Abstract
Modern imaging modalities, including magnetic resonance imaging (MRI), are valuable diagnostic and therapy monitoring tools in rheumatoid arthritis (RA). This article reviewed how these imaging modalities have greatly improved our understanding of pathogenic mechanisms in RA, namely the link between inflammation and damage. For example, traditional paradigms regarding the mechanisms of joint destruction, including the idea that synovitis and damage are uncoupled, have been challenged. As the power of MRI increases, there is a need to define normality since apparently normal joints occasionally exhibit MRI evidence of synovitis in the absence of symptoms.
Introduction: a historical perspective
Historically, subjects with rheumatoid arthritis (RA) presented relatively late for specialist rheumatology assessment following the failure of anti-inflammatory agents. Patients were eventually treated with disease-modifying therapies such as gold and penicillamine, which were of limited efficacy in comparison with modern therapeutic standards. Taken together, this delayed presentation and relative inefficacy of therapies meant that many patients had quite florid joint destruction at clinical presentation. The gold standard clinical imaging modality for RA is projection radiography (x-ray), in which periarticular osteopenia, joint space loss, and marginal erosion are noted to be commonplace. Of these abnormalities, marginal erosions came to be viewed as a specific and relatively sensitive diagnostic test and were adopted by the American College of Rheumatology as classification criteria for RA in 1987 [1].
Projection radiography therefore has placed the marginal erosive process centre stage in disease and has led to the idea that joint erosion and synovitis are often uncoupled (Table 1). This concept emerged because the relationship between joint swelling and joint destruction was not linear, since erosion progressed when synovitis was apparently treated [2-4]. Such clinical observations helped spur cellular and molecular investigations that revealed that an apparent uncoupling process was linked to synovial fibroblast transformation in which such cells were shown to destroy cartilage in a manner that was autonomous of inflammation [5]. At the dawn of the era of biological therapy in RA, this theory led to the concept of a dual-therapeutic strategy, including anti-inflammatory biological therapy, on one hand, and antineoplastic type drug strategies, including metallo-protease inhibition, on the other [6].
Table 1.
Traditional model for rheumatoid arthritis (RA) based mainly on radiographic findings and modern concepts emerging from magnetic resonance imaging in RA
| Traditional paradigms for RA | Modern imaging paradigms | |
| Erosions | A relatively late feature | Present in the majority at clinical presentation |
| Synovitis and damage | Synovitis not necessarily linked to damage – uncoupling | Linear link between synovitis with damage |
| Erosion mechanism | Immunologically mediated or autonomously by synovial fibroblasts | Major biomechanical contribution – such factors may mechanically uncouple synovitis from damage. |
| Cutting edge of erosions | Synovial fibroblasts and osteoclasts destroy tissue from outside in. | Recognition that magnetic resonance imaging bone oedema represents a diffuse osteitis – at least some of erosive process occurs within the bone. |
| Synovitis locations | Considered as diffuse | Regional variations within and between joints |
| Pathophysiological perspective | Erosion formation and cartilage pannus junction key to understanding RA | Erosion and pannus are inevitable and predictable consequences of chronic synovitis, RA being the most common cause. |
Despite the worldwide recognition of projection radiography as a relatively easily available imaging tool for RA, patients now present early, at the stage when projection radiography is normal in the majority of cases. This has driven the need for alternative imaging modalities for the assessment of early arthritis. Magnetic resonance imaging (MRI) has been recognised since the 1980s as a promising imaging tool in evaluating musculoskeletal disorders [7]. The remainder of this article deals with how MRI has challenged RA pathogenic concepts and how this has far-reaching implications.
How magnetic resonance imaging changed the way we view rheumatoid arthritis
Joint failure is the final common pathway of an array of inflammatory, crystal, and degenerative arthritis. The advantage of seeing patients early in the course of disease is that abnormalities evident in imaging are likely to be primary rather than secondary. Unlike projection radiography (which could essentially show only bone), MRI has unparalleled tomographic capabilities and can define different soft tissue structures within the joint, including the ability to clearly depict synovitis following the administration of the MRI contrast agent gadolinium-DTPA (Gd-DTPA) [8]. Ultrasound also brings with it excellent spatial resolution capabilities and the ability to visualise soft tissue and also erosion at quite high resolution, but unlike MRI, ultrasound is unable to ascertain pathologies taking place within the bone. Historically, papers dealing with MRI in rheumatology devoted considerable space to the technology and methodology because there were very few relevant data accrued using this modality. However, since there is now such a wealth of imaging data available from MRI, the present article will focus on it.
Magnetic resonance imaging determined bone erosion and bone oedema in rheumatoid arthritis
The original studies of MRI showed that it is more sensitive than projection radiography for the detection of erosions, particularly in the wrist joints [9,10]; however, at the outset, it was not clear whether radiographic and MRI erosions represented the same pathological processes. Not surprisingly, the tomographic nature of MRI has been used to show that erosions are more commonly detected compared with radiography at several sites, including the metacarpophalangeal (MCP) joints, shoulders, odontoid peg, knee, and feet [11-16]. Unlike erosion formation in small joints, studies to date using MRI have not proven to be beneficial for the assessment of articular cartilage loss in small joint disease in RA [17].
The advent of fat suppression MRI allowed rheumatologists to appreciate a new joint abnormality that was hitherto unrecognised in RA, namely periarticular bone oedema. Unlike erosions, this abnormality can be quite diffuse and may involve the entire MCP head. The administration of Gd-DTPA combined with fat suppression sequences strongly suggested that this represented an inflammatory process or an osteitis (Figure 1). Indeed, this has been confirmed histologically by evaluating tissue obtained at small joint arthroplasty in chronic RA where bone oedema lesions were identified prior to surgery [18].
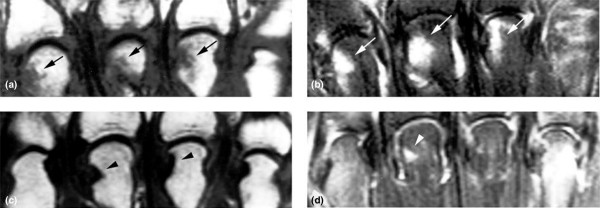
Figure 1.
Coronal magnetic resonance imaging (MRI) of the metacarpophalangeal (MCP) joints. (a) T1-weighted image from the MCP joints in early rheumatoid arthritis, and (b) corresponding fat suppression image at baseline. (c) Corresponding T1-weighted image at 6 months, and (d) fat suppression image at 6-month follow-up. The diffuse low signal at sites of MRI erosion in (a) (arrows) is better demarcated in (c) (arrowheads). The osteitis process at the 2nd, 3rd, and 4th MCP heads in (b) (arrows) has either improved or dramatically regressed in (d) (arrowhead). These images depict the inflammatory component to the MRI erosive process.
In practice, MRI bone erosions may be associated with an extensive halo of bone oedema. Historically, bone oedema is seen on fat suppression MRI sequences, but particularly severe bone oedema in which there has been extensive replacement of marrow fat may be evident on T1-weighted sequences. MRI erosion and bone oedema are intimately linked from the pathophysiological perspective. Bone oedema may regress following therapy with corticosteroids and methotrexate or following biological therapy. Hence, the regression of severe bone oedema lesions (evident even on T1-weighted imaging) has been taken as evidence for healing of erosions [19] (Figure 1). Strictly speaking, this is not true healing; this apparent repair (in reality, the resolution of inflammation) is in no way the same as healing of radiographic erosions where frank bone recortication may occur (Figure 2). These MRI features, in fact, depict the reversal of a preradiographic abnormality. However, the natural history of untreated MRI bone oedema is the subsequent development of radiographic erosions [20]. It has been fairly conclusively shown that MRI bone erosions are also associated with a cortical break as depicted by high-resolution ultrasound and computerised tomography confirming that they represent the same disease process [21,22].
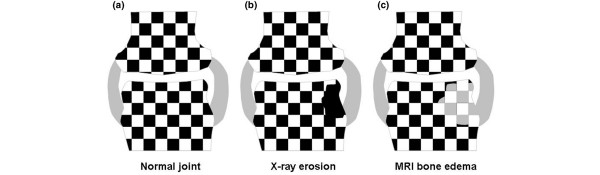
Figure 2.
Model for understanding the link between radiographic and magnetic resonance imaging (MRI) erosion. (a) A diagrammatic 'chessboard' model of a normal joint. The white squares depict the calcium-containing tissues on radiography. The black squares depict the soft tissues that are 'invisible' on radiography but that are visible on MRI due to their hydrogen atom content (fat or water on TI-weighted images and water on fat suppression images). (b) Radiographic erosion where bone cortex and trabecular bone are lost, hence the x-ray appearance of a 'hole' in the bone. Though not visible on x-ray, the erosion may be filled with stromal tissue. (c) Bone oedema on a fat suppression MRI. In this pre-erosive stage, the bone trabecular network is invisible to MRI (but is nevertheless present). The marrow soft tissues have an increased water content due to the osteitis that is seen as a high signal on fat suppression MRI (grey squares), as shown in (b). In the early stages, this will be associated with minimal bone trabecular destruction since it takes time from the inflammatory insult to end in osteoclast-mediated joint destruction. So the MRI pre-erosion lesion will not be evident on radiography. Because this is essentially inflammatory tissue, the resultant MRI lesions may appear to shrink or heal following therapy. Unlike radiographic erosions, this represents regression of inflammation rather than true bone repair. Nevertheless, the consequence of suppressing MRI erosions is that future radiographic damage may be prevented.
Synovitis and associated extracapsular changes in rheumatoid arthritis and other arthropathies
The ability of MRI to demonstrate synovitis in RA is the greatest strength of this method over projection radiography. The presence of synovitis can be inferred best on T2-weighted sequences based on increased joint fluid and confirmed using Gd-DTPA, which has greatly improved the accurate quantification of synovitis. Several studies have shown that MRI synovitis correlates extremely well with histological grades of synovitis, including tissue vascularity [23,24]. A number of approaches to assess synovitis can be used, including the quantitative measurement of synovitis by the evaluation of its thickness in millimetres, calculation of the volume of a given slice, or estimating the entire joint volume [25-27]. Alternative qualitative methods have also been used, including the dynamic measurement of the initial rate of Gd-DTPA enhancement and maximal enhancement, both of which are useful surrogates for the degree of synovial vascularity [28,29]. Generally, there is a correlation between the severity of synovitis and all of these parameters.
Whilst joint inflammation is conceptualised in relationship to synovitis in RA, studies have shown that severe synovitis is associated with extracapsular inflammation which likely relates to the nonspecific extension of a severe inflammatory reaction to the immediately adjacent tissues [30]. These extracapsular changes could be important for abnormalities, including ulnar drift and digital subluxation, but this needs to be assessed. Given that extracapsular abnormalities when present in systemic lupus erythematosus-associated hand disease are strongly linked to Jaccoud arthropathy, where disabling digital deviation is the norm, it seems that such extrasynovial changes are important. Extracapsular inflammatory changes are much more common in the spondylo-arthropathies (SpAs) and polymyalgia rheumatica (PMR)-associated hand disease, likely reflecting a different epicentre of the joint disease [31-33]. Also, clinical synovitis in osteoarthritis (OA) may be associated with florid extracapsular changes in small joint hand involvement [34,35]. The implications of these MRI observations are that clinically evident synovitis, in fact, may have a prominent nonsynovial component. From the practical perspective, this overlap in extra-capsular soft tissue pathology means that it is not feasible to use these changes as a diagnostic test in individual cases.
There is evidence that knee joint synovitis in psoriatic arthritis is more vascular as assessed arthroscopically and histologically compared with RA [36,37]. Again, MRI studies support this observation at the population level but it is not suitable as a diagnostic test [38]. However, studies in wrist and MCP joints have actually shown a more or equally vascular synovium in RA compared with SpA [39]. From MRI studies, the concept is emerging that there are certain site-specific differences in synovitis within and between diseases and the implications of this need further exploration.
It has been established that the suppression of synovitis in RA may be associated with some persistent disease at the histological level [40]. The same appears to hold true for MRI and ultrasound where synovial thickening may be evident in apparent clinical remission [41]. Since the synovium appears to be the primary target for the autoimmune process in autoantibody-associated RA, a reasonable goal should be its complete ablation. However, this raises the old question: where does normal end and disease begin? For example, some studies have shown that, on MRI, normal joints may occasionally have a small degree of enhancement [42]. Furthermore, OA may be associated with subclinical synovitis, and secondary OA is quite common in RA. This means that, in established disease with secondary damage, at least a component of the synovitis may not be autoimmune-driven in origin. Many important issues need addressing here, including how patients will respond to biological therapies for secondary degenerative-related synovitis, which could be erroneously interpreted as representing part of the primary autoimmune process.
It has also emerged that synovitis in RA, and indeed in other arthropathies, is not of equal magnitude within joints; for example, a greater volume of synovitis adjacent to the patella was found compared with remote sites in the suprapatellar pouch [38,43]. This could be of considerable consequence since it is much more difficult to ablate synovitis at this latter location. Whether this 'minimal residual synovitis' is prognostically relevant awaits further assessment.
The relationship between synovitis and bone erosion in rheumatoid arthritis
Radiographic studies have fuelled the notion that synovitis and joint erosion could be uncoupled. Our studies showed that synovitis was primary in RA and that erosions were seen only in joints where synovitis was present [44]. We subsequently demonstrated this in longitudinal studies and noted that, in patients with persistent synovitis, erosive disease continued in individual joints but that, where synovitis was suppressed to a very low level, the erosive process ceased [26]. An identical scenario was shown by other groups in the wrist joints [27]. It was also shown that joint erosion progression in joints without clinical synovitis was related to subclinical synovitis that could be detected on MRI [41]. In fact, radiographic progression of joint damage has been documented in patients who were in clinical remission [4]. However, in support of the theory that synovitis and bone erosion may be uncoupled are the findings from studies that show bone oedema to be a much stronger predictor of erosion than synovitis [20,45,46]. We propose that bone oedema is secondary to synovitis and consistent with this hypothesis is the observation that the magnitude of synovitis measured in a serial fashion is an independent predictor of MRI bone erosion [26,44]. Overall, MRI studies argue against the prevailing view that synovitis and erosion were uncoupled and are linked by an intermediate osteitis that is secondary to synovitis, and these observations are further appraised below.
Uncoupling of synovitis and erosion – but not as we know it
In MRI studies, to characterise the nature of erosion in RA, it was noted that bone erosions have a particular propensity to occur adjacent to the MCP joint collateral ligaments [47] (Figure 3). Actually, it had been known for many years, based on radiographic observation, that erosions were often 'compressive' in that they occurred adjacent to the small joint radial and ulnar collateral ligaments [48]. It is somewhat paradoxical that MRI does not support the uncoupling of inflammation from joint destruction concept since the presence of inflammation appears to be a sine qua non for erosion, but it does suggest that there is a biomechanical uncoupling of inflammation from damage. We have noted that the volume of synovitis in RA may be twice as great in the dorsal regions of the third MCP joints compared with the region adjacent to the radial collateral ligament in the fourth MCP joint, yet erosion formation was much more common in the latter site [47]. Coincidentally, it happens that these regions at the margin of the joints are the best visualised on projection radiography.
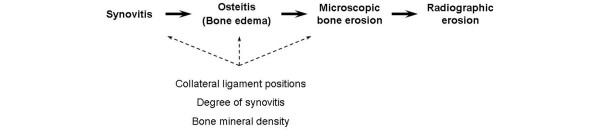
Figure 3.
Proposed series of events leading to bone erosion. The advent of magnetic resonance imaging (MRI) in early rheumatoid arthritis (RA) allows the demonstration of the early appearance of MRI bone oedema, histologically an osteitis, suggesting that the path to bone destruction in RA should be viewed as a close integration of synovitis and erosion. Modifying factors such as the position of joint collateral ligaments, the degree of synovitis, and bone mineral density may modify erosion formation.
Magnetic resonance imaging erosions in other arthropathies
Radiographic marginal erosions have been well recognised in other arthropathies, including psoriatic arthritis, and in erosive hand OA, usually in established or late disease [49,50]. As stated earlier, erosions have assumed a very important role in the diagnosis of RA, in predicting prognosis and for monitoring therapy. The demonstration that MRI erosive disease was commonplace in early RA fuelled the idea that erosion was fundamental, not just for diagnosis but also for understanding disease pathophysiology. Consequently, the idea has emerged that a scan of a patient with early RA showing MRI erosions places patients in a worse prognostic group. Indeed, there is evidence that MRI erosions in early RA predict subsequent radiographic erosions [19,51]. However, as explained below, the use of MRI for RA diagnosis based on 'MRI erosions' is potentially flawed.
As already stated, an early MRI bone erosion is not identical to mature radiographic erosion (Figure 2). Few would argue that PMR-associated hand synovitis is not associated with radiographic erosion development. However, PMR-associated hand disease has a similar degree of MRI bone erosion and bone oedema compared with early RA [31]. Furthermore, in the proof-of-concept study in question, the PMR group eventually went into complete remission. These findings underscore that early MRI erosions per se may not be prognostically relevant. What is the basis for these observations? It is likely that the dramatic response of PMR to corticosteroids leads to a dramatic suppression of synovitis and hence the erosive process is halted. Any bone cortical damage associated with PMR will consequently be less evident. This contrasts with RA, where synovitis has persisted despite therapy. These MRI observations are not confined just to PMR since hand erosive disease in early psoriatic arthritis that was selected on the basis of enthesitis pathology showed the same degree of erosion as RA [39]. Finally, a small proportion of hand OA patients have an erosive phenotype on radiography [49]. However, on high-resolution MRI, it is apparent that the erosive phenotype is the norm rather than the exception [34,35].
Conclusion
To date, MRI studies appear to confirm that autoantibody-associated RA is primarily a disorder of synovium. This emphasises the importance of the effective treatment of synovitis as being the only necessary and sufficient therapeutic goal for RA. It is clear that erosive disease is secondary and a predictable consequence of synovitis, with the added caveat that sites of joint compression may be more prone to erosion (Figure 3). However, we feel that the erosion concept and the link with poorer prognosis are so firmly engrained in the rheumatology community that MRI will be used erroneously for quite some time as a diagnostic or prognostic test for early RA. Also, MRI technology is advancing at a steady rate with improved resolution and an increasing emergent platform for undertaking molecular imaging in vivo in humans. The power of imaging to probe beyond the anatomical basis for RA and progressively delve to the cellular or molecular level of disease in humans is an exciting prospect.
Abbreviations
Gd-DTPA: gadolinium-DTPA; MCP: metacarpophalangeal; MRI: magnetic resonance imaging; OA: osteoarthritis; PMR: polymyalgia rheumatica; RA: rheumatoid arthritis; SpA: spondyloarthropathy.
Competing interests
The authors declare that they have no competing interests.
Note
The Scientific Basis of Rheumatology: A Decade of Progress
This article is part of a special collection of reviews, The Scientific Basis of Rheumatology: A Decade of Progress, published to mark Arthritis Research & Therapy's 10th anniversary.
Other articles in this series can be found at: http://arthritis-research.com/sbr
References
- Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Durez P, Malghem J, Nzeusseu Toukap A, Depresseux G, Lauwerys BR, Westhovens R, Luyten FP, Corluy L, Houssiau FA, Verschueren P. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum. 2007;56:3919–3927. doi: 10.1002/art.23055. [DOI] [PubMed] [Google Scholar]
- Berg WB van den. Uncoupling of inflammatory and destructive mechanisms in arthritis. Semin Arthritis Rheum. 2001;30:7–16. doi: 10.1053/sarh.2001.23704. [DOI] [PubMed] [Google Scholar]
- Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42. doi: 10.1002/art.11481. [DOI] [PubMed] [Google Scholar]
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- Castro-Rueda H, Kavanaugh A. Biologic therapy for early rheumatoid arthritis: the latest evidence. Curr Opin Rheumatol. 2008;20:314–319. doi: 10.1097/BOR.0b013e3282f5fcf6. [DOI] [PubMed] [Google Scholar]
- Terrier F, Hricak H, Revel D, Alpers CE, Reinhold CE, Levine J, Genant HK. Magnetic resonance imaging and spectroscopy of the periarticular inflammatory soft-tissue changes in experimental arthritis of the rat. Invest Radiol. 1985;20:813–823. doi: 10.1097/00004424-198511000-00009. [DOI] [PubMed] [Google Scholar]
- Konig H, Sieper J, Wolf KJ. Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology. 1990;176:473–477. doi: 10.1148/radiology.176.2.2367663. [DOI] [PubMed] [Google Scholar]
- Gilkeson G, Polisson R, Sinclair H, Vogler J, Rice J, Caldwell D, Spritzer C, Martinez S. Early detection of carpal erosions in patients with rheumatoid arthritis: a pilot study of magnetic resonance imaging. J Rheumatol. 1988;15:1361–1366. [PubMed] [Google Scholar]
- Foley-Nolan D, Stack JP, Ryan M, Redmond U, Barry C, Ennis J, Coughlan RJ. Magnetic resonance imaging in the assessment of rheumatoid arthritis – a comparison with plain film radiographs. Br J Rheumatol. 1991;30:101–106. doi: 10.1093/rheumatology/30.2.101. [DOI] [PubMed] [Google Scholar]
- Oostveen JC, Roozeboom AR, Laar MA van de, Heeres J, den Boer JA, Lindeboom SF. Functional turbo spin echo magnetic resonance imaging versus tomography for evaluating cervical spine involvement in rheumatoid arthritis. Spine. 1998;23:1237–1244. doi: 10.1097/00007632-199806010-00013. [DOI] [PubMed] [Google Scholar]
- Poleksic L, Zdravkovic D, Jablanovic D, Watt I, Bacic G. Magnetic resonance imaging of bone destruction in rheumatoid arthritis: comparison with radiography. Skeletal Radiol. 1993;22:577–580. doi: 10.1007/BF00197138. [DOI] [PubMed] [Google Scholar]
- Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf KJ, Raber H, Hamm B, Burmester GR, Bollow M. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999;42:1232–1245. doi: 10.1002/1529-0131(199906)42:6<1232::AID-ANR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hermann KG, Backhaus M, Schneider U, Labs K, Loreck D, Zühlsdorf S, Schink T, Fischer T, Hamm B, Bollow M. Rheumatoid arthritis of the shoulder joint: comparison of conventional radiography, ultrasound, and dynamic contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003;48:3338–3349. doi: 10.1002/art.11349. [DOI] [PubMed] [Google Scholar]
- Ejbjerg BJ, Vestergaard A, Jacobsen S, Thomsen HS, Ostergaard M. The smallest detectable difference and sensitivity to change of magnetic resonance imaging and radiographic scoring of structural joint damage in rheumatoid arthritis finger, wrist, and toe joints: a comparison of the OMERACT rheumatoid arthritis magnetic resonance imaging score applied to different joint combinations and the Sharp/van der Heijde radiographic score. Arthritis Rheum. 2005;52:2300–2306. doi: 10.1002/art.21207. [DOI] [PubMed] [Google Scholar]
- Alasaarela E, Suramo I, Tervonen O, Lahde S, Takalo R, Hakala M. Evaluation of humeral head erosions in rheumatoid arthritis: a comparison of ultrasonography, magnetic resonance imaging, computed tomography and plain radiography. Br J Rheumatol. 1998;37:1152–1156. doi: 10.1093/rheumatology/37.11.1152. [DOI] [PubMed] [Google Scholar]
- McQueen F, Lassere M, Edmonds J, Conaghan P, Peterfy C, Bird P, O'Connor P, Ejbjerg B, Klarlund M, Stewart N, Emery P, Shnier R, Genant H, Østergaard M. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Summary of OMERACT 6 MR Imaging Module. J Rheumatol. 2003;30:1387–1392. [PubMed] [Google Scholar]
- McQueen FM, Gao A, Ostergaard M, King A, Shalley G, Robinson E, Doyle A, Clark B, Dalbeth N. High-grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann Rheum Dis. 2007;66:1581–1587. doi: 10.1136/ard.2007.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen FM, Benton N, Crabbe J, Robinson E, Yeoman S, McLean L, Stewart N. What is the fate of erosions in early rheumatoid arthritis? Tracking individual lesions using x rays and magnetic resonance imaging over the first two years of disease. Ann Rheum Dis. 2001;60:859–868. [PMC free article] [PubMed] [Google Scholar]
- Hetland ML, Ejbjerg BJ, Hørslev-Petersen K, Jacobsen S, Vestergaard A, Jurik AG, Stengaard-Pedersen K, Junker P, Lottenburger T, Hansen I, Andersen LS, Tarp U, Skjødt H, Pedersen JK, Majgaard O, Svendsen AJ, Ellingsen T, Lindegaard HM, Christensen AF, Vallø J, Torfing T, Narvestad E, Thomsen HS, Ostergaard M. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2 year randomized controlled trial (CIMES-TRA) Ann Rheum Dis. 2008, Apr 3. [DOI] [PubMed]
- Døhn UM, Ejbjerg BJ, Court-Payen M, Hasselquist M, Narvestad E, Szkudlarek M, Møller JM, Thomsen HS, Østergaard M. Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res Ther. 2006;8:R110. doi: 10.1186/ar1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield RJ, Gibbon WW, Conaghan PG, O'Connor P, McGonagle D, Pease C, Green MJ, Veale DJ, Isaacs JD, Emery P. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000;43:2762–2770. doi: 10.1002/1529-0131(200012)43:12<2762::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Ostendorf B, Peters R, Dann P, Becker A, Scherer A, Wedekind F, Friemann J, Schulitz KP, Mödder U, Schneider M. Magnetic resonance imaging and miniarthroscopy of metacarpophalangeal joints: sensitive detection of morphologic changes in rheumatoid arthritis. Arthritis Rheum. 2001;44:2492–2502. doi: 10.1002/1529-0131(200111)44:11<2492::AID-ART429>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Gaffney K, Cookson J, Blades S, Coumbe A, Blake D. Quantitative assessment of the rheumatoid synovial microvascular bed by gadolinium-DTPA enhanced magnetic resonance imaging. Ann Rheum Dis. 1998;57:152–157. doi: 10.1136/ard.57.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, Shnier R, O'Connor P, Klarlund M, Emery P, Genant H, Lassere M, Edmonds J. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–1386. [PubMed] [Google Scholar]
- Conaghan PG, O'Connor P, McGonagle D, Astin P, Wakefield RJ, Gibbon WW, Quinn M, Karim Z, Green MJ, Proudman S, Isaacs J, Emery P. Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 2003;48:64–71. doi: 10.1002/art.10747. [DOI] [PubMed] [Google Scholar]
- Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, Lorenzen I. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:918–929. doi: 10.1002/1529-0131(199905)42:5<918::AID-ANR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Sonne-Holm S, Lorenzen I. Quantification of synovistis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn Reson Imaging. 1998;16:743–754. doi: 10.1016/S0730-725X(98)00008-3. [DOI] [PubMed] [Google Scholar]
- Reece RJ, Kraan MC, Radjenovic A, Veale DJ, O'Connor PJ, Ridgway JP, Gibbon WW, Breedveld FC, Tak PP, Emery P. Comparative assessment of leflunomide and methotrexate for the treatment of rheumatoid arthritis, by dynamic enhanced magnetic resonance imaging. Arthritis Rheum. 2002;46:366–372. doi: 10.1002/art.10084. [DOI] [PubMed] [Google Scholar]
- McGonagle D, Gibbon W, O'Connor P, Green M, Pease C, Ridgway J, Emery P. An anatomical explanation for good-prognosis rheumatoid arthritis. Lancet. 1999;353:123–124. doi: 10.1016/S0140-6736(05)76160-2. [DOI] [PubMed] [Google Scholar]
- Marzo-Ortega H, Rhodes LA, Tan AL, Tanner SF, Conaghan PG, Hensor EM, O'Connor P, Radjenovic A, Pease CT, Emery P, McGonagle D. Evidence for a different anatomic basis for joint disease localization in polymyalgia rheumatica in comparison with rheumatoid arthritis. Arthritis Rheum. 2007;56:3496–3501. doi: 10.1002/art.22942. [DOI] [PubMed] [Google Scholar]
- McGonagle D, Pease C, Marzo-Ortega H, O'Connor P, Gibbon W, Emery P. Comparison of extracapsular changes by magnetic resonance imaging in patients with rheumatoid arthritis and polymyalgia rheumatica. J Rheumatol. 2001;28:1837–1841. [PubMed] [Google Scholar]
- McGonagle D, Khan MA, Marzo-Ortega H, O'Connor P, Gibbon W, Emery P. Enthesitis in spondyloarthropathy. Curr Opin Rheumatol. 1999;11:244–250. doi: 10.1097/00002281-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Tan AL, Grainger AJ, Tanner SF, Emery P, McGonagle D. A high-resolution magnetic resonance imaging study of distal inter-phalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis Rheum. 2006;54:1328–1333. doi: 10.1002/art.21736. [DOI] [PubMed] [Google Scholar]
- Tan AL, Grainger AJ, Tanner SF, Shelley DM, Pease C, Emery P, McGonagle D. High-resolution magnetic resonance imaging for the assessment of hand osteoarthritis. Arthritis Rheum. 2005;52:2355–2365. doi: 10.1002/art.21210. [DOI] [PubMed] [Google Scholar]
- Veale D, Yanni G, Rogers S, Barnes L, Bresnihan B, Fitzgerald O. Reduced synovial membrane macrophage numbers, ELAM-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum. 1993;36:893–900. doi: 10.1002/art.1780360705. [DOI] [PubMed] [Google Scholar]
- Fiocco U, Cozzi L, Chieco-Bianchi F, Rigon C, Vezzù M, Favero E, Ferro F, Sfriso P, Rubaltelli L, Nardacchione R, Todesco S. Vascular changes in psoriatic knee joint synovitis. J Rheumatol. 2001;28:2480–2486. [PubMed] [Google Scholar]
- Rhodes LA, Tan AL, Tanner SF, Radjenovic A, Hensor EM, Reece R, O'Connor P, Emery P, McGonagle D. Regional variation and differential response to therapy for knee synovitis adjacent to the cartilage-pannus junction and suprapatellar pouch in inflammatory arthritis: implications for pathogenesis and treatment. Arthritis Rheum. 2004;50:2428–2432. doi: 10.1002/art.20444. [DOI] [PubMed] [Google Scholar]
- Marzo-Ortega H, Tanner SF, Rhodes LA, Tan AL, Conaghan PG, Hensor EMA, et al. Magnetic resonance imaging in the assessment of metacarpophalangeal joint disease in early psoriatic and rheumatoid arthritis. Scand J Rheumatol. 2008. [DOI] [PubMed]
- van Oosterhout M, Levarht EW, Sont JK, Huizinga TW, Toes RE, van Laar JM. Clinical efficacy of infliximab plus methotrexate in DMARD naive and DMARD refractory rheumatoid arthritis is associated with decreased synovial expression of TNF alpha and IL18 but not CXCL12. Ann Rheum Dis. 2005;64:537–543. doi: 10.1136/ard.2004.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, Wakefield RJ, O'Connor PJ, Emery P. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–3773. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- Ejbjerg B, Narvestad E, Rostrup E, Szkudlarek M, Jacobsen S, Thomsen HS, Østergaard M. Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum. 2004;50:1097–1106. doi: 10.1002/art.20135. [DOI] [PubMed] [Google Scholar]
- Rhodes LA, Conaghan PG, Radjenovic A, Grainger AJ, Emery P, McGonagle D. Further evidence that a cartilage-pannus junction synovitis predilection is not a specific feature of rheumatoid arthritis. Ann Rheum Dis. 2005;64:1347–1349. doi: 10.1136/ard.2004.033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D, Conaghan PG, O'Connor P, Gibbon W, Green M, Wakefield R, Ridgway J, Emery P. The relationship between synovitis and bone changes in early untreated rheumatoid arthritis: a controlled magnetic resonance imaging study. Arthritis Rheum. 1999;42:1706–1711. doi: 10.1002/1529-0131(199908)42:8<1706::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- McQueen FM, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S, McLean L, Stewart N. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1814–1827. doi: 10.1002/art.11162. [DOI] [PubMed] [Google Scholar]
- Haavardsholm EA, Boyesen P, Ostergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis. 2008;67:794–800. doi: 10.1136/ard.2007.071977. [DOI] [PubMed] [Google Scholar]
- Tan AL, Tanner SF, Conaghan PG, Radjenovic A, O'Connor P, Brown AK, Emery P, McGonagle D. Role of metacarpopha-langeal joint anatomic factors in the distribution of synovitis and bone erosion in early rheumatoid arthritis. Arthritis Rheum. 2003;48:1214–1222. doi: 10.1002/art.10963. [DOI] [PubMed] [Google Scholar]
- Martel W, Hayes JT, Duff IF. The pattern of bone erosion in the hand and wrist in rheumatoid arthritis. Radiology. 1965;84:204–214. doi: 10.1148/84.2.204. [DOI] [PubMed] [Google Scholar]
- Grainger AJ, Farrant JM, O'Connor PJ, Tan AL, Tanner S, Emery P, McGonagle D. MR imaging of erosions in interphalangeal joint osteoarthritis: is all osteoarthritis erosive? Skeletal Radiol. 2007;36:737–745. doi: 10.1007/s00256-007-0287-5. [DOI] [PubMed] [Google Scholar]
- Taylor WJ, Porter GG, Helliwell PS. Operational definitions and observer reliability of the plain radiographic features of psoriatic arthritis. J Rheumatol. 2003;30:2645–2658. [PubMed] [Google Scholar]
- Østergaard M, Hansen M, Stoltenberg M, Jensen KE, Szkudlarek M, Pedersen-Zbinden B, Lorenzen I. New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier. Arthritis Rheum. 2003;48:2128–2131. doi: 10.1002/art.11076. [DOI] [PubMed] [Google Scholar]