Abstract
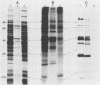
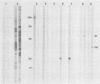
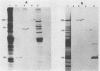
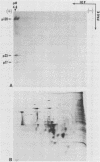
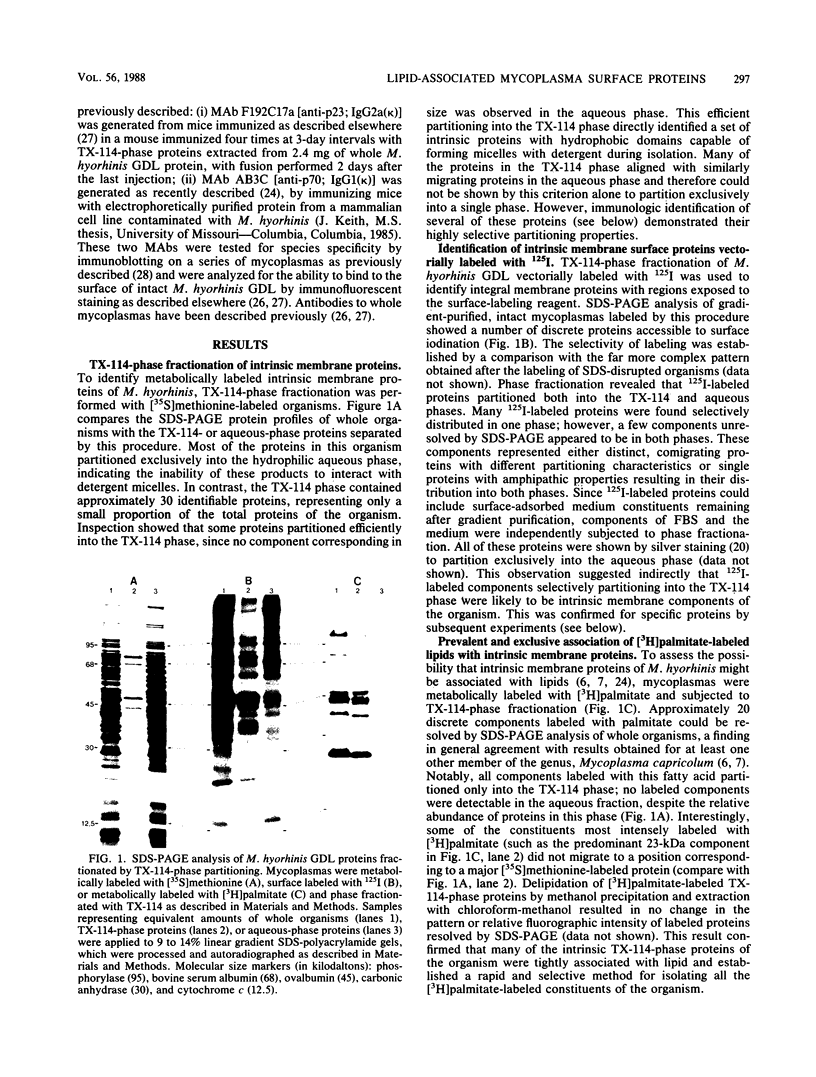
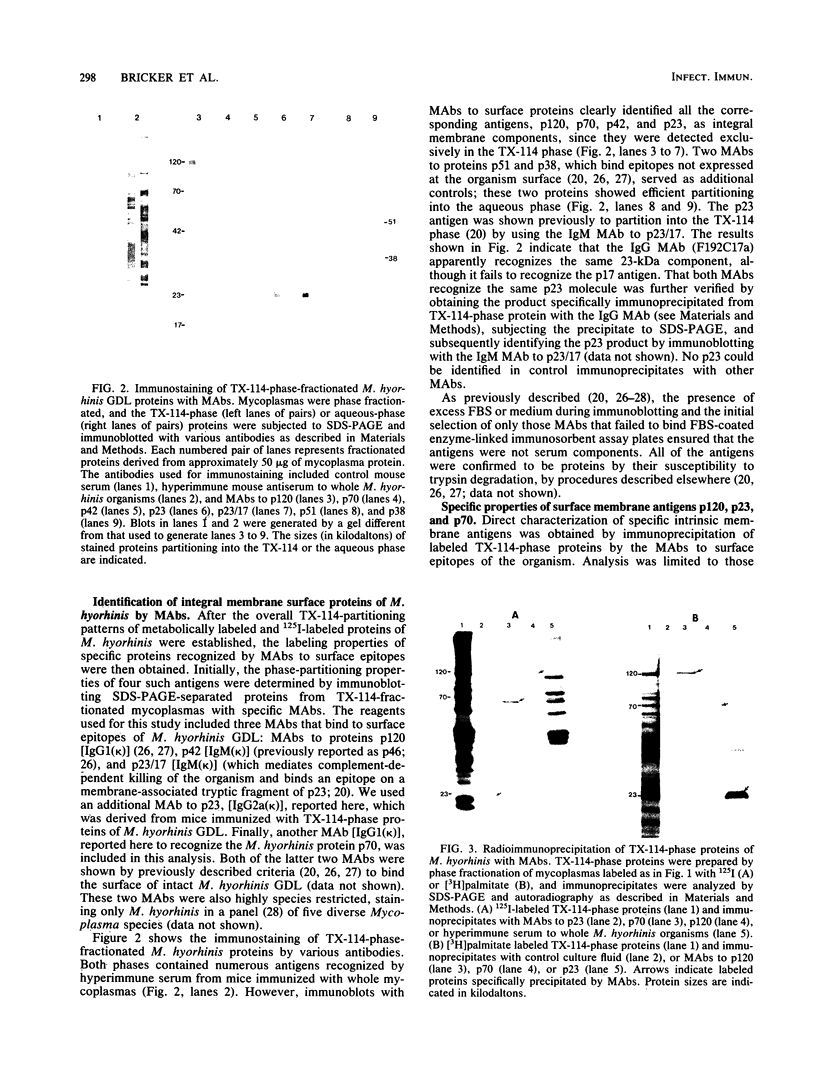
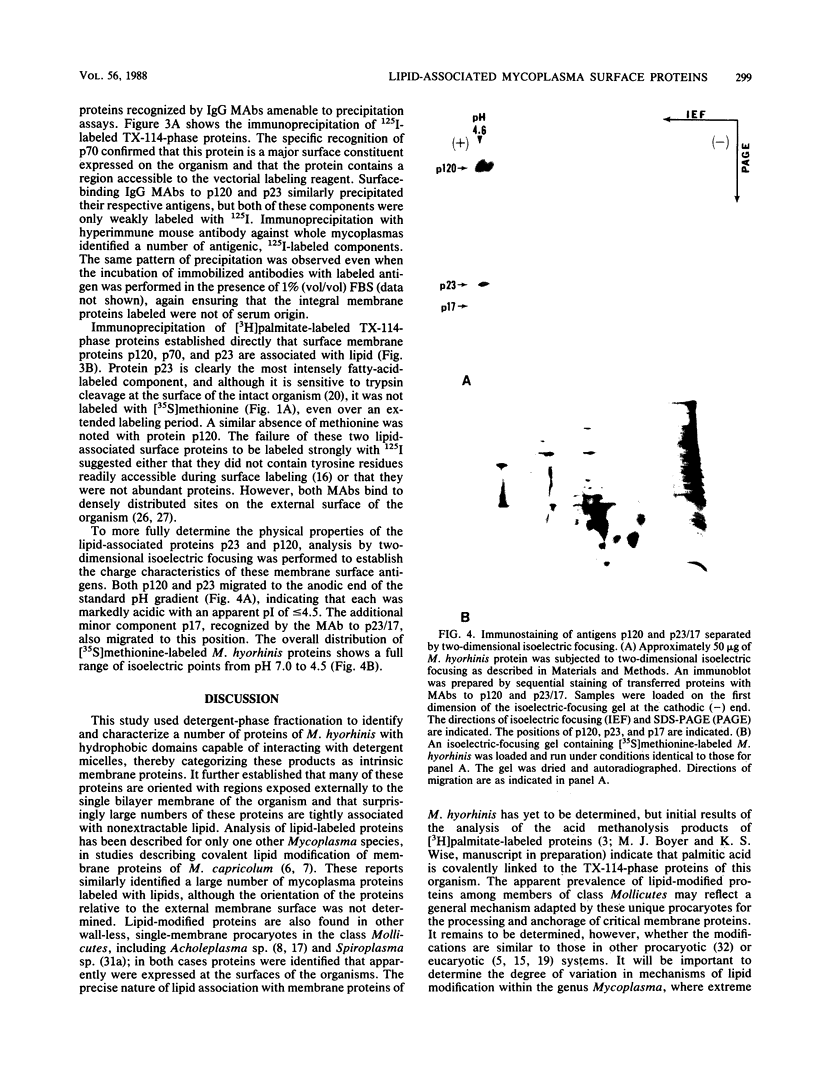
Triton X-114 (TX-114)-phase fractionation was used to identify and characterize integral membrane surface proteins of the wall-less procaryote Mycoplasma hyorhinis GDL. Phase fractionation of mycoplasmas followed by analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed selective partitioning of approximately 30 [35S]methionine-labeled intrinsic membrane proteins into the TX-114 phase. Similar analysis of [3H]palmitate-labeled cells showed that approximately 20 proteins of this organism were associated with lipid, all of which also efficiently partitioned as integral membrane components into the detergent phase. Immunoblotting and immunoprecipitation of TX-114-phase proteins from 125I-surface-labeled cells with four monoclonal antibodies to distinct surface epitopes of M. hyorhinis identified surface proteins p120, p70, p42, and p23 as intrinsic membrane components. Immunoprecipitation of [3H]palmitate-labeled TX-114-phase proteins further established that surface proteins p120, p70, and p23 (a molecule that mediates complement-dependent mycoplasmacidal monoclonal antibody activity) were among the lipid-associated proteins of this organism. Two of these proteins, p120 and p123, were acidic (pI less than or equal to 4.5), as shown by two-dimensional isoelectric focusing. This study established that M. hyorhinis contains an abundance of integral membrane proteins tightly associated with lipids and that many of these proteins are exposed at the external surface of the single limiting plasma membrane. Monoclonal antibodies are reported that will allow detailed analysis of the structure and processing of lipid-associated mycoplasma proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Clyde W. A., Jr, Hu P. C. Antigenic determinants of the attachment protein of Mycoplasma pneumoniae shared by other pathogenic Mycoplasma species. Infect Immun. 1986 Feb;51(2):690–692. doi: 10.1128/iai.51.2.690-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S., Bloch K. Proteolipid formation in Mycoplasma capricolum. Influence of cholesterol on unsaturated fatty acid acylation of membrane proteins. J Biol Chem. 1983 Oct 10;258(19):11814–11818. [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S. Phospholipids as acyl donors to membrane proteins of Mycoplasma capricolum. J Biol Chem. 1984 Sep 10;259(17):10771–10776. [PubMed] [Google Scholar]
- Dahl C. E., Sacktor N. C., Dahl J. S. Acylated proteins in Acholeplasma laidlawii. J Bacteriol. 1985 Apr;162(1):445–447. doi: 10.1128/jb.162.1.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gois M., Kuksa F., Franz J., Taylor-Robinson D. The antigenic differentiation of seven strains of Mycoplasma hyorhinis by growth-inhibition, metabolism-inhibition, latex-agglutination, and polyacrylamide-gel-electrophoresis tests. J Med Microbiol. 1974 Feb;7(1):105–115. doi: 10.1099/00222615-7-1-105. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Strominger J. L. Monoclonal antibodies reacting with immunogenic mycoplasma proteins present in human hematopoietic cell lines. J Immunol. 1982 Dec;129(6):2734–2738. [PubMed] [Google Scholar]
- Horowitz S. A., Garrett B., Davis J. K., Cassell G. H. Isolation of Mycoplasma pulmonis membranes and identification of surface antigens. Infect Immun. 1987 May;55(5):1314–1320. doi: 10.1128/iai.55.5.1314-1320.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Schaper U., Collier A. M., Clyde W. A., Jr, Horikawa M., Huang Y. S., Barile M. F. A Mycoplasma genitalium protein resembling the Mycoplasma pneumoniae attachment protein. Infect Immun. 1987 May;55(5):1126–1131. doi: 10.1128/iai.55.5.1126-1131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Nyström S., Johansson K. E., Wieslander A. Selective acylation of membrane proteins in Acholeplasma laidlawii. Eur J Biochem. 1986 Apr 1;156(1):85–94. doi: 10.1111/j.1432-1033.1986.tb09552.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Towler D. A., Glaser L. Specificity of fatty acid acylation of cellular proteins. J Biol Chem. 1985 Mar 25;260(6):3784–3790. [PubMed] [Google Scholar]
- Riethman H. C., Boyer M. J., Wise K. S. Triton X-114 phase fractionation of an integral membrane surface protein mediating monoclonal antibody killing of Mycoplasma hyorhinis. Infect Immun. 1987 May;55(5):1094–1100. doi: 10.1128/iai.55.5.1094-1100.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., Ferrell R. V., Wise K. S., McIntosh M. A. Identification of a repetitive genomic sequence that is distributed among a select group of mycoplasmas. Isr J Med Sci. 1987 May;23(5):368–373. [PubMed] [Google Scholar]
- Washburn L. R., Ramsay J. R., Roberts L. K. Characterization of the metabolism inhibition antigen of Mycoplasma arthritidis. Infect Immun. 1985 Aug;49(2):357–364. doi: 10.1128/iai.49.2.357-364.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Minion F. C., Cheung H. C. Translocation of Thy-1 antigen and a fluorescent lipid probe during lymphoblastoid cell interaction with Mycoplasma hyorhinis. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S210–S218. doi: 10.1093/clinids/4.supplement_1.s210. [DOI] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Antigenic mimicry of mammalian intermediate filaments by mycoplasmas. Infect Immun. 1985 May;48(2):587–591. doi: 10.1128/iai.48.2.587-591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Monoclonal antibodies to Mycoplasma hyorhinis surface antigens: tools for analyzing mycoplasma-lymphoid cell interactions. Yale J Biol Med. 1983 Sep-Dec;56(5-6):623–629. [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Mycoplasma hyorhinis GDL surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983 Sep;41(3):1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Ludwig W. What are mycoplasmas: the relationship of tempo and mode in bacterial evolution. J Mol Evol. 1984;21(4):305–316. doi: 10.1007/BF02115648. [DOI] [PubMed] [Google Scholar]
- Wroblewski H., Blanchard A., Nyström S., Wieslander A., Thomas D. Amphiphilic properties of spiralin, the major surface antigen of spiroplasmas. A preliminary report. Isr J Med Sci. 1987 May;23(5):439–441. [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Shirvan M. H., Barile M. F., Rottem S. The beta-subunit of the F1F0-ATPase is conserved in mycoplasmas. J Biol Chem. 1986 Jun 5;261(16):7109–7111. [PubMed] [Google Scholar]