., 1999). Moreover, disruption of the p38α gene results in defective transcription of genes that are regulated by ISREs and/or gamma interferon activated sequence elements (Li et al., 2004). The small GTPase Rac1 is activated in a type I IFN-dependent manner, and its function is required for downstream engagement of p38 pathway (Uddin et al., 2000). Type I IFNs also induce the activation of mitogen-activated kinase kinase (MKK) 3 and MKK6 that are essential for p38 activation (Li et al., 2005). Type I IFNs have also been shown to activate JNK for the induction of apoptosis in some lymphoma cells (Yanase et al., 2005), but the downstream signaling cascade that mediates type I IFN-induced JNK activation remains unknown. In addition, it has been reported that ERK2 is activated by type I IFNs and in response to viral infection, and the expression of dominant-negative (dn) ERK2 inhibits type I IFN-induced transcription (David et al., 1995; Wang et al., 2004). However, a recent study has shown that IFNβ does not induce ERK activation (Takada et al., 2005).
Filamins are nonmuscle actin-binding proteins that comprise a family of three members: filamin A, B, and C (Stossel et al., 2001; van der Flier and Sonnenberg, 2001). These filamin isoforms are large cytoplasmic proteins that play important parts in cross-linking cortical actin filaments into a dynamic three-dimensional structure. The N-terminal region of filamins contains an actin-binding domain (ABD), followed by a rod-like domain consisting of 24 tandem repeats (Gorlin et al., 1990). Dimerization of filamins through the last C-terminal repeat allows the formation of V-shaped flexible structure that is essential for function (Weihing, 1988; Gorlin et al., 1990). Filamins interact with >30 cellular proteins of great functional diversity (Stossel et al., 2001). For example, filamin A binds to numerous proteins involved in signal transduction, including the small GTPase RalA, RhoA, Rac1, and Cdc42 (Ohta et al., 1999); the guanine nucleotide exchange factor Trio (Bellanger et al., 2000); and TRAF2 (Leonardi et al., 2000). In addition, the interaction of CD28 with filamin A is required for the induction of T-cell cytoskeletal rearrangements and thus for the recruitment of lipid microdomains and signaling mediators into the immunological synapse (Tavano et al., 2006). Filamin A also serves as an adaptor protein that links human immunodeficiency virus-1 receptors to actin cytoskeleton remodeling machinery for facilitation of virus infection (Jimenez-Baranda et al., 2007). These diverse interactions suggest that filamins function as signaling scaffolds by connecting and coordinating a variety of cellular processes.
In the present study, we demonstrate for the first time that filamin B, but not filamin A, acts as a scaffold that tethers Rac1 and a JNK-specific MAPK module, recruits them to membrane ruffles, and mediates JNK activation in response to type I IFNs. Moreover, filamin B accelerates type I IFN-dependent apoptosis. These results demonstrate that filamin B plays a key role as a molecular scaffold in JNK signaling pathway for type I IFN-induced apoptosis.
MATERIALS AND METHODS
Antibodies and Reagents
Polyclonal anti-filamin A and anti-filamin B antibodies were raised in rabbits by injecting purified proteins. Antibodies against Xpress (Invitrogen, Carlsbad, CA), FLAG, β-actin (Sigma-Aldrich, St. Louis, MO), Rac1 (Millipore, Billerica, MA), hemagglutinin (HA; Roche Diagnostics, Indianapolis, IN), phospho (p)-JNK, JNK, MKK4, p-MKK4, poly(ADP-ribose) polymerase (PARP), c-Myc (Cell Signaling Technology, Danvers, MA), tumor necrosis factor-related apoptosis-inducing ligand-receptor 1 (R&D Systems, Minneapolis, MN), and MEKK1 and glutathione transferase (GST), green fluorescent protein (GFP) (Santa Cruz Biotechnology, Santa Cruz, CA), 6XHis (BD Biosciences, San Jose, CA) were used. Monoclonal anti-Myc antibody was produced from 9E10 hybridoma. Human recombinant IFNα was obtained from R&D Systems.
Plasmid Construction and Mutagenesis
cDNAs for filamin B and its deletion mutants were cloned into pcDNA4-HisMax. Expression vectors for the full-length or the C-terminal hinge-1 to repeat 24 (H1-R24) region of filamin A was also subcloned into pcDNA4-HisMax. Rac1, Rac1/G12V, Rac1/T17N, MEKK1, MEKK4, MKK4, MKK7, JNK1, and p38 cDNAs were subcloned into pcDNA3 containing a 5′-end Myc, FLAG, and/or HA tag sequences. MKK6 and JNK1 cDNAs were subcloned into pcDNA3.1-Myc/His. JNK1, MKK4, MEKK1Δ, and Rac1 cDNAs were subcloned into pGEX-4T-1. Dominant-negative JNK1 were generated by replacement of Thr183 and Tyr185 by Ala and Phe, respectively.
Sequences for Short Hairpin RNAs (shRNAs)
Two filamin B-specific shRNAs, Rac1-specific shRNA, MEKK1-specific shRNA, MKK4-specific shRNA, and their empty vectors (pSM2c or pLKO.1) were purchased from Open Biosystems (Huntsville, AL). Filamin B-specific shRNAs are tgc tgt tga cag tga gcg acc acc tac ttt gac atc tat ata gtg aag cca cag atg tat ata gat gtc aaa gta ggt ggg tgc cta ctg cct cgg a (shRNA-1) and tgc tgt tga cag tga gcg ccc agc cag cat cct ttg cta tta gtg aag cca cag atg taa tag caa agg atg ctg gct ggt tgc cta ctg cct cgg a (shRNA-2). Filamin A-specific shRNAs are tgc tgt tga cag tga gcg cgc cca ccc act tca cag taa ata gtg aag cca cag atg tat tta ctg tga agt ggg tgg gct tgc cta ctg cct cgg a (shRNA-1) and tgc tgt tga cag tga gcg ccc acc tac ttt gag atc ttt ata gtg aag cca cag atg tat aaa gat ctc aaa gta ggt ggt tgc cta ctg cct cgg a (shRNA-2). Rac1-specific shRNA is ccg gcg caa aca gat gtg ttc tta act cga gtt aag aac aca tct gtt tgc gtt ttt. MEKK1-specific shRNA is tgc tgt tga cag tga gcg agc ctt tcg tat ctc cat gaa ata gtg aag cca cag atg tat ttc atg gag ata cga aag gcc tgc cta ctg cct cgg a. MKK4-specific shRNA is ccg gct tct tat gga ttt gga tgt act cga gta cat cca aat cca taa gaa gtt ttt. MKK6-specific shRNA is ccg ggg cct aca tac cca gag cta act cga gtt agc tct ggg tat gta ggc ctt ttt. Cdc42-specific shRNA is ccg gcc ctc tac tat tga gaa act tct cga gaa gtt tct caa tag tag agg gtt ttt g.
Cell Culture and Transfections
HeLa and A549 cells were cultured in DMEM (JBI, Daegu, Korea) supplemented with 10% fetal bovine serum (FBS). M2 cells that were kindly provided by Dr. T. P. Stossel were grown in minimal essential medium supplemented with 8% normal calf serum and 2% FBS (Cunningham et al., 1992). Cells were transfected using Lipofectamine reagent in combination with PLUS reagent (Invitrogen). All transfection experiments were performed at least three times, and the efficiency of transfection was at least 65% in each experiment. For knockdown of proteins, HeLa cells were transfected with shControl or shRNAs by using jetPEI (Polyplus-transfection, New York, NY). After transfection, cells were treated with puromycin (3 μg/ml) for 1 wk to select transfected cells. Efficiency of protein knockdown was in range of 70–100% as verified by immunoblot and densitometric analysis.
Immunoprecipitation and Pull-Down Analysis
For immunoprecipitation, cells were lysed in 50 mM Tris-HCl, pH 8.0, containing 150 mM NaCl, 1% Triton X-100, or 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1× protease inhibitor cocktail (Roche Diagnostics). Cell lysates were incubated with appropriate antibodies for 2 h at 4°C and then with 50 μl of 50% slurry of protein A-Sepharose for 1 h. For pull-down analysis, cell lysates were prepared as described above and treated with nickel-nitrilotriacetic acid (Ni-NTA) agarose (QIAGEN) and glutathione (GSH)-Sepharose (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
In Vitro Binding Assay
GST-tagged Rac1, MEKK1Δ, MKK4, JNK, and GST were expressed in Escherichia coli, Rosetta (DE3) strain, and purified using GSH-Sepharose. The C-terminal R20-24 region of filamin B was subcloned into pET32a, expressed in Rosetta strain, and purified using NTA agarose. For NTA pull-down analysis, 3 μg of His-R20-24 was incubated 3 μg of GST or GST-tagged Rac1, MEKK1Δ, MKK4, or JNK in buffer A consisting of 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% NP-40, 10 mM Imidazole, 1 mM PMSF, and 1× protease inhibitor cocktail for 1 h at 4°C and then with 30 μl of 50% slurry of NTA resins for the next 1 h. Precipitated beads were washed three times with buffer A containing 20 mM imidazole. They were then subjected to SDS-polyacrylamide gel electrophoresis (PAGE) followed by immunoblot with anti-GST antibody. For GST-pull-down analysis, the GST- and His-tagged proteins were incubated as described above but in buffer B consisting of 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% NP-40, 1 mM PMSF, and 1× protease inhibitor cocktail for 1 h at 4°C and then with 30 μl of 50% slurry of GSH-Sepharose for the next 1 h. Precipitated beads were washed three times with buffer B containing 250 mM NaCl. They were then subjected to SDS-PAGE by immunoblot with anti-6XHis antibody.
In Vitro Kinase Assay
For assaying JNK activity, cell lysates were incubated for 5 h with GST-cJun bound to GSH-Sepharose beads. Precipitates were washed twice with buffer C consisting of 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF. They were again washed with buffer D consisting of 25 mM Tris-HCl, pH 7.5, 5 mM β-glycerophosphate, 2 mM DTT, 0.1 mM Na3VO4, and 10 mM MgCl2. After washing, precipitates were incubated with 0.2 mM ATP in buffer D for 30 min at 30°C. The samples were resolved by SDS-PAGE, and phosphoproteins were visualized by immunoblot with anti-p-cJun antibody.
For assaying MEKK1 activity, cell lysates were subjected to immunoprecipitation with anti-MEKK1 antibody. Precipitates were incubated with 2 μg of GST-MKK4 as a substrate in buffer D containing 0.2 mM ATP for 30 min at 30°C. The samples were then resolved by SDS-PAGE, and phosphoproteins were visualized by immunoblot with anti-p-MKK4 antibody.
Rac1 Activation Assay
Rac1 activation by IFNα was assayed by measuring its ability to interact with GST-fused GTPase-binding domain of Pak1 (GST-PBD) (Benard et al., 1999). Briefly, the GTPase-binding domain of human Pak1 was expressed in E. coli as a GST fusion protein. Cells were serum-starved for 3 h, treated with 10,000 U/ml IFNα for the next 0.5 h, and lysed in buffer C. Cell lysates were incubated for 1 h with 5 μg of GST-PBD–conjugated Sepharose beads. Bound proteins were pulled down, separated by SDS-PAGE, and subjected to immunoblot with anti-Rac1 antibody.
Immunocytochemistry
Cells were grown on coverslips. Two days after transfection, they were fixed by incubation for 10 min with 3.7% paraformaldehyde in phosphate-buffered saline (PBS). Cells were washed three times with PBS containing 0.1% Triton X-100, permeabilized with 0.5% Triton X-100 in PBS for 5 min, and treated with 3% bovine serum albumin (BSA) in PBS for 1 h. They were then incubated for 1 h with appropriate antibodies. After washing with PBS containing 0.1% Triton X-100, cells were incubated for 1 h with fluorescein isothiocyanate-, tetramethylrhodamine B isothiocyanate-, or Cy5-conjugated secondary antibody in PBS containing 3% BSA. To visualize F-actin, fixed cells were also stained with rhodamine-phalloidin. Cells were then observed using a confocal laser scanning microscope (LSM510; Carl Zeiss, Jena, Germany). Images were processed using Photoshop (Adobe Systems, Mountain View, CA).
RESULTS
Filamin B Is Required for Type I IFN-induced JNK Activation
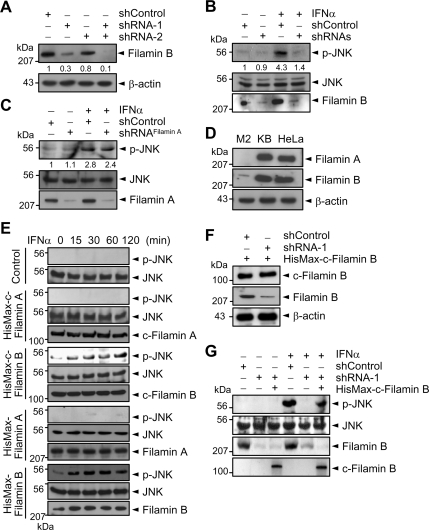
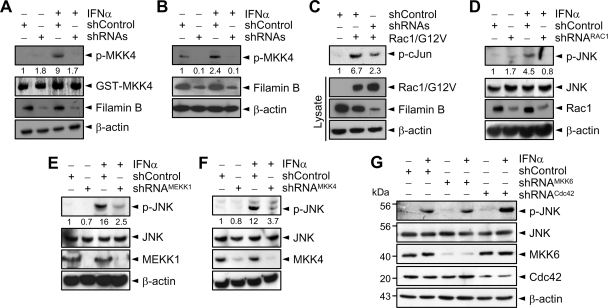
To determine whether filamin B is involved in type I IFN-induced activation of JNK, HeLa cells were transfected with filamin B-specific shRNA-1 (directed against N-terminal repeat 2), shRNA-2 (against C-terminal repeat 22), or both. Immunoblot analysis reveals that each shRNA, but not a negative control (shControl), could reduce the protein level of endogenous filamin B (Figure 1A). The filamin B level was further reduced upon transfection of both shRNA-1 and -2 (henceforth referred to as shRNAs). Furthermore, knockdown of endogenous filamin B by shRNAs markedly reduced IFNα-induced activation of JNK (Figure 1B). Because filamin A shows high sequence similarity with filamin B, we examined whether filamin A-specific shRNAs might also prevent IFNα-induced JNK activation. However, knockdown of endogenous filamin A showed little or no effect on JNK activation (Figure 1C). These results implicate a crucial role of filamin B, but not filamin A, in type I IFN-induced JNK signaling.
Figure 1.
Filamin B is required for type I IFN-induced JNK activation. (A) HeLa cells were transfected with either or both of filamin B-specific shRNA-1 and -2 or with shControl. Cell lysates were subjected to immunoblot with anti-filamin B or anti-β-actin antibody. (B) HeLa cells transfected with shRNAs or shControl were cultured for 1 h with or without IFNα. Cell lysates were subjected to immunoblot with anti-p-JNK or anti-JNK antibody. (C) Experiments were performed as in B but using filamin A-specific shRNAs or shControl. In A–C, the numerals indicate the densitometrically quantified band intensities relative to that seen with shControl only and are representative of three independent experiments. (D) Lysates from the indicated cells were subjected to immunoblot with anti-filamin A or anti-filamin B antibody. (E) M2 cells were transfected with a vector expressing HisMax-c-filamin B, HisMax-c-filamin A, HisMax-tagged full-length filamin A or filamin B, or an empty vector (Control). They were then cultured with IFNα. (F) HisMax-c-filamin B was expressed in HeLa cells transfected with shRNA-1 or shControl. Cell lysates were subjected to immunoblot with anti-filamin B or anti-Xpress antibody. (G) HeLa cells prepared as in F were cultured for 1 h with or without IFNα. Cell lysates were subjected to immunoblot with anti-p-JNK or anti-JNK antibody. The data in D–F are representative of three independent experiments, which showed similar results.
M2 is a human malignant melanoma cell line that does not express filamin A (Cunningham et al., 1992). Moreover, filamin B level in M2 cells was <5% of that in HeLa or KB cells (Figure 1D). To clarify further the involvement of filamin B in type I IFN-induced JNK activation, the C-terminal region from H1-R24 (see Figure 6A for the structural organization of filamin B) was expressed in M2 cells. Henceforth, the C-terminal H1-R24 region of filamin B was referred to as c-filamin B unless otherwise indicated. Likewise, the C-terminal H1-R24 region of filamin A was referred to as c-filamin A. IFNα treatment led to a marked increase in JNK activation in cells complemented with HisMax-c-filamin B (Figure 1E). In contrast, little or no p-JNK was formed in cells complemented with HisMax-c-filamin A. In addition, complementation of M2 cells with full-length filamin B, but not with full-length filamin A, could cause JNK activation in response to IFNα. These results indicate that filamin B, but not filamin A, specifically mediates type I IFN-induced JNK activation.
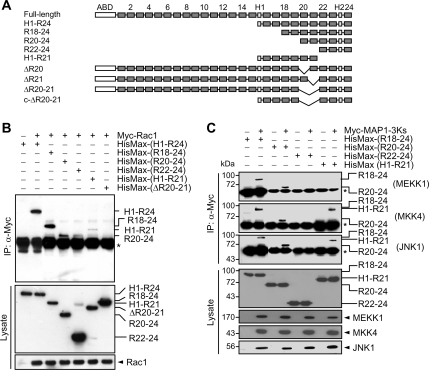
Figure 6.
Mapping of the binding regions for Rac1 and JNK cascade members within filamin B. (A) Serial and in-frame deletion mutants of filamin B were generated and subcloned into pcDNA4-HisMax vector. ABD denotes the actin-binding domain, H1 and H2 indicate the hinge regions, and the numerals show the repeat numbers. (B and C) HisMax-tagged deletions of filamin B were expressed in HeLa cells with Myc-Rac1 (B) or with Myc-tagged-MEKK1, MKK4, or JNK1 (C). Cell lysates were subjected to immunoprecipitation with anti-Myc antibody followed by immunoblot with anti-Xpress antibody. The asterisks indicate IgG heavy chain. Nearly the same data were obtained by two independent trials of each experiment.
Filamins are known to play an important role in three-dimensional arrangement of actin filaments (Stossel et al., 2001; van der Flier and Sonnenberg, 2001). To test a possibility that the abrogation of JNK activation by filamin B knockdown might have been indirectly caused by defects in actin organization, HisMax-c-filamin B lacking ABD was expressed in HeLa cells. Transfection of shRNA-1 (directed against N-terminal repeat 2) to the cells again caused a marked reduction in the level of endogenous filamin B, but showed little or no effect on that of HisMax-c-filamin B (Figure 1F). However, the reconstitution of HisMax-c-filamin B in cells depleted of endogenous filamin B restores IFNα-induced JNK activation (Figure 1G). These results suggest that the inhibition of JNK activation by shRNAs is not due to an indirect effect of filamin B knockdown on actin organization.
Filamin B Is Required for Type I IFN-induced Rac1 Activation
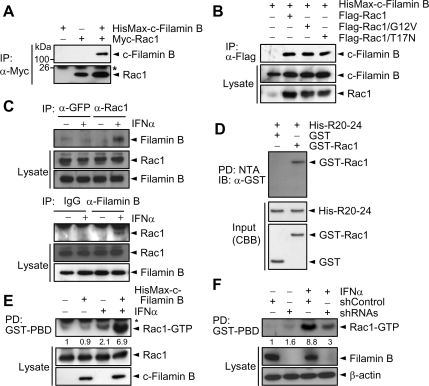
The Rho family of small GTPases interacts with various scaffold proteins that tether specific MAPK signaling cascades, thus acting as key nodes for signal integration and dissemination (Etienne-Manneville and Hall, 2002). To determine whether filamin B interacts with Rho GTPases, Myc-tagged Rac1 was expressed in HeLa cells with or without HisMax-c-filamin B. Immunoprecipitation analysis reveals that c-filamin B interacts with Rac1 (Figure 2A). To determine whether this interaction is dependent on the nucleotide-bound state of Rac1, we examined the ability of c-filamin B to interact with constitutively active GTP-bound (Rac1/G12V) or inactive dominant negative GDP-bound form of Rac1 (Rac1/T17N). Both forms of Rac1 could be coimmunoprecipitated with HisMax-c-filamin B (Figure 2B), suggesting that Rac1 interacts with filamin B independently of its nucleotide-bound state.
Figure 2.
Filamin B is required for type I IFN-induced Rac1 activation. (A) Myc-Rac1 was expressed in HeLa cells with or without HisMax-c-filamin (B) Cell lysates were subjected to immunoprecipitation with anti-Myc antibody followed by immunoblot with anti-Xpress or anti-Myc antibody. The asterisk indicates immunoglobulin G (IgG) light chain. (B) HisMax-c-filamin B was expressed with FLAG-Rac1, FLAG-Rac1/G12V, or FLAG-Rac1/T17N. Cell lysates were subjected to immunoprecipitation with anti-FLAG antibody followed by immunoblot with anti-Xpress. (C) HeLa cells were incubated with or without IFNα for 1 h. Cell lysates were subjected to immunoprecipitation with anti-Rac1 or anti-GFP antibody followed by immunoblot with anti-filamin B antibody. Reciprocal immunoprecipitation was carried out using anti-filamin B or preimmune serum (IgG) followed by immunoblot with anti-Rac1 antibody. (D) His-R20-24 incubated with GST-Rac1 was pulled down with NTA resins. Precipitates were subjected to SDS-PAGE followed by immunoblot with anti-GST antibody. Purified GST-Rac1 and His-R20-24 were subjected to SDS-PAGE followed by staining with Coomassie Blue R-250 (CBB). The data in A–D are representative of three independent experiments, which showed similar results. (E) HeLa cells transfected with an empty or HisMax-c-filamin B vector were serum-starved, treated with IFNα for 30 min, and lysed. The activation of Rac1 by IFNα was then assayed. (F) HeLa cells transfected with shRNAs or shControl were cultured for 1 h with or without IFNα. Cell lysates were then assayed for Rac1 activation. In E and F, the numerals indicate the quantified band intensities relative to that seen with no addition or shControl only, respectively, and are representative of three independent experiments.
To determine whether endogenous filamin B and Rac1 could interact with each other and type I IFNs influence this interaction, HeLa cells were incubated with or without IFNα. Filamin B was coprecipitated with Rac1 upon immunoprecipitation with anti-Rac1 antibody, but only when incubated with IFNα (Figure 2C, top). Reciprocally, Rac1 was coprecipitated with filamin B upon immunoprecipitation with anti-filamin B antibody, only when treated with IFNα (Figure 2C, bottom). These results indicate that the interaction between endogenous filamin B and Rac1 is IFNα-dependent. We then examined whether filamin B could physically interact with Rac1. Because Rac1 could interact with the C-terminal R20-24 region of filamin B under in vivo conditions (see below; Figure 6B), His-R20-24 and GST-Rac1 were expressed in E. coli and purified to apparent homogeneity. NTA pull-down analysis reveals that GST-Rac1, but not GST itself, could be coprecipitated with His-R20-24 (Figure 2D), indicating that filamin B directly binds Rac1.
To determine whether filamin B is involved in type I IFN-induced Rac1 activation, HeLa cells were transfected with a HisMax-c-filamin B vector, treated with IFNα, and subjected to assay for Rac1 activation. The level of activated Rac1 was increased by IFNα treatment, and this Rac1 activation was further enhanced by c-filamin B (Figure 2E). Furthermore, knockdown of filamin B by shRNAs led to a marked inhibition of IFNα-induced Rac1 activation (Figure 2F), implicating the role of filamin B in IFNα-induced Rac1 activation. These results also suggest that filamin B serves as a scaffold that recruits Rac1 for mediating type I IFN signaling.
Filamin B Colocalizes with Rac1 in Membrane Ruffles
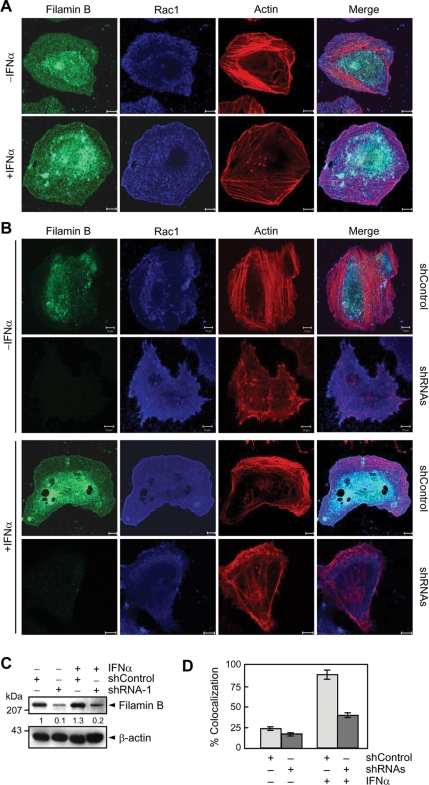
Because filamin B promotes type I IFN-induced Rac1 activation and because activated Rac1 is known to participate in membrane ruffle formation (Allen et al., 1997), we examined whether filamin B could be recruited to the ruffles in response to IFNα. Without IFNα, both endogenous filamin B and Rac1 showed a diffused cytoplasmic localization in HeLa cells (Figure 3A). In IFNα treated cells, a significant portion of them with F-actin was redistributed to and colocalized in the cell periphery, where membrane ruffles formed. These results suggest that type I IFNs promote not only the interaction between Rac1 and filamin B but also their colocalization in membrane ruffles. We then examined whether the knockdown of filamin B influences membrane ruffle formation. Transfection of filamin B-specific shRNA-1 led to a marked reduction in Rac1-mediated ruffle formation in IFNα-treated cells, whereas it showed little or no effect on the cytoplasmic distribution of Rac1 in untreated cells (Figure 3B). Figure 3C confirms the knockdown of filamin B by shRNA-1 in the cells used for the immunocytochemical analysis. In addition, the fractions of cells, showing colocalization of filamin B with Rac1 in membrane ruffles, were quantified (Figure 3D). These results suggest that filamin B promotes Rac1-mediated membrane ruffle formation in response to type I IFNs.
Figure 3.
Filamin B colocalizes with Rac1 in membrane ruffles. (A) HeLa cells incubated with or without IFNα for 30 min were stained with anti-filamin B or anti-Rac1 antibody or phalloidin. The bars indicate 10 μm. (B) Cells transfected with shRNA-1 or shControl were cultured and stained as in A. Bars, 10 μm. (C) Lysates were obtained from the same cells used in B and subjected to immunoblot with anti-filamin B or anti-β-actin antibody. The numerals indicate the quantified band intensities relative to that seen with shControl only and are representative of three independent experiments. (D) The fractions of cells showing colocalization of filamin B with Rac1 in membrane ruffles were quantified. Error bars are ±SEM of total 90 cells from at least three independent experiments.
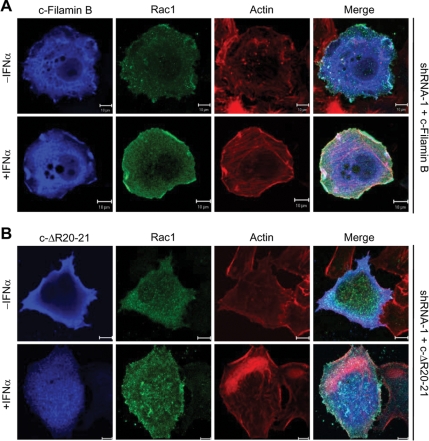
To demonstrate that the interaction of Rac1 with filamin B is required for the promotion of Rac1-mediated ruffle formation in response to type I IFNs, we generated an in-frame deletion of R20-21 in HisMax-c-filamin B for preventing its interaction with Rac1 (termed c-ΔR20-21; Figure 6A). HisMax-c-filamin B and HisMax-c-ΔR20-21 were expressed in HeLa cells that had been depleted of endogenous filamin B by shRNA-1. Reconstitution of c-filamin B, but not c-ΔR20-21, led to a marked increase in the formation of membrane ruffles, where c-filamin B and Rac1 colocalized with enriched F-actin in cells treated with IFNα (Figure 4). These results are consistent with our findings that HisMax-c-filamin B is capable of activating Rac1 in response to IFNα (Figure 2E). Thus, it seems clear that filamin B plays an important role in the promotion of Rac1-mediated ruffle formation through its interaction with and activation of Rac1. Because HisMax-c-filamin B lacks ABD, these results also suggest that ABD, which is known to play a role in actin organization, is not required for the colocalization of filamin B with Rac1 in membrane ruffles.
Figure 4.
Requirement of the interaction between filamin B and Rac1 for their colocalization in membrane ruffles. HisMax-c-filamin B (A) or HisMax-c-ΔR20-21 (B) was expressed in HeLa cells that had been transfected with shRNA-1. Cells were then incubated with or without IFNα for 30 min followed by staining with anti-Xpress or anti-Rac1 antibody or phalloidin. Bars, 10 μm.
Filamin B Interacts with JNK Cascade Members
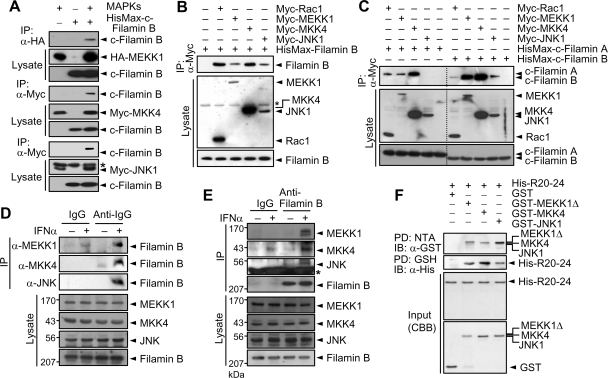
To determine whether filamin B interacts with a JNK-specific signaling module, HA- or Myc-tagged MAPK cascade members were expressed in HeLa cells with or without HisMax-c-filamin B. HisMax-c-Filamin B was coimmunoprecipitated with MEKK1, MKK4, and JNK1 (Figure 5A). However, HisMax-c-filamin B was not coprecipitated with MEKK4 or MKK6 that is a p38-specific activator (data not shown). HisMax-c-Filamin B was neither coprecipitated with MKK7 that also is a JNK activator (data not shown), suggesting that filamin B shows a binding selectivity even among MAP2Ks that activate JNK. In addition, all of Rac1, MEKK1, MKK4, and JNK1 could coprecipitate HisMax-tagged full-length filamin B (Figure 5B). Collectively, these results suggest that filamin B serves as a scaffold, tethering a JNK-specific MAPK module that is downstream to Rac1 (Rac1 → MEKK1 → MKK4 → JNK1). Marti et al. (1997) have reported that filamin A interacts with MKK4 and does not directly bind MEKK1 or JNK, and thereby suggested that MKK4 may form a ternary complex with filamin A and MEKK1. They also showed that filamin A-deficient M2 cells are defective in tumor necrosis factor-α-stimulated JNK activation and this defect could be recovered by complementation of filamin A. Therefore, it would be of interest to compare the ability of filamin A with that of filamin B in their interaction with JNK cascade members. HisMax-c-filamin A or HisMax-c-filamin B was expressed in HeLa cells with each of Myc-tagged MEKK1, MKK4, and JNK1 or Myc-Rac1. Immunoprecipitation analysis reveals that both c-filamin A and c-filamin B could interact with Rac1, MEKK1, and MKK4 (Figure 5C). However, c-filamin B showed a much higher affinity to MEKK1 than c-filamin A. Furthermore, c-filamin A, unlike c-filamin B, could not interact with JNK1. These results are consistent with our finding that filamin A could not promote type I IFN-induced JNK activation.
Figure 5.
Filamin B interacts with JNK cascade members. (A) HA-MEKK1, Myc-MKK4, or Myc-JNK1 was expressed in HeLa cells with HisMax-c-filamin B. Cell lysates were subjected to immunoprecipitation with anti-HA or anti-Myc antibody followed by immunoblot with anti-Xpress antibody. The asterisk in the bottom panel indicates IgG heavy chain. (B) Myc-tagged Rac1, MEKK1, MKK4, or JNK1 was expressed in HeLa cells with HisMax-tagged full-length filamin B. Cell lysates were subjected to immunoprecipitation with anti-Myc antibody followed by immunoblot with anti-Xpress antibody. The asterisk indicates a nonspecific band. (C) Myc-tagged Rac1, MEKK1, MKK4, or JNK1 was expressed in HeLa cells with HisMax-c-filamin A or HisMax-c-filamin B. Cell lysates were subjected to immunoprecipitation as described in B. (D) HeLa cells were incubated with or without IFNα for 1 h. Cell lysates were then subjected to immunoprecipitation with anti-MEKK1, anti-MKK4, or anti-JNK antibody or normal rabbit IgG (IgG) followed by immunoblot with anti-filamin B antibody. (E) Cells were incubated as in D, and their lysates were subjected to immunoprecipitation with anti-filamin B or preimmune serum (IgG) followed by immunoblot with anti-MEKK1, anti-MKK4 or anti-JNK antibody. (F) GST-tagged MEKK1Δ, MKK4, and JNK1 was incubated with His-R20-24 for 1 h at 4°C. His-R20-24 was pulled down with NTA resins, and precipitates were subjected to SDS-PAGE followed by immunoblot with anti-GST antibody (top). From the same incubation mixtures, GST-tagged proteins were pulled down with GSH-Sepharose, and precipitates were subjected to immunoblot with anti-His antibody (second panel). Purified GST-tagged proteins and His-R20-24 were subjected to SDS-PAGE followed by staining with CBB. Note that we used a deletion mutant of MEKK1 (MEKK1Δ) having the amino acid sequence of 397-750 for pull-down analysis, because its full-length form could not be expressed in E. coli. Similar data were obtained by at least three independent trials of each experiment set.
To determine whether endogenous filamin B could interact with endogenous JNK cascade members and type I IFNs affect this interaction, HeLa cells were incubated with or without IFNα. Immunoprecipitation analysis reveals that filamin B could be coprecipitated with MEKK1, MKK4, and JNK (Figure 5D) and vice versa (Figure 5E), but only when cells were incubated with IFNα. These results suggest that endogenous filamin B plays a role as a scaffold tethering JNK cascade members as well as Rac1 (Figure 2C) in response to type I IFNs. We then examined whether filamin B could directly bind JNK cascade members. His-R20-24 and GST-tagged MEKK1Δ, MKK4, and JNK1 were expressed in E. coli and purified to apparent homogeneity. NTA pull-down analysis shows that all of the JNK cascade members could be coprecipitated with His-R20-24 (Figure 5F). Reciprocally, pull-down analysis using GSH-Sepharose beads reveals that His-R20-24 could be coprecipitated with the JNK cascade members. Together, these results indicate that MEKK1, MKK4, and JNK1 physically interact with filamin B.
To map the regions within filamin B for the interaction with Rac1 and JNK cascade members, various deletions of filamin B were generated (Figure 6A) and expressed in HeLa cells with Myc-tagged Rac1, MEKK1, MKK4, or JNK1. HisMax-tagged R20-24 and H1-R21, but not R22-24, could be coimmunoprecipitated with Rac1 (Figure 6B). Moreover, HisMax-c-ΔR20-21 having in-frame deletion of R20-21 in HisMax-c-filamin B could not interact with Rac1, indicating that R20-21 is essential for the binding of Rac1. Alternatively, HisMax-tagged R18-24 and R20-24, but not H1-R21, could be coprecipitated with MEKK1 (Figure 6C), suggesting that the binding site of MEKK1 lies within R20-24. Both MKK4 and JNK1 interacted with R18-24, R20-24, and H1-R21. These results suggest that the binding site for MKK4 and JNK1 to filamin B resides within R20-21.
Filamin B Serves as a Scaffold for JNK Cascade Members
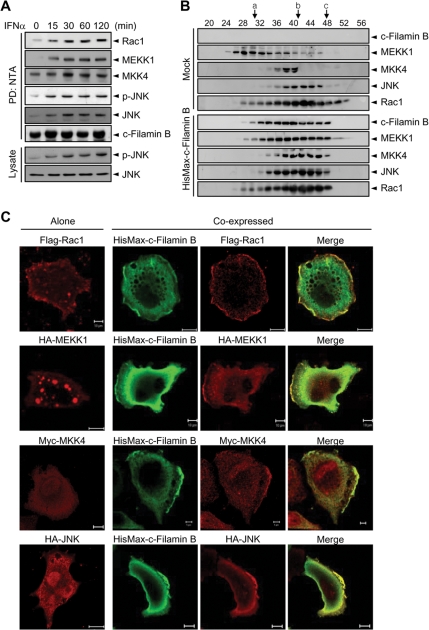
To determine whether filamin B indeed acts as a scaffold that tethers JNK cascade members for type I IFN-induced JNK activation, M2 cells were complemented with HisMax-c-filamin B and then treated with IFNα. NTA pull-down analysis shows that IFNα treatment leads to a dramatic increase in the interaction of c-filamin B with all of endogenous Rac1, MEKK1, MKK4, and JNK (Figure 7A). Moreover, immunoblot analysis of the same precipitates and lysates reveals that the level of p-JNK increases in parallel with the increase in the interaction of c-filamin B with JNK cascade members. Together with the data shown in Figure 5E, these results indicate that filamin B serves as a scaffold that tethers JNK cascade members and that type I IFNs promote the formation of filamin B scaffold complex. To clarify further whether filamin B can form a complex with endogenous JNK cascade members, HisMax-c-filamin B was expressed in M2 cells. After incubation with IFNα for 1 h, cell lysates were subjected to gel filtration. Immunoblot analysis reveals that all of the endogenous Rac1, MEKK1, MKK4, and JNK proteins cofractionate with c-filamin B when M2 cells were complemented with HisMax-c-filamin B, but not without it (Figure 7B). These results confirm that filamin B serves as a scaffold for Rac1 and the JNK cascade members.
Figure 7.
Filamin B serves as a scaffold for JNK cascade members. (A) M2 cells complemented with HisMax-c-filamin B were treated with IFNα. Cell lysates were subjected to pull-down with NTA resins followed by immunoblot analysis. The same precipitates and lysates were also probed with anti-p-JNK or anti-JNK antibody. (B) M2 cells transfected with an empty or HisMax-c-filamin B vector were cultured for 1 h with IFNα. Cell lysates were subjected to gel filtration on a Sephacryl S-300 column (0.7 × 28 cm) equilibrated with 20 mM Tris-HCl, pH 8.0, containing 150 mM NaCl and 7% glycerol. Fractions of 0.2 ml were collected and subjected to immunoblot with anti-Xpress antibody or antibody against Rac1, MEKK1, MKK4, or JNK. The size makers used were a, thyroglobulin (Mr = 669,000); b, β-amylase (200,000); and c, albumin (66,000). (C) FLAG-Rac1, HA-MEKK1, Myc-MKK4, or HA-JNK1 was expressed in HeLa cells with or without HisMax-c-filamin B. Cells were fixed and stained with anti-FLAG, anti-Xpress, anti-HA, or anti-Myc antibody. Bars, 10 μm. Similar data were obtained by at least three independent trials of each experiment set.
Of note was the finding that all of the proteins obtained from M2 cells without complementation of HisMax-c-filamin B ran on the column with sizes even larger than their original sizes. Particularly, MEKK1 behaved as a much larger protein than that from M2 cells complemented with HisMax-c-filamin B. MEKK1 is known to associate with vesicles and thus shows up as speckles (Fanger et al., 1997). Therefore, we examined whether the decrease in the apparent size of MEKK1 by filamin B complementation might be due to a change in its subcellular localization (i.e., dissociation from the vesicles and subsequent binding to filamin B in membrane ruffles). In accord with the previous report, MEKK1 showed up as speckles when expressed alone (Figure 7C). However, upon coexpression with HisMax-c-filamin B, a significant portion of MEKK1 was recruited to membrane ruffles where c-filamin B concentrated. Moreover, MKK4 and JNK1, which by themselves locate throughout the cytoplasm and the nucleus, were also recruited to membrane ruffles when coexpressed with HisMax-c-filamin B. Similarly, Rac1 that alone resides in the cytoplasm and cell periphery were concentrated in the ruffles upon HisMax-c-filamin B coexpression. Thus, the alteration in MEKK1 behavior on the gel filtration column seems to be due to the change in its subcellular localization upon c-filamin B complementation. Likewise, the changes in the chromatographic behavior of Rac1, MKK4, and JNK could be due to the alterations in their interaction partners under the same conditions. These results also implicate a critical role of filamin B as a scaffold that sequesters Rac1 and JNK cascade members in membrane ruffles for facilitating the type I IFN signaling.
Filamin B Promotes Sequential Activation of JNK Cascade Members
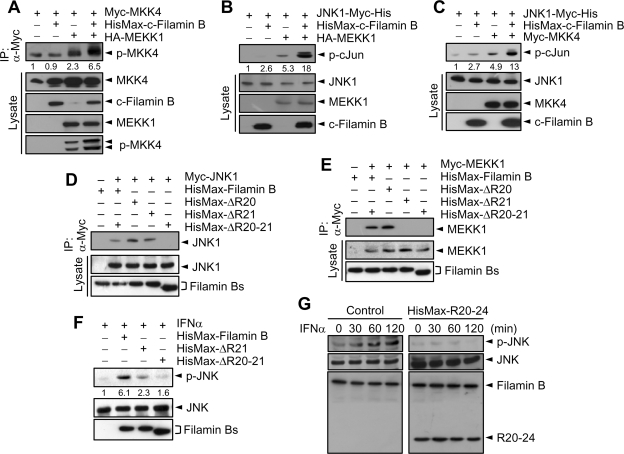
To determine the effect of filamin B on sequential activation of JNK cascade members, Myc-MKK4 was expressed in HeLa cells with or without HA-MEKK1. Immunoprecipitation analysis revealed that expression of MEKK1 promoted the phosphorylation of MKK4, and this MEKK1 activity was markedly enhanced by coexpression of HisMax-c-filamin B (Figure 8A). To determine the effect of filamin B on MEKK1- and MKK4-mediated activation of JNK1, JNK1-Myc-His was expressed in cells with HA-MEKK1 or Myc-MKK4. The level of p-cJun was increased by MEKK1 expression, and this increase in JNK1 activity was again enhanced by coexpression of HisMax-c-filamin B (Figure 8B). Likewise, expression of both MKK4 and HisMax-c-filamin B activated JNK1 to a greater extent than that of MKK4 alone (Figure 8C). These results indicate that filamin B is capable of promoting the MEKK1- and MKK4-mediated JNK1 activation by serving as a scaffold of the effector kinases.
Figure 8.
Filamin B promotes sequential activation of JNK cascade members. (A) Myc-MKK4 was expressed in HeLa cells with HisMax-c-filamin B, HA-MEKK1, or both. Cells lysates were subjected to immunoprecipitation with anti-Myc antibody followed by immunoblot with respective antibodies. (B) JNK1-Myc-His was expressed in HeLa cells with HA-MEKK1, HisMax-c-filamin B, or both. Cell lysates were assayed for the phosphorylation of cJun, followed by immunoblot with anti-p-cJun antibody. (C) JNK1-Myc-His was expressed in HeLa cells with Myc-MKK4, HisMax-c-filamin B, or both. (D and E) HisMax-tagged full-length filamin B or its mutant form having in-frame deletion of R20 (ΔR20), R21 (ΔR21), or both R20 and R21 (ΔR20-21) was expressed in HeLa cells with Myc-JNK1 (D) or Myc-MEKK1 (E). Cell lysates were then subjected to immunoprecipitation with anti-Myc antibody. (F) HisMax-tagged filamin B, ΔR21, or ΔR20-21 was complemented to M2 cells. After incubation with IFNα for 1 h, cell lysates were subjected to immunoblot with anti-p-JNK, anti-JNK, or anti-Xpress antibody. (G) HeLa cells were transfected with an empty (control) or HisMax-R20-24 vector. After incubating them with IFNα for various periods, cell lysates were subjected to immunoblot with anti-p-JNK, anti-JNK, anti-filamin B, or anti-Xpress antibody. In A–C, the numerals indicate the quantified band intensities relative to that seen with MKK4 or JNK alone. In F, the numerals indicate the quantified band intensities relative to that seen with transfection of an empty vector. All the numerals are representative of three independent experiments.
Deletion analysis of filamin B revealed that the binding site of MEKK1 lies within R20-24 and that of JNK1 and MKK4 resides in R20-21 (Figure 6C). To confirm the role of filamin B as a scaffold in JNK signaling pathway, in-frame deletions of full-length filamin B were generated in the regions that have the binding sites for MEKK1 and JNK1 (Figure 6A). We then examined their ability to interact with MEKK1 and JNK1 by expressing them in HeLa cells followed by immunoprecipitation analysis. Myc-JNK1 was coprecipitated with filamin B that lacks either R20 or R21 (HisMax-ΔR20 or HisMax-ΔR21, respectively), but not with that lacking both R20 and R21 (HisMax-ΔR20-21), indicating that JNK1 can bind either R20 or R21 (Figure 8D). Myc-MEKK1 was also coprecipitated with HisMax-ΔR20, but not with HisMax-ΔR21 or HisMax-ΔR20-21, indicating that R21, but not R20, is essential for the binding of MEKK1 to filamin B (Figure 8E). Consistently, in-frame deletion of R21 or both R20 and R21 led to a marked decrease in the ability of filamin B to promote IFNα-induced JNK activation (Figure 8F). Furthermore, overexpression of HisMax-R20-24 in HeLa cells dramatically inhibited the ability of endogenous filamin B to promote IFNα-induced JNK activation (Figure 8G), most likely by sequestering Rac1 and JNK cascade members into separate complexes. Collectively, these results confirm that filamin B plays an essential role as a scaffold in type I IFN-induced JNK signaling pathway.
Filamin B Is Required for IFNα-induced Activation of JNK Cascade Members
To determine whether endogenous filamin B indeed plays an essential role in the activation of JNK cascade members in response to type I IFNs, HeLa cells were transfected with shRNAs or shControl. IFNα caused a dramatic activation of endogenous Rac1 (Figure 2F), MEKK1 (Figure 9A), MKK4 (Figure 9B), and JNK (Figure 1B), and these activations were abrogated by transfection of filamin B-specific shRNAs. To determine whether filamin B serves as a link between activated Rac1 and JNK cascade for mediating type I IFN signaling, Rac1/G12V was expressed in cells transfected with shControl or shRNAs. Knockdown of filamin B led to a marked reduction in cJun phosphorylation (Figure 9C). These results indicate that filamin B serves as a scaffold that recruits endogenous Rac1 and JNK cascade members for efficient and facilitated activation of JNK in response to IFNα.
Figure 9.
Filamin B is required for IFNα-induced activation of JNK cascade. (A) HeLa cells transfected with shRNAs or shControl were incubated for 1 h with or without IFNα. Cell lysates were immunoprecipitated with anti-MEKK1 antibody. Precipitates were then subjected to in vitro kinase assay for MEKK1 by using GST-MKK4 as a substrate. (B) Cells were incubated as in A, and their lysates were immunoblotted with anti-p-MKK4. (C) A vector expressing Rac1/G12V was transfected to HeLa cells that had been transfected with shRNAs or shControl. Cell lysates were assayed for cJun phosphorylation. (D–F) HeLa cells transfected with shRNA specific for Rac1 (D), MEKK1 (E), or MKK4 (F) were incubated for 1 h with or without IFNα. Cell lysates were then subjected to immunoblot with anti-p-JNK or anti-JNK antibody. In A–F, the numerals indicate the quantified band intensities relative to that seen with shControl only and are representative of three independent experiments. (G) HeLa cells transfected with shRNA specific for MKK6 or Cdc42 were incubated for 1 h with or without IFNα. Cell lysates were then subjected to immunoblot with respective antibodies.
To determine whether the upstream effectors of JNK are indeed required for type I IFN-induced activation of JNK, HeLa cells transfected with shRNA specific to Rac1, MEKK1, or MKK4 were cultured with IFNα. IFNα markedly induced JNK activation in shControl-transfected cells, whereas little or no JNK activation could be seen in cells transfected with shRNA specific to Rac1, MEKK1, or MKK4 (Figure 9, D–F). As controls, cells were also transfected with MKK6- or Cdc42-specific shRNA. However, knockdown of MKK6 or Cdc42 did not show any effect on IFNα-induced JNK activation (Figure 9G). These results indicate that Rac1, MEKK1, and MKK4 are essential components of type I IFN-induced JNK signaling pathway.
Filamin B Accelerates Type I IFN-induced Apoptosis
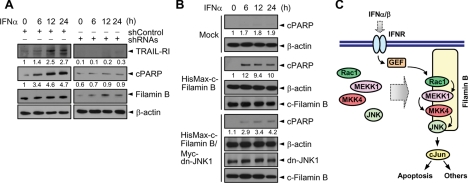
Type I IFNs induce apoptosis in part through up-regulation of TRAIL and its receptor TRAIL-R1/R2 (Oshima et al., 2001), and this up-regulation depends on IFN-induced JNK activation (Yanase et al., 2005). To determine whether filamin B promotes type I IFN-induced apoptosis, HeLa cells transfected with shRNAs or shControl were treated with IFNα. IFNα treatment led to an increase in the levels of TRAIL-R1 as well as in the cleavage of PARP in cells transfected with shControl (Figure 10A). However, knockdown of filamin B by shRNAs abrogated both IFNα-induced up-regulation of TRAIL-R1 and PARP cleavage, indicating that filamin B accelerates apoptosis. To determine whether filamin B-promoted apoptosis is indeed mediated by IFNα-induced JNK activation, HisMax-c-filamin B was expressed in M2 cells with or without dominant-negative form of JNK1 (dn-JNK1). Expression of dn-JNK1 strongly attenuated PARP cleavage (Figure 10B), indicating that filamin B accelerates IFNα-induced apoptosis through JNK activation. Together, these results demonstrate that filamin B accelerates type I IFN-induced apoptosis by promoting JNK activation.
Figure 10.
Filamin B accelerates type I IFN-induced apoptosis. (A) HeLa cells transfected with shRNAs or shControl were treated with IFNα. Cell lysates were immunoblotted with anti-TRAIL-R1, anti-PARP, anti-Xpress, or anti-β actin antibody. (B) HisMax-c-filamin B was expressed in M2 cells with or without Myc-dn-JNK1. After incubation with IFNα, cell lysates were subjected to immunoblot analysis. Mock indicates cells transfected with empty vectors. In A and B, the numerals indicate the ratio of quantified intensity of each band to that of β-actin, relative to that seen at the zero time of IFNα treatment, and they are representative of three independent experiments. (C) Binding of type I IFNs to their receptor (IFNR) induces the recruitment of filamin B to membrane ruffles and the binding of Rac1, MEKK1, MKK4, and JNK1 to filamin B scaffold. GEF, a putative guanine nucleotide exchange factor, may then specifically link IFN signal to Rac1 for the successive activation of downstream kinases and thereby for the generation of type I IFN-mediated biological responses, such as apoptosis.
DISCUSSION
In the present study, we have demonstrated that filamin B serves as a scaffold that tethers Rac1 and a JNK-specific MAPK module (Rac1 → MEKK1 → MKK4 → JNK) and mediates JNK activation in response to type I IFNs. Moreover, filamin B was shown to mediate type I IFN-induced apoptosis through the activation of JNK signaling pathway. These findings establish a novel function of filamin B as a scaffold in JNK signaling pathway for type I IFN-induced apoptosis. In contrast to filamin B, filamin A is known to mediate cell survival under stress conditions, such as stretch-induced physical stress (Glogauer et al., 1998) and genotoxic stresses (Yuan and Shen, 2001). In addition, filamin A has been shown to integrate with β1 integrins to mediate cell spreading and is required for adhesion-dependent cell survival (Kim et al., 2008). Although the molecular basis for the opposite biological effects of filamin A and filamin B remains unclear, a notable difference is that under both in vivo and in vitro conditions filamin A, unlike filamin B, is unable to bind JNK that plays a pivotal role in apoptosis. In addition, filamin A, unlike filamin B, does not physically bind MEKK1 (Marti et al., 1997), although both filamin isoforms can interact with MEKK1 under in vivo conditions albeit with marked different affinities (Figure 5C), suggesting that in vivo interaction between filamin A and MEKK1 is indirect. Thus, it is likely that filamin isoforms possess distinct functional specificity in serving as scaffolds by tethering different sets of context-specific signaling molecules in response to different stimuli.
An intriguing, but unanswered, question is on the mechanism by which Rac1, MEKK1, MKK4, and JNK1 interact with filamin B in an IFNα-dependent manner under in vivo conditions, despite our finding that they can directly bind to filamin B under in vitro conditions. Müller et al. (2001) have shown that KSR1 translocates from the cytoplasm to the cell surface in response to growth factors and this process is regulated by C-TAK1. In unstimulated cells, C-TAK1 associates with KSR1 and phosphorylates Ser392 to confer 14-3-3 binding and cytoplasmic sequestration of the scaffold protein. On stimulation, the phosphorylation state of Ser392 is reduced, allowing the KSR1 complex to colocalize with activated Ras and Raf-1 at the plasma membrane, thereby facilitating the activation of mitogen-activated protein kinase kinase and MAPK. Therefore, it is tempting to speculate that modification of filamin B, such as phosphorylation/dephosphorylation, in response to type I IFNs may be involved in the control of its ability to interact with Rac1 and JNK cascade members.
The existence of multiple JNK-specific MAP2Ks and MAP3Ks within a single cell raises an issue regarding the specificity of JNK activation. It has been suggested that the specificity of JNK activation may be achieved by forming signaling complexes on a specific scaffold protein. Indeed, a variety of scaffold proteins for JNK signaling pathway have been identified (Weston and Davis, 2002; Morrison and Davis, 2003; Dard and Peter, 2006). Examples include β-arrestin-2, CrkII, IKAP, JIP1–4, MKPX, POSH, and SKRP1. JIP1 and JIP2 are closely related proteins that bind to JNK, MKK7, and MLKs. Thus, it seems that in addition to MAPK cascade modules, their upstream effectors, such as small GTPases and guanine nucleotide-exchange factors (GEFs), play a critical role in conferring the specificity of JNK activation in response to specific signals.
The Rho family of small GTPases plays essential roles in the control of a variety of important cellular processes, including actin cytoskeleton rearrangement, cell cycle progression, and cellular transformation (Hall, 1998; Jaffe and Hall, 2002). Moreover, evidence has emerged that small GTPases are often organized into functional modules by scaffold proteins that interact with and tether key components of each signaling pathway. For examples, OSM scaffold, through its ability to bind the GTPase Rac, the actin filaments, and the upstream kinases MEKK3 and MKK3, activates p38 in response to osmostress (Uhlik et al., 2003). POSH also seems to function as a scaffold that links activated Rac1 and JNK cascade to promote neuronal cell death (Xu et al., 2003). In this study, we show that type I IFNs dramatically increase the interaction between endogenous filamin B and Rac1 and that this interaction leads to a marked enhancement of Rac1 activation. Furthermore, filamin B was shown to play a critical role as a scaffold that links between activated Rac1 and JNK cascade to promote apoptosis in response to type I IFNs. Thus, it seems that upon binding to filamin B, Rac1 serves as an upstream effector that mediates type I IFN signals to JNK signaling module for JNK activation.
Of note was the finding that Rac1 interacts with filamin B independently of its nucleotide-bound state. Several scaffold proteins are known to interact with specific GEFs for the activation of JNK pathway. The scaffold protein hCNK1 links Rho and RhoGEFs to JNK activation (Jaffe et al., 2005). Although JIPs do not seem to bind Rac1 or Cdc42, RacGEFs Tiam1 and Ras-GRF1 bind to JIP2 for selective stimulation of p38 upon Rac activation (Buchsbaum et al., 2002). These GEFs also bind to JIP1, which may lead to enhanced JNK activation in different cellular context (Morrison and Davis, 2003). Therefore, we propose that filamin B also binds a specific GEF that controls the activation state of Rac1 for transducing type I IFN signals from IFN receptors to JNK-specific cascade. That is, filamin B may provide a platform for GEF to turn on Rac1 so that GTP-bound Rac1 can initiate type I IFN-dependent JNK signaling pathway. In this regard, it would not be necessary for filamin B itself to distinguish between GDP- and GTP-bound forms of Rac1 for binding. In addition, MEKK1 was shown to regulate JNK pathway in a Cdc42/Rac-dependent manner (Fanger et al., 1997). Thus, MEKK1 may associate with GTP-bound Rac1 on filamin B scaffold for subsequent activation of downstream kinases.
Another possibility for the control of the activation state of Rac1 could be the involvement of upstream protein kinases that regulate the GEF activity. It has been reported that type I IFN-induced activation of Rac1 in p38 pathway could be regulated by upstream tyrosine kinases, such as JAKs or other IFNα-dependent kinases downstream to JAKs, through phosphorylation and activation of GEF for Rac1 (Mayer et al., 2001; Uddin et al., 2000). Among GEFs, Vav is tyrosine-phosphorylated in an IFNα-dependent manner (Micouin et al., 2000; Platanias and Sweet, 1994; Uddin et al., 1997). Furthermore, Vav has been shown to function as a GEF for Rac1 and via Rac1 activation, regulate the p38 and JNK signaling pathways (Crespo et al., 1997; Gringhuis et al., 1998; Salojin et al., 1999). Thus, Vav could be a potential candidate that regulates the activation state of Rac1 for transducing type I IFN-induced JNK signaling.
An important characteristic of scaffolds is their unique subcellular localization with specific signaling modules, thus allowing the facilitated interaction between signaling components and the efficient regulation of respective signaling pathway. Remarkably, expression of HisMax-c-filamin B led to the recruitment of Rac1, MEKK1, MKK4, and JNK1 to membrane ruffles (Figure 7C). Furthermore, IFNα treatment resulted in colocalization of endogenous filamin B and Rac1 in membrane ruffles and reconstitution of HisMax-c-filamin B to filamin B-depleted cells restored ruffle formation. These results suggest that filamin B plays an important role in the sequestration of Rac1 and JNK cascade members to membrane ruffles, where they form a complex with filamin B scaffold for efficient transduction of type I IFN signaling.
However, this finding raises a question how HisMax-c-filamin B (i.e., H1-R24) lacking ABD can promote ruffle formation that is known to require dynamic actin rearrangements. It has been established that Pak1 is activated by GTP-bound Rac1 and activated Pak1 plays a key in ruffle formation (Edwards et al., 1999). Filamin A is a substrate of Pak1 and required for Pak1-mediated ruffle formation. In retrospect, filamin A stimulates the Pak1 activity in response to growth factors, indicating that Pak1 and filamin A mutually influence the dynamic actin cytoskeletal structure (Vadlamudi et al., 2002). Furthermore, the Pak1-binding site resides in the C-terminal region of filamin A and a filamin A fragment lacking ABD can promote ruffle formation (Dyson et al., 2001; He et al., 2003). Similarly, HisMax-c-filamin B was found to interact with Pak1 (data not shown). Thus, it seems that H1-R24 stimulates ruffle formation by promoting type I IFN-induced Rac1 activation, which in turn activates Pak1, although it is unknown whether filamin B is phosphorylated by Pak1.
An additional question that needs to be answered is how filamin B, an actin-binding protein, carries out its signaling function without ABD. However, previous studies have shown that the actin-binding property mediated by ABD of filamin A is dispensable in numerous cases for its signaling function. For examples, complementation of N-terminally truncated filamin A in M2 cells could rescue transforming growth factor-β responsiveness (Sasaki et al., 2001), normal trafficking of large-conductance Ca2+-activated K+ channels to the plasma membrane (Kim et al., 2007), and μ opioid receptor down-regulation (Onoprishvili et al., 2008). Furthermore, C-terminal filamin A fragments could naturally be generated by proteolytic cleavage between R15 to R16, and the cleavage site is conserved in other filamin isoforms (Ozanne et al., 2000; Loy et al., 2003). In addition, generation of filamin A fragments could be regulated by a phosphorylation/dephosphorylation process (Jay et al., 2004; Garcia et al., 2006). Therefore, it has been proposed that proteolysis of scaffold proteins could provide a general mechanism to integrate cellular pathway. Together, it seems possible that type I IFNs induce the phosphorylation and cleavage of filamin B and the resulting C-terminal fragments (e.g., H1-R24) may participate in IFN-signaling pathway.
Until present, the only scaffold protein known to participate in type I IFN signaling is RACK1, which promotes the JAK-STAT pathway by forming a multiprotein complex consisting of the IFNα receptor, STAT1, JAK1, and TYK2 (Usacheva et al., 2003). Thus, filamin B represents the first scaffold that was shown in this study to mediate type I IFN-induced MAPK signaling. Type I IFNs are cytokines that exert important biological activities, including antiviral, antiproliferative, and immunomodulatory responses. These properties have led to the use of type I IFNs in the clinical treatment of certain malignancies, such as chronic myelogenous leukemia, hairy cell leukemia, lymphomas, and certain solid tumors (Platanias, 2003). The potent antiviral properties of IFNs have also ignited clinical studies, which have established their usefulness such as in the viral hepatitis syndromes in humans (Liang et al., 2000; Melian and Plosker, 2001; Regev and Schiff, 2001). Despite the clinical uses of IFNs and the substantial advances in the field of IFN signaling, the precise mechanism by which IFNs mediate their biological effects and relative contribution of each of the various IFN-activated signaling pathways in the induction of specific-IFN responses remain unclear. Therefore, our finding of filamin B that serves as a scaffold for accelerating type I IFN-induced apoptosis should contribute to the understanding of molecular mechanisms by which IFNs mediate the antitumor or antiviral activities of IFNs.
In summary, we propose a model for the role of filamin B in type I IFN-induced signaling pathway that leads to JNK activation as shown in Figure 10C. Binding of IFNα/β to type I IFN receptor activates an unknown GEF, resulting in the activation of Rac1. Activated Rac1 then plays a role in the activation of MEKK1 possibly through their interaction on filamin B scaffold, and thus for successive phosphorylation and activation of MKK4 and JNK. Activated JNK subsequently phosphorylates multiple downstream effectors, like cJun, for the generation of type I IFN-dependent biological responses, such as apoptosis. Together, our data establish that filamin B as a scaffold plays an essential role in type I IFN signaling pathway by mediating the formation of Rac1-MEKK1-MKK4-JNK phospho-relay module.
ACKNOWLEDGMENTS
We thank T. P. Stossel (Harvard Medical School) and T. Takafuta (University of Yamanashi) for providing the cDNAs for human full-length filamin A and filamin B, respectively. We thank S. G. Rhee (Ewha University) for critical reading of our manuscript. This work was supported by grants from the Korea Research foundation (KRF-2005-084-C00025) and the Korea Science and Engineering Foundation (M10533010001). J.S.C. was the recipient of the BK21 fellowship.
Abbreviations used:
- ABD
actin-binding domain
- GEF
guanine nucleotide-exchange factor
- GSH
glutathione
- H1
Hinge region 1
- IFN
interferon
- MAPK
mitogen-activated protein kinase
- PARP
poly(ADP-ribose) polymerase
- PBD
p21 (GTPase)-binding domain
- TRAIL-R1
tumor necrosis factor-related apoptosis-inducing ligand-receptor 1.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-06-0576) on September 24, 2008.
REFERENCES
- Allen W. E., Jones G. E., Pollard J. W., Ridley A. J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- Bellanger J. M., Astier C., Sardet C., Ohta Y., Stossel T. P., Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat. Cell Biol. 2000;2:888–892. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- Benard V., Bohl B. P., Bokoch G. M. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J. C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Buchsbaum R. J., Connolly B. A., Feig L. A. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol. Cell. Biol. 2002;22:4073–4085. doi: 10.1128/MCB.22.12.4073-4085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P., Schuebel K. E., Ostrom A. A., Gutkind J. S., Bustelo X. R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., Gorlin J. B., Kwiatkowski D. J., Hartwig J. H., Janmey P. A., Byers H. R., Stossel T. P. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- Dard N., Peter M. Scaffold proteins in MAP kinase signaling: more than simple passive activating platforms. Bioessays. 2006;28:146–156. doi: 10.1002/bies.20351. [DOI] [PubMed] [Google Scholar]
- David M., Petricoin E., 3rd, Benjamin C., Pine R., Weber M. J., Larner A. C. Requirement for MAP kinase (ERK2) activity in interferon α- and interferon β-stimulated gene expression through STAT proteins. Science. 1995;269:1721–1723. doi: 10.1126/science.7569900. [DOI] [PubMed] [Google Scholar]
- Dyson J. M., O'Malley C. J., Becanovic J., Munday A. D., Berndt M. C., Coghill I. D., Nandurkar H. H., Ooms L. M., Mitchell C. A. The SH2-containing inositol polyphosphate 5-phosphatase, SHIP-2, binds filamin and regulates submembraneous actin. J. Cell Biol. 2001;155:1065–1079. doi: 10.1083/jcb.200104005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. C., Sanders L. C., Bokoch G. M., Gill G. N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Fanger G. R., Johnson N. L., Johnson G. L. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E., Stracher A., Jay D. Calcineurin dephosphorylates the C-terminal region of filamin in an important regulatory site: a possible mechanism for filamin mobilization and cell signaling. Arch. Biochem. Biophys. 2006;446:140–150. doi: 10.1016/j.abb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Garrington T. P., Johnson G. L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- Glogauer M., Arora P., Chou D., Janmey P. A., Downey G. P., McCulloch C. A. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J. Biol. Chem. 1998;273:1689–1698. doi: 10.1074/jbc.273.3.1689. [DOI] [PubMed] [Google Scholar]
- Goh K. C., Haque S. J., Williams B. R. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 1999;18:5601–5608. doi: 10.1093/emboj/18.20.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin J. B., Yamin R., Egan S., Stewart M., Stossel T. P., Kwiatkowski D. J., Hartwig J. H. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J. Cell Biol. 1990;111:1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis S. I., de Leij L. F., Coffer P. J., Vellenga E. Signaling through CD5 activates a pathway involving phosphatidylinositol 3-kinase, Vav, and Rac1 in human mature T lymphocytes. Mol. Cell. Biol. 1998;18:1725–1735. doi: 10.1128/mcb.18.3.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- He H. J., Kole S., Kwon Y. K., Crow M. T., Bernier M. Interaction of filamin A with the insulin receptor alters insulin-dependent activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 2003;278:27096–27104. doi: 10.1074/jbc.M301003200. [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. Rho GTPases in transformation and metastasis. Adv. Cancer Res. 2002;84:57–80. doi: 10.1016/s0065-230x(02)84003-9. [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A., Schmidt A. Association of CNK1 with Rho guanine nucleotide exchange factors controls signaling specificity downstream of Rho. Curr. Biol. 2005;15:405–412. doi: 10.1016/j.cub.2004.12.082. [DOI] [PubMed] [Google Scholar]
- Jay D., Garcia E. J., de la Luz Ibarra M. In situ determination of a PKA phosphorylation site in the C-terminal region of filamin. Mol. Cell. Biochem. 2004;260:49–53. doi: 10.1023/b:mcbi.0000026052.76418.55. [DOI] [PubMed] [Google Scholar]
- Jimenez-Baranda S., et al. Filamin A regulates actin-dependent clustering of HIV receptors. Nat. Cell Biol. 2007;9:838–846. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- Kalvakolanu D. V. Interferons and cell growth control. Histol. Histopathol. 2000;15:523–537. doi: 10.14670/HH-15.523. [DOI] [PubMed] [Google Scholar]
- Kim E. Y., Ridgway L. D., Dryer S. E. Interactions with filamin A stimulate surface expression of large-conductance Ca2+-activated K+ channels in the absence of direct actin binding. Mol. Pharmacol. 2007;72:622–630. doi: 10.1124/mol.107.038026. [DOI] [PubMed] [Google Scholar]
- Kim H., Sengupta A., Glogauer M., McCulloch C. A. Filamin A regulates cell spreading and survival via β1 integrins. Exp. Cell Res. 2008;314:834–846. doi: 10.1016/j.yexcr.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Leonardi A., Ellinger-Ziegelbauer H., Franzoso G., Brown K., Siebenlist U. Physical and functional interaction of filamin (actin-binding protein-280) and tumor necrosis factor receptor-associated factor 2. J. Biol. Chem. 2000;275:271–278. doi: 10.1074/jbc.275.1.271. [DOI] [PubMed] [Google Scholar]
- Li Y., Batra S., Sassano A., Majchrzak B., Levy D. E., Gaestel M., Fish E. N., Davis R. J., Platanias L. C. Activation of mitogen-activated protein kinase kinase (MKK) 3 and MKK6 by type I interferons. J. Biol. Chem. 2005;280:10001–10010. doi: 10.1074/jbc.M410972200. [DOI] [PubMed] [Google Scholar]
- Li Y., Sassano A., Majchrzak B., Deb D. K., Levy D. E., Gaestel M., Nebreda A. R., Fish E. N., Platanias L. C. Role of p38α Map kinase in Type I interferon signaling. J. Biol. Chem. 2004;279:970–979. doi: 10.1074/jbc.M309927200. [DOI] [PubMed] [Google Scholar]
- Liang T. J., Rehermann B., Seeff L. B., Hoofnagle J. H. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann. Intern. Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- Loy C. J., Sim K. S., Yong E. L. Filamin A fragment localized to the nucleus to regulate androgen receptor and coactivator functions. Proc. Natl. Acad. Sci. USA. 2003;100:4562–4567. doi: 10.1073/pnas.0736237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti A., Luo Z., Cunningham C., Ohta Y., Hartwig J., Stossel T. P., Kyriakis J. M., Avruch J. Actin-binding protein-280 binds the stress-activated protein kinase (SAPK) activator SEK-1 and is required for tumor necrosis factor-α activation of SAPK in melanoma cells. J. Biol. Chem. 1997;272:2620–2628. doi: 10.1074/jbc.272.5.2620. [DOI] [PubMed] [Google Scholar]
- Mayer I. A., Verma A., Grumbach I. M., Uddin S., Lekmine F., Ravandi F., Majchrzak B., Fujita S., Fish E. N., Platanias L. C. The p38 MAPK pathway mediates the growth inhibitory effects of interferon-α in BCR-ABL-expressing cells. J. Biol. Chem. 2001;276:28570–28577. doi: 10.1074/jbc.M011685200. [DOI] [PubMed] [Google Scholar]
- Melian E. B., Plosker G. L. Interferon alfacon-1, a review of its pharmacology and therapeutic efficacy in the treatment of chronic hepatitis C. Drugs. 2001;61:1661–1691. doi: 10.2165/00003495-200161110-00009. [DOI] [PubMed] [Google Scholar]
- Micouin A., Wietzerbin J., Steunou V., Martyre M. C. p95(vav) associates with the type I interferon (IFN) receptor and contributes to the antiproliferative effect of IFN-α in megakaryocytic cell lines. Oncogene. 2000;19:387–394. doi: 10.1038/sj.onc.1203314. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Davis R. J. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Müller J., Ory S., Copeland T., Piwnica-Worms H., Morrison D. K. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell. 2001;8:983–993. doi: 10.1016/s1097-2765(01)00383-5. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Suzuki N., Nakamura S., Hartwig J. H., Stossel T. P. The small GTPase RalA targets filamin to induce filopodia. Proc. Natl. Acad. Sci. USA. 1999;96:2122–2128. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoprishvili I., Ali S., Andria M. L., Shpigel A., Simon E. J. Filamin a mutant lacking actin-binding domain restores mu opioid receptor regulation in melanoma cells. Neurochem. Res. 2008;33:2054–2061. doi: 10.1007/s11064-008-9684-y. [DOI] [PubMed] [Google Scholar]
- Oshima K., Yanase N., Ibukiyama C., Yamashina A., Kayagaki N., Yagita H., Mizuguchi J. Involvement of TRAIL/TRAIL-R interaction in IFN-α-induced apoptosis of Daudi B lymphoma cells. Cytokine. 2001;14:193–201. doi: 10.1006/cyto.2001.0873. [DOI] [PubMed] [Google Scholar]
- Ozanne D. M., Brady M. E., Cook S., Gaughan L., Neal D. E., Robson C. N. Androgen receptor nuclear translocation is facilitated by the F-actin crosslinking filamin. Mol. Endocrinol. 2000;14:1618–1626. doi: 10.1210/mend.14.10.0541. [DOI] [PubMed] [Google Scholar]
- Platanias L. C. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol. Ther. 2003;98:129–142. doi: 10.1016/s0163-7258(03)00016-0. [DOI] [PubMed] [Google Scholar]
- Platanias L. C. Mechanisms of type I- and type II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Platanias L. C., Sweet M. E. Interferon α induces rapid tyrosine phosphorylation of the vav proto-oncogene product in hematopoietic cells. J. Biol. Chem. 1994;269:3143–3146. [PubMed] [Google Scholar]
- Regev A., Schiff E. R. Drug therapy for hepatitis B. Adv. Intern. Med. 2001;46:107–135. [PubMed] [Google Scholar]
- Salojin K. V., Zhang J., Delovitch T. L. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1-/PAK-1/p38 MAPK signaling pathway. J. Immunol. 1999;163:844–853. [PubMed] [Google Scholar]
- Sasaki A., Masuda Y., Ohta Y., Ikeda K., Watanabe K. Filamin associates with Smads and regulates transforming growth factor-β signaling. J. Biol. Chem. 2001;276:17871–17877. doi: 10.1074/jbc.M008422200. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Weber M. J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C. Novel functions of interferon-induced proteins. Semin. Cancer Biol. 2000;10:93–101. doi: 10.1006/scbi.2000.0312. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Condeelis J., Cooley L., Hartwig J. H., Noegel A., Schleicher M., Shapiro S. S. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- Takada E., Shimo K., Hata K., Abiake M., Mukai Y., Moriyama M., Heasley L., Mizuguchi J. Interferon-β-induced activation of c-Jun NH2-terminal kinase mediates apoptosis through up-regulation of CD95 in CH31 B lymphoma cells. Exp. Cell Res. 2005;304:518–530. doi: 10.1016/j.yexcr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Takaoka A. A weak signal for strong responses: interferon-α/β revisited. Nat. Rev. Mol. Cell Biol. 2001;2:378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- Tavano R., Contento R. L., Baranda S. J., Soligo M., Tuosto L., Manes S., Viola A. CD28 interaction with filamin A controls lipid raft accumulation at the T-cell immunological synapse. Nat. Cell Biol. 2006;8:1270–1276. doi: 10.1038/ncb1492. [DOI] [PubMed] [Google Scholar]
- Uddin S., Lekmine F., Sharma N., Majchrzak B., Mayer I., Young P. R., Bokoch G. M., Fish E. N., Platanias L. C. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon α-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J. Biol. Chem. 2000;275:27634–27640. doi: 10.1074/jbc.M003170200. [DOI] [PubMed] [Google Scholar]
- Uddin S., Majchrzak B., Woodson J., Arunkumar P., Alsayed Y., Pine R., Young P. R., Fish E. N., Platanias L. C. Activation of the p38 mitogen-activated protein kinase by type I interferons. J. Biol. Chem. 1999;274:30127–30131. doi: 10.1074/jbc.274.42.30127. [DOI] [PubMed] [Google Scholar]
- Uddin S., Sweet M., Colamonici O. R., Krolewski J. J., Platanias L. C. The vav proto-oncogene product (p95vav) interacts with the Tyk-2 protein tyrosine kinase. FEBS Lett. 1997;403:31–34. doi: 10.1016/s0014-5793(97)00023-9. [DOI] [PubMed] [Google Scholar]
- Uhlik M. T., Abell A. N., Johnson N. L., Sun W., Cuevas B. D., Lobel-Rice K. E., Horne E. A., Dell'Acqua M. L., Johnson G. L. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat. Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- Usacheva A., Tian X., Sandoval R., Salvi D., Levy D., Colamonici O. R. The WD motif-containing protein RACK-1 functions as a scaffold protein within the type I IFN receptor-signaling complex. J. Immunol. 2003;171:2989–2994. doi: 10.4049/jimmunol.171.6.2989. [DOI] [PubMed] [Google Scholar]
- Vadlamudi R. K., Li F., Adam L., Nguyen D., Ohta Y., Stossel T. P., Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat. Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- van der Flier A., Sonnenberg A. Structural and functional aspects of filamins. Biochim. Biophys. Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- Wang F., Ma Y., Barrett J. W., Gao X., Loh J., Barton E., Virgin H. W., McFadden G. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 2004;5:1266–1274. doi: 10.1038/ni1132. [DOI] [PubMed] [Google Scholar]
- Wang X., Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006;18:753–760. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Weihing R. R. Actin-binding and dimerization domains of HeLa cell filamin. Biochemistry. 1988;27:1865–1869. doi: 10.1021/bi00406a011. [DOI] [PubMed] [Google Scholar]
- Weston C. R., Davis R. J. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Whitmarsh A. J., Davis R. J. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem. Sci. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- Widmann C., Gibson S., Jarpe M. B., Johnson G. L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Xu Z., Kukekov N. V., Greene L. A. POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J. 2003;22:252–261. doi: 10.1093/emboj/cdg021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase N., Hata H., Shimo K., Hayashida M., Evers B. M., Mizuguchi J. Requirement of c-Jun NH2-terminal kinase activation in interferon-α-induced apoptosis through upregulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in Daudi B lymphoma cells. Exp. Cell Res. 2005;310:10–21. doi: 10.1016/j.yexcr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Shen Z. Interaction with BRCA2 suggests a role for filamin-1 (hsFLNa) in DNA damage response. J. Biol. Chem. 2001;276:48318–48324. doi: 10.1074/jbc.M102557200. [DOI] [PubMed] [Google Scholar]