Abstract
Malaria parasite infection in anopheline mosquitoes induces nitrosative and oxidative stresses that limit parasite development, but also damage mosquito tissues in proximity to the response. Based on these observations, we proposed that cellular defenses in the mosquito may be induced to minimize self-damage. Specifically, we hypothesized that peroxiredoxins (Prxs), enzymes known to detoxify reactive oxygen species (ROS) and reactive nitrogen oxide species (RNOS), protect mosquito cells. We identified an Anopheles stephensi 2-Cys Prx ortholog of Drosophila melanogaster Prx-4783, which protects fly cells against oxidative stresses. To assess function, AsPrx-4783 was overexpressed in D. melanogaster (S2) and in A. stephensi (MSQ43) cells and silenced in MSQ43 cells with RNA interference before treatment with various ROS and RNOS. Our data revealed that AsPrx-4783 and DmPrx-4783 differ in host cell protection and that AsPrx-4783 protects A. stephensi cells against stresses that are relevant to malaria parasite infection in vivo, namely nitric oxide (NO), hydrogen peroxide, nitroxyl, and peroxynitrite. Further, AsPrx-4783 expression is induced in the mosquito midgut by parasite infection at times associated with peak nitrosative and oxidative stresses. Hence, whereas the NO-mediated defense response is toxic to both host and parasite, AsPrx-4783 may shift the balance in favor of the mosquito.
Keywords: Malaria, Mosquito, Peroxiredoxin, Plasmodium, Anopheles, Nitric oxide, Nitrosative stress, Free radicals
It has long been recognized that reactive oxygen species (ROS) and, more recently, reactive nitrogen oxide species (RNOS) function to defend hosts against pathogens [1]. Although ROS and RNOS are cytotoxic to pathogens and parasites, they also induce oxidative and nitrosative stresses in the host and can damage host tissues [1]. Host protection against oxidative and nitrosative stress, therefore, is vital for homeostasis and hence survival. Gene products that confer protection against ROS are well known. In contrast, gene products that confer protection against RNOS have been identified, but are less well studied. The peroxiredoxins (Prxs) are a recently discovered family of antioxidant peroxidases that can reduce hydrogen peroxide and alkyl hydroperoxides to water and the corresponding alcohols, respectively, and can protect cells from widely divergent organisms against a variety of nitrosative stress challenges [2–6].
Prxs do not show sequence homology to other known antioxidant enzymes. Crystal structures of PrxI, II, V, and VI have revealed that Prxs are novel members of the thioredoxin fold superfamily [7,8]. Unlike most peroxidases—which contain a heme ring at their active site (e.g., cytochrome c peroxidase) or a redox-sensitive moiety like selenocysteine (glutathione peroxidase; GPx), vanadium (algal bromoperoxide), or flavin (bacterial NADH peroxidase)—Prxs lack cofactor metal ions, prosthetic groups, or cofactors [9]. Prx activity is dependent upon a conserved cysteine, usually in a Val-Cys-Pro (VCP) motif, near the N-terminus [10]. Unlike other peroxidases, Prxs act as both peroxidase and cosubstrate because they are oxidized upon reaction with peroxide [11]. Four general types of Prxs are recognized and are defined according to the number and location of conserved cysteines.
Typical 2-Cys Prxs have two highly conserved cysteines and are among the most well-studied Prxs. This subfamily includes mammalian PrxI through IV and bacterial AhpC. A 2-Cys Prx initially reacts with hydroperoxides at the N-terminal Cys (–SH), thus oxidizing the residue to sulfenic acid (–SOH). This cysteinyl–sulfenic acid is unstable and attacks the C-terminal Cys of a second subunit forming an intermolecular disulfide bridge (–SS–) [8]; thus there are two disulfides in a head-to-tail dimer. To regenerate the active 2-Cys Prx, the disulfide must be reduced. The Prx/reductant relationship is highly specific. In Drosophila melanogaster, 2-Cys DmPrx-4783 uses only one of the two cytosolic thioredoxins as its substrate [12].
A family of five Prx genes was identified and characterized in D. melanogaster [13]. All DmPrxs were shown to have classical peroxidase activity[13,14]. Inducibility of the DmPrxs suggested that they function in oxidant defense [13]. When overexpressed in D. melanogaster S2 cells, each of the five DmPrxs conferred varying degrees of resistance to hydrogen peroxide as measured by cell viability [13]. Conversely, silencing of either of the 2-Cys Prxs, DmPrx-4783 or DmPrx-5037, increased the susceptibility of S2 cells to treatment with hydrogen peroxide and paraquat [14].
We have shown that Anopheles stephensi expresses a single-copy nitric oxide synthase (AsNOS) that is induced in response to infection with Plasmodium berghei and Plasmodium falciparum. We also demonstrated that AsNOS catalytic activity leads to an increase in midgut nitrosative stress [15–17]. Our data indicate that inflammatory levels of midgut RNOS limit or inhibit parasite growth [15,17]. These RNOS, however, also impose stress on the mosquito host (reviewed in [18]). Based on studies that implicate Prx family members in protection against nitrosative stress, along with observations that expression of 1-Cys, 2-Cys, and PrxV genes can be induced by a variety of stimuli [13,19], we sought to identify mosquito Prxs and to determine whether Prx-dependent protection against parasite-inducible nitrosative stress occurs in mosquito cells.
Here, we describe the identification and characterization of a 2-Cys Prx from A. stephensi. This 2-Cys Prx protects mosquito cells in vitro from ROS and RNOS that are generated in response to malaria parasite infection. Further, the pattern of induction of this novel Prx in parasite-infected A. stephensi suggests that this gene product protects the mosquito from self damage during peak periods of oxidative and nitrosative stresses associated with anti-parasite defense.
Materials and methods
Materials
TRIzol and anti-V5 antiserum were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Restriction enzymes (Promega brand), agarose, and proteinase K were purchased from Fisher Scientific (Fairlawn, NJ, USA). Glycogen, RNase cocktail, and DNase were purchased from Ambion (Austin, TX, USA). Cell culture medium and additives for MSQ43 cell maintenance were purchased from Mediatech (Herndon, VA, USA). PCR reagents and supplies were purchased from Applied Biosystems. Goat anti-mouse IgG was purchased from Pierce (Rockford, IL, USA). Peroxynitrite, Angeli's salt, and DEA-NO were purchased from Cayman Chemical (Ann Arbor, MI, USA). All chemicals were of at least analytical grade or molecular biology grade and were purchased from Sigma–Aldrich or Fisher Scientific unless otherwise noted.
Mosquito maintenance and infections
A. stephensi (Indian wild type) were reared at 27°C and 75% relative humidity under a 12-h light/dark cycle. The mosquitoes were fed once each life cycle on anesthetized Institute of Cancer Research (ICR) mice for egg production. All animal care procedures were performed according to the National Research Council Guide for the Care and Use of Laboratory Animals. For parasite infection, A. stephensi were allowed to feed on ICR mice previously inoculated intraperitoneally with thawed aliquots of P. berghei (ANKA strain). Parasitemias were determined from Giemsa-stained blood smears; mosquitoes were fed on mice with 12–17% parasitemias. Before and after blood feeding, mosquitoes were maintained on cotton pads soaked with 10% sucrose (w/v) in water. P. berghei-infected (I) mosquitoes were maintained at 21°C and 75% relative humidity. During time-course experiments non-blood-fed (NB) and blood-fed, uninfected (U) mosquitoes were also maintained at 21°C and 75% relative humidity.
Identification of A. stephensi and A. gambiae Prx sequences
To amplify and clone an A. stephensi 2-Cys Prx gene, degenerate oligonucleotide primers were designed against conserved encoded sequences that appear in most 2-Cys Prx proteins (forward primer, Fb, 5′-GGTGCTKTTCTTCTACCCG, and reverse primer, Rc, 5′-TCGATGATGAACAGGCC; where K is T or G). PCR was performed on genomic DNA prepared from fourth instar larvae and on cDNA prepared from adult female A. stephensi. Additional 5′ and 3′ sequences were obtained using rapid amplification of cDNA ends using the Marathon-Ready cDNA kit (Clontech) and the gene-specific primers P1, 5′-GGACTTTACCTTCGTCTGCCCGACCG, and P2, 5′-CGATGATGAACAGGCCACGGAATGG. The PCR amplimers were subcloned into pCR 2.1-TOPO and transformed into Top10 cells (Invitrogen). All cloned PCR amplimers were subjected to double-stranded DNA dideoxy dye terminator sequencing by UC Davis Sequencing to eliminate sequence ambiguities.
The A. gambiae genome database was searched using TBLASTN [20] with default parameters and all known D. melanogaster sequences as queries [13,21]. The D. melanogaster query sequences, indicated with accession numbers, included DmPrx-2540-1 (AF311879), DmPrx-2540-2 (AF311880), DmPrx-5037 (AF311747), DmPrx-6005 (AF311878), DmPrx-4156 (AF321614), DmPrx-4783-1 (AF321615), DmPrx-4783-2 (AF321616), and DmPrxV (NM_176513). Nucleotide sequences corresponding to the most significant hits to A. gambiae scaffolds were verified as peroxiredoxin-encoding sequences using BLASTN [22] analyses of the GenBank, EMBL, DDBJ, and PDB nucleotide databases (formerly the NCBI nonredundant nucleotide database). Putative nucleotide matches were translated in all reading frames to identify complete open reading frames, which were then verified as genes of interest using TBLASTN analyses [20] of all nonredundant GenBank CDS translations and PDB, SwissProt, PIR, and PRF peptide databases.
RNA extraction
Total RNA was extracted from dissected mosquito midguts using TRIzol (Invitrogen Life Technologies) according to the manufacturer's instructions. For RNA isolation from immortalized A. stephensi cells in culture, glycogen (250 μg/ml) was added to facilitate precipitation of the RNA. Total RNA was quantified spectrophotometrically. For Northern analyses, mRNA was purified from total RNA using the PolyATract mRNA isolation system (Promega).
Genomic Southern blotting analysis
Genomic DNA was prepared from fourth instar larvae of A. stephensi using a protocol adapted from Sambrook et al. [23]. Briefly, larvae were thoroughly rinsed in tepid water and crushed to a powder in liquid nitrogen. The powder was incubated at 37°C in extraction buffer (10 mM Tris, pH 8, 0.1 M EDTA, pH 8, 20 μg/ml pancreatic RNase, 0.5% SDS) for 1 h. Proteinase K (100 μg/ml) was added to the solution and the mixture was heated at 50°C for 3 h. After a phenol/chloroform extraction, the DNA was ethanol precipitated with ammonium acetate. To eliminate RNA contamination, the sample was treated with RNase cocktail (Ambion; 10 μg/ml). The isolated DNA was digested with EcoRI, KpnI, XhoI, or XmnI, electrophoretically separated through 0.8% agarose, and transferred to nylon membrane (Boehringer Mannheim) by capillary transfer.
Full-length A. stephensi 2-Cys Prx was amplified from A. stephensi cDNA using PCR and the following reagents and cycling parameters: forward primer, PWF, 5′-CAGCCTCCAAAAACACCATC; reverse primer, PWR, 5′-GAGGACTCTGGCTGGC; 1 cycle of 95°C for 10 min; 35 cycles of 95°C for 30 s, 57°C for 30 s, 72°C for 30 s; and 1 cycle of 72°C for 5 min. This PCR product was cloned into pCR 2.1-TOPO (Stratagene); this plasmid is hereafter referred to as “pTLP20.” For probe preparation, pTLP20 DNA was digested with EcoRI and electrophoretically separated through 0.8% agarose. The insert, consisting of the complete open reading frame, was purified using the Quantum Prep Freeze and Squeeze kit (Bio-Rad) and randomly labeled with [α-32P]dATP using the Strip-EZ DNA synthesis kit (Ambion).
Southern hybridization was performed overnight at 68°C followed by high-stringency washes (two washes at room temperature with 2× SSPE/0.5%, one wash at 55°C with 0.1 × SSPE/0.5% SDS, and one wash at 68°C with 0.1× SSPE/0.5% SDS). After being washed, the filter was exposed to photographic film (X-OMAT LS; Kodak Scientific Imaging Film) at −80°C.
Cell culture and transfection
D. melanogaster S2 cells were propagated in Drosophila expression system medium (Invitrogen) containing 10% fetal calf serum (FCS; Gibco or Mediatech) and antibiotics (Mediatech) and maintained at 27°C. The A. stephensi cell line MSQ43 (provided by the Department of Entomology, Walter Reed Army Institute of Research) was propagated in Eagle's minimum essential medium with Earl's salts (Cellgro) supplemented with l-glutamine, essential amino acids, glucose, antibiotics, and 5% FCS and maintained at 27°C and 5% CO2. For transfection, cells were plated in 24- or 6-well plates and plasmid or dsRNA was transfected into the cells using Effectene Transfection Reagent (Qiagen) as described by the manufacturer. Cell viability (%) was reported as the ratio of live cells to total cells normalized to the ratio without chemical challenge (to account for variable levels of background death).
Preparation of constructs for AsPrx overexpression
Full-length AsPrx was amplified from MSQ43 cell cDNA by PCR using primers that included the Kozak consensus sequence ([24]; underlined in ClonePrxF primer) and eliminated the stop codon to permit readthrough into plasmid sequence encoding epitope tags: ClonePrxF, 5′-ATCATGCCAGTTCCGGAG, and ClonePrxR, 5′-GTTAACAGCATTGAAGTATTCCTT. This product was cloned into the pMT/V5-His vector (Invitrogen) for copper-inducible expression in S2 cells and into the pcDNA3.1/V5-His vector (Invitrogen) for constitutive expression in MSQ43 cells. Hereafter, these plasmid constructs are referred to as “pTLP55” for S2 cell overexpression and “pTLP58” for MSQ43 cell overexpression. The pTLP55 and pTLP58 plasmid DNAs were isolated and purified from TOP10 Escherichia coli transformants (Qiagen). Correct orientation and sequence were verified for each clone.
Double-stranded RNA production and conditions for RNA interference (RNAi)
The complete open reading frame of AsPrx (591 bp) was amplified using the primers ClonePrxF1 and ClonePrxR1 and plasmid pTLP55 as template. Mouse cyclophilin was amplified using primers and template provided by the Lig'nScribe kit (Ambion). Both amplimers were purified and T7 adapters added using the Lig'nScribe kit. DNA templates for antisense and sense RNA synthesis were amplified using ClonePrxF1/adapter primer 2 and ClonePrxR1/adapter primer 1 for the adapted AsPrx templates and Cyc3′/adapter primer 1 and Cyc5′/adapter primer 1 for the adapted cyclophilin templates according to manufacturer's protocol. These templates were used to synthesize single-stranded RNA (ssRNA) using the MEGAscript/T7 in vitro transcription kit (Ambion). Template DNA was digested with DNase; the remaining ssRNA was purified using MEGAclear (Ambion) according to manufacturer's instructions. RNA yield was quantified using UV absorbance, and integrity was evaluated after electrophoretic separation through 1.4% Mops/formaldehyde agarose.
To prepare dsRNA, equal quantities of sense and antisense ssRNA were mixed in a 10- to 20-μl volume, heated in boiling water for 5 min, and cooled to room temperature (∼2 h). To ensure that dsRNA molecules were formed, dsRNA was resolved in 2% agarose with TBE after treatment with RNase A under both no-salt (0 M NaCl) and high-salt (0.3 M NaCl) conditions; dsRNA is protected from RNase A digestion under high-salt conditions, whereas ssRNA is not [25]. For silencing assays, 1 × 106 MSQ43 cells per well in six-well plates were transfected with 1 μg dsRNA using Effectene transfection reagent (Qiagen) according to the manufacturer's instructions. Efficiency of AsPrx-4783 silencing was determined using a two-step qRT-PCR. In the first step, an oligo(dT)16 primer was used for cDNA synthesis. After treatment with RNase, the cDNA was used for real-time PCR analysis. With this strategy, the potential for amplification from cDNA generated from dsRNA was eliminated and the reduction in transcript levels could be accurately quantified.
Experimentally induced stresses and analyses of cell viability
S2 cells were grown to confluency and then split 1:2 and allowed to recover overnight. Cell density was assessed using a hemocytometer, then 2.29 × 105 cells per well were seeded into 24-well culture plates and were transfected with pTLP55 using Effectene transfection reagent (Qiagen) at 18–26 h after seeding. Transfected gene expression was induced by exposure to 500 μM copper(II) sulfate. At 18–26 h after induction, transfected cells were exposed to one of the following conditions (with 0 h indicating immediately after treatment): 0, 10, or 20 mM hydrogen peroxide (Fisher) for 0, 3, 6, or 9 h; 0, 1.5, or 3 mM DEA-NO (NO donor; Cayman Chemical) for 0, 3, 6, or 9 h; 0, 200, or 500 μM peroxynitrite (PN) (Cayman Chemical) for 0, 3, 6, 9 h; or 0, 1, or 2 mM Angeli's salt (AS) (nitroxyl donor; Cayman Chemical) for 0, 2, 4, or 6 h. DEA-NO, AS, and PN concentrations were verified by spectroscopy, calculating concentration from the Beer–Lambert law and their respective extinction coefficients (ε250 = 9180 M−1 cm−1, ε237 = 6100 M−1 cm−1, and ε302 = 1670 M−1 cm−1).
MSQ43 cells were grown to confluency and seeded into 24-well plates for overexpression studies or 6-well plates for RNAi studies. Cells were seeded at 2.5 × 105 cells/ml and transfected using Effectene transfection reagent (Qiagen) at 18–26 h after seeding. Cells were transfected with the AsPrx overexpression construct (pTLP58), dsRNA for AsPrx, or dsRNA for cyclophilin or mock transfected, which followed the transfection protocol but omitted dsRNA. At 42–50 h after transfection, transfected cells were exposed to one of the following conditions (with 0 h indicating immediately after treatment): 0, 125, or 250 μM hydrogen peroxide (Fisher) for 0, 3, 6, or 9 h; 0, 350, or 700 μM DEA-NO for 0, 3, 6, or 9 h; 0, 75, or 150 μM PN for 0, 3, 6, 9 h; or 0, 50, or 100 μM AS for 0, 2, 4, or 6 h. Viability of S2 and MSQ43 cells after chemical challenge was assessed using trypan blue exclusion assay (Gibco). Approximately 400 cells were counted for each replicated treatment assay.
Northern blotting analyses of AsPrx-4783
One carton (∼250 female A. stephensi) was used for RNA isolations at 1 h and 7 days post-blood feeding (pBM) for both U and I groups. RNA was isolated from equal numbers of age-matched, NB individuals as a control. For each sample, 9 μg mRNA was electrophoretically separated through a 1% agarose NorthernMax (Ambion) gel. Separated transcripts were blotted onto nylon using capillary transfer. To visualize transferred mRNA, the blot was stained using a solution of 0.02% methylene blue in 0.3 M sodium acetate and then rinsed with 20% ethanol and finally destained in 0.2× SSPE and 1% SDS before hybridization.
For probe preparation, pTLP20 plasmid DNA was digested using KpnI. The purified insert was used for in vitro transcription from the T7 promoter in the presence of [α-32P]UTP to create a radiolabeled RNA probe (Ambion; Strip-EZ RNA; Strippable RNA Probe Synthesis & Removal Kit-T7). An amplimer encoding full-length A. stephensi S7 ribosomal protein (AsS7) was prepared from A. stephensi cDNA using PCR and the following reagents and cycling parameters: forward primer, S7F, 5′-GGCGATCATCATCTACGTGC; reverse primer, S7R, 5′-GTAGCTGCTGCAAACTTCGG; 1 cycle of 95°C for 10 min; 30 cycles of 95°C for 30 s, 53°C for 30 s, 72°C for 45 s; and 1 cycle of 72°C for 5 min. The amplimer was concentrated and washed in a 100,000 MW cutoff Microcon centrifugal filter (Millipore) and used to prepare a random-primed, radiolabeled probe in the presence of [α-32P]dATP.
Northern hybridizations were performed using Northern-Max PreHyb/Hyb solution (Ambion). For AsPrx Northern analyses, membranes were prehybridized for 2 h at 68°C and hybridized with the AsPrx probe for 20 h at 67°C. Blots were stripped using the Strip-EZ kit (Ambion) for posthybridization with the AsS7 probe. For AsS7 Northern analyses, membranes were prehybridized for 2 h at 68°C and the hybridized with the AsS7 probe for 21 h at 68°C. Autoradiographs were made by exposing blots to photographic film (X-OMAT LS; Kodak Scientific Imaging Film). Scanned autoradiographic images were analyzed using Kodak 1D Image Analysis Software (Eastman Kodak). Relative expression of AsPrx was calculated by dividing the mean intensity of the AsPrx hybridization signal by the mean intensity of the AsS7 hybridization signal.
Quantitative reverse transcription-polymerase chain reaction
For quantification of AsPrx expression in the midgut of A. stephensi, total RNA was isolated at selected time points using TRIzol (Invitrogen) according to the manufacturer's instructions. Expression was normalized against the expression of the S7 ribosomal protein gene as described [26]; the relative efficiencies of the AsS7 and AsPrx reactions were optimized such that the comparative threshold cycle (Ct) method of analysis could be used to quantify and normalize gene expression. PCR assays were performed using 10 ng of total RNA isolated from pools of 25 mosquito midguts and the following primers and probes for AsS7 or AsPrx: 300 nM s7f2 (5′-GAAGGCCTTCCAGAAGGTACAGA), 350 nM s7r2 (5′-CATCGGTTTGGGCAGAATG), and 200 nM TaqMan probe s7p (5′-VIC-AGAAGTTCTCCGGCAAGCACGTCGT-TAMRA) or 600 nM PrxF2 (5′-CTGCCCGACCGAAATCG), 600 nM PrxR2 (5′-TCGGCGAGCAGTGGAATC), and 200 nM TaqMan probe PrxP (5′-FAM-CTCGCGTGGATCAATGTACCGCG-TAMRA). All reactions, including controls with no added template, were duplicated and performed as one-step RT-PCRs as recommended (TaqMan Gold RT-PCR; PE Biosystems). Induction of AsPrx was calculated as the ratio of AsS7-normalized expression of AsPrx from P. berghei-infected mosquitoes to the AsS7-normalized expression of AsPrx-uninfected mosquitoes. Comparison of Ct values for reactions with and without RT was used to assess levels of genomic contamination: RNA samples with <1% genomic contamination (e.g., those with a difference of greater than 6.7 cycles between RT+ and RT− Ct values) were included in data analyses.
For validation of gene silencing by RNAi, two-step RT-PCR was performed on RNA collected from cultured cells. RNA was isolated using TRIzol and cDNA was synthesized by reverse transcribing 200 μg RNA with the TaqMan Universal PCR Master Mix Kit (Applied Biosystems). Oligo(dT)16 primers were used for first-strand cDNA synthesis, thereby selectively amplifying mRNA and excluding any contribution from dsRNA. One-microliter aliquots of cDNA were used as templates and reductions in transcript levels were evaluated using the comparative Ct method as described above.
Western blotting analyses for AsPrx overexpression validation
MSQ43 and S2 cell pellets were resuspended in 35–50 μl lysis buffer (20 mM Tris–HCl, pH 6, 1% Triton X-100, 137 mM NaCl) supplemented with 15% (vol/vol) protease inhibitor cocktail (Roche). For detection of the V5 epitope tag, Western blots were incubated with mouse anti-V5 primary antibody (Invitrogen) at 1:10,000 overnight at 4°C followed by a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Pierce) at 1:20,000 for 2 h at room temperature. For detection using anti-DmPrx-4783 antisera, Western blots were incubated with primary antibody at 1:2000 overnight at 4°C followed by a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Pierce) at 1:10,000 for 2 h at room temperature. Detection was based on the colorimetric VIP substrate (Vector) or chemiluminescent Supersignal substrate (Pierce).
Statistical analysis
Results with repeated measurements are expressed as means ± standard error of the mean (SEM). Data were analyzed using Student's t test.
Results
A. stephensi and A. gambiae Prx genes
Before sequencing of the A. gambiae genome and characterization of the D. melanogaster Prx family by Radyuk et al. [13], two thioredoxin peroxidases designated Jafrac1 and Jafrac2 were identified in D. melanogaster wing imaginal disc cells [27]. Based on sequence similarities between Jafrac1 and Jafrac2, degenerate primers were designed for PCR amplification of 2-Cys Prx gene fragments from A. stephensi cDNA and genomic DNA. The composite full-length cDNA sequence of AsPrx was 1223 bp and encoded a predicted protein of 196 amino acids with a calculated molecular mass and isoelectric point of 21.9 kDa and 5.95, respectively (http://scansite.mit.edu). BLAST (Basic Local Alignment Search Tool [20,22]) analysis predicted that AsPrx encoded a typical 2-Cys Prx.
Using the D. melanogaster Prx sequences as queries, five orthologous Prx sequences were identified in the A. gambiae genome (Fig. 1). In contrast to D. melanogaster, the genome of A. gambiae seems to encode single rather than multiple copies of each Prx gene. In addition, a sixth peroxiredoxin identified in both genomes was similar to vertebrate PrxV (Fig. 1). Orthologs of the bacterioferritin comigratory proteins (BCP), which form a discrete group associated with the Prx family [21], were not identified in the A. gambiae or D. melanogaster genomes.
Fig. 1.
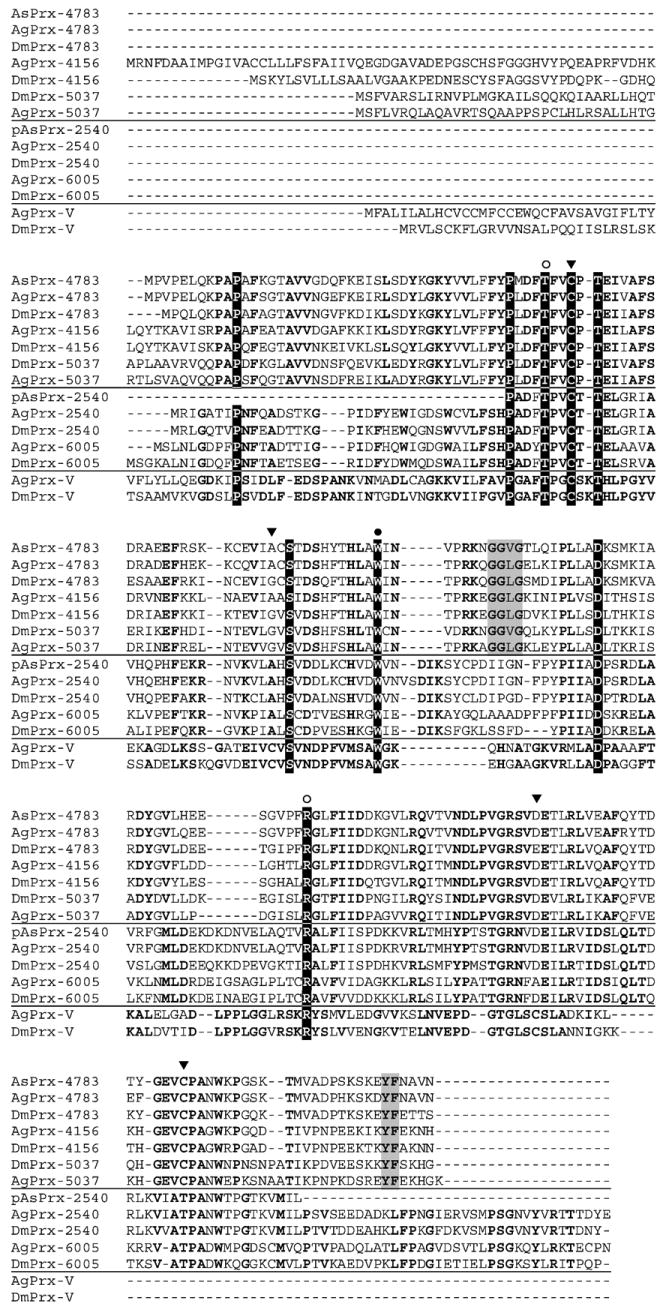
Alignment of Anopheles and Drosophila peroxiredoxins. Three of the four major Prx clades are represented: typical 2-Cys and 1-Cys and atypical 2-Cys (PrxV). Not shown are the BCP proteins because they were not identified in these insect genomes. Using the ClustalW method in conjunction with data from [29], the sequences were aligned and residues conserved among all three Prx types were shaded in black. Residues conserved in each clade are in bold. Redox-active cysteines are indicated with a black arrow (▼); residues proposed to be involved in the activation of the peroxidatic cysteine are identified with an open circle (○); residues believed to be involved in maintaining the dimeric structure of Prx are marked with a closed circle (●) [31]; residues highlighted in gray make up the signature sequence for 2-Cys Prx that is sensitive to hyperoxidation [30]. Abbreviations: Ag, Anopheles gambiae; As, Anopheles stephensi; and Dm, Drosophila melanogaster. The prefix “p” indicates that only a partial sequence was obtained.
Alignment of the predicted Prx proteins from A. stephensi, A. gambiae, and D. melanogaster (Fig. 1) revealed that the 2-Cys AsPrx shared the highest amino acid identity (78.1%) with DmPrx-4783. Hereafter, the A. stephensi 2-Cys Prx ortholog is referred to as AsPrx-4783. PCR amplification from both cDNA and genomic DNA of A. stephensi yielded identical amplimers (not shown), indicating that AsPrx-4783 is an intronless gene. The orthologous DmPrx-4783 and A. gambiae Prx-4783 (AgPrx-4783) are also intronless genes.
We predict that both AsPrx-4783 and AgPrx-4783 are localized to the cytoplasm, based on strong homology to the cytosolic DmPrx-4783 [13] and from sequence analyses using the PSORT II program [28]. Examination of the predicted amino acid sequences of A. stephensi and A. gambiae Prxs also revealed that residues associated with catalysis are conserved.
The peroxidatic cysteine of 1-Cys, 2-Cys, and BCP-like Prx proteins is conserved in the predicted AsPrx-4783 and corresponds to C49 (Fig. 1). Among the three subgroups, however, the consensus sequence around this C differs slightly. Like all 2-Cys Prx proteins, AsPrx-4783 also contains the resolving cysteine (C170), located within a conserved VCP sequence (Fig. 1). The peroxidatic and resolving cysteines are presumably involved in protein activation and dimerization. Fruitfly and mosquito 2-Cys Prx proteins contain the conserved GG (V/I/L)G-(X)n-YF sequence (Fig. 1), which is a signature for those Prxs that are sensitive to inactivation by hyperoxidation [29] (Fig. 1). Proteins of the three Prx subgroups also contain conserved residues corresponding to AsPrx-4783 T46, W84, and R125 (Fig. 1), which are required to activate the peroxidatic cysteine. Specifically, T46 hydrogen bonds to C49, whereas R125 interacts with the peroxidatic Cys to lower the pKa of the ionized form of thiol [30,31].
AsPrx-4783 Southern blotting analysis
To determine the copy number of AsPrx-4783 in the A. stephensi genome, Southern analysis was carried out using the complete open reading frame as a probe. Because sequence analysis indicated that AsPrx-4783 lacked consensus restriction sites for the enzymes used for digestion, we predicted that single bands would be evident after signal detection. However, EcoRI and XhoI digests revealed a doublet and two bands, respectively (Fig. 2, lanes 1 and 3), despite apparent complete digestion of the DNA (not shown) and high-stringency washes. High sequence identity of the AsPrx-4783 probe to other members of the Prx family may account for these observations. In the A. gambiae genome, the three members of the 2-Cys Prx subgroup, which are more similar to each other than to the PrxV or 1-Cys Prxs, are located on different chromosomes. Therefore, if the A. stephensi genome is similarly organized, at least two bands should be visible in all four lanes. In both the D. melanogaster and the A. gambiae genomes, however, the Prx-4783 genes are present as single copies. Because the DNA used for the analysis was pooled from a large number of A. stephensi females, unique sites of silent polymorphism within the AsPrx-4783 sequence could encode consensus restriction sites for XhoI and EcoRI that were not present in the cloned amplimer sequence, thereby altering the digest pattern. Based on gene number in A. gambiae and D. melanogaster, we predict that AsPrx-4783 is a single-copy gene.
Fig. 2.

Southern blot analysis of the AsPrx-4783 gene. Genomic DNA was isolated from adult A. stephensi and 12 μg of purified genomic DNA was digested with EcoRI (lane 1), KpnI (lane 2), XhoI (lane 3), or XmnI (lane 4); electrophoretically separated through 0.8% agarose; and transferred onto nylon membrane. The filter was hybridized with the 32P-labeled AsPrx-4783 coding region probe as described under Materials and methods. Molecular size markers (bp) are indicated on the left.
AsPrx-4783 expression in immortalized A. stephensi MSQ43 cells
In preparation for functional analyses of AsPrx-4783, gene expression and protein synthesis were examined in A. stephensi MSQ43 cells. Amplification of AsPrx-4783 from MSQ43 cells was confirmed by double-stranded sequencing of the cloned product (not shown). For Western blotting, AsPrx-4783 was detected with antiserum to DmPrx-4783 provided by Dr. William Orr (Southern Methodist University, Dallas, TX, USA) [13]. Protein lysate prepared from D. melanogaster S2 cells was used as a control. Prominent cross-reacting proteins of ∼28 kDa were observed in lysates from both MSQ43 and S2 cells under reducing conditions (Fig. 3A, lanes 1 and 3). Based on calculated molecular masses, mosquito and fruitfly monomeric Prx proteins should range from 21 to 22 kDa, but instead migrated with apparent molecular masses near 28 kDa. This observed increase in apparent molecular mass of monomeric Prx has been noted in the literature and seems to be a common characteristic of 2-Cys Prxs [32].
Fig. 3.

(A) Anti-DmPrx-4783 antiserum recognizes DmPrx-4783 and AsPrx-4783. Crude protein lysates (10 μg) from D. melanogaster S2 cells or from A. stephensi MSQ43 cells were subjected to 12% SDS–PAGE under reducing conditions (DTT), transferred to Immobilon-P membrane, and incubated with rabbit anti-DmPrx-4783 antiserum. Treatment conditions: (lane 1) untreated S2 cells, (lane 2) S2 cells stimulated with copper(II) sulfate for 24 h, (lane 3) untreated MSQ43 cells, (lane 4) MSQ43 cells mock transfected for 48 h, (lane 5) MSQ43 cells transfected with TLP58 for 48 h, and (lane 6) MSQ43 cells transfected with AsPrx-4783 dsRNA for 48 h. Molecular weight markers (kDa) are shown on the left. (B) Anti-V5 detects AsPrx-4783 overexpression and dimerization in D. melanogaster S2 cells. S2 cells were collected at various times after mock transfection or transfection with pTLP55, which encodes AsPrx-4783. Crude protein lysates (20 μg per lane) were electrophoretically separated through a 12% SDS–PAGE gel under reducing (with DTT) or nonreducing (without DTT) conditions, transferred to membrane, and probed with anti-V5 antiserum. Proteins with the expected masses of monomer and dimer are indicated. Anti-V5 antibody did not cross-react with proteins in cell extracts prepared from mock-transfected cells. Molecular weight markers (kDa) are shown on the left. (C) Anti-DmPrx-4783 detects AsPrx-4783 overexpression and mixed dimer formation in D. melanogaster S2 cells. Protein samples were isolated at 24 h after copper(II) sulfate treatment from S2 cells that were mock-transfected or transfected with plasmid TLP55. Crude protein lysates (20 μg per lane) were electrophoretically separated through a 12% SDS–PAGE gel under reducing (with DTT) or nonreducing (without DTT) conditions, transferred to membrane, and incubated with anti-DmPrx-4783 antiserum. Cross-reacting proteins correspond to possible AsPrx-4783 dimer and mixed AsPrx-4783/DmPrx-4783 dimer (a), endogenous dimeric DmPrx-4783 (b), fast migrating DmPrx-4783 (c), recombinant monomeric AsPrx-4783 (d), and endogenous monomeric DmPrx-4783 (e). Molecular weight markers (kDa) are shown on the left. (D) Anti-V5 detects AsPrx-4783 overexpression and dimerization in A. stephensi MSQ43 cells. MSQ43 cells were collected at various times after mock transfection or transfection with plasmid TLP58 encoding AsPrx-4783. Crude protein lysates (20 μg per lane) were electrophoretically separated through a 10% SDS–PAGE gel under nonreducing conditions, transferred to membrane, and probed with anti-V5 antibody. Proteins with the expected masses of monomer and dimer are indicated. Molecular weight markers (kDa) are shown on the left.
Overexpression of AsPrx-4783 in S2 and MSQ43 cells: assessment of protection against chemical challenge
Based on reports that overexpressed Prxs can protect heterologous host cells [3], we proposed to determine whether overexpressed AsPrx-4783 could protect D. melanogaster S2 cells from oxidative and nitrosative stress. The assays were designed to allow us to directly compare AsPrx-4783 function with previous reports of DmPrx-4783 function using the same assay system [14].
Recombinant AsPrx-4783 was overexpressed in D. melanogaster S2 cells after transfection with pMT/V5-His TOPO pTLP55. In control untransfected cells, induction with copper(II) sulfate did not increase expression of endogenous DmPrx-4783 (Fig. 3A, compare lanes 1 and 2). In transfected cells, overexpression of AsPrx-4783 was confirmed with anti-V5 Western blots using protein lysates prepared 6–72 h after induction with copper(II) sulfate (Fig. 3B). Dimerization and reduction of dimers were not prevented by the V5 tag (Fig. 3B), confirming that the overexpressed AsPrx-4783 was likely to be functional.
In order to confirm that the cross-reacting protein(s) represented different redox-induced conformations of AsPrx-4783, S2 cell extracts from pTLP55- and mock-transfected cells were electrophoretically separated under reducing and nonreducing conditions and then examined by Western blot using anti-DmPrx-4783 to visualize both DmPrx-4783 and overexpressed AsPrx-4783 (Fig. 3C). In the absence of DTT, anti-DmPrx-4783 detected three bands in pTLP55-transfected cells (Fig. 3C, lane 1): a prominent dimer (b), a slightly faster migrating band (c), and a slower migrating diffuse band (a). Relative to mock-transfected cells (Fig. 3C, lane 2), decreased intensity of the main dimeric band (b) together with the appearance of a slower migrating diffuse band (a) in transfected cells may indicate mixed dimer formation (Fig. 3C, lane 1). A reductive dissociation of both the endogenous DmPrx-4783 and the overexpressed AsPrx-4783 was observed when S2 cell protein lysate was incubated with 100 mM DTT (Fig. 3C, lanes 1 and 3). The apparent molecular mass of overexpressed AsPrx-4783 (d) was higher than that of the endogenous DmPrx-4783 (e) due to the epitope tag (Fig. 3C, lane 3). These experiments confirmed that apparent dimeric forms of AsPrx-4783, like those of DmPrx-4783, were reduced by treatment with DTT, indicating that the epitope tag did not seem to interfere with dimer formation and reduction of AsPrx-4783.
To determine whether AsPrx-4783 could protect S2 cells from ROS- and RNOS-associated damage, viability of pTLP55-transfected and mock-transfected S2 cells was assessed after exposure to varying concentrations of hydrogen peroxide, DEA-NO (NO donor), AS (nitroxyl donor), and PN. Treatment conditions were based on chemical doses that killed ∼50–70% of S2 cells in preliminary studies (not shown). At each time point, cell viability was normalized against nonchallenged S2 cells to account for variability in background cell death. Unexpectedly, overexpressed AsPrx-4783 did not protect S2 cells against hydrogen peroxide (Fig. 4A). Similarly, AsPrx-4783 did not protect S2 cells against DEA-NO (Fig. 4B). However, overexpressed AsPrx-4783 significantly enhanced S2 cell viability after treatment with 200 and 500 μM PN and with 1 mM AS, relative to mock-transfected cells (Figs. 4C and 4D), suggesting that AsPrx-4783 provided RNOS-specific protection to S2 cells.
Fig. 4.

Overexpressed AsPrx-4783 protects transfected D. melanogaster S2 cells from RNOS-specific cytotoxicity. D. melanogaster S2 cells were mock transfected (control cells) or transfected with the pTLP55 plasmid encoding AsPrx-4783 (PRX cells). Protection due to overexpression of AsPrx-4783 was assessed by trypan blue exclusion assay for cell viability. Cells were challenged with (A) 0, 10, or 20 mM hydrogen peroxide, (B) 0, 1500, or 3000 μM DEA-NO, (C) 0, 200, or 500 μM peroxynitrite, and (D) 0, 1, or 2 mM Angeli's salt as described under Materials and methods. Values represent means ± SEM of three independent replicates. Significance versus control is designated: *p < 0.05; Student's t test.
In a subsequent series of experiments, we attempted to determine whether AsPrx-4783 could protect A. stephensi cells from ROS- and RNOS-associated damage. Although the metallothionein promoter can be induced by 100–200 μM copper(II) sulfate in two Aedes albopictus cell lines [33,34], copper induction was not feasible in MSQ43 cells because the concentration required to drive transcription caused significant cell death even in the absence of transfection (not shown). Therefore, for overexpression in A. stephensi cells, a plasmid was constructed to drive constitutive expression of AsPrx-4783 under control of a cytomegalovirus (CMV) promoter (pcDNA3.1/His-V5 TOPO, pTLP58). In control experiments, MSQ43 cells showed nearly 100% transfection efficiency as detected after transfection and detection of an identical lacZ-expressing control plasmid (pcDNA3.1/lacZ-His TOPO; not shown). Overexpression of AsPrx-4783 in MSQ43 cells was confirmed with anti-V5 Western blots using protein lysate collected 6–96 h after transfection (Fig. 3D). In addition, both endogenous and slower migrating, overexpressed AsPrx-4783 were detected using anti-DmPrx-4783 (Fig. 3A, lane 5). Maximal protein synthesis was observed at 48–96 h after transfection (Fig. 3D), thus transfected cells were challenged during this time period.
Both pTLP58-transfected and mock-transfected MSQ43 cells were exposed to concentrations of hydrogen peroxide, DEA-NO, AS, and PN before assessment of cell viability using trypan blue exclusion. MSQ43 cells were more sensitive to chemical treatment than were S2 cells, thus the concentrations used were lower than were used in the S2 cell study. For all chemical treatments, overexpression of AsPrx-4783 under the CMV promoter enhanced MSQ43 cell viability relative to mock-transfected treated cells (Fig. 5).
Fig. 5.

Overexpressed AsPrx-4783 protects transfected A. stephensi MSQ43 cells from RNOS-specific cytotoxicity. A. stephensi MSQ43 cells were mock transfected without plasmid (control cells) or transfected with the pTLP58 plasmid encoding AsPrx-4783 (PRX cells). Protection due to overexpression of AsPrx-4783 was assessed by trypan blue exclusion assay for cell viability. Cells were challenged with (A) 0, 125, or 250 μM hydrogen peroxide, (B) 0, 300, or 700 μM DEA-NO, (C) 0, 75, or 150 μM peroxynitrite, and (D) 0, 50, or 100 μM Angeli's salt as described under Materials and methods. Values are the means ± SEM of the results from three independent replicates. Significance versus control is designated: *p < 0.05; Student's t test.
RNAi-mediated gene silencing of AsPrx-4783 in MSQ43 cells: assessment of protection against chemical challenge
In organisms ranging from yeast to insects to human cells, Prx gene silencing has resulted in decreased cell survival when deficient cells are exposed to toxic oxidative and nitrosative stress [4,14,35]. Therefore, to provide additional supporting data that AsPrx-4783 protected A. stephensi cells against ROS- and RNOS-associated damage, we used dsRNA-mediated RNA interference to silence AsPrx-4783 expression in A. stephensi cells.
In replicated assays, AsPrx-4783 expression was decreased by >90% at 24–72 h and by >80% at 5 days after transfection of AsPrx-4783 dsRNA (Fig. 6). Specificity of AsPrx-4783 silencing was demonstrated by transfection of control A. stephensi cells with dsRNA for mouse cyclophilin. Expression of AsPrx-4783 was not affected by transfection of this heterologous dsRNA (Fig. 6). In addition to the significant effect of silencing on AsPrx-4783 mRNA levels, transfection of dsRNA significantly reduced AsPrx-4783 protein levels in MSQ43 cells (Fig. 3A, compare lanes 4 and 6). Despite the efficiency and apparent specificity of our gene silencing protocol, we are uncertain whether AsPrx-4783 silencing affects other Prx family members in A. stephensi cells.
Fig. 6.

dsRNA-mediated AsPrx-4783 silencing in A. stephensi MSQ43 cells is specific and persistent. MSQ43 cell cultures were collected 24–120 h after mock transfection (no dsRNA) or transfection with double-stranded RNA for AsPrx-4783 (dsPRX) or murine cyclophilin (dsCyc). Total RNA was isolated (TRIzol) and cDNA was synthesized using oligo(dT)16 (Applied Biosystems) for use in two-step RT-PCR. Relative AsPrx-4783 transcript levels were normalized to AsS7. Relative levels of AsPrx-4783 are shown as normalized ratios in cells transfected with dsPrx or dsCyc; no impact of transfection on AsPrx-4783 would be equivalent to a relative expression level of 1.0. Addition of 400 ng dsPRX resulted in an 81–96% decrease in AsPrx-4783 transcript levels relative to both dsCyc and no dsRNA controls.
Transfected and mock-transfected MSQ43 cells were exposed to varying concentrations of hydrogen peroxide, DEA-NO, AS, and PN, then cell viability was evaluated using the trypan blue exclusion assay. For each transfection, qRT-PCR analysis was used to verify that AsPrx-4783 transcript levels were significantly reduced in dsRNA-treated cells (not shown). Based on replicated assays, AsPrx-4783 silencing significantly reduced MSQ43 viability compared to mock-transfected cells for all four chemicals tested (Fig. 7). Together, AsPrx-4783 overexpression and gene silencing data indicate that this gene product protects A. stephensi cells against ROS- and RNOS-associated cell damage and death.
Fig. 7.

Silencing of AsPrx-4783 increased susceptibility of A. stephensi MSQ43 cells to RNOS-specific cytotoxicity. A. stephensi MSQ43 cells were mock transfected (control) or transfected with double-stranded AsPrx-4783 RNA (dsPRX). After transfection, cells were challenged with (A) 0, 125, or 250 μM hydrogen peroxide, (B) 0, 300, or 700 μM DEA-NO, (C) 0, 75, or 150 μM peroxynitrite, and (D) 0, 50, or 100 μM Angeli's salt as described under Materials and methods. Cell viability was assessed by the trypan blue exclusion assay. Values represent means ± SEM from three independent replicates. Significance versus control is designated: *p < 0.05; Student's t test.
Expression of AsPrx-4783 in vivo
Northern blot analysis using full-length AsPrx-4783 as a probe revealed a single prominent transcript of ∼1200 bp (Fig. 8A), which corresponded to the size of the cDNA amplimer from MSQ43 cells obtained with PCR. Blood-feeding-induced expression in whole uninfected insects relative to non-blood-fed insects (U/NB) was observed at 7 days but not at 1 h pBM (relative induction of 4.0 for 7 days and 1.2 for 1 h; Fig. 8A). However, we did not detect significant induction of AsPrx-4783 expression in infected insects relative to uninfected insects (I/U) at 1 h or 7 days pBM (relative expression values of 0.9 for 1 h and 1.3 for 7 days; Fig. 8A).
Fig. 8.
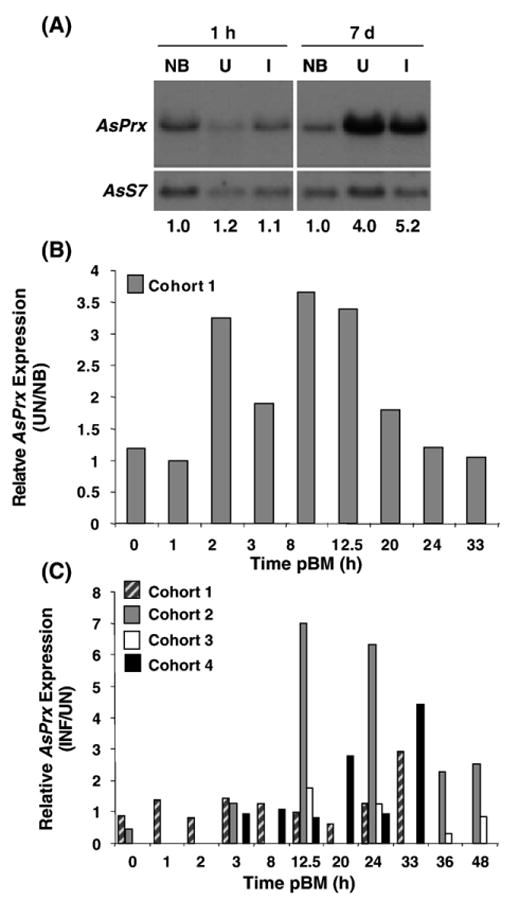
Northern blotting analysis and qRT-PCR of AsPrx-4783 expression in vivo. (A) Messenger RNA was collected from whole non-blood-fed (NB), P. berghei-infected (I), and uninfected (U) A. stephensi at 1 h and 7 days after blood feeding. Aliquots of 9 μg mRNA were separated on a 1% agarose NorthernMax (Ambion) gel and transferred to a nylon membrane. RNA probe was prepared from cloned template containing full-length coding sequence for AsPrx-4783. The cross-hybridizing AsPrx-4783 transcript has an estimated size of 1194 bp, which corresponds to the predicted transcript size of 1223 bp based on sequence analysis. Densitometry was used to quantitate transcript abundance. AsPrx-4783 transcript levels were normalized against AsS7 ribosomal protein gene transcript levels to correct for loading differences; these values are indicated at the bottom. (B, C) Quantitative RT-PCR as described under Materials and methods was performed on total RNA prepared from midguts, carcasses (body tissues minus midguts), and whole bodies of non-blood-fed (NB) mosquitoes and from the same tissues of mosquitoes after ingestion of an uninfected (UN) or P. berghei-infected (INF) blood meal. A single cohort of mosquitoes was used to determine the effects of blood feeding alone (B), whereas four cohorts of mosquitoes with overlapping time points were used to determine the effects of parasite infection on AsPrx-4783 expression (C). Relative induction of AsPrx-4783 expression in the A. stephensi midgut is shown as the ratio of UN to NB expression (B) and as the ratio of INF to UN expression (C). No induction of AsPrx-4783 expression was apparent in the carcass or whole body after blood feeding or infection (not shown).
Based on a preliminary indication of induction of AsPrx-4783 expression in whole insects in response to blood feeding from Northern analyses (Fig. 8A) and protection provided by AsPrx-4783 against ROS and RNOS (Figs. 5 and 7), we examined expression of AsPrx-4783 more closely in dissected tissues of A. stephensi using qRT-PCR. Analyses of midgut RNA isolated from uninfected and matched non-blood-fed A. stephensi revealed that AsPrx-4783 expression was induced up to 3.7-fold relative to non-blood-fed controls at 2–20 h pBM (Fig. 8B).
Analyses of midgut RNA isolated from four cohorts of P. berghei-infected and matched uninfected A. stephensi revealed that infection with P. berghei induced AsPrx-4783 expression in the midguts of three of four cohorts of A. stephensi >2- to 7-fold at 12.5–48 h pBM (Fig. 8C). In contrast, expression in mosquito carcasses (whole body minus midgut) was not induced by parasite infection as measured by qRT-PCR (not shown). The observation that AsPrx-4783 expression is limited to the midgut is further supported by the fact that 10-fold greater amounts of RNA from carcasses (100 ng) were assayed by qRT-PCR than were assayed from midguts (10 ng). It is likely that variability in AsPrx-4783 induction is due to differences in parasite infection intensity as was demonstrated for As60A expression in the mosquito midgut [26]. Together with Northern blot results, qRT-PCR analyses indicate that induction of AsPrx-4783 expression in the midgut in response to parasite infection does not begin until 12.5 h pBM and is limited to the period of blood digestion (≤48 h pBM).
Discussion
Numerous studies have demonstrated that Prxs can protect cells against a variety of nitrosative stresses. The majority of work has focused on the roles of pathogen and parasite Prxs in protection against host defenses. In contrast, a smaller number of studies have demonstrated that Prxs of eukaryotic hosts can be induced in response to viral, bacterial, and fungal infections [36–38]. Here, we investigated whether the 2-Cys Prx AsPrx-4783 can protect A. stephensi cells against ROS- and RNOS-associated cell damage and death that are consistent with conditions known to exist during malaria parasite infection of A. stephensi.
Overexpression of AsPrx-4783 protected MSQ43 cells against nitroxyl, DEA-NO, hydrogen peroxide, and PN (Fig. 5). These stresses are relevant to midgut conditions in A. stephensi and in the closely related A. gambiae after feeding and during parasite infection. In previous studies, we reported that AsNOS was upregulated in a step-wise pattern after feeding and parasite infection from a few hours after blood feeding to the completion of blood digestion [15,17]. This induction leads to the synthesis of inflammatory levels of nitrogen oxides, which are likely produced from reactions involving NO and ROS [17]. Like AsNOS, A. gambiae NOS is induced in response to parasite infection [39]. In addition, elevated levels of superoxide and/or hydrogen peroxide, perhaps generated by inducible host enzymes, have been detected in A. gambiae at 24 h after blood feeding [40] and in Anopheles albimanus in response to the presence of malaria parasites [41], suggesting that protection against ROS and RNOS is necessary for homeostasis in anopheline mosquitoes.
Protection against PN is particularly critical for maintaining host tissue integrity. DNA is a physiological target of PN; oxidation reactions cause base modifications, mutations, and single- and double-strand breaks [42]. PN can also oxidize sulfhydryls, leading to thiyl radical formation [43]. Major nitration targets of PN include phenols such as tyrosine residues in proteins; indeed, nitrotyrosine formation has been associated with a variety of inflammatory conditions [44] and has been observed in ookinete-invaded cells of the A. gambiae midgut [45].
In response to these stresses, both prokaryotic and eukaryotic organisms have evolved selective defenses against PN. For example, GPx, a selenium-containing enzyme structurally similar to the thioredoxin peroxidases, has PN reductase activity in bovine erythrocytes [46]. This PN reductase activity is more efficient when the pH is alkaline, such as the slightly alkaline conditions in the midgut of a mosquito. For full activity, GPx requires reduced glutathione (GSH); glutathione reductase (GR) catalyzes the conversion of glutathione disulfide to GSH. Insects seem to lack GPx [47] and GR [12,48] and instead substitute a thioredoxin system for similar chemical reactions [12,48]. High levels of NO synthesis in insects, such as we have observed in A. stephensi, may have been a strong selective pressure for this trait because NO inactivates GPx in a dose-dependent manner [49].
In contrast to GPx, the Prxs have received more attention for their roles as PN reductases. The majority of reductase activity has been attributed to 2-Cys Prx orthologs, with the exception of the bovine 1-Cys (PrxVI) [50] and human PrxV [5]. A related tryparedoxin peroxidase from the protozoan parasite Trypanosoma cruzi can also function as a PN reductase [6]. It is perhaps not surprising, therefore, that the 2-Cys Prx AsPrx-4783 would protect A. stephensi cells from PN-associated cell death. Interestingly, a bacterial Prx reduces PN seven times faster than does the endogenous GPx [2], which if true for the insect Prx orthologs, would have significant implications for protecting A. stephensi from its own anti-parasite defenses.
Overexpression of AsPrx-4783 in MSQ43 cells protected against nitroxyl-associated cell damage and death (Fig. 5). Whereas we suspect that this RNOS is toxic to developing parasites, midgut nitroxyl may also be associated with rapid activation of ingested human latent transforming growth factor-β1, which regulates parasite development in A. stephensi [17]. As such, AsPrx-4783 seems to protect mosquito cells against an RNOS that likely participates directly in parasite killing and indirectly in the regulation of parasite killing.
Nitroxyl (HNO/NO−) induces double- and single-strand DNA breaks and base oxidation [51]. It is widely accepted that HNO and singlet oxygen can react to form PN [52]. Thus, the ability of AsPrx-4783 to protect against HNO may be due in part to PN reductase activity of AsPrx-4783. However, the chemistries of HNO and PN differ, and some studies have noted that toxicities of these compounds derive from different reactive intermediates [53]. For example, HNO has a high affinity for GSH [54]; exposure of cells to millimolar concentrations of AS can dramatically reduce intracellular GSH through the production of GSNHOH [51,53]. As such, Prxs may function to protect cells through direct recycling of oxidized thiols to maintain cellular homeostasis [55] in mosquito cells.
In contrast to protection of A. stephensi MSQ43 cells against all oxidants tested, overexpression of AsPrx-4783 protected D. melanogaster S2 cells against AS and PN, but not against DEA-NO or hydrogen peroxide (Fig. 4). Radyuk et al. [13] found that overexpressed DmPrx-4783 protected S2 cells from death due to treatment with 20 mM hydrogen peroxide for up to 4 h. There are several possible explanations for these conflicting results. First, it would be reasonable to assume that DmPrx-4783 and AsPrx-4783 are not identical in catalytic activity; other A. stephensi Prxs may function more efficiently to reduce peroxides. Given the sequence signatures of AsPrx-4783, it is also possible that AsPrx-4783 is over-oxidized by hydrogen peroxide, a problem that could be compounded if S2 cells cannot provide sufficient reductants to recycle and reactivate the oxidized AsPrx-4783. Finally, the pool of heterodimeric (fruitfly and mosquito) Prx-4783 in transfected cells may be less efficient at peroxide reduction than homodimeric DmPrx-4783. Heterodimer formation and cooperativity between heterologous monomers of 2-Cys Prxs have been observed and provide additional regulation of activity [4,56,57]. However, it is possible that the interspecies heterodimer is less efficient than either homodimer in protecting S2 cells from damage due to treatment with hydrogen peroxide and DEA-NO. Our observations would also imply that the mechanism for protection against NO and peroxides differs from that for nitroxyl and PN in S2 cells.
In addition to potential differences in activity of AsPrx-4783 homo- and heterodimers, differences in physiology of S2 and MSQ43 cells, as well as in the overexpression protocols used for these cell lines, may account for differences in AsPrx-4783 protection against oxidative and nitrosative stresses. For all stresses tested, A. stephensi cell lines were more sensitive to oxidative and nitrosative stress than were the D. melanogaster S2 cells. These results suggest that the roles of Prxs in these cell types must also differ. For overexpression in S2 cells, copper(II) sulfate was used to induce transcription from a metallothionein promoter in the plasmid construct. This induction would also be expected to induce expression of the endogenous metallothionein in S2 cells, which can protect these cells from some oxidative and nitrosative stresses [58,59] and potentially confound analyses of AsPrx-4783. Data in support of this hypothesis came from a preliminary experiment in which S2 cells were mock transfected and challenged with hydrogen peroxide or DEA-NO with or without copper(II) sulfate pretreatment. In these assays, S2 cells pretreated with copper(II) sulfate exhibited enhanced viability relative to cells that were not pretreated (not shown). Western analyses of these cells showed similar levels of DmPrx-4783 protein in the presence and absence of copper(II) sulfate pretreatment (Fig. 3A, lanes 1 and 2). Thus, the added protection was not the result of increased endogenous DmPrx-4783 protein expression.
Based on the protection against ROS and RNOS afforded by AsPrx-4783 (Figs. 5 and 7), we hypothesized that AsPrx-4783 expression may be induced in the midgut of adult female A. stephensi after blood feeding. In support of our hypothesis, we observed that AsPrx-4783 was induced approximately two- to fourfold at 2–20 h pBM and fourfold at 7 days pBM relative to non-blood-fed mosquitoes. In A. gambiae, AgPrx-4783 is significantly expressed in the ovaries and fat body as well as the midgut at 24 h pBM [60], suggesting that elevation in AsPrx-4783 expression by Northern analysis at 7 days pBM (Fig. 8A) may be due to contributions from several tissues. The induction of AsPrx-4783 at 7 days pBM suggests a role at extended times postdigestion. Consistent with such a role, we observed that AsNOS is induced in the midguts of blood-fed insects relative to nonfed insects at 6 and 9 days pBM and that nitrate/nitrite levels in circulating hemolymph are elevated at 7 days pBM and then gradually decrease in uninfected insects through 14 days pBM [15]. Thus, elevated AsPrx-4783 would be available to protect against ROS and RNOS generated during and after blood digestion.
Induction of Prx-4783 expression after blood feeding in A. gambiae suggests that this 2-Cys Prx may provide similar protection in a closely related species. DNA microarray analyses of A. gambiae [60] have revealed that several AgPrxs, including AgPrx-4783, were upregulated in whole insects from 24 to 96 h after blood feeding. Another study reported that AgPrx-4783 was upregulated at least twofold at 30 h pBM in abdomens of A. gambiae relative to nonfed insects [61]. Blood-feeding-induced expression of two thioredoxins and a thioredoxin reductase in A. gambiae at 24 h pBM [62] indicates that reduction machinery would be available to support reduction and recycling of oxidized AgPrx-4783.
In addition to AgPrx-4783, SOD and catalase gene expression is induced by blood feeding in A. gambiae [40]. Specifically, SOD expression is induced by blood feeding, depending on the strain of A. gambiae, to as high as 3.65-fold at 12 h pBM and to 2.5-fold at 24 h pBM relative to nonfed controls [40]. By comparison, catalase expression was also induced by blood feeding in A. gambiae, but in some cases, induction was associated with rising rather than falling H2O2 levels in mosquito hemolymph [40]. In general, these observations suggest that a suite of antioxidants is mobilized to protect the mosquito host against oxidative and nitrosative stresses associated with blood feeding.
In addition to mosquito-generated ROS and RNOS, the ingested blood itself may be a source of radical chemistry that contributes to observed induction of AsPrx-4783. Mosquitoes ingest hemoglobin in volumes greater than their body weight. Within 20 min of feeding, erythrocyte hemolysis frees 1 to 10% of the total ingested hemoglobin in the midgut lumen [63]. The globins are dissociated from heme and digested, whereas the heme groups are polymerized to insoluble hematin under oxygenated, slightly alkaline conditions [64]. Polymerization, however, is not instantaneous, nor does it completely abolish radical chemistry [65]. Thus, significant quantities of heme and hematin can react with iron and oxygen in the blood meal to form damaging ROS that may be detoxified by AsPrx-4783 in the mosquito midgut.
Although AsPrx-4783 was induced by blood feeding alone, our data indicate that stronger induction of AsPrx-4783 occurred in the midgut in response to parasite infection. We previously observed a similar pattern of induction of AsNOS expression in A. stephensi. Specifically, AsNOS is expressed at low levels before blood feeding, at higher levels in response to blood ingestion, and at the highest levels in response to parasite infection [15]. Hence, the step-wise increases in AsPrx-4783 expression mirror the step-wise increases in AsNOS induction in the A. stephensi midgut.
At 12.5–48 h pBM, AsPrx-4783 expression was induced in the A. stephensi midgut to levels two- to sevenfold higher in malaria parasite-infected insects than in uninfected insects. This induction was specific to parasite infection and is independent of the effects of blood feeding alone, because the latter response is common to both infected and uninfected insects. As observed for blood digestion, the timing and degree of infection-specific induction of AsPrx-4783 were consistent with previous observations of oxidative and nitrosative stress induced in response to parasite infection. Previously, we reported that AsNOS was induced by parasite infection in the midgut greater than twofold at 6 and at 36 and 48 h pBM relative to uninfected blood-fed insects [17]. This biphasic induction leads to the synthesis of nitrogen oxides in the midgut lumen range from 75 to >160 μM at 12–24 h pBM [17]. Values in excess of 75 μM are consistent with inflammatory levels of nitrite/nitrate reported in human serum under conditions of sepsis [66,67] and therefore indicate that the NO-mediated response of A. stephensi to Plasmodium infection is an inflammatory response. In addition to this extracellular accumulation of toxic RNOS, strong, localized tyrosine nitration is correlated with advanced stages of apoptosis in ookinete-invaded A. stephensi midgut cells [45], confirming that the NO-mediated response to parasite infection is deleterious to the host. However, AsNOS induction is often uncoupled from tyrosine nitration in parasite-invaded cells [45]. Given the protection afforded by AsPrx-4783 against nitrating species and other damaging RNOS, we propose that induction of AsPrx-4783 in the midgut epithelium of A. stephensi to levels as high as sevenfold relative to control insects is perhaps one of several mechanisms to limit self-induced damage (e.g., nitration of tyrosine) that is a by-product of the NO-mediated defense against parasite development.
In sum, our observations indicate that, under direct insult from parasite development, AsPrx-4783 can protect mosquito cells through reduction of hostile ROS and RNOS. In addition to direct detoxification, however, AsPrx-4783 may also mediate cellular responses to ROS and RNOS indirectly. Specifically, 2-Cys Prx can regulate several proteins and pathways that regulate apoptotic cell death directly, including the tyrosine kinase c-Abl, caspases, nuclear factor-κB, and activator protein-1 [35]. In addition, the human 2-Cys PrxII can alternate between functioning as a peroxidase and functioning as a protein chaperone to protect HeLa cells from hydrogen peroxide-induced cell death [68]. As such, it is possible that AsPrx-4783 has dual functions in detoxification of ROS and RNOS as well as in maintenance of homeostasis in A. stephensi through regulation of apoptosis and elimination of cells damaged in the course of parasite infection [69]. Further study of AsPrx-4783 and other mosquito Prxs is necessary to determine how these gene products might be manipulated to increase protection of insect cells in the context of enhancing anti-parasite resistance.
Acknowledgments
Funding for this work was provided by NIH NIAID AI50663. We thank Dr. William Orr (Southern Methodist University, Dallas, TX, USA) for antisera to DmPrx-4783, Dr. Theresa Dellinger (Virginia Tech) for assistance with cell viability assays, and Dr. Yoram Vodovotz (University of Pittsburgh) and Dr. Shankar Mondal (UC Davis) for critical reviews of the manuscript.
Abbreviations
- AS
Angeli's salt, sodium α-oxyhyponitrite with a molecular formula of Na2(ONNO2)
- BCP
bacterioferritin comigratory protein
- CMV
cytomegalovirus
- Ct
threshold cycle
- DEA-NO
diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate with a molecular formula of
- dsRNA
double-stranded RNA
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- GPx
glutathione peroxidase
- GSH
glutathione
- GR
glutathione reductase
- HNO/NO−
nitroxyl
- ICR
Institute of Cancer Research
- I
malaria parasite-infected
- lacZ
β-galactosidase
- Mops
3-(N-morpholino)propanesulfonic acid
- MSQ43
Anopheles stephensi immortalized cell line
- NB
non-blood-fed
- NOS
nitric oxide synthase
- pBM
post-blood-feeding
- PN
peroxynitrite
- Prx
peroxiredoxin
- qRT-PCR
quantitative reverse transcriptase-polymerase chain reaction
- RNAi
RNA interference
- RNOS
reactive nitrogen oxide species
- ROS
reactive oxygen species
- S2
Drosophila melanogaster immortalized cell line
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SEM
standard error of the mean
- SSPE
3.0 M sodium chloride, 0.2 M sodium hydrogen phosphate, 0.02 M EDTA, pH 7.4
- U
uninfected
References
- 1.Fang FC, editor. Nitric oxide and infection. Kluwer Academic/Plenum; New York: 1999. [Google Scholar]
- 2.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Xie QW, Nathan C. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol Cell. 1998;1:795–805. doi: 10.1016/s1097-2765(00)80079-9. [DOI] [PubMed] [Google Scholar]
- 4.Wong CM, Zhou Y, Ng RW, Kung HF, Jin DY. Cooperation of yeast peroxiredoxins Tsa1p and Tsa2p in the cellular defense against oxidative and nitrosative stress. J Biol Chem. 2002;277:5385–5394. doi: 10.1074/jbc.M106846200. [DOI] [PubMed] [Google Scholar]
- 5.Dubuisson M, Vander Stricht D, Clippe A, Etienne F, Nauser T, Kissner R, Koppenol WH, Rees JF, Knoops B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett. 2004;571:161–165. doi: 10.1016/j.febslet.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 6.Trujillo M, Budde H, Pineyro MD, Stehr M, Robello C, Flohe L, Radi R. Trypanosoma brucei and Trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols. J Biol Chem. 2004;279:34175–34182. doi: 10.1074/jbc.M404317200. [DOI] [PubMed] [Google Scholar]
- 7.Declercq JP, Evrard C, Clippe A, Stricht DV, Bernard A, Knoops B. Crystal structure of human peroxiredoxin 5, a novel type of mammalian peroxiredoxin at 1.5 Å resolution. J Mol Biol. 2001;311:751–759. doi: 10.1006/jmbi.2001.4853. [DOI] [PubMed] [Google Scholar]
- 8.Hirotsu S, Abe Y, Okada K, Nagahara N, Hori H, Nishino T, Hakoshima T. Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product. Proc Natl Acad Sci USA. 1999;96:12333–12338. doi: 10.1073/pnas.96.22.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K, Kim IH, Lee KY, Rhee SG, Stadtman ER. The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem. 1988;263:4704–4711. [PubMed] [Google Scholar]
- 10.Chae HZ, Uhm TB, Rhee SG. Dimerization of thiol-specific antioxidant and the essential role of cysteine 47. Proc Natl Acad Sci USA. 1994;91:7022–7026. doi: 10.1073/pnas.91.15.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabilloud T, Heller M, Gasnier F, Luche S, Rey C, Aebersold R, Benahmed M, Louisot P, Lunardi J. Proteomics analysis of cellular response to oxidative stress: evidence for in vivo overoxidation of peroxiredoxins at their active site. J Biol Chem. 2002;277:19396–19401. doi: 10.1074/jbc.M106585200. [DOI] [PubMed] [Google Scholar]
- 12.Bauer H, Kanzok SM, Schirmer RH. Thioredoxin-2 but not thioredoxin-1 is a substrate of thioredoxin peroxidase-1 from Drosophila melanogaster: isolation and characterization of a second thioredoxin in D. melanogaster and evidence for distinct biological functions of Trx-1 and Trx-2. J Biol Chem. 2002;277:17457–17463. doi: 10.1074/jbc.M200636200. [DOI] [PubMed] [Google Scholar]
- 13.Radyuk SN, Klichko VI, Spinola B, Sohal RS, Orr WC. The peroxiredoxin gene family in Drosophila melanogaster. Free Radic Biol Med. 2001;31:1090–1100. doi: 10.1016/s0891-5849(01)00692-x. [DOI] [PubMed] [Google Scholar]
- 14.Radyuk SN, Sohal RS, Orr WC. Thioredoxin peroxidases can foster cytoprotection or cell death in response to different stressors—A study of thioredoxin peroxidase under- and over-expression in Drosophila cells. Biochem J. 2003;371:743–752. doi: 10.1042/BJ20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luckhart S, Rosenberg R. Gene structure and polymorphism of an invertebrate nitric oxide synthase gene. Gene. 1999;232:25–34. doi: 10.1016/s0378-1119(99)00121-3. [DOI] [PubMed] [Google Scholar]
- 17.Luckhart S, Crampton AL, Zamora R, Lieber MJ, Dos P, Santos C, Peterson TML, Emmith N, Lim J, Wink DA, Vodovotz Y. Mammalian transforming growth factor-β1, activated after ingestion by Anopheles stephensi, modulates mosquito immunity. Infect Immun. 2003;71:3000–3009. doi: 10.1128/IAI.71.6.3000-3009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Barillas-Mury C. Ookinete-induced midgut peroxidases detonate the time bomb in anopheline mosquitoes. Insect Biochem Mol Biol. 2005;35:721–727. doi: 10.1016/j.ibmb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signaling. 2005;7:768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- 20.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdoucq L, Vignols F, Jacquot JP, Chartier Y, Meyer Y. In vivo characterization of a thioredoxin H target protein defines a new peroxiredoxin family. J Biol Chem. 1999;274:19714–19722. doi: 10.1074/jbc.274.28.19714. [DOI] [PubMed] [Google Scholar]
- 22.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 24.Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodin MM, Schlagnhaufer B, Weir T, Romaine CP. Characterization of an RNA-dependent RNA polymerase activity associated with La France isometric virus. J Virol. 1997;71:2264–2269. doi: 10.1128/jvi.71.3.2264-2269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crampton A, Luckhart S. The role of As60A, a TGF-beta homolog, in Anopheles stephensi innate immunity and defense against Plasmodium infection. Infect Genet Evol. 2001;1:131–141. doi: 10.1016/s1567-1348(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez J, Agudo M, Van Damme J, Vandekerckhove J, Santaren JF. Polypeptides differentially expressed in imaginal discs define the peroxiredoxin family of genes in Drosophila. Eur J Biochem. 2000;267:487–497. doi: 10.1046/j.1432-1327.2000.01022.x. [DOI] [PubMed] [Google Scholar]
- 28.Horton P, Nakai K. Better prediction of protein cellular localization sites with the k nearest neighbors classifier. Proc Int Conf Intell Syst Mol Biol. 1997;5:147–152. [PubMed] [Google Scholar]
- 29.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 30.Flohe L, Budde H, Bruns K, Castro H, Clos J, Hofmann B, Kansal-Kalavar S, Krumme D, Menge U, Plank-Schumacher K, Sztajer H, Wissing J, Wylegalla C, Hecht HJ. Tryparedoxin peroxidase of Leishmania donovani: molecular cloning, heterologous expression, specificity, and catalytic mechanism. Arch Biochem Biophys. 2002;397:324–335. doi: 10.1006/abbi.2001.2688. [DOI] [PubMed] [Google Scholar]
- 31.Choi HJ, Kang SW, Yang CH, Rhee SG, Ryu SE. Crystal structure of a novel human peroxidase enzyme at 2.0 Å resolution. Nat Struct Biol. 1998;5:400–406. doi: 10.1038/nsb0598-400. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto A, Tsukamoto S, Yamamoto H, Ueda-Hashimoto M, Takahashi M, Suzuki H, Morikawa H. Functional complementation in yeast reveals a protective role of chloroplast 2-Cys peroxiredoxin against reactive nitrogen species. J Plant. 2003;33:841–851. doi: 10.1046/j.1365-313x.2003.01669.x. [DOI] [PubMed] [Google Scholar]
- 33.Kovach MJ, Carlson JO, Beaty BJ. A Drosophila metallothionein promoter is inducible in mosquito cells. Insect Mol Biol. 1992;1:37–43. doi: 10.1111/j.1365-2583.1993.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu CC, Fallon AM. Evaluation of a heterologous metallothionein gene promoter in transfected mosquito cells. Comp Biochem Physiol B Biochem Mol Biol. 1997;116:353–358. doi: 10.1016/s0305-0491(96)00265-9. [DOI] [PubMed] [Google Scholar]
- 35.Butterfield LH, Merino A, Golub SH, Shau H. From cytoprotection to tumor suppression: the multifactorial role of peroxiredoxins. Antioxid Redox Signaling. 1999;1:385–402. doi: 10.1089/ars.1999.1.4-385. [DOI] [PubMed] [Google Scholar]
- 36.Do HM, Hong JK, Jung HW, Kim SH, Ham JH, Hwang BK. Expression of peroxidase-like genes, H2O2 production, and peroxidase activity during the hypersensitive response to Xanthomonas campestris pv. vesicatoria in Capsicum annuum. Mol Plant Microbe Interact. 2003;16:196–205. doi: 10.1094/MPMI.2003.16.3.196. [DOI] [PubMed] [Google Scholar]
- 37.Rouhier N, Gelhaye E, Sautiere PE, Brun A, Laurent P, Tagu D, Gerard J, de Fay E, Meyer Y, Jacquot JP. Isolation and characterization of a new peroxiredoxin from poplar sieve tubes that uses either glutaredoxin or thioredoxin as a proton donor. Plant Physiol. 2001;127:1299–1309. [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KS, Kim SR, Park NS, Kim I, Kang PD, Sohn BH, Choi KH, Kang SW, Je YH, Lee SM, Sohn HD, Jin BR. Characterization of a silkworm thioredoxin peroxidase that is induced by external temperature stimulus and viral infection. Insect Biochem Mol Biol. 2005;35:73–84. doi: 10.1016/j.ibmb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Dimopoulos G, Seeley D, Wolf A, Kafatos FC. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO J. 1998;17:6115–6123. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci USA. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanz-Mendoza H, Hernandez-Martinez S, Ku-Lopez M, Rodriguez Mdel C, Herrera-Ortiz A, Rodriguez MH. Superoxide anion in Anopheles albimanus hemolymph and midgut is toxic to Plasmodium berghei ookinetes. J Parasitol. 2002;88:702–706. doi: 10.1645/0022-3395(2002)088[0702:SAIAAH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Szabo C, Ohshima H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide. 1997;1:373–385. doi: 10.1006/niox.1997.0143. [DOI] [PubMed] [Google Scholar]
- 43.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls: the cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 44.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S, Gupta L, Han YS, Barillas-Mury C. Inducible peroxidases mediate nitration of Anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J Biol Chem. 2004;279:53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 46.Sies H, Sharov VS, Klotz LO, Briviba K. Glutathione peroxidase protects against peroxynitrite-mediated oxidations: a new function for selenoproteins as peroxynitrite reductase. J Biol Chem. 1997;272:27812–27817. doi: 10.1074/jbc.272.44.27812. [DOI] [PubMed] [Google Scholar]
- 47.Sohal RS, Arnold L, Orr WC. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mech Ageing Dev. 1990;56:223–235. doi: 10.1016/0047-6374(90)90084-s. [DOI] [PubMed] [Google Scholar]
- 48.Kanzok SM, Fechner A, Bauer H, Ulschmid JK, Muller HM, Botella-Munoz J, Schneuwly S, Schirmer R, Becker K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291:643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- 49.Asahi M, Fujii J, Suzuki K, Seo HG, Kuzuya T, Hori M, Tada M, Fujii S, Taniguchi N. Inactivation of glutathione peroxidase by nitric oxide: implication for cytotoxicity. J Biol Chem. 1995;270:21035–21039. doi: 10.1074/jbc.270.36.21035. [DOI] [PubMed] [Google Scholar]
- 50.Peshenko IV, Shichi H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic Biol Med. 2001;31:292–303. doi: 10.1016/s0891-5849(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 51.Wink DA, Feelisch M, Fukuto J, Chistodoulou D, Jourd'heuil D, Grisham MB, Vodovotz Y, Cook JA, Krishna M, DeGraff WG, Kim S, Gamson J, Mitchell JB. The cytotoxicity of nitroxyl: possible implications for the pathophysiological role of NO. Arch Biochem Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 52.Kirsch M, de Groot H. Formation of peroxynitrite from reaction of nitroxyl anion with molecular oxygen. J Biol Chem. 2002;277:13379–13388. doi: 10.1074/jbc.M108079200. [DOI] [PubMed] [Google Scholar]
- 53.Miranda KM, Espey MG, Yamada K, Krishna M, Ludwick N, Kim S, Jourd'heuil D, Grisham MB, Feelisch M, Fukuto JM, Wink DA. Unique oxidative mechanisms for the reactive nitrogen oxide species, nitroxyl anion. J Biol Chem. 2001;276:1720–1727. doi: 10.1074/jbc.M006174200. [DOI] [PubMed] [Google Scholar]
- 54.Wong PS, Hyun J, Fukuto JM, Shirota FN, DeMaster EG, Shoeman DW, Nagasawa HT. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 55.Monteiro G, Kowaltowski AJ, Barros MH, Netto LE. Glutathione and thioredoxin peroxidases mediate susceptibility of yeast mitochondria to Ca(2+)-induced damage. Arch Biochem Biophys. 2004;425:14–24. doi: 10.1016/j.abb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Wong CM, Siu KL, Jin DY. Peroxiredoxin-null yeast cells are hypersensitive to oxidative stress and are genomically unstable. J Biol Chem. 2004;279:23207–23213. doi: 10.1074/jbc.M402095200. [DOI] [PubMed] [Google Scholar]
- 57.Jin DY, Chae HZ, Rhee SG, Jeang KT. Regulatory role for a novel human thioredoxin peroxidase in NF-κB activation. J Biol Chem. 1997;272:30952–30961. doi: 10.1074/jbc.272.49.30952. [DOI] [PubMed] [Google Scholar]
- 58.Schwarz MA, Lazo JS, Yalowich JC, Allen WP, Whitmore M, Bergonia HA, Tzeng E, Billiar TR, Robbins PD, Lancaster JR, Jr, Pitt BR. Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric oxide. Proc Natl Acad Sci USA. 1995;92:4452–4456. doi: 10.1073/pnas.92.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang GW, Schuschke DA, Kang YJ. Metallothionein-over-expressing neonatal mouse cardiomyocytes are resistant to H2O2 toxicity. Am J Physiol. 1999;276:H167–H175. doi: 10.1152/ajpheart.1999.276.1.H167. [DOI] [PubMed] [Google Scholar]
- 60.UC Irvine: Anopheles gambiae Gene Expression Profile. http://www.angagepuci.bio.uci.edu. [Google Scholar]
- 61.Dana AN, Hong YS, Kern MK, Hillenmeyer ME, Harker BW, Lobo NF, Hogan JR, Romans P, Collins FH. Gene expression patterns associated with blood-feeding in the malaria mosquito Anopheles gambiae. BMC Genom. 2005;6:5. doi: 10.1186/1471-2164-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro JM. A catalogue of Anopheles gambiae transcripts significantly more or less expressed following a blood meal. Insect Biochem Mol Biol. 2003;33:865–882. doi: 10.1016/s0965-1748(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 63.Chege GM, Beier JC. Blood acquisition and processing by three Anopheles (Diptera: Culicidae) species with different innate susceptibilities to Plasmodium falciparum. J Med Entomol. 1998;35:319–323. doi: 10.1093/jmedent/35.3.319. [DOI] [PubMed] [Google Scholar]
- 64.Berner R, Rudin W, Hecker H. Peritrophic membranes and protease activity in the midgut of the malaria mosquito, Anopheles stephensi (Liston) (Insecta: Diptera) under normal and experimental conditions. J Ultrastruct Res. 1983;83:195–204. doi: 10.1016/s0022-5320(83)90077-1. [DOI] [PubMed] [Google Scholar]
- 65.Dix TA, Fontana R, Panthani A, Marnett LJ. Hematin-catalyzed epoxidation of 7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by polyunsaturated fatty acid hydroperoxides. J Biol Chem. 1985;260:5358–5365. [PubMed] [Google Scholar]
- 66.Spack L, Havens PL, Griffith OW. Measurements of total plasma nitrite and nitrate in pediatric patients with the systemic inflammatory response syndrome. Crit Care Med. 1997;25:1071–1078. doi: 10.1097/00003246-199706000-00027. [DOI] [PubMed] [Google Scholar]
- 67.Wong HR, Carcillo JA, Burckart G, Shah N, Janosky JE. Increased serum nitrite and nitrate concentrations in children with the sepsis syndrome. Crit Care Med. 1995;23:835–842. doi: 10.1097/00003246-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Jang HH, Lee KO, Chi YH, Jung BG, Park SK, Park JH, Lee JR, Lee SS, Moon JC, Yun JW, Choi YO, Kim WY, Kang JS, Cheong GW, Yun DJ, Rhee SG, Cho MJ, Lee SY. Two enzymes in one: two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Han YS, Barillas-Mury C. Implications of Time Bomb model of ookinete invasion of midgut cells. Insect Biochem Mol Biol. 2002;32:1311–1316. doi: 10.1016/s0965-1748(02)00093-0. [DOI] [PubMed] [Google Scholar]


