Abstract
Finger tapping tasks are one of the most common paradigms used to study the human motor system in functional neuroimaging studies. These tasks can vary both in the presence or absence of a pacing stimulus as well as in the complexity of the tapping task. A voxel-wise, coordinate-based meta-analysis was performed on 685 sets of activation foci in Talairach space gathered from 38 published studies employing finger tapping tasks. Clusters of concordance were identified within the primary sensorimotor cortices, supplementary motor area, premotor cortex, inferior parietal cortices, basal ganglia, and anterior cerebellum. Subsequent analyses performed on subsets of the primary set of foci demonstrated that the use of a pacing stimulus resulted in a larger, more diverse network of concordance clusters, in comparison to varying the complexity of the tapping task. The majority of the additional concordance clusters occurred in regions involved in the temporal aspects of the tapping task, rather than its execution. Tapping tasks employing a visual pacing stimulus recruited a set of nodes distinct from the results observed in those tasks employing either an auditory or no pacing stimulus, suggesting differing cognitive networks when integrating visual or auditory pacing stimuli into simple motor tasks. The relatively uniform network of concordance clusters observed across the more complex finger tapping tasks suggests that further complexity, beyond the use of multi-finger sequences or bimanual tasks, may be required to fully reveal those brain regions necessary to execute truly complex movements.
Keywords: meta-analysis, finger tapping, motor, activation likelihood estimation, ALE, paced finger tapping, auditory stimulus, visual stimulus, self-paced movement, movement complexity, sequential finger movements, bimanual
INTRODUCTION
Finger tapping tasks are commonly used to study the human motor system in functional neuroimaging studies. Tapping tasks have the advantage of being simple enough to use in the study of both normal control subjects as well as those with neuropathologies affecting the motor system, while being flexible enough to accommodate numerous modifications. These tasks can vary across studies both by the use or lack of a pacing stimulus and in the relative complexity of the tapping task.
Pacing stimuli are used to ensure that all subjects uniformly perform a given finger tapping task at a predetermined rate. The stimuli are usually in the form of a regularly paced, repetitive auditory or visual cue, such as that produced by a metronome (e.g. Catalan 1998; Colebatch 1991; Sadato 1996a) or blinking light (e.g. Indovina 2001; Jäncke 2000b), respectively. Such finger tapping tasks performed in the presence of a pacing stimulus are referred to as externally guided or externally generated. In contrast, the task can be performed in the absence of any pacing stimulus (i.e. self-paced). Such self-paced tapping tasks are referred to as internally guided or internally generated. The results from studies investigating the effects of auditory and visual pacing stimuli have reported different networks of active brain regions, however, these results are not consistent across different studies.
Pacing stimuli are also often used in conjunction with more complex finger tapping tasks such as multi-finger sequential or bimanual tapping tasks. For the purposes of the ensuing analyses, multi-finger sequential tapping tasks were taken to be complex in terms of the increased number of fingers involved in the task; factors such as the rate of movement and the length of the sequence were not specifically considered. Bimanual tasks were taken to be any task involving the tapping of fingers on both hands, regardless of the symmetry. These types of complex finger tapping tasks are often employed to elicit neural activation that is more representative of what would be observed in typical, everyday manual movements that may not be practical to complete within the confines of a MRI or PET scanner. The use of complex finger tapping tasks also allows for the further study of secondary and tertiary neural motor regions that may not be active during a simple, unimanual index finger tapping task.
Results from studies employing finger tapping tasks can be divergent due to variations in the experimental paradigms used, making them difficult to interpret across studies. Additionally, studies can choose to focus on a few specific neural regions (e.g. Jäncke 2000a; Colebatch 1991; De Luca 2005), resulting in partial descriptions of the underlying neural network involved in a given tapping task. A quantitative meta-analysis technique, such as that proposed independently by Turkeltaub et. al. (2002) and Chein et. al. (2002) provides a method to assess the degree of concordance across multiple studies. The results of such an analysis can be useful in determining a more complete network of neural regions involved in a given task or paradigm as well as in forming new hypotheses and interpreting results from subjects with neurological impairments.
This present study was not the first to use quantitative meta-analysis techniques to assess concordance across studies examining the human motor system. Chouinard and Paus (2006) employed a similar technique to further elucidate the roles of the primary motor and premotor cortices in various motor tasks. Four motor-related tasks – movement response selection, movement response to a stimulus, execution of object-related hand movements, and observation of object-related hand movements – were chosen to map out the roles of the dorsal and ventral premotor cortices in these tasks. Chouinard and Paus were successful in utilizing meta-analysis to identify several distinct nonprimary motor areas within the motor cortex.
In the present meta-analysis, our aim was to isolate the corpus of published literature for simple hand movements (i.e., finger tapping), and identify the entire network of brain regions associated with this type of motor task. Our intent was to examine agreement across studies not only in the motor cortex, but also throughout all cortical, subcortical, and cerebellar regions. In addition, meta-analysis was used to differentiate the brain regions that are active during the most common variations of finger tapping tasks: auditorially paced, visually paced, self-paced, single index finger, unimanual, dominant hand (RH) multi-finger sequence, and bimanual, as well as to compare these networks among the tapping task variations. We hypothesized that the choice of finger tapping task variation would have a strong influence on the observed network of active brain regions.
METHODS
Several literature searches were performed in Medline to find the published corpus of literature prior to July 2006 involving finger tapping tasks in unimpaired, right-handed subjects. References from all relevant papers were also examined. From these search results, only those papers which reported activations as coordinates in stereotactic space (x,y,z) were considered. Papers directly addressing motor learning or using over-trained subjects such as professional musicians were excluded. Results from 38 papers (22 fMRI and 16 PET; Table 1) were selected (685 foci), and three analyses were performed. The first pooled the results from all of the included studies. For the second, studies were divided into three groups based on the type or lack of pacing stimulus employed: auditory stimulus (22 papers; 403 foci), visual stimulus (7 papers; 109 foci), and no stimulus (13 papers; 173 foci). The final analysis divided the studies into three groups determined by the complexity of the tapping task used: right hand index finger (23 papers; 311 foci), RH multi-finger sequence (15 papers; 242 foci), and bimanual (5 papers; 90 foci). All MNI coordinates were transformed to Talairach space using the icbm2tal transform (Lancaster 2007), which has shown to provide improved fit over the mni2tal transform (Brett 2001,2002). When applying the Lancaster transform, software-specific versions were used for FSL (icbm_fsl2tal) and SPM (icbm_spm2tal) coordinates, to correct for varying normalization methods within each software package. Activation likelihood estimate (ALE) maps were created for each grouping by modeling each focus as a three-dimensional Gaussian function with a FWHM of 12 mm (Turkeltaub 2002). Statistical significance was assessed using a permutation test with 5000 permutations, corrected for multiple comparisons using the false discovery rate (FDR) (Laird 2005). The resultant maps were thresholded at P < 0.05.
Table 1.
Published Studies Employing Finger Tapping Tasks Used in the Meta-Analyses. A total of 38 studies with 74 contrasts and 685 foci were included in the finger tapping meta-analyses (listed in alphabetical order by first author). The type or lack of pacing stimulus and the level of complexity as well as whether the task was bimanual are indicated for each contrast.
| Publication | Contrast | Foci | Stimulus | Complexity | Bimanual |
|---|---|---|---|---|---|
| Aoki, 2005 | Index finger vs Rest | 1 | auditory | RH index | |
| Ring finger vs Rest | 7 | auditory | |||
| Double finger tapping vs Rest | 12 | auditory | |||
| Aramaki, 2006 | Parallel vs Rest | 18 | auditory | bimanual | |
| Mirror vs Rest | 7 | auditory | bimanual | ||
| Blinkenberg, 1996 | Finger tapping vs Rest | 10 | auditory | RH index | |
| Boecker, 1998 | Motor sequences vs Rest | 20 | auditory | sequence | |
| Calautti, 2001 | RH tapping vs Rest, young subjects | 10 | auditory | RH index | |
| LH tapping vs Rest, young subjects | 10 | auditory | |||
| RH tapping vs Rest, older subjects | 4 | auditory | RH index | ||
| LH tapping vs Rest, older subjects | 10 | auditory | |||
| Catalan, 1998 | Sequence-12 vs Rest | 9 | auditory | sequence | |
| Catalan, 1999 | Sequence-16 vs Rest, controls | 12 | auditory | sequence | |
| Colebatch, 1991 | Index vs Rest | 3 | auditory | RH index | |
| Opposition vs Rest | 8 | auditory | sequence | ||
| De Luca, 2005 | RH finger tapping, controls | 4 | visual | RH index | |
| Denslow, 2005 | Volitional movement vs sham TMS | 18 | auditory | RH index | |
| Fox, 2004 | Finger movement vs sham TMS | 1 | auditory | RH index | |
| Gelnar, 1999 | Finger opposition vs Rest | 8 | none | sequence | |
| Gerardin, 2000 | Motor Execution vs Rest | 24 | auditory | ||
| Gosain, 2001 | Finger-tapping vs Rest | 2 | none | RH index | |
| Hanakawa, 2003 | Finger movement vs Rest | 25 | visual | sequence | |
| Indovina, 2001 | Move vs Rest | 15 | visual | RH index | |
| Jäncke, 1999 | RH, 1 Hz vs Rest | 2 | auditory | sequence | |
| RH, 3 Hz vs Rest | 2 | auditory | sequence | ||
| LH, 1 Hz vs Rest | 2 | auditory | sequence | ||
| LH, 3 Hz vs Rest | 2 | auditory | sequence | ||
| Both Hands, 1 Hz vs Rest | 2 | auditory | sequence | bimanual | |
| Both Hands, 3 Hz vs Rest | 2 | auditory | sequence | bimanual | |
| Jäncke, 2000a | Right fast, Left slow | 3 | none | bimanual | |
| Right slow, Left fast | 3 | none | bimanual | ||
| Right fast | 1 | none | RH index | ||
| Right slow | 2 | none | RH index | ||
| Left fast | 1 | none | |||
| Left slow | 2 | none | |||
| Jäncke, 2000b | Auditory synchronization vs Rest | 12 | auditory | RH index | |
| Auditory continuation vs Rest | 11 | none | RH index | ||
| Visual synchronization vs Rest | 13 | visual | RH index | ||
| Visual continuation vs Rest | 12 | none | RH index | ||
| Joliot, 1998 | Finger tapping vs Rest | 13 | none | RH index | |
| Joliot, 1999 | Finger tapping vs Rest - PET | 11 | none | RH index | |
| Finger tapping vs Rest - fMRI-average | 16 | none | RH index | ||
| Finger tapping vs Rest - fMRI-correlation | 20 | none | RH index | ||
| Kawashima, 1999 | Finger movements vs Rest | 3 | none | RH index | |
| Kawashima, 2000 | Memory timed finger movement vs Rest | 10 | none | RH index | |
| Visually cued finger movement vs Rest | 14 | visual | RH index | ||
| Kuhtz-Buschbeck, 2003 | Motor execution, Complex RH vs Baseline | 8 | auditory | sequence | |
| Motor execution, Complex LH vs Baseline | 12 | auditory | sequence | ||
| Larsson, 1996 | Self paced movements vs Rest | 12 | none | RH index | |
| Visually triggered movements vs Rest | 14 | visual | RH index | ||
| Lehericy, 2006 | Simple vs Rest | 8 | auditory | RH index | |
| Scale vs Rest | 11 | auditory | sequence | ||
| Complex vs Rest | 27 | auditory | sequence | ||
| Lerner, 2004 | Tapping vs Rest, Normals | 9 | auditory | RH index | |
| Lutz, 2000 | Random vs Rest | 17 | visual | RH index | |
| Regular vs Rest | 7 | visual | RH index | ||
| Mattay, 1998 | Non-dominant hand, simple motor | 8 | none | sequence | |
| Dominant hand, simple motor | 12 | none | sequence | ||
| Dominant hand, random motor | 15 | none | sequence | ||
| Müller, 2002 | Finger tapping vs Rest, healthy subjects | 4 | none | ||
| Ramsey, 1996 | fMRI Finger tapping vs Rest, Mean | 1 | none | sequence | |
| PET Finger tapping vs Rest, Mean | 1 | none | sequence | ||
| Riecker, 2006 | Main effects during index finger movement, young subjects | 6 | auditory | RH index | |
| Main effects during index finger movement, older subjects | 8 | auditory | RH index | ||
| Rounis, 2005 | Main effects of movement | 17 | auditory | RH index | |
| Sadato, 1996 | Various length sequences | 6 | auditory | sequence | |
| Sadato, 1997 | Mirror vs Rest | 13 | auditory | sequence | bimanual |
| Parallel vs Rest | 15 | auditory | sequence | bimanual | |
| Right unimanual vs Rest | 3 | auditory | RH index | ||
| Left unimanual vs Rest | 6 | auditory | |||
| Bimanual mirror vs Rest | 12 | auditory | bimanual | ||
| Bimanual parallel vs Rest | 13 | auditory | bimanual | ||
| Seitz, 2000 | Irregularly paced right finger movement | 4 | auditory | RH index | |
| Wilson, 2004 | Moving fingers | 2 | none | sequence | bimanual |
| Yoo, 2005 | Group-level finger tapping activation | 17 | auditory | sequence |
RESULTS
The ALE map for the main effects of all finger tapping task variations included in this study is shown in Figure 1. Common, robust concordance was seen in bilateral sensorimotor cortices (L: −38,−26,50; R: 36,−22,54), supplementary motor area (SMA) (−4,−8,52), left ventral premotor cortex (−54,−2,32), bilateral inferior parietal cortices (L: −50, −26,20; R: 40,−42,44), bilateral basal ganglia (L: −22, −8,4; R: 22,−10,6), and bilateral anterior cerebellum (L: −22,−52,22; R: 16,−50,−20). Smaller clusters of concordance were seen in the right dorsolateral prefrontal cortex (34,32,34), right inferior frontal gyrus (54,4,22), and left occipital lobe (−44,−66,4). The appearance of this latter trio of brain regions will later be shown to have more to do with the inclusion of visually-paced finger tapping tasks, than in these regions playing a role in the performance of finger tapping tasks in general.
Figure 1.
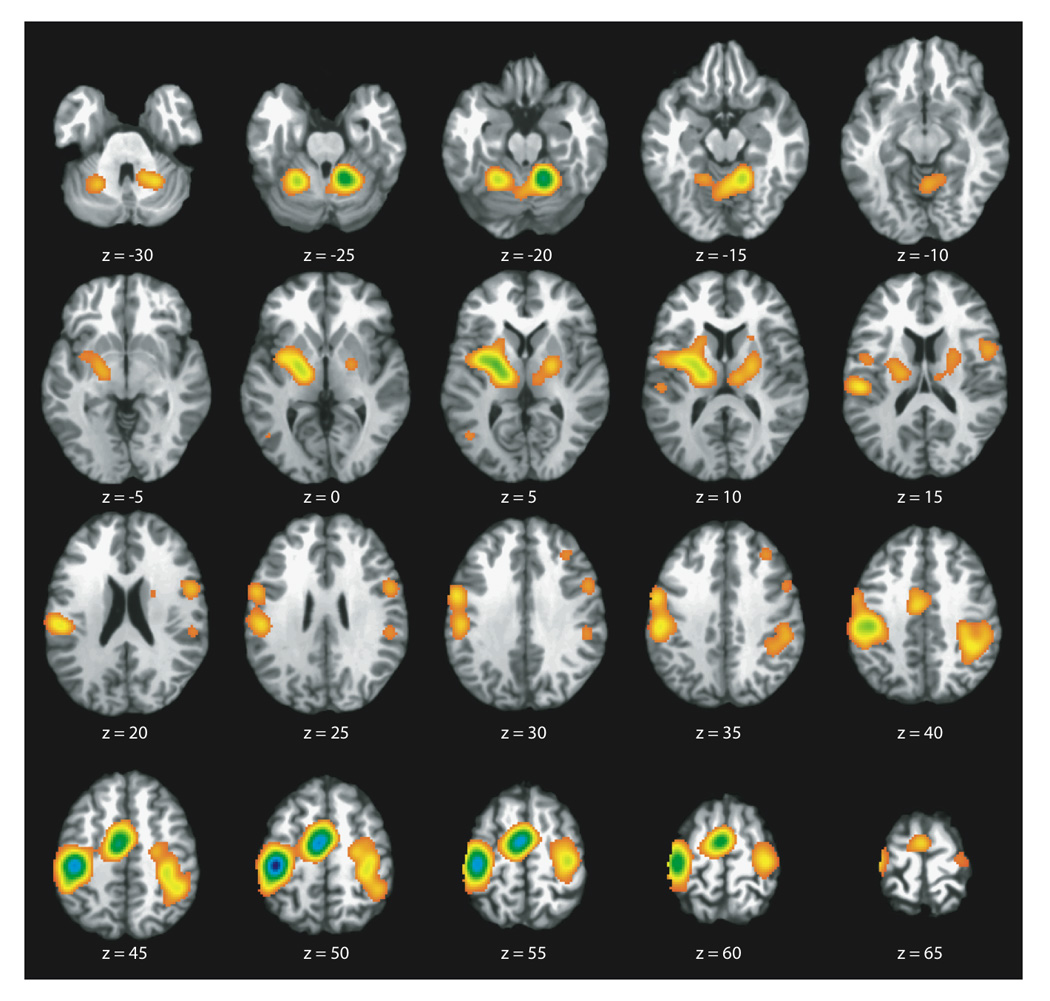
ALE Map of the Main Effects (All Finger Tapping Task Variations). Axial slices representing full brain coverage are shown (P < 0.05; FDR corrected). Robust concordance is seen in bilateral sensorimotor cortices, supplementary motor area, left ventral premotor, bilateral inferior parietal cortices, bilateral basal ganglia, and bilateral anterior cerebellum.
Figure 2 (A–G) shows representative axial slices of the combined ALE map for the three groups of studies involving the use or absence of pacing stimuli - auditorially-paced, visually-paced, and self-paced. In addition to the executive areas noted above for the main effects, the ALE map for the pacing stimulus group showed clusters of concordance, which were unique to one or more of the task variations in this grouping. All three variations within this group showed concordance within the dorsal premotor cortices (R: 47,1,50; 38,−10,54), with laterality depending on task variation. The visually- and self-paced groups exhibited concordance within the right dorsolateral prefrontal cortex (42,34,32; 32,30,32) and right inferior parietal lobe (46,−44,44). The auditorially-and self-paced groups shared concordance in bilateral claustrum (L: −32,−4,6; R: 34, −8,14), again with laterality depending on task variation. The visually-paced group had exclusive concordance in bilateral insula (L: −48,10,0; R: 48,10,2), right inferior frontal gyrus (50,8,32), bilateral occipital lobe (L: −42,−66,4; −10,−82,−2; R: 44,−66,−2; 4,−76,0), and left posterior cerebellum (−28,−62,−28). Finally, the auditorially-paced group had exclusive concordance in Brodmann’s area 44 (56,3,20) and the self-paced group in the left ventral premotor cortex (−52,−2,10) and right posterior cerebellum (10,−66,−32).
Figure 2.
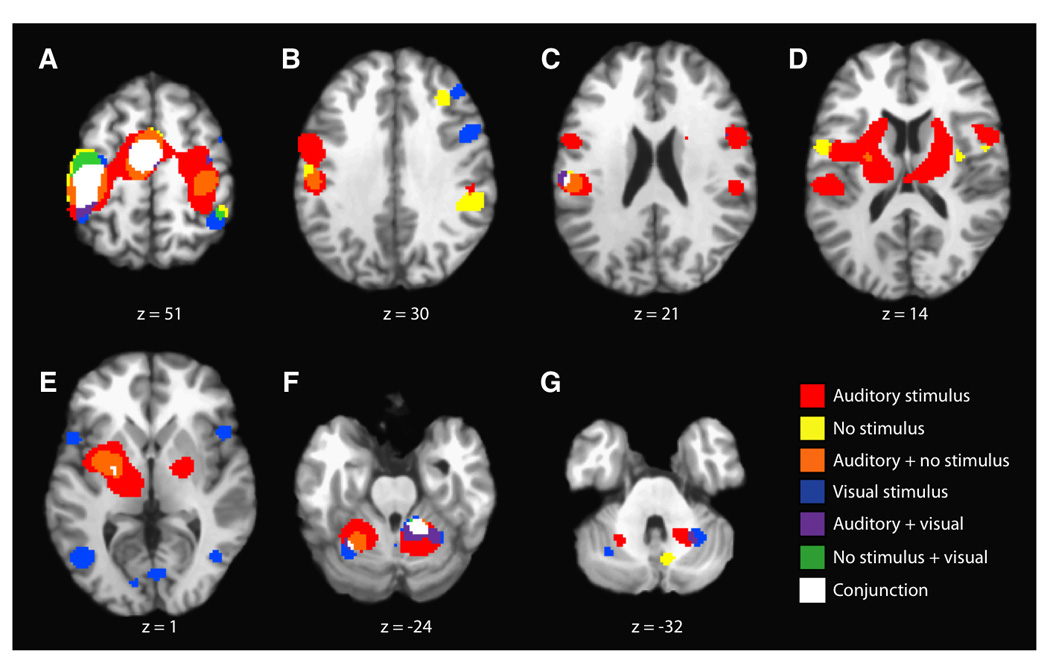
ALE Results Segregated By Pacing Stimuli. Representative axial slices are shown (P < 0.05; FDR corrected) for finger tapping activations when paced by auditory, visual, or no stimuli. Overlap (conjunction) among all three task variations is seen in the same executive regions noted for the main effects in Figure 1, except the basal ganglia. The results also show many clusters of concordance that are unique to one or more of the task variations.
In order to determine the relative effect of tapping rate in the meta-analytic comparisons of stimulus modality, we performed a comparison of study characteristics for auditory vs. visual stimuli and paced vs. self-paced tapping tasks. The tapping rate was recorded for each experiment that was included in the meta-analyses. An unpaired t test was performed on the tapping rates for auditory (mean rate = 1.716 Hz) and visual (mean rate = 1.241 Hz) studies in which no statistical difference was found between groups (P = 0.182). A subsequent test was performed to determine if a rate effect existed between paced (mean rate = 1.642 Hz) and self-paced (mean rate = 1.795 Hz) studies; however, this test also revealed no difference (P = 0.5301). Note that this result may potentially be biased by the fact that no observed tapping rate was reported in 6 of 24 self-paced experiments; however, given the high P value this is unlikely.
To determine the effect of tapping difficulty on the observed meta-analysis results, studies were reviewed and assigned scores of tapping difficulty according to a five-point rating system: 1 – unimanual single finger, 2 – bimanual single finger or unimanual simple sequence, 3 – bimanual simple sequence or unimanual complex sequence, 4 – bimanual mirrored tapping of a complex sequence, 5 – bimanual parallel tapping of a complex sequence. Unpaired t tests were carried out on comparisons of auditory (mean score = 1.919) vs. visual (mean score = 1.125) studies and paced (mean score = 1.794) vs. self-paced (mean score = 1.708) studies. Again, no difference was found between either group (P = 0.059 and P = 0.760, respectively). Arguably, the test of auditory vs. visual nearly achieved significance, and therefore some potential bias may have been introduced into the meta-analysis as a result of this. Future imaging studies will be useful in dissociating the degree of interaction between auditory vs. visual stimuli and tapping difficulty.
Figure 3 (A–G) shows representative axial slices of the combined ALE map for the three groups of studies involving tapping task complexity - RH index finger, RH multi-finger sequence, and bimanual. Unlike for the meta-analyses results for the pacing stimulus group, the combined ALE map for the complexity group suggested that there are fewer unique brain regions required to complete more complex finger tapping tasks that are not already active during the execution of finger tapping tasks of all variations. All three task variations within this grouping shared concordance within the dorsal (L: −16,−20,48; R: 34,−8,52; 46,1,51) and ventral (−56,−4,34; −54,2,10; −50,0,34) premotor cortices. The RH multi-finger sequence and RH index finger tapping task variations showed concordance in the right inferior parietal cortex (46,−44,44; 38,−40,44). The RH multi-finger sequence tapping task group exhibited concordance within bilateral posterior parietal cortices (L: −26,−62,48; R: 16,−72,42), and the bimanual group within the left posterior cerebellum (−4,−66,−18). The RH index finger tapping task group showed a small cluster of concordance within the left DLPFC (−37,30,32), in addition to sharing several clusters of concordance with the externally paced tasks, including the right insula (48,12,4), right inferior frontal gyrus (52,6,28), bilateral claustra (L: −32,−4,4; R: 34,14,6), and right DLPFC (34,30,32).
Figure 3.
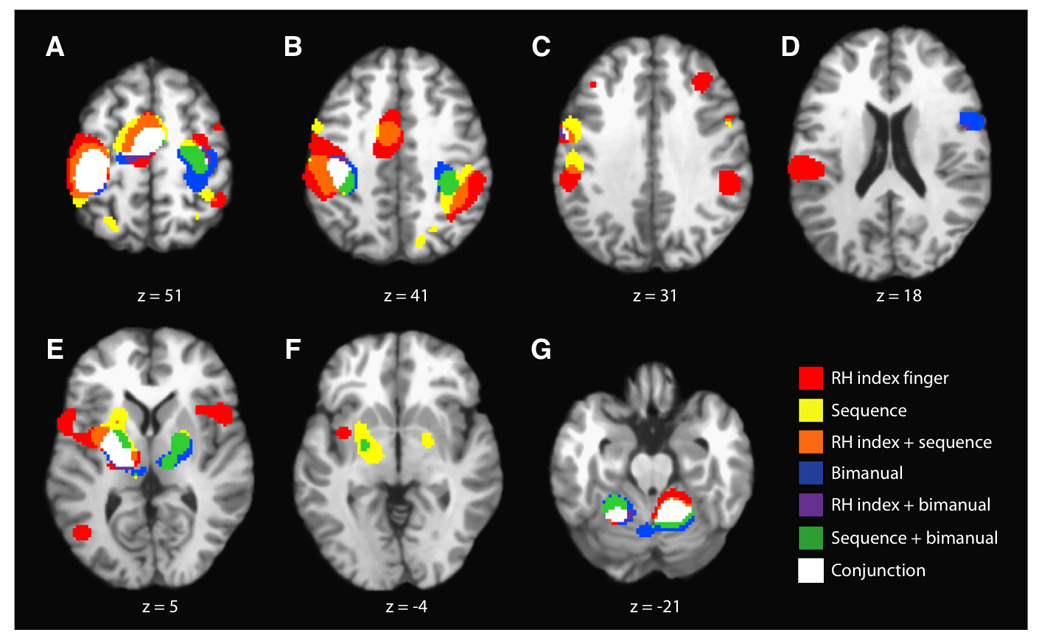
ALE Results Segregated By Task Complexity. Representative axial slices are shown (P < 0.05; FDR corrected). Overlap (conjunction) among all three task variations (RH index, RH multi-finger sequence, and bimanual tapping) is seen in the same executive regions noted for the main effects in Figure 1. Compared with the results from the effects of a pacing stimulus, there are fewer concordance clusters unique one or more of the task variations, not observed in the main effects.
DISCUSSION
An ALE meta-analysis was performed to quantify the motor system during finger tapping, a common task used in functional imaging studies. Results allowed for detailed description of the motor networks involved in single finger, unimanual, dominant hand multi-finger, and bimanual movement sequences, varying in the presence or absence of a pacing (auditory or visual) stimulus.
MAIN EFFECTS
The results for the main effects analysis across all finger tapping tasks exhibited clusters of concordance in regions commonly associated with the performance of motor tasks, including the primary sensorimotor cortex (SM1), supplementary motor area (SMA), basal ganglia (BG), and cerebellum. The primary sensorimotor cortex has traditionally been considered the main executive locus for simple voluntary movements (Gerloff 1998), however, recent studies have implicated this region in the processing of complex sequential tapping tasks (Kawashima 1993,1994a,b; Kim 1993a,b; Rao 1993; Chen 1997) as well as the processing of bimanual movements (Kermadi 2000). The SMA has also been found to be an executive area for simple voluntary movements (Colebatch 1991; Matteli 1993; Sadato 1995). Additionally, activity in the SMA has been linked to higher motor processing functions such as the initiation of movement, motor programming (Roland 1980b), motor planning (Orgogozo 1979; Grafton 1992a; Rao 1993), readiness to move (Fox 1985), motor learning (Roland 1989; Seitz 1990; Grafton 1992a), complexity of movement (Shibasaki 1993), bimanual coordination (Goerres 1998; Immisch 2001; Jäncke 2000a; Meyer-Lindenberg 2002; Sadato 1997; Stephan 1999a,b; Toyokura 1999,2002; Ullén 2003), and responsiveness to internal cueing of movement (Halsband 1993) or to the selection of movement (Deiber 1991). Activation in the basal ganglia has been associated with both the performance of simple repetitive movements (Lehéricy 1998; Maillard 2000), as well as more complex sequential movements (Roland 1982; Shibasaki 1993; Jenkins 1994a; Jueptner 1997a; Gordon 1998; Boecker 1998; Catalan 1998; Haslinger 2002). Activity in both the SMA and BG has been preferentially linked to internally generated movements over externally generated movements in non-human primates (Mushiake 1990,1991; Tanji 1994; Tanji and Shima 1994,1996; Brinkman 1984; Chen 1995, Taniwaki 2003; Romo 1987; Middleton 2000), however this distinction has not been consistently reported in humans (Menon 1998; Cunningon 2002). Regions in the cerebellum have been shown to be active during the preparation, execution, and timing of both simple and complex movements (Habas 2004a), and while cerebellar activity has been traditionally associated with externally cued movements, it has been observed in motor tasks driven by both external and internal cues (Grafton 1992b).
In addition to the above regions, clusters of concordance are observed in regions that have been traditionally reported to be preferentially active during one or more specific finger tapping task variations, namely the premotor and parietal cortices. For both the externally cued and complex tapping tasks, activity has been reported within the premotor and parietal cortices. The premotor cortex has been shown to play an important role in the transformation of sensory information into appropriate motor behavior (Crammond 1996; Godschalk 1981; Johnson 1996; Mushiaki 1991; Wise 1997; Halsband 1993; Harrington 2000; Grafton 1993; Kawashima 1995; Passingham 1985; Samuel 1997; Roland 1984), especially in regards to sequential movements (Harrington 2000; Catalan 1998; Grafton 1992a; Shibasaki 1993). The parietal cortex has also been shown to be active during both auditorily- and visually-cued movements (Deiber 1991; Grafton 1992b; Jenkins 1994b; Huttunnen 1996; Forss 1998; Linn 2002), also particularly during both the execution and production of complex, sequential motor tasks (Gordon 1998; Sadato 1996a; Honda 1998; Jenkins 1994b; Rauch 1995; Sakai 1998; Boecker 1998). The observed concordance in these two regions suggests that they may play a more general, executive role in motor tasks, in addition to their more task variation specific roles discussed below.
PACING STIMULUS EFFECTS
In line with the ALE map for the main effects of all finger tapping task variations (Figure 1), conjunction analysis revealed that the three groups (auditorially-, visually-, and self-paced) shared common, robust concordance in the primary sensorimotor cortex, supplementary motor area, and anterior cerebellum (Figure 2). This shared concordance is in agreement with the body of literature on externally and internally generated motor tasks, suggesting that these areas are involved in the execution motor tasks under both conditions (Weeks 2001; Grafton 1992b). Within the basal ganglia, there was no robust concordance among all three groups. The auditorially- and self-paced groups demonstrated concordance within the putamen and thalamus. The use of visually-paced tapping tasks only revealed concordance within the left globus pallidus external (GPe). These results suggest that the basal ganglia may be active during both externally and internally generated motor tasks but only for those externally generated tasks driven by an auditory cue.
The concordance within the dorsal premotor cortex (PMd; BA 6) was lateralized based on task variation, with the visually-paced group showing bilateral concordance, the auditorially-paced group in the right PMd, and the self-paced group in the left PMd. With the exception of the visually-paced group’s clusters in the right PMd, none showed distinct clusters of concordance in this region. Rather, the clusters comprising the primary sensorimotor cortices extended anterior enough to encompass the cortical region usually defined as the dorsal premotor cortex, and the location of this extension in the left hemisphere was in agreement with the region of the left PMd identified through a previous meta-analysis study (Chouinard 2006) as being involved in the execution of simple movements in response to an external stimulus. The premotor cortex, in general, has traditionally been associated with the execution of movements under sensory guidance (Goldberg 1985). Removal of the PMd in humans, in particular, has been shown to disrupt the ability to use arbitrary cues to withhold or perform a particular movement (Petrides 1982,1985; Halsband 1982,1985). More recently, Schubotz and von Cramon (2003) posited a general trend for lateral premotor cortex dominance over externally guided movements and medial premotor cortex dominance over internally guided movements, implying that the premotor cortex is involved in movement execution regardless of the presence of absence of an external cue, which is confirmed by concordance within this region for both the externally and internally paced tasks.
From Figure 2.B, one can observe a definite somatotopy to the concordance cluster within the right dorsolateral prefrontal cortex (DLPFC; BA 9), with the visually-paced group exhibiting concordance in the lateral aspect and the self-paced group in the more medial aspect. The DLPFC has been consistently linked to self-paced movements. Jahanshahi et. al. (1995) found greater activation within the DLPFC during self-initiated movements. This study also noted, in line with previous results (Deiber 1991; Frith 1991; Playford 1992) that the DLPFC was the area that significantly distinguished internally generated movements from externally triggered movements, especially in regards to internal temporal processing (Rao 2001; Thaut 2003), so its observed concordance for visually-paced movements is not in line with previous results. However, this area is also associated with sustained attention (Pardo 1991). It may be that externally generated finger tapping tasks that use a visual pacing stimulus may not be as automatic as those the make use of an auditory pacing stimulus.
The inferior parietal lobe (LPi; BA 40) has been linked to the encoding of sequence-specific information (Honda 1998; Jenkins 1994b; Rauch 1995; Sakai 1998) as well as sensorimotor integration processes (Huttunnen 1996; Forss 1998; Linn 2002). Vaillancourt et. al. (2006) showed increased activation in right LPi, in conjunction with right PMd and right PMv, in the presence of frequent visual pacing stimulus during a visuomotor task. Its increased activity observed during internally generated movements has been hypothesized to be a consequence of increased attentional demands directed towards somatosensory input from the limbs (Debaere 2003).
As with the dorsal premotor cortices, the concordance in the bilateral claustra was also lateralized based on task variation, with the cluster in the left hemisphere corresponding to the auditorially-paced group and that in the right hemisphere to the self-paced group. Activity in the claustrum has been linked to sensorimotor integration (Edelstein 2004). Neuroanatomical studies have shown that the non-human primate claustrum shares reciprocal connections with structures in the frontal lobe, including the motor, premotor, and cingulate cortices; the visual cortices in the occiplital lobe; the temporal cortex; the parieto-occipital and posterior parietal cortices; and somatosensory areas (Crick 2005). A prior PET study in humans revealed involvement of the claustrum, along with the insula, in cross-model matching tasks that require the simultaneous evaluation of information from more than one sensory domain (Hadjikhani 1998). The results from this study, in particular, lend support to the hypothesis that the claustrum serves to combine and bind different attributes of objects, both within and across modalities. Crick and Koch (2005) further suggest that the claustrum may contain specialized mechanisms that permit information within its own extent to synchronize different perceptual, cognitive, and motor modalities. That these two different pacing modalities activated the left and right claustra separately suggests a potential segregation of sensory processing within the claustrum.
The concordance seen in bilateral insula, right inferior frontal gyrus (IFG), Brodmann’s areas 18 and 37, and left posterior cerebellum, specifically Crus I (Schmahmann 1999) can all be related to the processing of the visual pacing stimuli. Areas 18 and 37 are commonly associated with the processing of visual information, and activity in Crus I has been linked to visuomotor processing in the presence of frequent but not infrequent visual stimuli (Vaillancourt 2006). Anatomic studies have shown that the insular cortex (BA 13) has numerous afferent and efferent connections with a diverse array of brain structures including the motor and somatosensory cortices (Augustine 1996). With regards to its motor-related connections, researchers have suggested that the insula plays an important role as a motor association area involved in the movement of the upper limbs, including the hands (Chollet 1991; Weiller 1992) and in saccadic movements of the eyes (Petit 1993). This region has also been shown to be involved in a variety of timing tasks including interval sequence encoding (Schubotz 2000) and sensorimotor synchronization (Rubia 2000). Cerasa et. al. (2006) proposed that this region, along with the right IFG (BA 9), may guide the timing of sequential movements through both the internal subvocalization of the interval duration and multi-modal integration. This hypothesis is supported by observed insular and right IFG activity during both acoustically (Rao 1997; Riecker 2002) and visually (Cerasa 2005; Penhune 1998) stimulated timing tasks.
The self-paced group exhibited small clusters of concordance within the left inferior ventral premotor cortex (BA 6) and the right cerebellar pyramis (VIII-IX; Schmahmann 1999). The role of the ventral premotor cortex is discussed more fully below in relation to its observed concordance for the complex finger tapping tasks, but as it is most often associated with the execution of visually guided movements (Kurata 1993,1994a; Rizzolatti 1996b; Debaere 2003), its apparent concordance for the self-paced tapping task group represents a deviation from the accepted role of the PMv. This suggests a role of the PMv in movement beyond sensorimotor integration. There is little published about the right cerebellar pyramis, however, Rijntjes et. al. (1999) provided evidence that it may be the location of a third homunculus. If this is true, it is uncertain why activation was not seen in this area for all finger tapping task variations.
Brodmann’s area 44 (frontal opercular cortex; inferior frontal gyrus) has been connected to finger movements (Binkofski 1999; Harrington 2000; Schlaug 1994), motor imagination (Decety 1994; Grafton 1996; Stephan 1995), motor learning (Seitz 1992), and motor observation (Haslinger 2002; Chouinard 2006). Rao et. al. (1997) found this region to be active during auditory continuation tasks, suggesting it may play a role in the internal timing of movements. Studies have further found the right inferior fronal gyrus along with the right superior temporal gyrus form a network associated with the retrieval and rehearsal of auditory information, particularly in the absence of any external stimulus (Zatorre 1996), making this network key in the subvocal rehearsal systems (Paulesu 1993). Additional studies have proposed that this region, along with the lateral aspect of BA 6, forms the human ventral premotor cortex (Tomaiuolo 1999). Activity in the frontal opercular and ventral premotor cortices has been linked to the learning of implicit and explicit motor sequences (Hazeltine 1997; Rauch 1995; Seitz 1992) and novel visuomotor associations (Toni 2001; Toni 2002). In non-human primates, studies have shown that the ventral premotor cortex plays an important role during visually guided movements (Kurata 1994c), so its concordance for the auditorially-paced tasks - many of which are sequential tapping tasks - may have more to do with the performance of the sequential task as opposed to the modality of the pacing sequence.
The use or lack of a pacing stimulus has an effect on the network of brain regions observed to be active during finger tapping tasks. The use of a visual stimulus requires the recruitment of a number of brain regions distinct from either the use of an auditory stimulus or no stimulus. This network includes the bilateral insula, right inferior frontal gyrus (IFG), Brodmann’s areas 18 and 37, and left posterior cerebellum. The self-paced tasks appear to be more demanding than the auditorily-paced tasks, necessitating more complex cognitive control, as evidenced by observed concordance in several frontal and prefrontal regions including more lateral aspects of the premotor and parietal cortices, as well as the dorsolateral pretrontal cortex. The auditorily-paced tasks did not appear to recruit many additional regions beyond those observed in the main effects (Figure 1). However, the observed concordance in Brodmann’s Area 44 is of interest, as while it this region has previously been associated with the internal timing system, its observed concordance during the auditorily-paced tasks, suggests a role in both the internal timing system as well as the sensorimotor integration of external, auditory pacing stimuli. Taken together, these results suggest that there is a stronger distinction between the visually-paced tasks and either the auditorily- or self-paced tasks than between the auditorily- and self-paced tasks, as evidenced by greater overlap of the ALE results for the auditorily- and self-paced tasks.
TASK COMPLEXITY EFFECTS
Comparing the studies based on the complexity of the tapping task employed - RH index finger, RH multi-finger sequence, and bimanual – conjunction analysis yielded common concordance in the primary sensorimotor cortex, SMA, basal ganglia, and anterior cerebellum. The concordance was contralateral to the dominant hand for the RH index finger tapping task group in all areas except the anterior cerebellum, in which case it was bilateral. The concordance within these executive regions was bilateral for the bimanual tapping group, which was expected. It was also bilateral for the sequence tapping group, even though the majority of the studies included in this group used unimanual sequence tasks.
Concordance within the dorsal premotor cortices was again lateralized based on task variation, with the RH index finger group exhibiting bilateral clusters of concordance, the sequence group right hemispherical, and the bimanual group left hemispherical. The PMd, in addition to its afore-described role in integrating sensory information into movements, has also been shown to be involved in modulating movement frequency and complexity (Nakai 2003; Debaere 2004; Ullén 2003). Kermadi (2000) demonstrated that this region contains bimanual specific neurons, with the left PMd playing a fundamental role in the control of bimanual movements in right-handed subjects (Hlustík 2002). The right PMd has been shown to play a role in the execution of motor sequences, even those performed with the dominant hand in right-handed subjects (Sadato 1996a). The cluster of concordance centered on the right primary sensorimotor cortex for the sequence tapping task group extended anterior into the cortical area traditionally considered to be the PMd, supporting this conclusion. However, the only definite concordance within the right PMd was seen in the results for the RH index finger tapping group. Since the two clusters of concordance were in similar locations to those seen for the auditorily- and visually-paced groups (and likewise with the anterior extension of the left sensorimotor cluster), their appearance in the RH index finger results may have more to do with that group’s inclusion of externally paced tapping tasks, as opposed to the region’s role in simple, dominant hand index finger tapping. Particularly, the location of the anterior extension of the left sensorimotor cortex into the left PMd - as discussed above for in reference to the use of pacing stimuli - is in agreement with the locus for simple movements in response to an external stimulus (Chouinard 2006).
The clusters of concordance in the ventral aspect of the premotor cortex were located in the left hemisphere (BA 6) for the RH index finger and sequence groups; the bimanual group also exhibited a small cluster in the left hemisphere (BA 6), in addition to a larger cluster in the right hemisphere (BA 44), where the role of BA 44 in motor tasks and the PMv has been previously addressed in reference to its observed concordance for the auditorily-paced tapping task group. As stated above, the ventral premotor cortex has consistently been shown to be active during visually guided movements (Kurata 1993,1994a; Rizzolatti 1996; Debaere 2003), especially during reaching and grasping tasks (Kurata 1994b,1994c,1999; Rizzolatti 1987,1990). Fogassi et. al. (1992) posited that the PMv represents a body-centered frame of reference which is involved in manipulating movement direction towards a target location. Since all of the studies used in these meta-analyses involved finger-tapping tasks, it is more likely that the observed concordance within this region supports the results of Stephan et. al. (1995) who found activation within the left PMv, in particular, during the execution of upper limb movements. This suggests that PMv activity may constitute part of the normal physiologic processes during finger movement. In addition to its role in sensorimotor integration, the left PMv in particular has been implicated speech and language production, as its extent has been shown to encompass part of Broca’s region (Binkofski 2004), and as one of the key structures in the human mirror neuron system, demonstrating activation during observation, execution, and imitation of action (Iacoboni 1999,2006).
As mentioned above, the inferior parietal lobe has been implicated in the performance of sequential finger movements (Jenkins 1994a), confirmed by the concordance observed in the right hemisphere for the RH multi-finger sequence group. A large cluster of concordance was also observed in the right LPi for the RH index finger tapping group, suggesting that, in addition to this region’s role in sensorimotor integration and sequence performance, it may play a role in the execution of movements in general. There was also an apparent somatotopy within the right LPi, with the region controlling sequence production and performance located more anteriorly to that controlling sensorimotor integration and general movement production. Such a functional dichotomy within this region has not yet, to the best of the authors’ knowledge, been reported.
The roles of the insular cortex, right inferior frontal gyrus, claustrum, and right dorsolateral prefrontal cortex have been previously discussed in terms of their roles in externally paced finger tapping tasks. The observed concordance within these regions for the RH index finger group is more likely due to the inclusion of these studies using external pacing cues in the RH index finger tapping task group, as opposed any specific role these regions have in simple, right hand index finger tapping. However in regards to the insular cortex, its role in movement timing would seem to suggest that this region should exhibit concordance for more than just the visually-paced studies and RH index finger studies, especially since this region is also implicated in such timing tasks as interval sequence encoding (Schubotz 2000) and sensorimotor synchronization (Rubia 2000). The concordance cluster within the left DLPFC, observed for the RH index finger group, cannot be fully explained, as this region has been implicated in motor preparation for imitative tasks as well as in the selection and combination of individual motor elements into new motor tasks (Buccino 2004), and the RH index finger tapping tasks employed by the studies were neither imitative nor novel.
Only the ALE maps for the RH multi-finger sequence tapping task group showed concordance within bilateral posterior parietal cortex (BA7, precuneus). Neurons in the posterior parietal cortex (PPC) have been shown to be responsive to hand manipulation and movements in extra-personal space (Mountcastle 1975), and the region itself is thought of as an integrative system involved in the processing of spatial aspects of movements (Boecker 1998; Sadato 1996a; Roland 1980a). Since finger tapping tasks do not necessitate extensive movements in extra-personal space, the observed concordance within the PPC may suggest that this area is also involved in the temporal aspects of the task, the integration of sensory information into the movement sequence, as well as the production of movement sequences in general (Gordon 1998s).Studies have shown that this region, particularly the right PPC, is active during complex sequential motor tasks (Boecker 1998; Jenkins 1994a; Wenderoth 2005; Grafton 1992b). Further studies have demonstrated the role of the PPC in the integration of both auditory and visual cues into movement selection and execution (Deiber 1991; Grafton 1992b; Jenkins 1994a). In addition to serving as a higher-order motor structure, activity within the PPC has been linked to the executive processing of working memory, in particular, updating, order, and manipulation tasks (Wager 2003). Further studies have also demonstrated this region to be active during memory retrieval (Buckner 1996; Smith 1997; Krause 1999; Schmidt 2002; Shannon and Buckner 2004), suggesting that activity in the posterior parietal cortex may be related to the retrieval of a memorized sequence and its translation into a plan of execution (Sakai 1998) in addition to its above described role in the temporal aspects and sensory integration of movement sequences.
Concordance for the bimanual tapping task group is observed in the posterior cerebellum, vermal lobe VI/declive (Schmahmann 1999). Activity in the posterior cerebellum has been most often linked to the temporal aspects of motor and visuospatial working memory tasks (Geier 2007; Jantzen 2007; Simmonds 2007). Additionally, Habas et. al. (2004b) demonstrated, specifically, that vermal lobe VI may play a role in both simple and complex bimanual movements. These results suggest that bimanual movements may require additional temporal processing compared with unimanual tasks.
EFFECTS OF TAPPING RATE AND PROFICIENCY
Although we included coordinates from studies specifically employing tapping tasks of varying rates (Aramaki 2006; Blinkenberg 1996; Jäncke 1999; Jäncke 2000a; Kawashima 1999; Lehéricy 2006; Riecker 2006; Rounis 2006), and previous studies have shown that the so called “rate effect” can have an impact on the degree of both cortical (Deiber 1999; Jäncke 1998; Lutz 2005) and subcortical (Lutz 2005) activations, it would be difficult for us to draw any conclusions regarding the effect of varying the frequency of a tapping task would have on the observed clusters of concordance. For all studies reporting a movement frequency, the frequencies ranged from 0.25 to 4 Hz (average = 1.73 Hz; mode = 2 Hz). There, as yet, does not appear to be a consensus as to at what frequency or frequencies of movement the rate effect becomes important (Deiber 1999). Sadato et. al. (1996b) found peak SMA activation at 0.5 Hz, with decreasing activation at higher frequencies. Blinkenberg et. al. (1996), in contrast, found peak SMA activation at 1 Hz, with decreasing activation at higher frequencies less than 2 Hz; a second peak was reported at 4 Hz. Finally, in regards to self-paced, repetitive simple movements in young, healthy subjects, Diciotti et. al. (2007) demonstrated that the rate effect did was not a relevant source of variability in fMRI signal for task frequencies ranging from 0.2 to 2 Hz. Since the average and mode frequencies of movement reported were both approximately 2 Hz, it is unlikely that the rate effect had any significant impact on the results of our meta-analyses.
Enhanced training on motor tasks has also been shown to have an effect on the level of activation observed in cortical motor structures, particularly the primary sensorimotor cortex (Jäncke 2000c; Koeneke 2006a, 2006b). Prior functional neuroimaging studies have demonstrated an initial decrease in primary sensorimotor cortical activation contralateral to the moving hand during motor skill acquisition, followed by an enlargement in activation in this same region during the course of motor training, which has been shown to be sustained for up to 4 weeks post-training (Jäncke 2000c). Motor skill acquisition has been suggested to occur in two discrete stages: the first being a fast learning, initial, within-session improvement phase and the second being a slow learning phase, consisting of delayed, incremental gains in performance during continued practice (Koeneke 2006a). Only one study included (Boecker 1998) reported using an extended training period of 2 weeks, in an effort to allow the complex tapping sequences employed to become more automatic. Of the remaining studies who reported training (21 studies), all used training sessions just prior to scanning. Although the majority of these sutides merely indicated that training had occurred, several studies did report the duration of these training sessions, which lasted from a few minutes to up to an hour. As there is no conclusive neurophysiological data demonstrating a clear effect of the first stage of motor skill learning (Koeneke 2006a), the lack of consistency in reporting details on pre-scan training makes it again difficult to assess what effect, if any this may have had on our results.
CONCLUSIONS
From the results of the meta-analyses performed, it appears that the choice or lack of a pacing stimulus has a greater effect on the network of brain regions consistently reported to be active than the choice of a more complex tapping task. For all of the task variations considered, though, the additional regions reported to be active, beyond those involved in the general motor execution, seem to be involved preferentially in the temporal aspects of the tapping task. The use of a visual pacing stimulus seems to require a network of brain regions that is distinct from either that observed during the use of an auditory or no pacing stimulus. This suggests that the network of brain regions necessary to integrate a visual pacing stimulus into a simple motor task is separate from that for an auditory pacing stimulus, however, further study examining the effects of pacing stimuli on the performance of simple motor tasks is warranted. The lack of diversity in brain networks consistently reported during the two complex finger tapping task variations examined here suggests that further complexity, such as movement in extra-personal space, may be required to fully elucidate the network of brain regions necessary to execute truly complex motor tasks.
ACKNOWLEDGMENTS
ARL was supported by the Human Brain Project of the NIMH (R01-MH074457-01A1). STW was supported by the Vilas (William F) Trust Estate: Vilas Life Cycle Professorship and NIH grant 1-R01-CA118365-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aoki T, Tsuda H, Takasawa M, Osaki Y, Oku N, Hatazawa J, Kinoshita H. The effect of tapping finger and mode differences on cortical and subcortical activities: a PET study. Exp. Brain Res. 2005;160:375–383. doi: 10.1007/s00221-004-2008-9. [DOI] [PubMed] [Google Scholar]
- Aramaki Y, Honda M, Okada T, Sadato N. Neural correlates of the spontaneous phase transition during bimanual coordination. Cerebral Cortex. 2006;16:1338–1348. doi: 10.1093/cercor/bhj075. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Brain Res. Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolattie G, Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur. J. Neurosci. 1999;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G. Motor functions of the Broca’s region. Brain and Language. 2004;89:362–369. doi: 10.1016/S0093-934X(03)00358-4. [DOI] [PubMed] [Google Scholar]
- Blinkenberg M, Bonde C, Holm S, Svarer C, Andersen J, Paulson OB, Law I. Rate dependence of regional cerebral activation during performance of a repetitive motor task: a PET study. J. Cereb. Blood Flow Metab. 1996;16:794–803. doi: 10.1097/00004647-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Boecker H, Dagher A, Ceballos-Baumann AO, Passingham RE, Samuel M, Friston KJ, Poline J, Dettmers C, Conrad B, Brooks DJ. Role of the human rostral supplementary motor area and the basal ganglia in motor sequence control: investigations with H2 15O PET. J. Neurophysiol. 1998;79:1070–1080. doi: 10.1152/jn.1998.79.2.1070. [DOI] [PubMed] [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster J. Using the Talairach atlas with the MNI template. NeuroImage. 2001;13:S85. [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat. Rev. Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Brinkman C. Supplementary motor area of the monkey’s cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J. Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, Petersen SE. Functional anatomic studies of memory retrieval for auditory words and visual pictures. J. Neurosci. 1996;16:6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C, Serrati C, Baron JC. Effects of age on brain activation during auditory-cued thumb-to-index opposition: A positron emission tomography study. Stroke. 2001;32:139–146. doi: 10.1161/01.str.32.1.139. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain. 1998;121:253–264. doi: 10.1093/brain/121.2.253. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain. 1999;122:483–495. doi: 10.1093/brain/122.3.483. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Hagberg GE, Bianciardi M, Sabatini U. Visually cued motor synchronization: modulation of fMRI activation patterns by baseline condition. Neurosci. Lett. 2005;373:32–37. doi: 10.1016/j.neulet.2004.09.076. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Hagberg GE, Peppe A, Bianciardi M, Gioia MC, Costa A, Castriota-Scanderbeg A, Caltagirone C, Sabatini U. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res. Bull. 2006;71:259–269. doi: 10.1016/j.brainresbull.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fissell K, Jacobs S, Fiez JA. Functional heterogeneity within Broca’s area during verbal working memory. Physiol. Behav. 2002;77:635–639. doi: 10.1016/s0031-9384(02)00899-5. [DOI] [PubMed] [Google Scholar]
- Chen YC, Thaler D, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. II. The timing and selection of learned movements. Exp. Brain Res. 1995;102:461–473. doi: 10.1007/BF00230650. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen LG. Involvement of the ipsilateral motor cortex in finger movements of different complexities. Ann. Neurol. 1997;41:247–254. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann. Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. The Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Deiber MP, Passingham RE, Friston KJ, Frackowiak RS. Region cerebral blood flow during voluntary arm and hand movements in human subjects. J. Neurophysiol. 1991;65:1392–1401. doi: 10.1152/jn.1991.65.6.1392. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Differential relation of discharge in primary motor cortex and premotor cortex to movements versus actively maintained postures during a reaching task. Exp. Brain Res. 1996;108:45–61. doi: 10.1007/BF00242903. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. What is the function of the claustrum? Philos. Trans. R. Soc. Lond., B, Bio. Sci. 2005;360:1271–1279. doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. NeuroImage. 2002;15:373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- De Luca M, Smith S, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp. Brain Res. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs. external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. NeuroImage. 2003;19:764–776. doi: 10.1016/s1053-8119(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Cerebellar and premotor function in bimanual coordination: parametric neural response to spatiotemporal complexity and cycling frequency. NeuroImage. 2004;21:1416–1427. doi: 10.1016/j.neuroimage.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta JC, Fazio F. Mapping motor representations with positron emission tomography. Nature. 1994;371:600–602. doi: 10.1038/371600a0. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp. Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibañez V, Sadato N, Hallet M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: Effect of movement type and rate. J. Neurophysiol. 1999;81:3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- Denslow S, Lomarev M, George MS, Bohning DE. Cortical and subcortical brain effects of transcranial magnetic stimulation (TMS)-induced movement: an interleaved TMS/functional magnetic resonance imaging study. Biol. Pyschiatry. 2005;57:752–760. doi: 10.1016/j.biopsych.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Diciotti S, Gavazzi C, Della Nave R, Boni E, Ginestroni A, Paoli L, Cecchi P, De Stefano N, Mascalchi M. Self-paced frequency of a simple motor task and brain activation: An fMRI study in healthy subjects using an on-line monitor device. NeuroImage. 2007;38:402–412. doi: 10.1016/j.neuroimage.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Edelstein LR, Denaro FJ. The clastrum: a historical review of its anatomy, physiology, cytochemistry and functional significance. Cell. Mol. Biol. (Noisy-le-grand) 2004;50:675–702. [PubMed] [Google Scholar]
- Fogassi L, Gallese V, di Pellegrino G, Fadiga L, Gentilucci J, Luppino G, Matelli M, Pedotti A, Rizzolatti G. Space coding by premotor cortex. Exp. Brain Res. 1992;89:686–690. doi: 10.1007/BF00229894. [DOI] [PubMed] [Google Scholar]
- Forss N, Jousmaki V. Sensorimotor integration in human primary and secondary somatosensory cortices. Brain Res. 1998;781:259–267. doi: 10.1016/s0006-8993(97)01240-7. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Thach WT. Functional mapping of the human cerebellum with positron emission tomography. Proc. Natl. Acad. Sci. USA. 1985;82:7462–7466. doi: 10.1073/pnas.82.21.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Sandoval H, Fox SP, Kochunov P, Lancaster JL. Column-based model of electric field excitation of cerebral cortex. Human Brain Mapping. 2004;22:1–14. doi: 10.1002/hbm.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RS. Willed action and prefrontal cortex in man: a study with PET. Proc. Biol. Sci. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. NeuroImage. 2007;35:904–915. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV. A comparative fMRI study of cortical representations for thermal painful, vibrotactial, and motor performance tasks. NeuroImage. 1999;10:460–482. doi: 10.1006/nimg.1999.0482. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Sirigu A, Lehéricy S, Poline JB, Gaymard B, Marsault C, Agid Y, Le Bihan D. Partially overlapping neural networks for real and imagined hand movements. Cereb. Cortex. 2000;10:1093–1104. doi: 10.1093/cercor/10.11.1093. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. The role of the human motor cortex in the control of complex and simple finger movement sequences. Brain. 1998;121:1695–1709. doi: 10.1093/brain/121.9.1695. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Lemon RN, Nijs HG, Kuypers HG. Behaviour of neurons in monkey periarcuate and precentral cortex before and during visually guided arm and hand movements. Exp. Brain Res. 1981;44:113–116. doi: 10.1007/BF00238755. [DOI] [PubMed] [Google Scholar]
- Goerres GW, Samuel M, Jenkins IH, Brooks DJ. Cerebral control of unimanual and bimanual movements: an H2(15)O PET study. Neuroreport. 1998;9:3631–3638. doi: 10.1097/00001756-199811160-00014. [DOI] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function: review and hypotheses. Behav. Brain Sci. 1985;8:567–616. [Google Scholar]
- Gordon AM, Lee JH, Flament D, Ugurbil K, Ebner TJ. Functional magnetic resonance imaging of motor, sensory, and posterior parietal cortical areas during performance of sequential typing movements. Exp. Brain Res. 1998;121:153–166. doi: 10.1007/s002210050447. [DOI] [PubMed] [Google Scholar]
- Gosain AK, Birn RM, Hyde JS. Localization of the cortical response to smiling using new imaging paradigms with functional magnetic resonance imaging. Plast. Reconstr. Surg. 2001;108:1136–1144. doi: 10.1097/00006534-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Woods RP, Phelps ME. Human functional anatomy of visually guided finger movements. Brain. 1992a;115:565–587. doi: 10.1093/brain/115.2.565. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presky S, Friston KJ, Frackowiak RS, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J. Neurosci. 1992b;12:2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Tyszka M. Functional imaging of procedural motor learning: Relating cerebral blood flow with individual subject performance. Human Brain Mapping. 1993;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Woods RP, Arbib MA. Functional anatomy of pointing and grasping in humans. Cereb. Cortex. 1999;6:226–237. doi: 10.1093/cercor/6.2.226. [DOI] [PubMed] [Google Scholar]
- Habas C, Axelrad H, Cabanis EA. The cerebellar second homunculus remains silent during passinve bimanual movements. Neuroreport. 2004a;15:1571–1574. doi: 10.1097/01.wnr.0000133970.53139.e3. [DOI] [PubMed] [Google Scholar]
- Habas C, Axelrad H, Nguyen TH, Cabanis EA. Specific neocerebellar activation during out-of-phase bimanual movements. Neuroreport. 2004b;15:595–599. doi: 10.1097/00001756-200403220-00005. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Roland PE. Cross-modal transfer of information between the tactile and the visual representations in the human brain: A positron emission tomographic study. J. Neurosci. 1998;18:1072–1084. doi: 10.1523/JNEUROSCI.18-03-01072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsband U, Passingham RE. The role of premotor and parietal cortex in the direction of action. Brain Res. 1982;240:368–372. doi: 10.1016/0006-8993(82)90239-6. [DOI] [PubMed] [Google Scholar]
- Halsband U, Passingham RE. Premotor cortex and the conditions for movement in monkeys (Macaca fascicularis) Behav. Brain Res. 1985;18:269–277. doi: 10.1016/0166-4328(85)90035-x. [DOI] [PubMed] [Google Scholar]
- Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116:243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M. Functional properties of brain areas associated with motor execution and imagery. J. Neurophysiol. 2003;89:989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Rao SM, Haaland KY, Bobholz JA, Mayer AR, Binderx JR, Cox RW. Specialized neural systems underlying representations of sequential movements. J. Cogn. Neurosci. 2000;12:56–77. doi: 10.1162/08989290051137602. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Weilke F, Ceballos-Baumnann AO, Bartenstein P, von Einsiedel HG, Schwaiger M, Conrad B, Boecker H. The role of lateral premotor-cerebellar-parietal circuits in motor sequence control: a parametric fMRI study. Brain Res. Cogn. Brain Res. 2002;13:159–168. doi: 10.1016/s0926-6410(01)00104-5. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Hlustík P, Solodkin A, Gullapalli RP, Noll DC, Small SL. Functional lateralization of the human premotor cortex during sequential movements. Brain and cognition. 2002;49:54–62. doi: 10.1006/brcg.2001.1483. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibáñez V, Pascual-Leone A, Zhuang P, Hallett M. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain. 1998;121:2159–2173. doi: 10.1093/brain/121.11.2159. [DOI] [PubMed] [Google Scholar]
- Huttenen J, Wikström H, Korvenoja A, Seppäläinen AM, Aronen H, Ilmoniemi RJ. Significance of the second somatosensory cortex in sensorimotor integration: enhancement of sensory responses during finger movements. Neuroreport. 1996;7:1009–1012. doi: 10.1097/00001756-199604100-00011. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequence of its dysfunction. Nat. Rev. Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Immisch I, Walvogel D, van Gelderen P, Hallett M. The role of the medial wall and its anatomical variations for bimanual antiphase and in-phase movements. NeuroImage. 2001;14:674–684. doi: 10.1006/nimg.2001.0856. [DOI] [PubMed] [Google Scholar]
- Indovina I, Sanes JN. Combined visual attention and finger movement effects on human brain representations. Exp. Brain Res. 2001;140:265–279. doi: 10.1007/s002210100796. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jantzen KJ, Oullier O, Marshall M, Steinberg FL, Kelso JAS. A parametric fMRI investigation of context effects in sensorimotor timing and coordination. Neuropsychologia. 2007;45:673–684. doi: 10.1016/j.neuropsychologia.2006.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Specht K, Mirzazade S, Loose R, Himmelbach M, Lutz K, Shah NJ. A parametric analysis of the ‘rate effect’ in the sensorimotor cortex: a functional magnetic resonance imaging analysis in human subjects. Neurosci. Lett. 1998;252:37–40. doi: 10.1016/s0304-3940(98)00540-0. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Specht K, Mirzazade S, Peters M. The effect of finger-movement speede of the dominant and the subdominant hand on cerebellar activation: A functional magnetic resonance imaging study. NeuroImage. 1999;9:497–507. doi: 10.1006/nimg.1998.0426. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Peters M, Himmelbach M, Nösselt T, Shah J, Steimetz H. fMRI study of bimanual coordination. Neuropsychologia. 2000a;38:164–174. doi: 10.1016/s0028-3932(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Loose R, Lutz K, Specht K, Shah NJ. Cortical activations during paced finger-tapping applying visual and auditory pacing stimuli. Brain Res. Cogn. Brain Res. 2000b;10:51–66. doi: 10.1016/s0926-6410(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Shah NJ, Peters M. Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Cogn. Brain Res. 2000c;10:177–183. doi: 10.1016/s0926-6410(00)00028-8. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. J. Neurosci. 1994a;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Brown R, Frackowiak RSJ, Marsden CD, Passingham RE, Brooks DJ. Impaired activation of mesial frontal-cortex during self-paced movements in Parkinsons-disease. Neurology. 1994b;44:A353. [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb. Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Joliot M, Crivello F, Badier JM, Diallo B, Tzourio N, Mazoyer B. Anatomical congruence of metabolic and electromagnetic activation signals during a self-paced motor task: a combined PET-MEG study. NeuroImage. 1998;7:337–351. doi: 10.1006/nimg.1998.0333. [DOI] [PubMed] [Google Scholar]
- Joliot M, Papathanassiou D, Mellet E, Quinton O, Mazoyer N, Courtheoux P, Mazoyer B. FMRI and PET of self-paced finger movement: comparison of intersubject stereotaxic averaged data. NeuroImage. 1999;10:430–447. doi: 10.1006/nimg.1999.0483. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J. Neurophysiol. 1997;77:1325–1337. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Yamada K, Kinomura S, Yamaguchi T, Matsui H, Yoshioka S, Fukuda H. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res. 1993;623:33–40. doi: 10.1016/0006-8993(93)90006-9. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Fukuda H. Functional organization of the human primary motor area: an update on current concepts. Rev. Neurosci. 1994a;5:347–354. doi: 10.1515/revneuro.1994.5.4.347. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Roland PE, O’Sullivan BT. Activity in the human primary motor cortex related to ipsilateral hand movements. Brain Res. 1994b;663:251–256. doi: 10.1016/0006-8993(94)91270-x. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Roland PE, O’Sullivan BT. Functional anatomy of reaching and visuomotor learning: a positron emission tomography study. Cereb. Cortex. 1995;5:111–122. doi: 10.1093/cercor/5.2.111. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Inoue K, Sugiura M, Okada K, Ogawa A, Fukuda H. A positron emission tomography study of self-paced finger movements at difference frequencies. Neuroscience. 1999;92:107–112. doi: 10.1016/s0306-4522(98)00744-1. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Okuda J, Umetsu A, Sugiura M, Inoue K, Suzuki K, Tabuchi M, Tsukiura T, Narayan SL, Nagasaka T, Yanagawa I, Fujii T, Takahashi S, Fukuda H, Yamadori A. Human cerebellum plays an important role in memory-timed finger movement: an fMRI study. J. Neurophysiol. 2000;83:1079–1087. doi: 10.1152/jn.2000.83.2.1079. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Rouiller EM. Do bimanual motor actions involve the dorsal premotor (PMd), cingulated (CMA) and posterior parietal (PPC) cortices? Comparison with primary and supplementary motor cortical areas. Somatosensory & motor research. 2000;17:255–271. doi: 10.1080/08990220050117619. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Georgopoulos AP, Merkle H, Ellermann JM, Menon RS, Ogawa S, Ugurbil K. Functional imaging of human motor cortex at high magnetic field. J. Neurophsyiol. 1993a;69:297–302. doi: 10.1152/jn.1993.69.1.297. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993b;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Lutz K, Herwig U, Ziemann U, Jäncke L. Extensivve training of lementary finger tapping movements changes the pattern of motor cortex excitability. Exp. Brain Res. 2000a;174:199–209. doi: 10.1007/s00221-006-0440-8. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Lutz K, Esslen M, Jäncke L. How finger tapping practice enhances efficiency of motor control. NeuroReport. 2006b;17:1565–1569. doi: 10.1097/01.wnr.0000234748.80936.1d. [DOI] [PubMed] [Google Scholar]
- Krause BJ, Schmidt D, Mottaghy FM, Taylor J, Halsband U, Herzog H, Tellmann L, Müller-Gärtner HW. Episodic retrieval activates the precuneus irrespective of the imagery content of word pair associates. A PET study. Brain. 1999;122:255–263. doi: 10.1093/brain/122.2.255. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Mahnkopf C, Holzknecht C, Siebner H, Ulmer S, Jansen O. Effector-independent representations of simple and complex imagined finger movements: a combined fMRI and TMS study. Eur. J. Neurosci. 2003;18:3375–3387. doi: 10.1111/j.1460-9568.2003.03066.x. [DOI] [PubMed] [Google Scholar]
- Kurata K. Premotor cortex of monkeys: set- and movement-related activity reflecting amplitude and direction of wrist movements. J. Neurophysiol. 1993;69:187–200. doi: 10.1152/jn.1993.69.1.187. [DOI] [PubMed] [Google Scholar]
- Kurata K. Information processing for motor control in primate premotor cortex. Behav. Brain Res. 1994a;61:135–142. doi: 10.1016/0166-4328(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Kurata K. Site of origin of projections from the thalamus to dorsal versus ventral aspects of the premotor cortex of monkeys. Neurosci. Res. 1994b;21:71–76. doi: 10.1016/0168-0102(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoffman DS. Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J. Neurophysiol. 1994c;71:1151–1164. doi: 10.1152/jn.1994.71.3.1151. [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoshi E. Reacquisition deficits in prism adaptation after muscimol microinjection into the ventral premotor cortex of monkeys. J. Neurophysiol. 1999;81:1927–1938. doi: 10.1152/jn.1999.81.4.1927. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Gulyás B, Roland PE. Cortical representation of self-paced finger movement. Neuroreport. 1996;7:463–468. doi: 10.1097/00001756-199601310-00021. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, van de Moortele PF, Lobel E, Paradis AL, Vidailhet M, Frouin V, Neveu P, Agid Y, Marsault C, Le Bihan D. Somatotopical organization of striatal activation during finger and toe movement: a 3-T functional magnetic resonance imaging study. Ann. Neurol. 1998;44:398–404. doi: 10.1002/ana.410440319. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Bardinet E, Tremblay L, van de Moortele PF, Pochon JB, Dormont D, Kim DS, Yelnik J, Ugurbil K. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb. Cortex. 2006;16:149–161. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- Lerner A, Shill H, Hanakawa T, Bushara K, Goldfine A, Hallett M. Regional cerebral blood flow correlates of the severity of writer's cramp symptoms. NeuroImage. 2004;21:904–913. doi: 10.1016/j.neuroimage.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Lin YY, Forss N. Functional characterization of human second somatosensory cortex by magnetoencephalography. Behav. Brain Res. 2002;135:141–145. doi: 10.1016/s0166-4328(02)00143-2. [DOI] [PubMed] [Google Scholar]
- Lutz K, Specht K, Shah NJ, Jäncke L. Tapping movements according to regular and irregular visual timing signals investigated with fMRI. Neuroreport. 2000;11:1301–1306. doi: 10.1097/00001756-200004270-00031. [DOI] [PubMed] [Google Scholar]
- Lutz K, Koeneke S, Wüstenberg T, Jäncke L. Asymmetry of cortical activation during maximum and convenient tapping speed. Neurosci. Lett. 2005;373:61–66. doi: 10.1016/j.neulet.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Maillard L, Ishii K, Bushara K, Waldvogel D, Schulman AE, Hallett M. Mapping the basal ganglia: fMRI evidence for somatotopic representation of face, hand, and foot. Neurology. 2000;55:377–383. doi: 10.1212/wnl.55.3.377. [DOI] [PubMed] [Google Scholar]
- Matelli M, Rizzolatti G, Bettinardi V, Gilardi MC, Perani D, Rizzo G, Fazio F. Activation of precentral and mesial motor areas during the execution of elementary proximal and distal arm movements: a PET study. Neuroreport. 1993;4:1295–1298. doi: 10.1097/00001756-199309150-00002. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Santha AK, Van Horn JD, Tallent KA, Frank JA, Weinberger DR. Hemispheric control of motor function: a whole brain echo planar fMRI study. Psychiatry Res. 1998;83:7–22. doi: 10.1016/s0925-4927(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Menon V, Glover GH, Pfefferbaum A. Differential activation of dorsal basal ganglia during externally and self paced sequences of arm movements. Neuroreport. 1998;9:1567–1573. doi: 10.1097/00001756-199805110-00058. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Ziemann U, Hajak G, Cohen L, Berman KF. Transitions between dynamical states of differing stability in the human brain. Proc. Natl. Acad. Sci. USA. 2002;99:10948–10953. doi: 10.1073/pnas.162114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Brain Res. Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J. Neurophsyiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Selective coding of motor sequence in the supplementary motor area of the monkey cerebral cortex. Exp. Brain Res. 1990;82:208–210. doi: 10.1007/BF00230853. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J. Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- Müller JL, Röder CH, Schuierer G, Klein H. Motor-induced brain activation in cortical, subcortical and cerebellar regions in schizophrenic inpatients A whole brain fMRI fingertapping study. Prog. Neuropsychopharmocol. Biol. Psychiatry. 2002;26:421–426. doi: 10.1016/s0278-5846(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Nakai T, Kato C, Glover GH, Toma K, Moriya T, Matsuo K. A functional magnetic resonance imaging study of internal modulation of an external visual cue for motor execution. Brain Res. 2003;968:238–247. doi: 10.1016/s0006-8993(03)02249-2. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Larsen B. Activation of the supplementary motor area during voluntary movement in man suggests it works as a supramotor area. Science. 1979;206:847–850. doi: 10.1126/science.493986. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Premotor cortex: sensory cues and movement. Behav. Brain Res. 1985;18:175–185. doi: 10.1016/0166-4328(85)90073-7. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zattore RJ, Evans AC. Cerebellar contributions to motor timing: a PET study of auditory and visual rhythm reproduction. J. Cogn. Neurosci. 1998;10:752–765. doi: 10.1162/089892998563149. [DOI] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Salamon G, Mazoyer B, Berthoz A. PET study of voluntary saccadic eye movements in humans: basal ganglia-thalamocortical system and cingulated cortex involvement. J. Neurophsyiol. 1993;69:1009–1017. doi: 10.1152/jn.1993.69.4.1009. [DOI] [PubMed] [Google Scholar]
- Petrides M. Motor conditional associative-learning after selective prefrontal lesions in the monkey. Behav. Brain Res. 1982;5:407–413. doi: 10.1016/0166-4328(82)90044-4. [DOI] [PubMed] [Google Scholar]
- Petrides M. Deficits in non-spatial conditional associated learning after periarcuate lesions in the monkey. Behav. Brain Res. 1985;1985:95–101. doi: 10.1016/0166-4328(85)90085-3. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann. Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Kirkby BS, van Gelderen P, Berman KF, Duyn JH, Frank JA, Mattay VS, Van Horn JD, Esposito G, Moonen CT, Weinberger DR. Functional mapping of human sensorimotor cortex with 3D BOLD fMRI correlates highly with H2(15)O PET rCBF. J. Cereb. Blood Flow Metab. 1996;16:755–764. doi: 10.1097/00004647-199609000-00001. [DOI] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J. Neurosci. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Naure Neurosci. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Brown HD, Curran T, Alpert NM, Kendrick A, Fischman AJ, Kosslyn SM. A PET investigation of implicit and explicit sequence learning. Human Brain Mapping. 1995;3:271–286. [Google Scholar]
- Riecker A, Wildgruber D, Dogil G, Grodd W, Ackermann H. Hemispheric lateralization effects of rhythm implementation during syllable repetitions: an fMRI study. NeuroImage. 2002;16:169–176. doi: 10.1006/nimg.2002.1068. [DOI] [PubMed] [Google Scholar]
- Riecker A, Gröschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A. Functional significance of age-related differences in motor activation patterns. NeuroImage. 2006;32:1345–1354. doi: 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Dettmers C, Büchel C, Kiebel S, Frackowiak RS, Weiller C. A blueprint for movement: functional and anatomical representations in the human motor system. J. Neurosci. 1999;19:8043–8048. doi: 10.1523/JNEUROSCI.19-18-08043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Gentilucci M, Fogassi L, Luppino G, Matelli M, Ponzoni-Maggi S. Neurons related to goal-directed motor acts in inferior act 6 of the macaque monkey. Exp. Brain Res. 1987;67:220–224. doi: 10.1007/BF00269468. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Gentilucci M, Camarda RM, Gallese V, Luppino G, Metlli M, Fogassi L. Neurons related to reaching-grasping arm movements in the rostral part of 6 (area 6a beta) Exp. Brain Res. 1990;82:337–350. doi: 10.1007/BF00231253. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res. Cogn. Brain Res. 1996;3:1131–1141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Roland PE, Skinhøj E, Lassen NA, Larsen B. Different cortical areas in man in organization of voluntary movements in extrapersonal space. J. Neurophysiol. 1980a;43:137–150. doi: 10.1152/jn.1980.43.1.137. [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J. Neurophysiol. 1980b;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Roland PE, Meyer E, Shibasaki T, Yamamoto YL, Thompson CJ. Region cerebral blood flow changes in cortex and basal ganglia during voluntary movements in normal human volunteers. J. Neurophysiol. 1982;48:467–480. doi: 10.1152/jn.1982.48.2.467. [DOI] [PubMed] [Google Scholar]
- Roland PE. Organization of motor control by the normal human brain. Human Neurobiology. 1984;2:205–216. [PubMed] [Google Scholar]
- Roland PE, Eriksson L, Widén L, Stone-Elander S. Changes in regional cerebral oxidative metabolism induced by tactile learning and recognition in man. Eur. J. Neurosci. 1989;1:3–18. doi: 10.1111/j.1460-9568.1989.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Romo R, Schultz W. Neuronal activity preceding self-initiated or externally timed arm movements in area 6 of monkey cortex. Exp. Brain Res. 1987;67:656–662. doi: 10.1007/BF00247297. [DOI] [PubMed] [Google Scholar]
- Rounis E, Lee L, Siebner HR, Rowe JB, Friston KJ, Rothwell JC, Frackowiak RSJ. NeuroImage. 2005;26:164–176. doi: 10.1016/j.neuroimage.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci. Biobehav. Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Sadato N, Zerffior TA, Campbell G, Konishi J, Shibasaki H, Hallett M. Regional cerebral blood flow changes in motor cortical areas after transient anesthesia of the forearm. Ann. Neurol. 1995;37:74–81. doi: 10.1002/ana.410370114. [DOI] [PubMed] [Google Scholar]
- Sadato N, Campbell G, Ibáñez V, Deiber M, Hallett M. Complexity affects regional cerebral blood flow change during sequential finger movements. J. Neurosci. 1996a;16:2691–2700. doi: 10.1523/JNEUROSCI.16-08-02691.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Ibáñez V, Deiber MP, Campbell G, Leonardo M, Hallett M. Frequency-dependent changes in regional cerebral blood flow during finger movements. J. Cereb. Blood Flow Metab. 1996b;16:23–33. doi: 10.1097/00004647-199601000-00003. [DOI] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y. Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J. Neurosci. 1997;17:9667–9674. doi: 10.1523/JNEUROSCI.17-24-09667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Pütz B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J. Neurosci. 1998;18:1827–1840. doi: 10.1523/JNEUROSCI.18-05-01827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, Brooks DJ. Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain. 1997;120:963–976. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Schalug G, Knorr U, Seitz R. Inter-subject variability of cerebral activations in acquiring a motor skill: a study with positron emission tomography. Exp. Brain Res. 1994;98:523–534. doi: 10.1007/BF00233989. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Krause BJ, Mottaghy FM, Halsband U, Herzog H, Tellmann L, Müller-Gärtner HW. Brain systems engaged in encoding and retrieval of word-pair associates independent of their imagery content or presentation modalities. Neuropsychologia. 2002;40:457–470. doi: 10.1016/s0028-3932(01)00102-6. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Friederici AD, von Cramon DY. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. NeuroImage. 2000;11:1–12. doi: 10.1006/nimg.1999.0514. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Frunctional-anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. NeuroImage. 2003;20:S5120–S5131. doi: 10.1016/j.neuroimage.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Roland E, Bohm C, Greitz T, Stone-Elander S. Motor learning in man: a positron emission tomographic study. Neuroreport. 1990;1:57–60. doi: 10.1097/00001756-199009000-00016. [DOI] [PubMed] [Google Scholar]
- Seitz RJ. Learning of sequential finger movements in man: a combined kinematic and positron emission tomography (PET) study. Eur. J. Neurosci. 1992;4:154–165. doi: 10.1111/j.1460-9568.1992.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Stephan KM, Binkofski F. Control of action as mediated by the human frontal lobe. Exp. Brain Res. 2000;133:71–80. doi: 10.1007/s002210000402. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J. Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikeda A, Miyazaki M. Both primary motor cortex and supplementary area play an important role in complex finger movement. Brain. 1993;116:1387–1398. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cogn Psychology. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J. Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Binkofski F, Halsband U, Dohle C, Wunderlich G, Schnitzler A, Tass P, Posse S, Herzog H, Surm V, Zilles K, Seitz RJ, Freund HJ. The role of ventral medial wall motor areas in bimanual coordination. A combined lesion and activation study. Brain. 1999a;122:351–368. doi: 10.1093/brain/122.2.351. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Binkofski F, Posse S, Seitz RJ, Freund HJ. Cerebral midline structures in bimanual coordination. Exp. Brain Res. 1999b;128:243–249. doi: 10.1007/s002210050844. [DOI] [PubMed] [Google Scholar]
- Taniwaki T, Okayama A, Yoshiura T, Nakmura Y, Goto Y, Kira J, Tobimatsu S. Reappraisal of the motor role of basal ganglia: a functional magnetic resonance image study. J. Neurosci. 2003;23:3432–3438. doi: 10.1523/JNEUROSCI.23-08-03432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J. The supplementary motor area in the cerebral cortex. Neurosci. Res. 1994;19:251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K. Contrast of neuronal activity between the supplementary motor area and other cortical motor areas. In: Lüders HO, editor. Advances in Neurology. Supplementary Sensorimotor Area. Philadelphia: Lippincott-Raven; 1996. pp. 95–103. [PubMed] [Google Scholar]
- Thaut MH. Neural basis of rhythmic timing networks in the human brain. Ann. N.Y. Acad. Sci. 2003;999:364–373. doi: 10.1196/annals.1284.044. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald D, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrids M. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur. J. Neurosci. 1999;11:3033–3046. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Toni I, Ramnani H, Josephs O, Ashburner J, Passingham RE. Learning arbitrary visuomotor association: temporal dynamic of brain activity. NeuroImage. 2001;14:1048–1057. doi: 10.1006/nimg.2001.0894. [DOI] [PubMed] [Google Scholar]
- Toni I, Rowe J, Stephan KE, Passingham RE. Changes of cortico-striatal effect connectivity during visuomotor learning. Cereb. Cortex. 2002;12:1040–1047. doi: 10.1093/cercor/12.10.1040. [DOI] [PubMed] [Google Scholar]
- Toyokura M, Muro I, Komiya T, Obara M. Relation of bimanual coordination to activation in the sensorimotor cortex and supplementary motor area: analysis using functional magnetic resonance imaging. Brain Res. Bull. 1999;48:211–217. doi: 10.1016/s0361-9230(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Toyokura M, Muro I, Komiya T, Obara M. Activation of pre-supplementary motor area (SMA) and SMA proper during unimanual and bimanual complex sequences: an analysis using functional magnetic resonance imaging. J. Neuroimaging. 2002;12:172–178. doi: 10.1111/j.1552-6569.2002.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Ullén F, Forssberg H, Ehrsson HH. Neural networks for the coordination of the hands in time. J. Neurophsyiol. 2003;89:1126–1135. doi: 10.1152/jn.00775.2002. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Corcos DM. Intermittent visuomotor processing in the human cerebellum, parietal cortex, and premotor cortex. J. Neurophysiol. 2006;95:922–931. doi: 10.1152/jn.00718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Weeks RA, Honda M, Catalan MJ, Hallett M. Comparison of auditory, somatosensory, and visually instructed and internally generated finger movements: a PET study. NeuroImage. 2001;14:219–230. doi: 10.1006/nimg.2001.0780. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarcation in man. Ann. Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulated cortex and precuneus in the coordination of motor behaviour. Eur. J. Neurosic. 2005;22:235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Ann. Rev. Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Wei X, Dickey CC, Guttmann CR, Panych LP. Long-term reproducibility analysis of fMRI using hand motor task. Int. J. Neurosci. 2005;115:55–77. doi: 10.1080/00207450490512650. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Halpern AR, Perry DW, Meyer E, Evans AC. Hearing in the mind's ear: a PET investigation of musical imagery and perception. J. Cogn. Neurosci. 1996;8:29–46. doi: 10.1162/jocn.1996.8.1.29. [DOI] [PubMed] [Google Scholar]


