Abstract
The cytoplasmic translation factor eEF1A has been implicated in the nuclear export of tRNA species in lower eukaryotes. Here we demonstrate that eEF1A plays a central role in nuclear export of proteins in mammalian cells. TD-NEM (transcription-dependent nuclear export motif), a newly characterized nuclear export signal, mediates efficient nuclear export of several proteins including the von Hippel-Lindau (VHL) tumor suppressor and the poly(A)-binding protein (PABP1) in a manner that is dependent on ongoing RNA polymerase II (RNA PolII)-dependent transcription. eEF1A interacts specifically with TD-NEM of VHL and PABP1 and disrupting this interaction, by point mutations of key TD-NEM residues or treatment with actinomycin D, an inhibitor of RNA PolII-dependent transcription, prevents assembly and nuclear export. siRNA-induced knockdown or antibody-mediated depletion of eEF1A prevents in vivo and in vitro nuclear export of TD-NEM–containing proteins. Nuclear retention experiments and inhibition of the Exportin-5 pathway suggest that eEF1A stimulates nuclear export of proteins from the cytoplasmic side of the nuclear envelope, without entering the nucleus. Together, these data identify a role for eEF1A, a cytoplasmic mediator of tRNA export in yeast, in the nuclear export of proteins in mammalian cells. These results also provide a link between the translational apparatus and subcellular trafficking machinery demonstrating that these two central pathways in basic metabolism can act cooperatively.
INTRODUCTION
In eukaryotes, the formation of the nuclear envelope resulted in the segregation of the cell into two distinct cellular compartments, the nucleus and the cytoplasm. This division necessitated the concomitant evolution of regulated nuclear-cytoplasmic transport pathways in order to maintain rapid and specific communication between these cellular compartments. The regulated and timely bidirectional trafficking of RNA and protein cargoes into and out of the nucleus is a prerequisite for basic biological processes. Disruption of such nuclear-cytoplasmic transport pathways results in deregulation of cellular processes and may lead to various diseases (Smith and Koopman, 2004; Terry et al., 2007; Truant et al., 2007).
Nuclear-cytoplasmic transport of all molecules, such as proteins and RNA species, across the nuclear envelope occurs through channels formed by macromolecular structures known as nuclear pore complexes (NPCs; Wente, 2000; Rout and Aitchison, 2001). Although differences exist between protein and RNA transport, the same fundamental sequence of events are conserved; essentially, cargoes bind to soluble transporters in the donor compartment, are transported through NPCs, and are released in the target compartment. A general theme in the export of proteins from the nucleus to the cytoplasm is that specialized export receptors (exportins) of the β-karyopherin family of transporters recognize cargoes harboring specific export signals, such as the recognition of the classical leucine-rich nuclear export signal (NES) by the CRM1 exportin (Fischer et al., 1995; Wen et al., 1995; Fornerod et al., 1997; Fukuda et al., 1997; Stade et al., 1997; Gorlich and Kutay, 1999). Exportins form complexes with substrates in the nucleus with the aid of a small GTPase, Ran (Moore and Blobel, 1993; Melchior and Gerace, 1998; Moore, 1998). The loading and release of substrates with exportins is dependent on a concentration gradient of RanGTP across the nuclear envelope (Izaurralde et al., 1997; Richards et al., 1997). Exportins preferentially bind their substrate at high nuclear RanGTP levels and exit the nucleus as exportin-cargo–RanGTP complexes (Richards et al., 1997; Macara, 2001). Substrates are then released in the cytoplasm upon hydrolysis of RanGTP to RanGDP and exportins return to the nucleus for another round of export.
In recent years it has become apparent that several proteins, unrelated to exportins, play important roles in the nuclear export of proteins and RNAs by exerting their effects from the cytoplasmic side of the nuclear envelope. Current advances in understanding RNA export have revealed a crucial role for several cytoplasmic proteins in the export of mRNA. The DEAD-box protein Dbp5, an ATPase that binds to the cytoplasmic filaments of the NPC, was found to be essential for mRNA export and was postulated to act as a molecular motor to pull mRNAs through the nuclear pore (Tseng et al., 1998; Hodge et al., 1999; Schmitt et al., 1999; Weirich et al., 2004; Cole and Scarcelli, 2006). Mutations of the DBP5 gene or altering the interaction between Dbp5 and Nup159, a nucleoporin of the cytoplasmic filament of the NPC, lead to a dramatic and rapid block of mRNA export (Snay-Hodge et al., 1998; Tseng et al., 1998; Hodge et al., 1999; Schmitt et al., 1999). In addition, the ATPase activity of Dbp5 is stimulated by Gle1, an essential mRNA export factor that is also primarily located at the cytoplasmic face of the NPC (Alcazar-Roman et al., 2006; Weirich et al., 2006). Gle1 mutant strains have been previously shown to exhibit strong defects in mRNA export. In accordance with these observations it has been recently shown that a mutant variant of Gle1 that fails to interact with cytoplasmic Dbp5 considerably reduced the ability of mRNAs to export form the nucleus (Alcazar-Roman et al., 2006; Weirich et al., 2006). Also, the GTPase-activating protein RanGAP1 and its stimulatory factor RanBP1, which are crucial for the hydrolysis of RanGTP to RanGDP, are exclusively cytoplasmic proteins (Coutavas et al., 1993; Bischoff et al., 1994; Bischoff et al., 1995a,b; Matunis et al., 1996; Richards et al., 1996; Mahajan et al., 1997). Mislocalization of either RanGAP1 or RanBP1 to the nuclear compartment through microinjection experiments inhibited major nuclear export of proteins and certain RNA species (Izaurralde et al., 1997).
In yeast, the involvement of cytoplasmic factors has also been demonstrated for nuclear export of tRNA. Both the translation elongation factor eEF1A and the newly characterized protein Cex1p have been described as cytoplasmic components of the nuclear aminoacylation-dependent tRNA export pathway (Grosshans et al., 2000a,b; Kohler and Hurt, 2007; McGuire and Mangroo, 2007; Hopper and Shaheen, 2008). eEF1A and Cex1p were found to copurify with one another and to interact directly with aminoacylated tRNA (Grosshans et al., 2000a; McGuire and Mangroo, 2007). In a yeast genetic system, strains with reduced levels of, or mutated, eEF1A exhibited strong accumulation of mature tRNAs in the nuclear compartment (Grosshans et al., 2000a; McGuire and Mangroo, 2007). Depletion of Cex1p also reduced the efficiency of nuclear tRNA export (McGuire and Mangroo, 2007). However, cells with reduced levels of both eEF1A and Cexp1 showed a significantly increased level of nuclear tRNA retention. These observations support the idea that eEF1A and Cexp1 function in the same pathway, as cytoplasmic export factors involved in tRNA export. In fact, because Cex1p interacts with the NPC it was postulated that Cex1p accepts aminoacyl-tRNAs from the nucleus at the cytoplasmic side of the NPC and delivers them to eEF1A through a channeling mechanism (McGuire and Mangroo, 2007). Altogether, these examples provide evidence that proteins residing in the cytoplasm orchestrate events on the cytoplasmic side of the nuclear envelope that are equally as important as those occurring in the nucleus during nuclear export of proteins and RNA species.
We have recently identified a novel nuclear export sequence, TD-NEM (transcription-dependent nuclear export motif), that mediates efficient nuclear export of proteins such as the von Hippel-Lindau (VHL) tumor suppressor and the poly(A)-binding protein PABP1 (Khacho et al., 2008). Nuclear export through TD-NEM, which is encoded by the consensus sequence DxGx2Dx2L, requires ongoing RNA Polymerase II (RNA PolII)-mediated transcription and operates independently of the classical CRM1/NES-mediated nuclear export pathway (Khacho et al., 2008). Here we report the identification of the translation elongation factor eEF1A as a cytoplasmic factor involved in TD-NEM–mediated nuclear export. These results suggest that eEF1A, a mediator of tRNA export in yeast, is also involved in the nuclear export of proteins in mammalian cells. These findings further argue that factors limited to the cytoplasmic compartment can be essential mediators of the nuclear export pathway.
MATERIALS AND METHODS
Cell Culture, Transfections, Drug Treatments, and Hypoxia Treatment
786-0 (VHL-negative) renal carcinoma cells, MCF-7 cells and A549 cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained in DMEM supplemented with 5% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P-S) in a 37°C and 5% CO2 environment. Transient transfections in MCF-7 and A549 cells were conducted with Effective transfection reagent (QIAGEN, Chatsworth, CA). Transfected cells were incubated for 24 h before any manipulations or drug treatment. Where indicated cells were treated at 37°C with a final concentration of 2 μM actinomycin D (ActD) or 10 μM leptomycin B (LMB) for 1 h before photobleaching experiments and 8 μM ActD, 25 μg/ml DRB (5,6-dichloro-1-β-d-ribofuranosylbenzimidazole), or 10 μM LMB for 3 h before live cell fluorescence imaging or harvesting for immunoprecipitation. Where indicated, cells were treated with 100 μg/ml cycloheximide for 2 h. For SD or AP conditions a buffer-free medium (DME; Invitrogen, Carlsbad, CA) was freshly prepared and supplemented with 5% (vol/vol) FBS and 1% P-S. NaHCO3, 44 mM, was added and the pH was adjusted to 7.2 (SD) or 6.6 (AP) with HCL. Air was bubbled into both types of media to stabilize the pH at 7.2. In hypoxia the SD media remained stable and the AP media reverted slowly to a pH of 6.6.
Plasmids and Adenoviruses
VHL and deletion mutants were cloned into pcDNA3.1 between an NH2-terminal Flag-tag and a COOH-terminal green fluorescent protein (GFP) tag, as previously described (Lee et al., 1999; Bonicalzi et al., 2001; Groulx and Lee, 2002). F-VHL-GFP-NLS and F-GFP were previously described in Groulx et al. (2000) and Lee et al. (1999). Human full-length VHL and deletion mutants, the strong nuclear export sequence (NES) from the human immunodeficiency virus REV were inserted into a F-PK-GFP-NLS construct that was previously described in Khacho et al. (2008) and Groulx et al. (2000). cDNAs corresponding to VHL(114–138) and PABP1(296–317) that encode TD-NEM sequences were inserted into F-GFP or F-PK-GFP-NLS. For F-eEF1A-GFP-NLS the human eEF1A cDNA was inserted between Flag and GFP-NLS using ApaI and XhoI restriction sites. The human PABP1 was fused to GFP-F to produce the GFP-F-PABP1 fusion protein. F-VHL-GFP, F-ΔC157-GFP, and F-GFP adenoviruses were produced using the Cre-lox recombination system (Lee et al., 1999; Groulx and Lee, 2002). PK-GFP-NoDSH+ and PK-GFP-NLS-NoDSH+ were previously described in Mekhail et al. (2007).
Small Interfering RNA
For small interfering RNA (siRNA), experiments cell were transfected with 100 nM of either of eEF1A siRNA (2991, 2804; Ambion, Austin, TX), Exp5–1 siRNA (Lund et al., 2004), eEF2 siRNA (10791; Ambion), control siRNA (Ambion), or Effectene alone (mock) for 48 h before photobleaching experiments or 72 h before. Where indicated siRNA-transfected cells were treated with 8 μM ActD for 3 h after a 72-h incubation period with siRNA and before live cell fluorescence imaging. For photobleaching experiments, siRNA-transfected cells were treated with 2 μM ActD for 1 h after a 48-h incubation period with siRNA and before FLIP (fluorescent loss in photobleaching) analysis.
Live Cell Fluorescence Imaging
Images of living cells transiently expressing GFP from experiments where photobleaching was not utilized were imaged with an Axiovert S100TV microscope (Carl Zeiss MicroImaging, Thornwood, NY) equipped with a 40×/1.2 C-Apochromat water immersion objective using a digital charged-coupled device camera (Empix Imaging, Mississauga, Ontario, Canada). Cell nuclei were stained with Hoechst 33342 (Sigma, St. Louis, MO). Images were captured using the Northern Eclipse software package (Empix Imaging).
Photobleaching and Microscopy
Cells were cultured and transfected directly onto 35-mm dishes with coverslip bottoms (MatTek, Ashland, MA). Photobleaching and live cells microscopy was performed using a confocal microscope (LSM5 Pascal Laser Scanning Microscope, Carl Zeiss, Toronto, Ontario, Canada). In all experiments cells were maintained at 37°C in an environmental chamber. A 63× plan Apo oil immersion lens with a 1.4 NA was used for bleaching and imaging. Indicated areas were exposed to three rapid pulses of a 488-nm argon laser at 100%, and image acquisition was at 1% of full laser power. A highly quantitative live cell nuclear export assay utilizing FLIP (fluorescent loss in photobleaching) technology was used to measure nuclear export activity. For these cytoplasmic FLIP experiments, a fusion protein consisting of the large and amorphous pyruvate kinase (PK), which does not encode localization determinants, GFP, and the nuclear localization signal (NLS; Kalderon et al., 1984) derived from the simian virus large T antigen SV40 (PK-GFP-NLS) was used. Cells expressing a PK-GFP-NLS–tagged fusion protein were repeatedly bleached in a small cytoplasmic region and imaged at 30-s intervals. Small bleached areas for cytoplasmic FLIPs were kept consistent in terms of size and distance from the nucleus. Fluorescence loss in the unbleached areas (nuclei) was quantified as previously described (Phair and Misteli, 2000; Mekhail et al., 2005) using the following equation: Irel = (I(t)/I(0))*(N(0)/N(t)), where I(t) is the average intensity of the unbleached nucleus or cell at time point t, I(0) is the average prebleached intensity of the nucleus or cell of interest, and N(0) and N(t) are the average nuclear or cellular fluorescence intensity of a neighboring cell in the same field of vision at prebleach or at time t, respectively. This calculation accounts for any losses in fluorescence by normalizing the fluorescence of the cell of interest to that of a neighboring cell of approximate equal size and fluorescent intensity. Pseudocolor images were generated to highlight differences in GFP fluorescence: red represents high fluorescent intensity and light blue represents low fluorescent intensity. The quantification graphic was generated by a FLIP software. For all bleaching experiments ∼10 datasets were analyzed for each result. Pseudocoloring for bleaching experiments was achieved by applying the gradient map function of Photoshop (Adobe, San Jose, CA) to a montage of picture frames prepared with ImageJ software (http://rsb.info.nih.gov/ij/; National Institutes of Health, Bethesda, MD). The Northern Eclipse (Empix Imaging), Excel (Microsoft, Redmond, WA), and FreeHand (Macromedia, San Francisco, CA) software packages were also used to capture images, analyze the data, and generate graphs.
Immunoprecipitation, Silver Staining, and Immunoblotting
Cells were lysed in lysis buffer containing 0.5% Igepal CA630, 100 mM NaCl, 20 mM Tris-HCl (pH 7.6), 5 mM MgCl2, and 1 mM sodium orthovanadate with 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 1 μg/ml pepstatin. Cell lysates were incubated with anti-Flag M2 beads (Sigma) overnight while tumbling at 4°C. Beads were washed several times and eluted with Flag peptides (Sigma). For total cell lysates, cells were washed several times in phosphate-buffered saline (PBS), lysed with 4% SDS in PBS, and boiled for 5 min, and the DNA was sheared by passage through a 19-gauge needle. Protein concentration was quantified using the bicinchoninic acid (BCA) method (Pierce, Rockford, IL). Samples were separated on denaturing polyacrylamide gels. Silver-stained gels were performed according to manufacturer's protocol (Bio-Rad Laboratories, Richmond, CA). Western blot gels were transferred onto PVDF membranes and blocked in skimmed milk powder in PBS containing 0.2% Tween 20 (PBST) before incubation with Flag-M2 (Sigma), eEF1A (Santa Cruz Biotechnology, Santa Cruz, CA), actin (Sigma), eEF2 (Cell Signaling Technology, Beverly, MA) or Expotin-5 (a kind gift from Ian Macara, University of Virginia, Charlottesville, VA) antibodies. Membranes were washed with 0.2% Tween-PBS and blotted with a secondary antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA) and detected by Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer, Waltham, MA).
Immunofluorescence
Cells were seeded onto coverslips and fixed with prechilled methanol for 10 min at −20°C followed by prechilled acetone for 1 min at −20°C. Anti-PABP1 mAb was used (Upstate Biotechnology, Lake Placid, NY). Cells were incubated for 1 h with a primary antibody solution containing 10% FBS and 1% Triton-X-100 (vol/vol) at room temperature in a humidified chamber. Cells were then washed several times in PBS before a 1-h incubation with a secondary Texas Red–labeled antibody (Jackson ImmunoResearch) at room temperature in a dark humidified chamber. Hoechst stain 33342 (Sigma) was added to visualize nuclei and coverslips were mounted using Fluoromount G (EMS, Hatfield, PA).
Radioisotope Labeling
Cells were plated on 35-mm dishes after which they were transfected with 100 nM eEF1A, eEF2, or scrambled siRNA (mock) for 48 and 72 h. At the indicated times cells were incubated for 30 min in glutamine-, methionine-, and cysteine-free DMEM and then labeled with 10 μCi/ml [35S]Met for 30 min. Cells were harvested and lysed for 30 min at 4°C with modified RIPA lysis buffer containing 50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, and a cocktail of protease inhibitors. Total cellular lysates were separated on a denaturing polyacrylamide gel. The gel was transferred onto a PVDF membrane, and [35S]Met labeling was visualized by autoradiography. The membrane was washed three times in PBST and blocked in skimmed milk powder in PBST before incubation with eEF1A and eEF2 antibodies.
In Vitro Nuclear Export Assay
The in vitro export assay was performed as described in Groulx et al. (2000). Briefly, cells were plated and grown on a 35-mm coverslip plate. Cells were washed with transport buffer (TB) containing 20 mM HEPES, pH 7.3, 110 mM KOAc, 5 mM NaOAc, 2 mM Mg(OAc)2 and permeabilized at 4°C for 5 min with TB containing 50 μg/ml digitonin and a protease inhibitor mixture (Hoechst stain 33258; Sigma) was used to monitor the permeabilization). After several washes with TB at 4°C, cells were incubated for 30–45 min at 20°C in the presence of a standard mixture that included MCF-7 cellular lysate, TB, 2 mM ATP, 2 mM GTP, and an ATP-regenerating system (5 mM creatine phosphate and 20 U/ml creatine phosphokinase) to a final volume of 1 ml. To obtain the MCF-7 cellular lysate, first MCF-7 cells were incubated in RSB hypotonic buffer containing; 10 mM HEPES, pH 6.2, 10 mM NaCl and 1.5 mM MgCl2, for 15 min at 4°C. Cells were then homogenized in a tight pestle homogenizer. Protein concentrations were quantified using the BCA protocol. For experiments using eEF1A- or eEF2-depleted MCF-7 cellular lysates, cells were homogenized as described above. Lysate containing 0.5 mg of proteins was incubated in the presence of 10 μg of eEF1A antibody or 10 μg of eEF2 antibody and a cocktail of protease inhibitors for 1 h while rotating at 4°C. Undepleted lysates were incubated with 10 μg of irrelevant Flag antibody as a control. This was followed by incubation with 20 μl protein A/G PLUS-agarose beads (Santa Cruz Biotechnology) for 3 h at 4°C. Cells were then spun to remove beads bound to immunoprecipitated eEF1A of eEF2. This was followed by another round of eEF1A or eEF2 depletion using 10 μg of eEF1A antibody or 10 μg of eEF2 antibody and 20 μl protein A/G PLUS-agarose beads; however the immunoprecipitation was performed overnight. The lysate was spun and an aliquot of the supernatant immunoblotted to verify depletion of eEF1A or eEF2. Actin was used as a control. Lysates that were successfully depleted of eEF1A or eEF2 were used in the export assay in the same manner as described above.
RESULTS
The Translation Elongation Factor eEF1A Is a Novel VHL and PABP1-interacting Protein
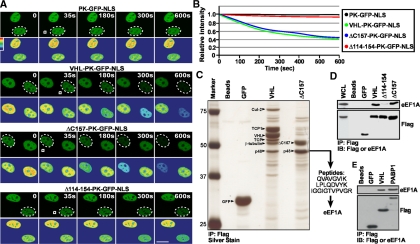
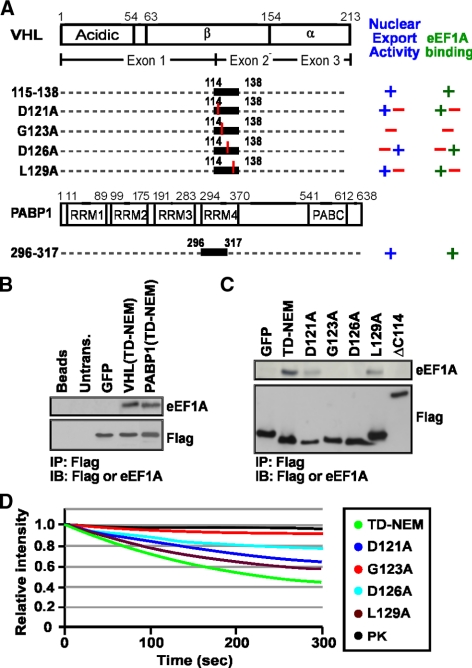
The VHL tumor suppressor, a recognition motif of a Cullin-2–containing E3 ubiqutin ligase complex, and PABP1, a general RNA metabolism and translation initiation factor, engage in a nuclear export pathway that is dependent on ongoing RNA PolII activity and a novel nuclear export motif, TD-NEM (Afonina et al., 1998; Lee et al., 1999; Groulx and Lee, 2002; Khacho et al., 2008). As previously reported, addition of RNA PolII inhibitors, such as ActD, inhibits nuclear export of both VHL and PABP1. In contrast, nuclear export of VHL and PABP1 is not affected by leptomycin B (LMB), a compound that abolishes CRM1-dependent nuclear export of NES-containing proteins (Afonina et al., 1998; Kudo et al., 1999; Lee et al., 1999; Khacho et al., 2008). This observation indicates that nuclear export of these proteins is independent of the classical CRM1/NES nuclear export pathway. Because nuclear export of VHL and PABP1 requires ongoing RNA PolII-dependent transcription, we considered the possibility that TD-NEM–mediated nuclear export occurs via an mRNA export pathway. However, inhibiting mRNA export using either the vesicular stomatitis virus (VSV; von Kobbe et al., 2000; Faria et al., 2005) or the influenza virus (Satterly et al., 2007), did not affect nuclear export of VHL (data not shown). Also, we were not able to identify any mRNA or tRNA species associated with VHL (data not shown), suggesting that nuclear export of VHL does not occur through an mRNA or tRNA export pathway. To further investigate the mechanism involved in nuclear export of VHL and PABP1, we produced adenoviruses that express Flag-tagged VHL and ΔC157, a C-terminal truncation mutant of VHL. ΔC157 fails to bind to core E3 ubiquitin ligase components but retains the ability to mediate nuclear export of the PK-GFP-NLS reporter in a FLIP nuclear export assay, which measures whether or not a protein can exit from the nucleus (see Materials and Methods), compared with PK-GFP NLS alone that does not encode any nuclear export signals (Figure 1, A and B), and maintains sensitivity to ActD (Lee et al., 1999; Bonicalzi et al., 2001; Khacho et al., 2008). Immunoprecipitation of VHL followed by silver staining of the gel revealed several previously identified associated proteins including the core ubiquitin ligase component Cullin-2 (Cul-2) and elongins B and C (Figure 1C and data not shown; Pause et al., 1997; Feldman et al., 1999; Hergovich et al., 2003). In addition, we observed a previously unidentified but highly abundant band that migrated at 48 kDa (Figure 1C). ΔC157 only assembled with the 48-kDa protein but not with the core ubiquitin ligase component Cullin-2 or other associated proteins, as expected (Figure 1C and Ohh et al., 2000; Bonicalzi et al., 2001). The p48 kDa band was the only major protein found associated with ΔC157 even in higher or lower percentage gels. Peptide sequence analysis identified p48 as the eukaryotic elongation factor 1A (eEF1A), a key component of the translational machinery (Andersen et al., 2003).
Figure 1.
Identification of a novel VHL and PABP1 interacting protein. (A and B) Full-length VHL and the truncation mutant ΔC157 mediate nuclear export of a PK-GFP-NLS reporter protein; however Δ114–154 is nuclear export deficient. MCF-7 cells transiently expressing VHL, ΔC157 or Δ114–154 fused to the PK-GFP-NLS reporter protein were subjected to the live cell FLIP nuclear export assay. The PK-GFP-NLS reporter consists of the large and amorphous pyruvate kinase (PK) to ensure that movement across the NPC is not due to diffusion, GFP to provide fluorescence, and a strong nuclear localization signal (NLS) to ensure all fusion proteins exhibit similar rates of nuclear import and to obtain a strong steady-state nuclear localization. In this assay a small region of the cytoplasm (white squares) within specific cells (dashed circles outline cell nuclei) was repeatedly photobleached. Kinetics for the loss of nuclear fluorescence, which indicates nuclear export activity, from images in A were calculated and plotted on a graph (B). (A) Pseudocolored panels are included to better illustrate subtle changes in fluorescence intensity (red represents highest intensity; blue represents lowest intensity). Scale bar, 10 μm. (C) Identification of a novel VHL-interacting protein. VHL−/− 786-0 cells were infected with Flag-tagged GFP, VHL-GFP, or ΔC157-GFP adenoviruses. Anti-Flag beads were incubated with lysis buffer (Beads lane) or lysates from infected cells. Immunoprecipitates were run on a 10% SDS-PAGE gel and subjected to silver staining. Bands were excised and analyzed by mass spectrometry. VHL associated with known interacting proteins, such as Cullin-2 (Cul-2), the cytosolic chaperonin TCP-1 and β-tubulin. p48 was identified as the eukaryotic elongation factor 1 alpha (eEF1A). Lanes referred to as “Beads” indicate that the Flag-beads were incubated with lysis buffer alone. (D) eEF1A can interact with VHL and ΔC157 but not with Δ114–154. 786-0 cells infected with adenoviruses to express Flag and GFP-tagged VHL, ΔC157, Δ114–154, or GFP alone were immunoprecipitated with anti-Flag beads and immunoblotted with anti-Flag or anti-eEF1A antibodies. Whole cell lysates (WCL) were obtained from 786-0 cells infected with F-VHL-GFP. (E) eEF1A is also a novel PABP1-interacting protein. MCF-7 cells were transiently transfected with Flag and GFP-tagged VHL, PABP1, or GFP alone. Immunoprecipitation and Western blot analysis was the same as in D.
Assembly of VHL and ΔC157 with endogenous eEF1A was confirmed by immunoprecipitation and Western blot analysis using anti-eEF1A antibodies (Figure 1D). Interestingly, endogenous eEF1A was not able to interact with a deletion mutant of VHL, Δ114–154, that does not encode a TD-NEM and fails to engage in nuclear export (Lee et al., 1999; Bonicalzi et al., 2001; Khacho et al., 2008), suggesting that eEF1A may play a role in nuclear export of VHL. More importantly PABP1 also interacted with endogenous eEF1A (Figure 1E). Because ΔC157 retains transcription-dependent nuclear export activity and only binds to eEF1A, we decided to further characterize this new VHL and PABP1-associated protein for its potential involvement in nuclear export.
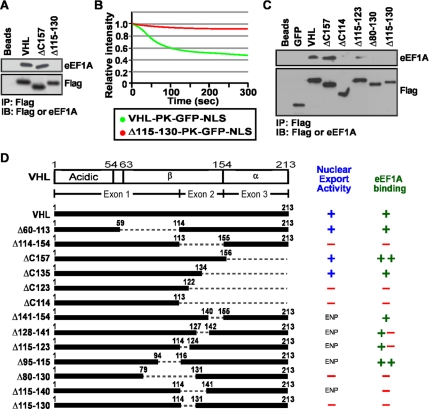
We reasoned that if eEF1A is involved in transcription-dependent nuclear export, its interaction with VHL would be dependent on the presence of TD-NEM, which is encoded within residues 115–130. We found that the nuclear export-defective VHL Δ115–130 (Khacho et al., 2008), which lacks the DxGx2Dx2L motif that encodes TD-NEM, fails to bind to eEF1A (Figure 2A and B). Truncation and deletion analysis revealed a clear correlation between VHL mutants that are able to export the PK-GFP-NLS reporter from the nucleus, in an in vivo nuclear export assay, and interact with endogenous eEF1A (Figure 2, C and D). These results point to a possible role for eEF1A in TD-NEM–mediated nuclear export of proteins.
Figure 2.
TD-NEM of VHL is required for interaction with eEF1A. (A and B) The transcription-dependent nuclear export sequence of VHL, encoded within residues 115–130, is required to interact with eEF1A and mediate nuclear export. (A) Cellular lysates from cells transiently expressing Flag-tagged VHL-GFP, Δ157-GFP, or Δ115–130-GFP were immunoprecipitated with anti-Flag beads and immunoblotted with anti-Flag and anti-eEF1A antibodies. Lanes referred to as “Beads” indicate that the Flag-beads were incubated with lysis buffer alone. (B) MCF-7 cells transiently expressing PK-GFP-NLS–tagged VHL or Δ115–130 were submitted to the live cell FLIP nuclear export assay. A small cytoplasmic region of the cell was photobleached repetitively and the loss of nuclear fluorescence, which is indicative of nuclear export activity, was monitored over time. The graph represents the relative loss of nuclear fluorescence over time. (C and D) Mapping the eEF1A-binding region within VHL. (C) Cellular lysates from cells transiently expressing the indicated constructs were immunoprecipitated and immunoblotted the same as in A. (D) Schematic diagram indicates deletion and truncation mutants of VHL that were submitted to immunoprecipitation with anti-Flag beads and immunoblotted with anti-Flag or anti-eEF1A antibodies. These mutants of VHL were also fused to PK-GFP-NLS and submitted to the live cell FLIP nuclear export assay. (+), (+ −), (− +), and (−) indicate, in a decreasing order, the ability of the fusion proteins to interact with eEF1A or export from the nucleus. ENP, experiment was not performed.
eEF1A Is Required for Nuclear Export of Proteins Encoding a TD-NEM
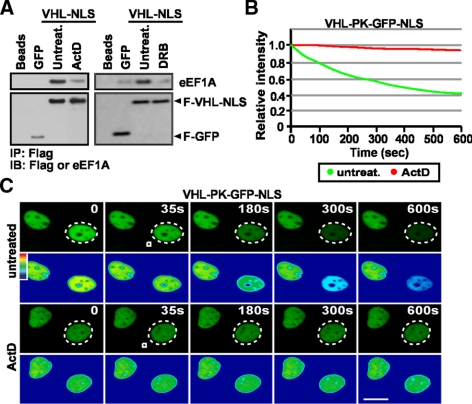
The requirement for eEF1A to mediate nuclear export of TD-NEM–containing proteins was assessed using different approaches. First, we tested the effect of inhibitors of RNA PolII-dependent transcription, such ActD and DRB, which cause nuclear accumulation of VHL and PABP1 (Lee et al., 1999; Bonicalzi et al., 2001; Groulx and Lee, 2002; Khacho et al., 2008) and abrogate TD-NEM–mediated nuclear export (Khacho et al., 2008). We wanted to test the possibility that ActD alters nuclear export by interfering with the TD-NEM/eEF1A interaction. Because ActD and DRB cause a nuclear shift in the localization of VHL, we fused VHL-GFP to a strong nuclear localization signal (NLS) in order to start with equal nuclear levels of protein in treated versus untreated cells. Addition of ActD or DRB, which inhibit TD-NEM activity (Figure 3, B and C, and Khacho et al., 2008) and cause nuclear accumulation of VHL and PABP1 (Lee et al., 1999; Groulx and Lee, 2002; Khacho et al., 2008), partially prevented assembly between VHL-GFP-NLS and eEF1A (Figure 3A). Because this experiment most likely captures an in vitro interaction it provides a link between transcription-dependent nuclear export and eEF1A. These data demonstrate a correlation between inhibition of TD-NEM nuclear export and eEF1A binding and suggest the possibility that ActD and DRB may block nuclear export of VHL by altering its interaction with eEF1A.
Figure 3.
Inhibitors of RNA PolII-dependent transcription block the interaction between VHL and eEF1A. (A) ActD and DRB hinder the interaction between eEF1A and VHL. MCF-7 cells transiently expressing F-GFP or F-VHL-GFP-NLS were untreated or treated with 8 μM ActD or 25 μg/ml DRB for 3 h before harvesting cells. Lysates were immunoprecipitated with anti-Flag beads and immunoblotted with Flag or eEF1A antibodies. (B and C) ActD inhibits TD-NEM–mediated nuclear export of VHL. MCF-7 cells transiently expressing VHL-PK-GFP-NLS were either untreated or treated with 2 μM ActD for 1 h before being submitted to cytoplasmic FLIP analysis. Cells were repeatedly bleached in a small cytoplasmic region (white squares) within cells (dotted circles outline the nuclei of bleached cells). The loss of nuclear fluorescence was monitored (C), calculated, and plotted on a graph (B). Scale bar, 10 μm.
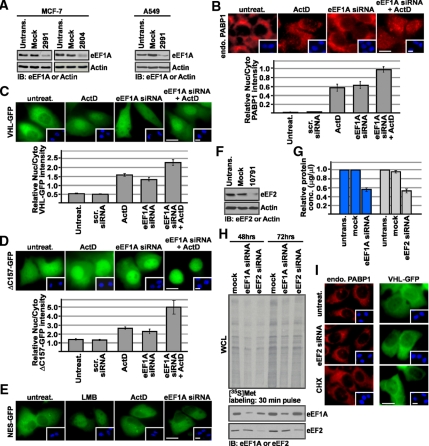
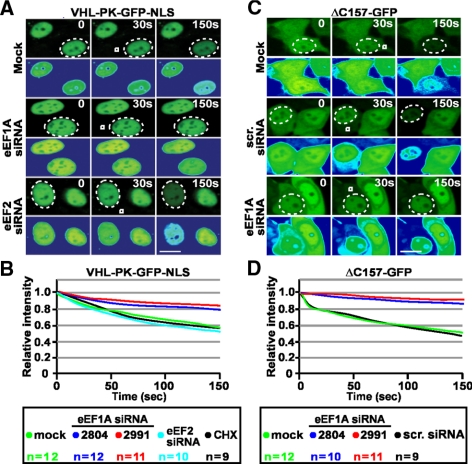
Second, we tested the effect of silencing endogenous eEF1A using RNA interference. Silencing of eEF1A with two independent siRNAs markedly reduced the levels of eEF1A protein (Figure 4A) but did not result in measurable cell death as quantified by propidium iodide (PI) exclusion (data not shown). Supposing that eEF1A is required for transcription-dependent nuclear export, we reasoned that silencing of its protein levels should alter the steady state localization of TD-NEM-containing proteins in a manner reminiscent to the nuclear accumulation induced by ActD or DRB. Silencing eEF1A led to nuclear accumulation of endogenous PABP1 and transiently expressed VHL-GFP and ΔC157-GFP, which was similar to that induced by ActD treatment, when compared with untreated cells or those transfected with a scrambled siRNA (Figure 4, B–D). However, localization of the soluble cytoplasmic fusion protein, GFP-NES, which is altered by LMB, was not affected by eEF1A knockdown or ActD treatment (Figure 4E). Because eEF1A is highly abundant it is difficult to achieve a complete knockdown of its protein levels, most likely resulting in residual eEF1A. We hypothesized that silencing eEF1A and treating with ActD, which partially inhibits interaction between eEF1A and VHL (Figure 3A), should result in an additive effect because the residual eEF1A would be blocked by ActD. Indeed, transfecting cells with eEF1A siRNA followed by ActD treatment resulted in a significant increase in the nuclear/cytoplasmic ratio of endogenous PABP1 and transiently expressed VHL-GFP and ΔC157-GFP (Figure 4, B–D). In fact, we observed a complete nuclear shift of ΔC157-GFP (Figure 4D), a protein that fails to interact with most VHL-binding proteins (Figure 1C). To eliminate the possibility that nuclear accumulation is due to a block in translation elongation, we silenced the elongation factor eEF2 (Figure 4F) using RNA interference to achieve a comparable decrease in protein synthesis and translational activity as that obtained with eEF1A silencing (Figure 4, G and H). Silencing eEF2 did not have an effect on the steady-state localization of either endogenous PABP1 or transiently expressed VHL-GFP (Figure 4I). Treatment with cycloheximide, an inhibitor of protein translation, also had no effect (Figure 4I), confirming that the change in steady-state localization was not due to a requirement of ongoing protein synthesis.
Figure 4.
siRNA-mediated silencing of eEF1A causes nuclear accumulation of TD-NEM–containing proteins. (A) eEF1A-specific siRNA reduces eEF1A protein level. MCF-7 and A549 cells were either mock-transfected or transfected with 100 nM eEF1A siRNA (2991 or 2804) for 72 h and then subjected to Western blot analysis using anti-eEF1A antibody. Actin was used as a loading control. (B) eEF1A knockdown and ActD treatment cause nuclear accumulation of endogenous PABP1. MCF-7 cells were transiently transfected with 100 nM control scrambled siRNA, 100 nM eEF1A siRNA (2991 or 2804), or untransfected for 72 h. Where indicated cells were transfected with 100 nM eEF1A siRNA for 72 h followed by treatment with 8 μM ActD for 3 h. Localization of endogenous PABP1 was determined by immunofluorescence using a PABP1-specific antibody. Scale bar, 10 μm. (C–E) eEF1A knockdown and ActD treatment results in nuclear accumulation of transiently expressed VHL-GFP and ΔC157-GFP, but does not affect GFP-NES. MCF-7 cells were cotransfected with 100 nM eEF1A siRNA (2991 or 2804) or control scrambled siRNA and VHL-GFP, ΔC157-GFP, or GFP-NES. Where indicated, cells were treated with 8 μM ActD or 10 μM LMB for 3 h after the 72-h incubation after transfection. Insets in B–E show Hoechst staining of DNA; scale bars, 10 μm. Graphs in B–D represent relative nuclear:cytoplasmic ratios of fluorescence intensity. (F–I) Nuclear accumulation of TD-NEM–containing proteins in the absence of eEF1A is not due to an overall decrease in protein translation. (F) A549 cells were either mock-transfected or transfected with 100 nM eEF2 siRNA (10791) for 72 h and then subjected to Western blot analysis using anti-eEF2 antibody. Actin was used as a loading control. (G) A549 cells were untransfected, mock-transfected, or transfected with eEF1A or eEF2 siRNA for 72 h. Total cellular protein levels were measured using a standard protein quantification method. The decrease in protein levels in mock- or siRNA-transfected cells was calculated relative to protein levels in untransfected cells. (H) A549 cells were mock-transfected or transfected with eEF1A or eEF2 siRNA for 48 and 72 h. At the indicated times, cells were pulse-labeled for 30 min with [35S]Met. Labeled whole cell lysates (WCL) were separated on a 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane, and translational activity was visualized by autoradiography (top panel). The two bottom panels show immunoblots from the same membrane, using eEF1A and eEF2 antibodies. (I) For determining the effect of eEF2 silencing, cells were transfected with only 100 nM eEF2 siRNA or cotransfected with VHL-GFP and 100 nM eEF2 siRNA for 72 h. Endogenous PABP1 was detected by immunofluorescence using a PABP1 antibody. For determining the effect of cycloheximide, cells were either untransfected or transiently transfected with VHL-GFP followed by treatment with cycloheximide for 2 h. Insets, Hoechst staining of DNA; scale bars, 10 μm.
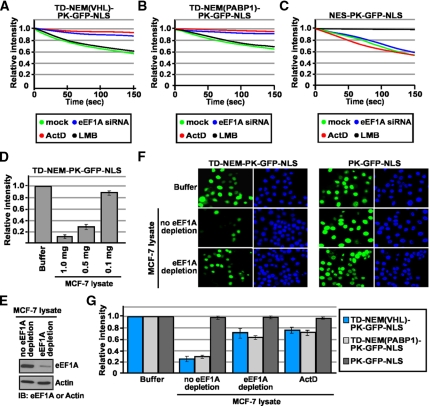
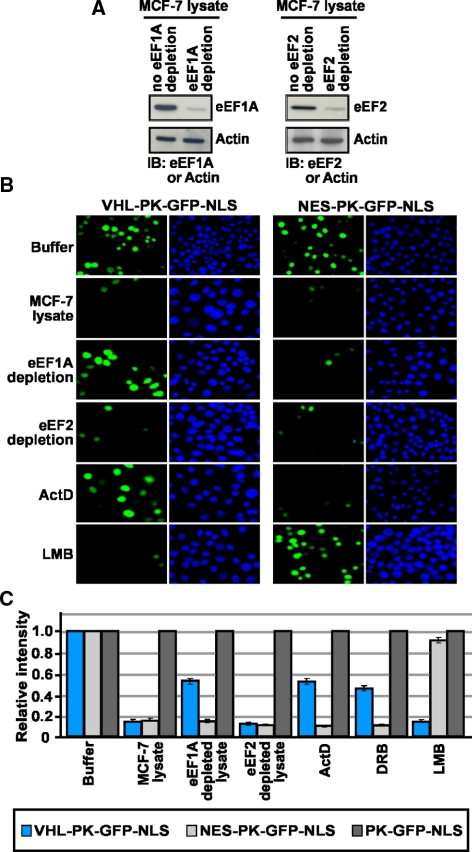
siRNA-mediated silencing of eEF1A protein decreased the nuclear export rate of VHL-PK-GFP-NLS and ΔC157-GFP to levels similar to those observed upon treatment with ActD when compared with mock or control scrambled siRNA (Figure 5, A–D). However, silencing of eEF1A did not have an effect on the nuclear export rate of a classical nuclear export sequence (NES-PK-GFP-NLS; refer to Figure 9C). This indicates that silencing this translational factor does not alter the nuclear-cytoplasmic trafficking in general. Likewise, silencing eEF2 or treatment with cycloheximide confirmed that the reduced rate of export of VHL-PK-GFP-NLS was not due to the requirement of ongoing protein synthesis (Figure 5, A and B) but specifically from the decreased levels of endogenous eEF1A. To further uncouple the function of eEF1A in translation from its role in nuclear export and to provide additional evidence for a role in TD-NEM mediated nuclear export, we performed in vitro nuclear export assays. This assay serves to; 1) uncouple ongoing translation with nuclear export activity of eEF1A because there is negligible ongoing translation in the in vitro export assay and 2) eliminate the possibility for the loss of unknown factor(s) due to the partial arrest in translation in siRNA treatment for 48 and 72 h. Addition of cellular lysate depleted from eEF1A, using eEF1A specific antibodies (Figure 6A and see Materials and Methods), resulted in a significant decrease in nuclear export of VHL-PK-GFP-NLS in digitonin-permeabilized cells when compared with nondepleted lysate or lysate incubated with an irrelevant Flag antibody (Figure 6, B and C). Addition of ActD or DRB to living cells before digitonin treatment resulted in a similar decrease in nuclear export of VHL-PK-GFP-NLS (Figure 6, B and C). However, addition of cellular lysate depleted of eEF2 (Figure 6A) did not have an effect (Figure 6, B and C). In vitro nuclear export of NES-PK-GFP-NLS, which is sensitive to LMB (Figure 6, B and C), was not affected by either eEF1A or eEF2 depletion or by ActD and DRB treatment (Figure 6, B and C). These observations verify the results obtained with siRNA-mediated silencing of eEF1A and demonstrate that indeed eEF1A is required for nuclear export of TD-NEM. These results demonstrate that eEF1A binds to and is involved in nuclear export of TD-NEM–containing proteins and are consistent with a previous report showing that eEF1A is required for export of molecules in a yeast genetic system (Grosshans et al., 2000a).
Figure 5.
eEF1A is required for nuclear export of TD-NEM–containing proteins in living cells. (A–D) Silencing of eEF1A alters nuclear export of TD-NEM–containing proteins. MCF-7 cells were either mock-transfected or cotransfected with 100 nM eEF1A siRNA (2991 or 2804) or control siRNA and VHL-PK-GFP-NLS (A and B) or ΔC157-GFP (C and D) for 48 h before being submitted to the live cell FLIP nuclear export assay. In B cells were also treated with cycloheximide (CHX) for 1 h before cytoplasmic FLIP analysis. Cells were repetitively photobleached in a small cytoplasmic area (white box) within a cell of interest (dotted circle outlines the cell nucleus). The loss of nuclear fluorescence was monitored and plotted on a graph. The line graphs represent mean values obtained from the indicated number of cells used (n) per condition. Scale bars, 10 μm.
Figure 9.
eEF1A is required for TD-NEM–mediated nuclear export. (A-C) TD-NEM nuclear export requires eEF1A and is independent of the CRM1/NES nuclear export pathway. MCF-7 cells were transiently cotransfected with the indicated constructs and 100 nM eEF1A siRNA for 48 h before being assessed by the live cell FLIP nuclear export assay. Where indicated, cells were transfected with the indicated constructs then treated with either 2 μM ActD or 10 μM LMB for 1 h before cytoplasmic FLIP. The loss of nuclear fluorescence was monitored and graphed. (D–G) eEF1A is required for nuclear export of TD-NEM in an in vitro export assay. (D) Protein levels in an MCF-7 cellular lysate were quantified using standard protein quantification methods. Buffer alone or cellular lysate containing different protein amounts in the presence of the ATP-, GTP-, and ATP-regenerating system were added to digitonin-permeabilized cells transiently expressing TD-NEM-PK-GFP-NLS. Nuclear export was monitored by loss of nuclear fluorescence. Relative loss in nuclear fluorescence was calculated and plotted on a graph. (E) MCF-7 lysate containing 0.5 mg of protein was depleted of eF1A using eEF1A specific antibody (see Materials and Methods for details). The depletion of eEF1A was assessed by Western blot analysis. Actin was used as a control. (F and G) MCF-7 cells transiently expressing the indicated constructs were permeabilized with digitonin and incubated with transport buffer alone, MCF-7 lysate, or MCF-7 lysate that was depleted of eEF1A. Where indicated cells were pretreated with 8 μM ActD for 3 h before permeabilization with digitonin and the nuclear export assay was performed using undepleted MCF-7 lysate containing 8 μM ActD. All conditions were performed in the presence of ATP-, GTP-, and ATP-regenerating system. Nuclear export was monitored by loss of nuclear fluorescence (F). Relative loss in nuclear fluorescence was calculated and plotted on a graph (G).
Figure 6.
eEF1A is required for nuclear export of TD-NEM–containing proteins in vitro. (A) MCF-7 lysate containing 0.5 mg of protein (see Figure 9D) was depleted of eEF1A or eEF2 using eEF1A or eEF2 specific antibodies (see Materials and Methods for details). The depletion of eEF1A and eEF2 was assessed by Western blot analysis. Actin was used as a control. (B and C) MCF-7 cells transiently expressing the indicated constructs were permeabilized with digitonin and incubated with transport buffer alone, MCF-7 lysate, or MCF-7 lysate that was depleted of eEF1A or eEF2. Where indicated cells were pretreated with 8 μM ActD, 25 μg/ml DRB, or 10 μM LMB for 3 h before permeabilization with digitonin, and the nuclear export assay was performed using undepleted MCF-7 lysate containing the same concentrations of drugs as was used in the pretreatment. All conditions were performed in the presence of ATP-, GTP-, and ATP-regenerating system. Nuclear export was monitored by loss of nuclear fluorescence (B). Relative loss in nuclear fluorescence was calculated and plotted on a graph (C).
eEF1A Mediates Nuclear Export from the Cytoplasmic Side of the Nuclear Envelope
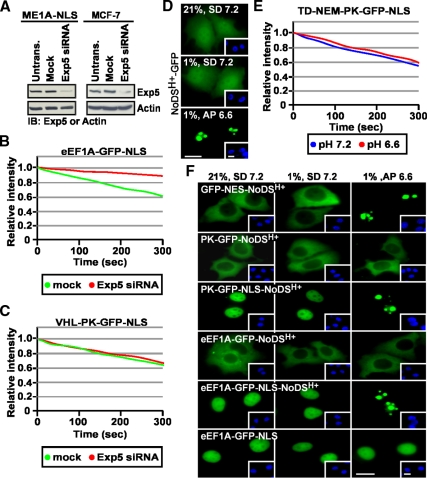
We examined the role of Exportin-5 (Exp5) in TD-NEM–mediated nuclear export since this exportin plays a role in nuclear export of eEF1A. siRNA-mediated silencing of endogenous Exp5 (Figure 7A) essentially abolished the ability of an eEF1A-GFP-NLS fusion protein to engage in nuclear export (Figure 7B) as previously shown by other groups (Bohnsack et al., 2002; Calado et al., 2002), though, in our cellular system, the steady-state localization of this fusion protein was mainly nuclear (see Figure 7F). In contrast, silencing of Exp5 did not disturb the nuclear export rate of VHL-PK-GFP-NLS (Figure 7C). These data suggest that the export pathway utilized by TD-NEM is independent of Exp5 and, consistent with data obtained with ActD and DRB, argues the possibility that tRNA may play a more minor role in this process.
Figure 7.
The function of eEF1A in nuclear export of TD-NEM–containing proteins is Exp5-independent and exerted from the cytoplasmic side of the nuclear envelope. (A–C) Nuclear export by TD-NEM is independent of Exp5. (A) ME1A-NLS (MCF-7 cells stably expressing eEF1A-GFP-NLS) and MCF-7 cells were transfected with 100 nM Exp5 siRNA for 48 h and then lysed and subjected to Western blot analysis using an anti-Exp5 antibody. Actin was used as a loading control. ME1A-NLS cells (B) or MCF-7 cells transiently expressing VHL-PK-GFP-NLS (C) were mock-transfected or transfected with 100 nM Exp5 siRNA and incubated for 48 h after which nuclear export was analyzed by the live cell FLIP nuclear export assay, as previously described. Loss of nuclear fluorescence was plotted on a graph (B and C). (D–F) eEF1A is not targeted to the nucleolus by the NoDSH+. (D) MCF7 cells were cultured in standard media (SD) and transiently transfected to express the indicated GFP-tagged constructs. Cells were replenished with either fresh standard media (SD, pH 7.2) or acidification permissive media (AP, initial pH 7.2) that allows maximal extracellular acidification to pH 6.6 (see Materials and Methods). Cells either remained in normoxia (21% O2) or transferred to hypoxia (1% O2) for 18 h. Extracellular pH at the endpoint is indicated on each panel. (E) MCF-7 cells transiently expressing TD-NEM fused to PK-GFP-NLS were treated the same as in D. Cytoplasmic FLIP was performed to assess nuclear export activity. Loss of nuclear fluorescence was monitored and plotted on a graph. (F) MCF-7 cells transiently expressing the indicated constructs were incubated in the same conditions as in D. The localization of GFP-tagged fusion proteins was assessed by fluorescent microscopy. Insets in D and F show Hoechst staining of DNA; scale bars, 10 μm.
This data raised the possibility that eEF1A may promote nuclear export of TD-NEM–containing proteins without having to enter the nucleoplasm. This would be consistent with a model proposed by Grosshans et al. (2000) and McGuire et al. (2007) that eEF1A can stimulate nuclear export of tRNA from the cytoplasmic side of the nuclear envelope and that eEF1A ongoing nuclear import rate, if any, is very slow (Bohnsack et al., 2002; Calado et al., 2002). To test this, we designed an assay based on the nucleolar detention properties of the previously identified NoDSH+ (nucleolar detention signal mediated by H+), which targets proteins for static detention by the nucleolar architecture of acidotic cells (Mekhail et al., 2005, 2006, 2007). We reasoned that, upon NoDSH+ activation, an eEF1A-NoDSH+ fusion protein would be captured and detained by the nucleolus only if eEF1A is able to enter the nucleoplasm. NoDSH+-GFP displays a diffuse nucleocytoplasmic localization pattern similar to GFP alone in neutral pH but accumulated in the nucleolus in acidotic cells, as previously described (Figure 7D and Mekhail et al., 2005, 2006). Similarly, NES-NoDSH+-GFP, which can diffuse into the nucleus but has a steady-state cytoplasmic localization due the presence of a strong and classical NES, is targeted to the nucleolus during acidosis (Figure 7F). PK-GFP-NoDSH+ failed to accumulate in the nucleolus of acidotic cells since it cannot import into the nucleoplasm, in contrast to the same fusion protein with an NLS (Figure 7F). eEF1A-GFP-NoDSH+ did not accumulate in the nucleolus suggesting that eEF1A does not import into the nucleoplasm (Figure 7F). Addition of a NLS caused the complete nucleolar sequestration of eEF1A-NoDSH+ in acidosis indicating that this protein can be captured by the nucleolus once imported into the nucleus (Figure 7F). NoDSH+-containing proteins that display a similar cytoplasmic distribution at steady state to eEF1A, but can import into the nucleoplasm, are captured by the nucleolus in acidic pH (Figure 7F and see Mekhail et al., 2005). The rate of nuclear export of TD-NEM–containing proteins is similar regardless of the extracellular pH (Figure 7E) providing evidence that eEF1A can function in nuclear export of TD-NEM–containing proteins without having to enter the nucleus.
eEF1A Specifically Interacts with the TD-NEM Nuclear Export Sequence
As expected, we found that both residues 114–138 of VHL and residues 296–317 of PABP1 alone, which encode TD-NEM and mediate transcription-dependent nuclear export activity, were sufficient to bind to eEF1A (Figure 8, A and B). Single amino acid substitutions of key residues in the DxGx2Dx2L motif of VHL markedly reduced binding to eEF1A and nuclear export activity (Figure 8, A, C, and D). These data further demonstrate a clear correlation between nuclear export activity of TD-NEM and its ability to interact with eEF1A.
Figure 8.
TD-NEM is sufficient for eEF1A interaction. (A) Schematic diagram depicts the TD-NEM of VHL encoded within residues 115–138 and alanine substitution of the key residues of TD-NEM. Schematic diagram also depicting the TD-NEM of PABP1 encoded between residues 296 and 317. (+), (+ −), (− +), and (−) indicate, in a decreasing order, the ability of the fusion proteins to interact with eEF1A or export from the nucleus. (B) The transcription-dependent nuclear export sequence, TD-NEM, of VHL and PABP1 is sufficient to interact with endogenous eEF1A. MCF-7 cells transiently expressing GFP alone or the TD-NEM sequences of VHL or PABP1 tagged with Flag and GFP were submitted to immunoprecipitation with anti-Flag beads and immunoblotted with anti-Flag or anti-eEF1A antibodies. TD-NEM refers to residues 114–138 of VHL and residues 298–317 of PABP1. (C) Conserved residues in TD-NEM are required for eEF1A interaction. TD-NEM refers to residues 114–138 of VHL. Flag and GFP-tagged D121A, G123A, D126A, and L129A indicate the amino acid that was substituted to alanine within residues 114–138 of VHL. Immunoprecipitation and Western blot was as described above. (D) Conserved residues in TD-NEM are required for efficient nuclear export. MCF-7 cells transiently expressing TD-NEM, D121A, G123A, D126A, or L129A fused to PK-GFP-NLS or expressing PK-GFP-NLS alone were subjected to the live cell FLIP nuclear export assay. The loss of nuclear fluorescence during cytoplasmic FLIP was monitored and graphed.
eEF1A Is Required for Nuclear Export of TD-NEM
To verify the involvement of eEF1A during transcription-dependent nuclear export our last criterion was to test the requirement for eEF1A in the nuclear export of the minimal TD-NEM sequence. We have previously shown that TD-NEM of both VHL and PABP1 mediate nuclear export of a PK-GFP-NLS reporter protein in a manner that is sensitive to ActD, but insensitive to LMB, an inhibitor of the classical NES/CRM1 nuclear export pathway (Khacho et al., 2008). In contrast, nuclear export of the classical NES is inhibited by LMB but is unaffected by ActD (Khacho et al., 2008). This demonstrates that nuclear export through TD-NEM operates independently of the classical CRM1/NES-mediated nuclear export pathway. siRNA-mediated silencing of eEF1A results in a decreased nuclear export rate of TD-NEM from both VHL and PABP1 to levels similar to those observed upon treatment with ActD (Figure 9, A and B). However, silencing of eEF1A did not have an effect on nuclear export of NES (Figure 9C) further demonstrating that the classical NES/CRM1 nuclear export pathway operates independently of eEF1A. These results also support the involvement of eEF1A in TD-NEM–mediated nuclear export because the decreased export of TD-NEM upon silencing of eEF1A is not due to a decrease in protein synthesis or a general effect on nuclear-cytoplasmic trafficking.
Next we tested the requirement of eEF1A to mediate nuclear export of TD-NEM in an in vitro nuclear export assay. Addition of decreasing amounts of cellular lysate to digitonin-permeabilized cells expressing TD-NEM-PK-GFP-NLS demonstrated the presence of a limiting factor in the lysate, which is required for mediating TD-NEM nuclear export (Figure 9D). Addition of cellular lysate depleted from eEF1A, using eEF1A specific antibodies (Figure 9E and see Materials and Methods), resulted in a significant decrease in nuclear export of TD-NEM of VHL and PABP1 in digitonin-permeabilized cells when compared with nondepleted lysate or lysates incubated with an irrelevant Flag antibody (Figure 9F and G). Addition of ActD to living cells before digitonin treatment resulted in a similar decrease in nuclear export (Figure 9G). These observations verify the results obtained with siRNA-mediated silencing of eEF1A and demonstrate that indeed eEF1A is required for nuclear export of TD-NEM.
DISCUSSION
VHL and PABP1 share the common characteristic of engaging in a nuclear export pathway that is dependent on ongoing RNA PolII activity, but independent of the classical CRM1/NES nuclear export pathway. We have previously shown that transcription-dependent nuclear export of VHL and PABP1 is mediated by a novel nuclear export motif, TD-NEM. Here we report that TD-NEM–mediated nuclear export involves an essential member of the translational machinery, the translation elongation factor eEF1A. eEF1A is a novel VHL and PABP1 interacting protein that binds specifically to the TD-NEM sequence. siRNA-induced silencing or antibody-mediated depletion of eEF1A decreases TD-NEM nuclear export kinetics. Inhibition of RNA PolII-dependent transcription impairs the TD-NEM/eEF1A interaction thereby decreasing TD-NEM–mediated nuclear export. Inhibition of eEF1A did not effect NES-mediated nuclear export of proteins, suggesting that eEF1A and CRM1 operate independently. Because eEF1A exhibits a predominantly cytoplasmic localization, we propose that its functional role is orchestrated from the cytoplasmic side of the nuclear envelope. The results presented here highlight the essential role of cytoplasmic export factors in the coordination of events that lead to export from the nuclear to the cytoplasmic compartment. Meanwhile, these results also provide a link between the translational apparatus and the subcellular trafficking machinery demonstrating that these two central pathways in basic metabolism can cooperate to sustain the highly dynamic nature of cellular processes.
Regulated and signal-mediated transport of proteins between the nucleus and the cytoplasm is critical for many cellular processes. Several studies have reported the need for ongoing RNA PolII activity to actively shuttle proteins between the nuclear and cytoplasmic compartments. Most studies have focused on the role of ongoing transcription in nuclear import of proteins such as hnRNPs (Pinol-Roma and Dreyfuss, 1991, 1992, 1993; Siomi et al., 1997). However, there are also proteins that necessitate RNA PolII-mediated transcription to efficiently export from the nucleus, including VHL and PABP1 (Afonina et al., 1998; Lee et al., 1999; Groulx and Lee, 2002; Griffis et al., 2004; Zhang et al., 2005; Khacho et al., 2008). In an attempt to understand the mechanism of transcription-dependent nuclear export we set out to identify components involved in this nuclear export pathway. In doing so, we have identified a member of the translational machinery, eEF1A, as a factor involved in the transcription-dependent nuclear export pathway. The correlation between binding and nuclear export activity argues that the interaction between TD-NEM and eEF1A is biochemically relevant. Removal of TD-NEM from full-length VHL totally abolished binding to eEF1A. TD-NEM alone from both VHL and PABP1, which consists of only a few residues, bound to eEF1A. However, mutations of key residues within the DxGx2Dx2L consensus sequence of TD-NEM, particularly G123A, measurably hindered the ability of TD-NEM to bind to eEF1A and mediate export of reporter proteins. Yet, it is the data obtained with the functional assays that substantiates a role for eEF1A in TD-NEM–dependent nuclear export. Silencing of endogenous eEF1A alters the steady-state localization of full-length endogenous PABP1 and transiently expressed VHL, and the nuclear export competent VHL truncation mutant ΔC157. The reduction of nuclear export rate in a FLIP nuclear export assay of cells treated with siRNA against eEF1A argues that eEF1A has a direct role in the nuclear export activity of TD-NEM in living cells though a potential role of cytoplasmic retention cannot be formally excluded. However, depletion of endogenous eEF1A from a cellular lysate resulted in a decrease in nuclear export activity of TD-NEM–containing proteins in an in vitro nuclear export assay arguing that eEF1A is directly involved in nuclear export. Treatment with ActD or DRB partially abrogated binding of eEF1A with TD-NEM, providing a possible explanation for their ability to block TD-NEM activity and nuclear export of VHL and PABP1. Although TD-NEM–mediated nuclear export does not appear to occur via mRNA export, we cannot exclude the possibility that other RNA PolII-dependent RNA species may be involved. Put together, these data, in addition to the binding results, support a role for eEF1A in TD-NEM–dependent nuclear export.
Several lines of evidence suggest that eEF1A itself is a key element in the coordination of TD-NEM nuclear export, rather than its contribution to protein translation as a member of the translational machinery. First, acute inhibition of protein translation by cycloheximide did not have an effect on TD-NEM–mediated nuclear export. Second, chronic inhibition of translation was tested by silencing of another translation elongation factor, eEF2, such that a comparable decrease in steady-state protein levels and translational activity, as in eEF1A silencing, was achieved. Knockdown of eEF2 did not have an effect on the localization or the nuclear export activity of TD-NEM–containing proteins compared with silencing of eEF1A. In addition, these experiments suggest that blockage of protein synthesis during the period of the experiments did not deplete a protein whose synthesis would have been required for nuclear export of TD-NEM–containing proteins. Finally, the role of eEF1A in TD-NEM–mediated nuclear export was addressed using in vitro nuclear export assays. This assay uncouples ongoing translation with nuclear export activity of eEF1A and it eliminates the possibility for the loss of unknown factor(s) required for nuclear export. Together these results aid to uncouple the role of eEF1A in TD-NEM–mediated nuclear export and protein synthesis.
Work conducted by several groups, including the present study, have failed to detect endogenous or exogenous eEF1A in the nuclear compartment. In fact, Calado et al. (2002) proposed the Exp5 pathway as a way to exclude eEF1A from nuclei if it were ever to enter, such as after cell division. In this study we demonstrate that TD-NEM–mediated nuclear export is independent of the Exp5 pathway. Also, the nucleolar retention experiments shown here, in addition to data from other groups, suggest that eEF1A may exert its function in TD-NEM–dependent nuclear export from the cytoplasmic side of the nuclear envelope. Because the nucleolar retention experiments were performed using exogenous eEF1A, we cannot formally exclude the possibility that a small population of endogenous eEF1A is capable of entering the nuclear compartment. In fact, this is an intriguing possibility that would easily explain the role of eEF1A in nuclear export of RNA species and TD-NEM proteins. It is difficult to assess whether this is the case. Consistent with a possible cytoplasmic role for eEF1A, is the work by Grosshans et al. (2000) that reported a role for cytoplasmic eEF1A in nuclear export of tRNAs in yeast, where nuclear accumulation of mature tRNAs was observed in strains with reduced amounts or mutated eEF1A. Because eEF1A was undetectable in the nuclear compartment, Grosshans et al. postulated that eEF1A may function at the cytoplasmic face of the nuclear pore to facilitate the release of charged tRNAs from the aminoacyl-tRNA synthase. Interestingly, another cytoplasmic protein, Cexp1, has been recently identified as a component of the tRNA export pathway. In fact, given that Cexp1 associates with the NPC by interacting with Nup116p, it was proposed that Cexp1 collects aminoacyl-tRNAs from nuclear export receptors at the cytoplasmic side of the NPC and transfer them to eEF1A using a channeling mechanism (McGuire and Mangroo, 2007). Now we have evidence to support a role for eEF1A in nuclear export of TD-NEM–containing proteins. Because a common theme for cytoplasmic nuclear export factors is their involvement in the final steps of transport, it is possible that eEF1A functions in receiving proteins as they pass through the NPC. In this sense, eEF1A may utilize its GDP/GTP binding properties to release proteins in the cytoplasm, similar to the manner by which RanGTP hydrolysis to RanGDP results in the release of exportin-cargo complexes.
Previously, cytoplasmic eEF1A has been implicated in the nuclear export pathway of tRNA in yeast. We propose that eEF1A is a cytoplasmic component of the TD-NEM–dependent nuclear export pathway in mammalian cells. The fact that eEF1A may function only from the cytoplasmic side of the nuclear envelope implies the existence a yet unidentified nuclear exporter that would interact with TD-NEM–containing cargo in the nucleus to facilitating its passage though the NPC. However we cannot rule out the possibility that the TD-NEM/eEF1A system operates independently of known classical nuclear export pathways. Future work in unraveling other components of this export pathway will further uncover the mechanism of transcription-dependent nuclear export of proteins and clarify the precise role of eEF1A during this process.
ACKNOWLEDGMENTS
We thank Ian Macara for providing the Exportin-5 antibody. We thank Josianne Payette for technical support. This work is supported by a grant from the Canadian Institutes of Health Research (CIHR) to S. Lee. S. Lee. is the recipient of the National Cancer Institute of Canada Harold E. Johns Award. K. Mekhail is a recipient of CIHR fellowship.
Abbreviations used:
- ActD
actinomycin D
- CRM1
Chromosome Region Maintenance (exportin 1)
- Dbp5
the DEAD box RNA helicase Rat8p/Dbp5p
- eEF1A
eukaryotic translation elongation factor 1 alpha
- FLIP
fluorescence loss in photobleaching
- LMB
leptomycin B
- NES
nuclear export sequence
- NLS
nuclear localization signal
- NoDSH+
nucleolar detention signal regulated by [H+]
- PABP1
poly(A)-binding protein 1
- PK
pyruvate kinase
- TD-NEM
transcription-dependent nuclear export motif
- VHL
von Hippel-Lindau.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-06-0562) on September 17, 2008.
REFERENCES
- Afonina E., Stauber R., Pavlakis G. N. The human poly(A)-binding protein 1 shuttles between the nucleus and the cytoplasm. J. Biol. Chem. 1998;273:13015–13021. doi: 10.1074/jbc.273.21.13015. [DOI] [PubMed] [Google Scholar]
- Alcazar-Roman A. R., Tran E. J., Guo S., Wente S. R. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat. Cell Biol. 2006;8:711–716. doi: 10.1038/ncb1427. [DOI] [PubMed] [Google Scholar]
- Andersen G. R., Nissen P., Nyborg J. Elongation factors in protein biosynthesis. Trends Biochem. Sci. 2003;28:434–441. doi: 10.1016/S0968-0004(03)00162-2. [DOI] [PubMed] [Google Scholar]
- Bischoff F. R., Klebe C., Kretschmer J., Wittinghofer A., Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc. Natl. Acad. Sci. USA. 1994;91:2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Krebber H., Kempf T., Hermes I., Ponstingl H. Human RanGTPase-activating protein RanGAP1 is a homologue of yeast Rna1p involved in mRNA processing and transport. Proc. Natl. Acad. Sci. USA. 1995a;92:1749–1753. doi: 10.1073/pnas.92.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Krebber H., Smirnova E., Dong W., Ponstingl H. Co-activation of RanGTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1. EMBO J. 1995b;14:705–715. doi: 10.1002/j.1460-2075.1995.tb07049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack M. T., Regener K., Schwappach B., Saffrich R., Paraskeva E., Hartmann E., Gorlich D. Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm. EMBO J. 2002;21:6205–6215. doi: 10.1093/emboj/cdf613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonicalzi M. E., Groulx I., de Paulsen N., Lee S. Role of exon 2-encoded beta -domain of the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 2001;276:1407–1416. doi: 10.1074/jbc.M008295200. [DOI] [PubMed] [Google Scholar]
- Calado A., Treichel N., Muller E. C., Otto A., Kutay U. Exportin-5-mediated nuclear export of eukaryotic elongation factor 1A and tRNA. EMBO J. 2002;21:6216–6224. doi: 10.1093/emboj/cdf620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Scarcelli J. J. Transport of messenger RNA from the nucleus to the cytoplasm. Curr. Opin. Cell Biol. 2006;18:299–306. doi: 10.1016/j.ceb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Coutavas E., Ren M., Oppenheim J. D., D'Eustachio P., Rush M. G. Characterization of proteins that interact with the cell-cycle regulatory protein Ran/TC4. Nature. 1993;366:585–587. doi: 10.1038/366585a0. [DOI] [PubMed] [Google Scholar]
- Faria P. A., Chakraborty P., Levay A., Barber G. N., Ezelle H. J., Enninga J., Arana C., van Deursen J., Fontoura B. M. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol. Cell. 2005;17:93–102. doi: 10.1016/j.molcel.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Feldman D. E., Thulasiraman V., Ferreyra R. G., Frydman J. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol. Cell. 1999;4:1051–1061. doi: 10.1016/s1097-2765(00)80233-6. [DOI] [PubMed] [Google Scholar]
- Fischer U., Huber J., Boelens W. C., Mattaj I. W., Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- Fornerod M., Ohno M., Yoshida M., Mattaj I. W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Asano S., Nakamura T., Adachi M., Yoshida M., Yanagida M., Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Gorlich D., Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- Griffis E. R., Craige B., Dimaano C., Ullman K. S., Powers M. A. Distinct functional domains within nucleoporins Nup153 and Nup98 mediate transcription-dependent mobility. Mol. Biol. Cell. 2004;15:1991–2002. doi: 10.1091/mbc.E03-10-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans H., Hurt E., Simos G. An aminoacylation-dependent nuclear tRNA export pathway in yeast. Genes Dev. 2000a;14:830–840. [PMC free article] [PubMed] [Google Scholar]
- Grosshans H., Simos G., Hurt E. Review: transport of tRNA out of the nucleus-direct channeling to the ribosome? J. Struct. Biol. 2000b;129:288–294. doi: 10.1006/jsbi.2000.4226. [DOI] [PubMed] [Google Scholar]
- Groulx I., Bonicalzi M. E., Lee S. Ran-mediated nuclear export of the von Hippel-Lindau tumor suppressor protein occurs independently of its assembly with cullin-2. J. Biol. Chem. 2000;275:8991–9000. doi: 10.1074/jbc.275.12.8991. [DOI] [PubMed] [Google Scholar]
- Groulx I., Lee S. Oxygen-dependent ubiquitination and degradation of hypoxia-inducible factor requires nuclear-cytoplasmic trafficking of the von Hippel-Lindau tumor suppressor protein. Mol. Cell. Biol. 2002;22:5319–5336. doi: 10.1128/MCB.22.15.5319-5336.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A., Lisztwan J., Barry R., Ballschmieter P., Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat. Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- Hodge C. A., Colot H. V., Stafford P., Cole C. N. Rat8p/Dbp5p is a shuttling transport factor that interacts with Rat7p/Nup159p and Gle1p and suppresses the mRNA export defect of xpo1–1 cells. EMBO J. 1999;18:5778–5788. doi: 10.1093/emboj/18.20.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Shaheen H. H. A decade of surprises for tRNA nuclear-cytoplasmic dynamics. Trends Cell. Biol. 2008;18:98–104. doi: 10.1016/j.tcb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Izaurralde E., Kutay U., von Kobbe C., Mattaj I. W., Gorlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Khacho M., Mekhail K., Pilon-Larose K., Payette J., Lee S. Cancer-causing mutations in a novel transcription-dependent nuclear export motif of VHL abrogate oxygen-dependent degradation of hypoxia-inducible factor. Mol. Cell. Biol. 2008;28:302–314. doi: 10.1128/MCB.01044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A., Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat. Rev. 2007;8:761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- Kudo N., Matsumori N., Taoka H., Fujiwara D., Schreiner E. P., Wolff B., Yoshida M., Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Neumann M., Stearman R., Stauber R., Pause A., Pavlakis G. N., Klausner R. D. Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor suppressor protein. Mol. Cell. Biol. 1999;19:1486–1497. doi: 10.1128/mcb.19.2.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Guttinger S., Calado A., Dahlberg J. E., Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- Macara I. G. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 2001;65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R., Delphin C., Guan T., Gerace L., Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- Matunis M. J., Coutavas E., Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 1996;135:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire A. T., Mangroo D. Cex1p is a novel cytoplasmic component of the Saccharomyces cerevisiae nuclear tRNA export machinery. EMBO J. 2007;26:288–300. doi: 10.1038/sj.emboj.7601493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhail K., Khacho M., Carrigan A., Hache R. R., Gunaratnam L., Lee S. Regulation of ubiquitin ligase dynamics by the nucleolus. J. Cell Biol. 2005;170:733–744. doi: 10.1083/jcb.200506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhail K., Rivero-Lopez L., Al-Masri A., Brandon C., Khacho M., Lee S. Identification of a common subnuclear localization signal. Mol. Biol. Cell. 2007;18:3966–3977. doi: 10.1091/mbc.E07-03-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhail K., Rivero-Lopez L., Khacho M., Lee S. Restriction of rRNA synthesis by VHL maintains energy equilibrium under hypoxia. Cell Cycle. 2006;5:2401–2413. doi: 10.4161/cc.5.20.3387. [DOI] [PubMed] [Google Scholar]
- Melchior F., Gerace L. Two-way trafficking with Ran. Trends Cell Biol. 1998;8:175–179. doi: 10.1016/s0962-8924(98)01252-5. [DOI] [PubMed] [Google Scholar]
- Moore M. S. Ran and nuclear transport. J. Biol. Chem. 1998;273:22857–22860. doi: 10.1074/jbc.273.36.22857. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Ohh M., Park C. W., Ivan M., Hoffman M. A., Kim T.-Y., Huang L. E., Pavletich N., Chau V., Kaelin W. G. Ubiquitination of hypoxia-inducible factor requires direct binding to the [bgr]-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Pause A., Lee S., Worrell R. A., Chen D. Y., Burgess W. H., Linehan W. M., Klausner R. D. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc. Natl. Acad. Sci. USA. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair R. D., Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature. 2000;404:604–609. doi: 10.1038/35007077. [DOI] [PubMed] [Google Scholar]
- Pinol-Roma S., Dreyfuss G. Transcription-dependent and transcription-independent nuclear transport of hnRNP proteins. Science. 1991;253:312–314. doi: 10.1126/science.1857966. [DOI] [PubMed] [Google Scholar]
- Pinol-Roma S., Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- Pinol-Roma S., Dreyfuss G. hnRNP proteins: localization and transport between the nucleus and the cytoplasm. Trends Cell Biol. 1993;3:151–155. doi: 10.1016/0962-8924(93)90135-n. [DOI] [PubMed] [Google Scholar]
- Richards S. A., Carey K. L., Macara I. G. Requirement of guanosine triphosphate-bound ran for signal-mediated nuclear protein export. Science. 1997;276:1842–1844. doi: 10.1126/science.276.5320.1842. [DOI] [PubMed] [Google Scholar]
- Richards S. A., Lounsbury K. M., Carey K. L., Macara I. G. A nuclear export signal is essential for the cytosolic localization of the Ran binding protein, RanBP1. J. Cell Biol. 1996;134:1157–1168. doi: 10.1083/jcb.134.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout M. P., Aitchison J. D. The nuclear pore complex as a transport machine. J. Biol. Chem. 2001;276:16593–16596. doi: 10.1074/jbc.R100015200. [DOI] [PubMed] [Google Scholar]
- Satterly N., Tsai P. L., van Deursen J., Nussenzveig D. R., Wang Y., Faria P. A., Levay A., Levy D. E., Fontoura B. M. Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc. Natl. Acad. Sci. USA. 2007;104:1853–1858. doi: 10.1073/pnas.0610977104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C., von Kobbe C., Bachi A., Pante N., Rodrigues J. P., Boscheron C., Rigaut G., Wilm M., Seraphin B., Carmo-Fonseca M., Izaurralde E. Dbp5, a DEAD-box protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pore complex via a conserved interaction with CAN/Nup159p. EMBO J. 1999;18:4332–4347. doi: 10.1093/emboj/18.15.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi M. C., Eder P. S., Kataoka N., Wan L., Liu Q., Dreyfuss G. Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins. J. Cell Biol. 1997;138:1181–1192. doi: 10.1083/jcb.138.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Koopman P. A. The ins and outs of transcriptional control: nucleocytoplasmic shuttling in development and disease. Trends Genet. 2004;20:4–8. doi: 10.1016/j.tig.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Snay-Hodge C. A., Colot H. V., Goldstein A. L., Cole C. N. Dbp5p/Rat8p is a yeast nuclear pore-associated DEAD-box protein essential for RNA export. EMBO J. 1998;17:2663–2676. doi: 10.1093/emboj/17.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Ford C. S., Guthrie C., Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Terry L. J., Shows E. B., Wente S. R. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- Truant R., Atwal R. S., Burtnik A. Nucleocytoplasmic trafficking and transcription effects of huntingtin in Huntington's disease. Prog. Neurobiol. 2007;83:211–227. doi: 10.1016/j.pneurobio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Tseng S. S., Weaver P. L., Liu Y., Hitomi M., Tartakoff A. M., Chang T. H. Dbp5p, a cytosolic RNA helicase, is required for poly(A)+ RNA export. EMBO J. 1998;17:2651–2662. doi: 10.1093/emboj/17.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kobbe C., van Deursen J. M., Rodrigues J. P., Sitterlin D., Bachi A., Wu X., Wilm M., Carmo-Fonseca M., Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell. 2000;6:1243–1252. doi: 10.1016/s1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Weirich C. S., Erzberger J. P., Berger J. M., Weis K. The N-terminal domain of Nup159 forms a beta-propeller that functions in mRNA export by tethering the helicase Dbp5 to the nuclear pore. Mol. Cell. 2004;16:749–760. doi: 10.1016/j.molcel.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Weirich C. S., Erzberger J. P., Flick J. S., Berger J. M., Thorner J., Weis K. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat. Cell Biol. 2006;8:668–676. doi: 10.1038/ncb1424. [DOI] [PubMed] [Google Scholar]
- Wen W., Meinkoth J. L., Tsien R. Y., Taylor S. S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Wente S. R. Gatekeepers of the nucleus. Science. 2000;288:1374–1377. doi: 10.1126/science.288.5470.1374. [DOI] [PubMed] [Google Scholar]
- Zhang T., Delestienne N., Huez G., Kruys V., Gueydan C. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J. Cell Sci. 2005;118:5453–5463. doi: 10.1242/jcs.02669. [DOI] [PubMed] [Google Scholar]