Abstract
Osteoporosis and disorders of bone fragility are highly heritable, but despite much effort the identities of few of the genes involved has been established. Recent developments in genetics such as genome-wide association studies are revolutionizing research in this field, and it is likely that further contributions will be made through application of next-generation sequencing technologies, analysis of copy number variation polymorphisms, and high-throughput mouse mutagenesis programs. This article outlines what we know about osteoporosis genetics to date and the probable future directions of research in this field.
Introduction
Ninety years ago a major debate took place between the Mendelians and the Biometricians. Mendel's laws of inheritance (with their clear phenotype-genotype correlation) were inadequate to explain heritable and normally distributed quantitative traits such as height, bone mineral density (BMD), and weight. The elegant solution to this problem was that both parties were right; single genes cannot underlie inheritance of complex quantitative traits, but such traits arise due to the action of multiple genes, each inherited in Mendelian fashion and each exerting their individual effect upon the ultimate phenotype. Over the past century many monogenic diseases with classical Mendelian inheritance have successfully been mapped, but progress in dissection of quantitative trait loci has – until very recently – been frankly disappointing. Now, quantum leaps in genotyping and bioinformatics capacity have at last provided an opportunity to unravel the genetic basis of quantitative traits that underlie human disease.
Osteoporosis represents a paradigm in this area: a common and disabling disease in which the phenotype is caused by the effects of multiple quantitative trait loci. Approaches to identify genes in which rare mutations have a large phenotypic effect had been extremely successful in mapping monogenic bone diseases (for example, osteoporosis-pseudoglioma), and certainly such genetic studies identified hitherto unexpected pathways that also contribute to osteoporosis. However, until very recently, there had been little return from extensive efforts to identify common genetic polymorphisms in the multiple genes, each of small individual effect, that ultimately result in the phenotype of osteoporosis. It is therefore illuminating to review the genetics of osteoporosis not only in its specifics but also as a model for the dissection of other complex genetic disorders – from genetic epidemiology, candidate gene association studies, and linkage studies to whole-genome association studies – and to consider future directions.
The problem
Osteoporosis is a common condition of elderly men and women, which manifests clinically by minimal trauma fractures, particularly vertebral and hip fracture. Almost a quarter of European women aged over 50 years are osteoporotic according to World Health Organization criteria for BMD (t-score < -2.5), and the remaining lifetime risk for any osteoporotic hip and vertebral fracture in 50-year-old Caucasian women is 39% [1]. Osteoporosis is not confined to women, as is evident particularly in older age groups, in which up to 40% of hip fractures occur in men [2].
The economic and social costs of osteoporosis represent a huge drain of health resources. In 2005 there were approximately 2 million osteoporotic fractures in the USA, with health care costs estimated at US$17 billion [3]. This cost was expected to rise by 50% by 2025. In Sweden osteoporotic fracture is responsible for more hospital bed-days than breast cancer and prostate cancer combined [4]. Osteoporosis is not just a problem for the developed world. Rapid population growth and aging populations in both developed and developing countries mean that worldwide osteoporotic fracture rates are expected to increase. The brunt of these costs will be faced by developing countries that are least equipped to cope.
Currently, most therapeutic options retard the rate of bone loss but they do not convert osteoporosis back to normal bone mass. Only a few anabolic agents exist, although generally such options are too costly to be practicable, even for wealthy countries. Current screening methods to identify at-risk individuals have only moderate predictive capacity and as such are not suitable for general population use. The only hope to reverse the oncoming worldwide hip fracture tsunami will be if radical changes are made in our understanding, prevention, and treatment of osteoporosis. Genetics research offers the potential to elucidate the disease process more fully, to identify new targets for therapeutic intervention, and to refine prognostic tests in order to improve targeting of primary prevention measures to those most in need.
Genetic epidemiology
The first step in any condition thought to have an underlying genetic aetiology is to establish whether a trait (such as low BMD or fracture) really is heritable. From there, modeling can predict the likely mode of inheritance, demonstrate the appropriate method for investigation (for instance, family versus general population, selected versus nonselected population), and inform power calculations to ensure that an appropriate study of adequate size is performed.
Twin and family studies have demonstrated that osteoporosis is highly familial, and that the tendency of the condition to run in families is predominantly due to genetic factors. This is true of a wide range of osteoporosis-related phenotypes, including BMD, bone turnover, and skeletal dimensions associated with growth and fracture risk [5-8], as well as fracture risk itself [9].
There has been extensive debate and research within the bone research community about the optimal phenotype to study. The ultimate goal of research in osteoporosis genetics is to identify genes that increase bone fragility. It would therefore seem enticing to study fracture as the primary outcome variable. However, fractures can occur for a wide variety of reasons, some of which are unrelated to bone fragility, and it is likely to prove genetically more complex than intermediate bone phenotypes, such as BMD.
BMD (as measured using dual energy x-ray absorptiometry) is the screening tool most commonly used to identify patients with osteoporosis and who are at increased risk for low-trauma fracture. The heritability of BMD, measured using a variety of methods in twin and intergenerational studies, has been shown to be very high. Studies of female twins have shown heritability of BMD to be 57% to 92% [10-12], including studies of postmenopausal twins [13]. Estimates from intergenerational family studies have also identified substantial heritability of BMD (44% to 67%) [14-16]. Several segregation studies, in families drawn from the general population, and ascertained with probands with more severe phenotypes, have demonstrated that the majority of the heritability of BMD is polygenic [14,16-20]. In specific populations substantial monogenic effects have been observed, but this has always been on the background of predominantly polygenic effects [19,21-23].
Therefore most genetic studies in osteoporosis to date have focused on the phenotype of BMD, because it is highly heritable, easy to measure, and has an established strong relationship with fracture risk. However, areal BMD (bone quantity per unit bone area measured) does not provide information regarding bone distribution (between cortical and cancellous compartments) or bone microarchitecture. Large and small bones with different volumetric BMD (bone quantity per unit bone volume measured) and fracture risk may have similar areal BMD. Methods to determine bone architectural measures and bone fragility indices from areal BMD scans make inappropriate assumptions about similarity of bone shape between individuals, and therefore have not proven better predictors of fracture risk than areal BMD itself.
There has been significant interest in noninvasive assessment of bone microarchitecture. Data from murine studies in particular indicate that although a large proportion of genetic variants can be identified by studies of BMD alone, significant additional information can be obtained with use of more informative bone imaging modalities, such as quantitative computed tomography scanning and magnetic resonance imaging. These methods are still in development, however, and will not be suitable for large-scale genetic studies until there is better standardization of measures, and until their genetic epidemiology and clinical significance are better established in humans.
Fracture risk is known to run in families, with the relative risk ratio of a fracture in a first-degree relative ranging from 1.3 to 2.4, varying according to the type of relative pair and site of fracture [24,25]. Fracture heritability studies in twins and families have generally found more limited heritability than for BMD, with the possible single exception of hip fracture in younger cohorts (age < 69 years). In a national Finnish cohort of 15,098 twins [26], no significant increase in monozygotic twin concordance for fracture was observed. The findings of this study have been debated, and a reanalysis suggested that the data were consistent with a 35% heritability of fracture liability (significance level not reported) [27]. A study of 6,750 British twins [28] found significant heritability of 54% for Colles' fracture in women. Michaelson and coworkers [29] studied 33,432 Swedish twins and reported age-adjusted heritability of any fracture of 16%, osteoporotic fractures of 27%, and hip fracture of 46%. A significant age interaction was observed, with the heritability of fracture being highest when the fracture occurred at a younger age (heritability of 68% at age < 69 years), and no heritability observed at older ages, when most hip fractures occur (heritability 3% at age > 79 years). Deng and colleagues [25] demonstrated low heritability of Colles' fracture in a study of 6,274 sisters or mothers of women who had had a previous Colles' fracture (heritability 25.4%; significance level not reported). In a separate study of 50 Caucasian families [9], they demonstrated no significant heritability of wrist and spinal fractures, and heritability of hip fracture was only of marginal significance (P = 0.048, uncorrected for the multiple, albeit correlated, phenotypes studied). That study, and the British twin study referred to above, suggested that the genetic correlation between hip fracture and BMD was low. This appears to contradict several seminal reports on the genetic epidemiology of osteoporosis, demonstrating that premenopausal daughters of mothers with osteoporotic fracture have low BMD [30-32].
Overall, the fracture studies suggest that fracture has a lower heritability than BMD, particularly among the elderly. Thus, although it is clearly important to determine whether BMD-associated polymorphisms influence bone fragility (the ultimate question), the most powerful approach is likely to be initial screens targeting genes that affect BMD, with subsequent testing to determine the relevance of such genes to fracture. The obvious disadvantage of this approach is that if genes influence bone fragility and fracture risk independent of BMD, then this approach will not identify them. However, the evidence from BMD and fracture associated genes to date is that nearly all BMD-associated genes are also fracture associated.
What genes are known to cause osteoporosis?
Until the development of genome-wide association studies, researchers employed family-based linkage techniques and conducted candidate gene association studies in their valiant attempts to identify osteoporosis genes. Monogenic skeletal diseases affecting BMD are summarized in Table 1; genetic associations with general community BMD are summarized elsewhere [33]. As was the general experience with these approaches in other complex genetic diseases, the signal-to-noise ratio was not sufficient to permit robust identification of any particular genes involved, with one notable exception – the gene encoding lipoprotein-related receptor protein 5 (LRP5).
Table 1.
Major monogenic high and low bone mass syndromes
| Gene | Encoded protein | Bone disorder | OMIM ref. |
| COL1A1 | Col1a1 chain of type 1 collagen | Osteogenesis imperfecta | 166200 |
| COL1A2 | Col1a2 chain of type 1 collagen | Osteogenesis imperfecta | 120160 |
| CRTAP | Cartilage associated protein | Osteogenesis imperfecta type VII | 605497 |
| LEPRE1 | Prolyl 3-hydroxylase | Osteogenesis imperfecta type VIII | 610339 |
| PLOD2 | Lysyl hydroxylase-2 | Bruck syndrome (osteogenesis imperfecta with joint contractures) type 2 | 601865 |
| CA2 | carbonic anhydrase II | Osteopetrosis (autosomal recessive) | 611492 |
| TCIRG1 | Vacuolar proton pump | Osteopetrosis (autosomal recessive) | 604592 |
| CLCN7 | Chloride channel 7 | Osteopetrosis (both autosomal recessive and autosomal dominant forms) | 602727 |
| OSTM1 | Osteopetrosis-related transmembrane protein 1 | Osteopetrosis (autosomal recessive) | 607649 |
| LRP5 | Low density lipoprotein-receptor related protein 5 | Osteoporosis-pseudoglioma syndrome | 603506 |
| LRP5 | Low density lipoprotein-receptor related protein 5 | High bone mass syndrome | |
| SOST | Inhibitor of Wnt signalling to osteoblasts | von Buchem disease and sclerosteosteosis | 605740 |
| OPG (TNFRSF11B) | Osteoprotegerin | Juvenile Paget's disease (hereditary hyperphosphatasia) | 602643 |
| RANK (TNFRSF11A) | RANK | Familial expansile osteolysis | 603499 |
| ALPL | Tissue-nonspecific (bone/liver/kidney) alkaline phosphatase | Hypophosphatasia | 171760 |
| CASR | Calcium-sensing receptor | Neonatal hyperparathyroidism | 601199 |
| CTSK | Cathepsin K | Pyknodysostosis | 601105 |
The role played by this gene in bone was first identified from rare monogenic diseases, using classical linkage approaches followed by fine mapping and candidate gene screening. Inactivating mutations cause the autosomal-recessive condition osteoporosis-pseudoglioma, with low BMD observed in obligate carriers [34]. Activating mutations result in the autosomal-dominant conditions of high bone mass syndrome [35,36]. Subsequent studies rapidly demonstrated that the gene played a significant role in the general population [37,38], a finding also confirmed in Asian populations [39-41]. Association was also observed with fracture risk [42,43]. The association of LRP5 with bone density is apparent even in childhood, indicating a likely effect on bone accrual [37,44]. Carriers of LRP5 variants have BMD 0.17 to 0.57 standard deviations away from the population mean [45,46].
As discussed below, two studies [46,47] recently demonstrated association of LRP5 with BMD, achieving genome-wide significance (P < 10-7). The importance of these studies is not just in confirming the significance of LRP5, which was already established: rather, they serve as proof-of-concept that whole-genome-wide association approaches successfully identify quantitative trait loci that underlie BMD and osteoporosis.
These genetic findings have stimulated major research programs into the LRP5/Wnt signaling pathway as a major pathway in skeletal development and as a potential therapeutic target for osteoporosis. Of particular interest are treatments targeting sclerostin (encoded by the gene SOST), which is thought to inhibit LRP5. Mutations in SOST cause a high bone mass syndrome and van Buchem disease, which is a form of osteopetrosis with low fracture risk [48,49]. Common polymorphisms of SOST have also been demonstrated to be associated with general population variation in BMD [45,50], although this has been less well studied than LRP5. Anti-sclerostin antibodies are currently in clinical trials and are showing promise as anabolic agents in osteoporosis. Thus, new therapeutic modalities are already in place as a direct consequence of genetic research in osteoporosis.
A large number of other candidate genes have been implicated in one study or another as being associated with osteoporosis. Many of these are likely to be true positive findings, but in our opinion few have sufficiently robust evidence to be considered 'established', without needing further confirmation. As such, their significance is currently hard to judge. Similarly, although several areas have been linked with BMD in family studies, in no case has the evidence of linkage been sufficiently strong as to be considered robust, and to date no clear candidate gene has been identified from this approach as contributing to BMD in the general population. Consequently, research in osteoporosis genetics has moved to the more powerful and comprehensive approach of genome-wide association studies to make progress.
Genome-wide association studies and osteoporosis
Several groups worldwide are currently performing genome-wide association studies in osteoporosis, mostly studying general population cohorts, particularly focusing on BMD. An early screen from the Framingham study [51] lacked sufficient marker density and statistical power, and no findings of genome-wide significance were reported. Two recent studies, examining larger cohorts and using denser marker sets, have been more successful.
deCODE Genetics [52] studied 5,861 men and women from the general population, initially testing more than 300,000 single nucleotide polymorphisms (SNPs), and then following up 74 SNPs in a further cohort of 7,925 Icelandic, Australian, and Danish individuals. Five regions were identified that achieved genome-wide significance for association with BMD. In two cases, these SNPs were in genes that are known to be involved in bone development or turnover, including RANKL (encoding receptor activator of nuclear factor-κ) and its antagonist OPG (encoding osteoprotegerin). Two novel regions included an area on chromosome 1p36 close to the gene ZBTB40 (encoding zinc finger and ETB domain containing 40) and, somewhat surprisingly, the major histocompatibility complex. Significant association was also seen near to ESR1 (encoding estrogen receptor-α), a gene previously associated with low BMD. However, all bar one of the associated markers lie not in ESR1 itself but in an open reading frame gene C6orf97, which is currently of unknown expression and function. This may prove to be the primary associated gene.
Notable results were also seen for SNPs in a number of other candidate genes previously studied in osteoporosis, although not achieving genome-wide significance in this study. These included SNPs in SOST, in the glucocorticoid receptor gene NR3C1 (in the top 500 BMD-associated SNPs overall), and in the vitamin D receptor gene and LRP5 (in the top 1,000 SNPs). It is therefore likely that other true osteoporosis-associated SNPs will be identified among these less strongly associated markers.
The study also investigated association with fracture, in a cohort including a total of 4,406 fracture cases and 36,785 control individuals [52]. No gene achieved genome-wide significance for fracture, but moderate levels of association (P = 10-3 to 10-4) were seen for the 1p36 region, the major histocompatibility complex, RANK, and two regions not initially detected through association with BMD, namely 2p16 and 11p11 (the latter containing the gene LRP4). When tested in the overall BMD cohort, these regions did achieve moderate level association with BMD (2p16, P = 8 × 10-7; LRP4, P = 3 × 10-4). Thus, no gene was identified to have significant association with fracture but not with BMD. This lends support to the approach of studying BMD as the primary phenotype.
One further point to note illustrates the importance of adequately powered studies of sufficient marker density. ESR1 variants associated with BMD were not associated with fracture; this is in disagreement with a prospective meta-analysis of 18,917 individuals performed by the GENOMOS consortium [53], which identified association with fracture but not BMD. The meta-analysis studied two intronic SNPs in ESR1, neither of which exhibited any association in the deCODE study. The difference in the findings probably relates to the low coverage of ESR1 genetic variation in the GENOMOS study, which was estimated at only about 30% [54].
The effect size of the fracture associated variants in the deCODE study [52] was small, with risk ratios ranging between 1.06 to 1.15. Individually, they are not of great use in prediction of fracture risk, which will probably require computation of risk from combinations of markers. The current capacity of these tests to predict fracture is illustrated in Figure 1. Using the findings from the discovery component of the study, we calculated the posterior probability of a fracture for allele carriers of the five SNPs most strongly associated with fracture (assuming a dominant model, Hardy-Weinberg equilibrium, and no interaction between markers [that their effects are additive]). This combination was associated with a likelihood ratio of fracture of 2.25 (the risk of fracture was increased by 2.25 in carriers of all five SNPs) and a likelihood of fracture of 0.75 in those who did not carry the SNPs. The combination of carriage of all five SNPs was expected to be present in 50% of fracture cases and 47.5% of control individuals, and thus is informative for a large proportion of the population. With increasing numbers of markers available, better predictive performance will be possible, although larger combinations of markers will be relevant to smaller numbers of people. How such genetic tests interact with traditional osteoporosis risk factors (such as BMD) has yet to be established.
Figure 1.
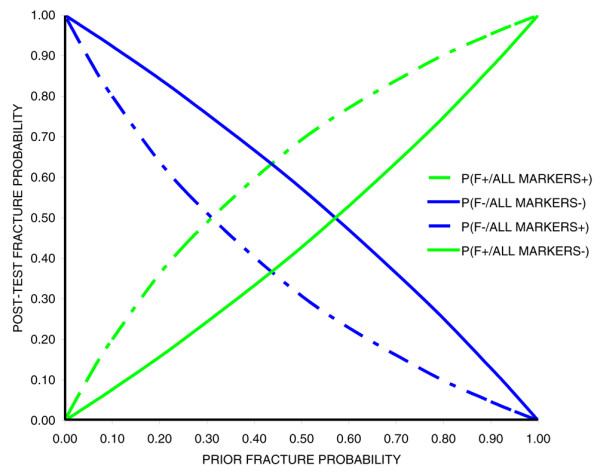
Fracture risk given genetic marker findings. Presented is the post-test probability of fracture given the pre-test risk and findings at five most strongly fracture-associated SNPs in deCODE osteoporosis genome-wide association study [52]. P(F+/MARKERS+) indicates the probability of fracture in carriers of all five SNP risk alleles. P(F-/MARKERS-) indicates the probability of no fracture in individuals negative for all five SNP risk alleles. P(F-/MARKERS+) indicates the probability of fracture in individuals negative for all five SNP risk alleles. P(F+/MARKERS-) indicates the probability of fracture in carriers of all five SNP risk alleles. SNP, single nucleotide polymorphism.
In the other genome-wide association study recently published [47], 2,094 twins from the TwinsUK cohort were examined, and then a two-phase replication study performed in further BMD cohorts (n = 4,877 total) and for association with fracture (n = 660 fractures, n = 6,639 nonfracture controls). Two genes reached genome-wide significance, namely LRP5 and OPG. Marginal fracture association was also observed (P = 0.006) in carriers of risk alleles at both genes, but the effect size of this association was large (odds ratio = 1.33) and combination common (22%), suggesting that it may be a useful prognostic test if the fracture association can be confirmed.
These studies illustrate the massive sample sizes required to identify osteoporosis genes, particularly if fracture is used as the study end-point. Studies of younger fracture cohorts are likely to be more fruitful, given the greater heritability suggested for hip fracture in younger cases, but these will be harder to recruit, because most fractures occur in older age cohorts. The small effect size seen with the fracture-associated variants indicates that future studies will need to be adequately powered to detect variants with odds ratios lower than those observed here, and will thus need to be extremely large. For example, assuming an equal number of cases and controls, an SNP with minor allele frequency of 0.25 in linkage disequilibrium with a fracture-associated SNP with D' = 0.8, and a statistical threshold for significance of P = 10-7, for a marker with an additive relative risk of 1.1 more than 17,000 fracture cases will be required. The genetic assumptions underlying this calculation are actually optimistic. Much larger numbers will be required if the associated variants are less common, if the mapping SNP and fracture-associated SNP have different allele frequencies, or if gene-gene interactions are involved. In reality, the numbers required will be beyond the reach of individual studies, and large consortia and meta-analytical methods will be required to achieve adequate power. The genetics community is well aware of this, and the recently established European Union funded 'Genetic Factors in Osteoporosis' (GEFOS) consortium has rapidly established itself as the central organization for such efforts worldwide.
Future directions
There is little doubt that genome-wide association studies will identify more genes that are involved in bone fragility than those that have been reported so far. Genome-wide association analysis in unselected populations has proven to be a powerful method with which to identify common genes of moderate effect size. The studies performed to date are not sufficiently powered to identify genes of smaller population effect, and may not identify some forms of human genetic variation that are likely to influence bone fragility. No single approach is likely to identify all bone fragility genes, and a variety of different methods are either being developed or are in use to tackle the problem.
The usual mantra for complex diseases is that larger studies are needed and are likely to make many further contributions to what we know. Size isn't everything, however; it is equally likely that more efficient study designs of selected cohorts aimed at maximizing the power to detect association will make further significant discoveries, and at considerably lower genotyping cost than simply increasing the sample size. In particular, cohorts recruited to minimize genetic heterogeneity are likely to be valuable. The genetic control over skeletal development is known to vary between sites and sexes, and it is likely that genes make different contributions at different ages. Thus, cohorts recruited to investigate osteoporosis genetics focusing on a particular site, age, and sex are likely to have greater power to identify genes than studies of cohorts recruited unselected from the general population. Our group recently demonstrated this with a proof-of-principle study [45], which easily confirmed the known association of LRP5 with BMD in a cohort of just 320 postmenopausal women selected for extreme BMD at the hip.
Meta-analysis may also produce findings that individual screens have missed. Although in the past competition between groups hindered data sharing for meta-analysis, there is a solid recognition among osteoporosis researchers that collaboration and open data sharing will be essential both for gene discovery and for replication.
Genetics research is technology driven. Genome-wide association analysis was made possible by chip-based SNP genotyping technology. A further genetics revolution is being brought about by the development of next-generation sequencers capable of producing up to 20 gigabases per run, which has reignited interest in monogenic diseases. It is likely that in a high proportion of individuals with extreme phenotypes (such as extreme high or low BMD in humans) a monogenic – usually rare – mutation underlies their extreme phenotype, as has been demonstrated, for example, with osteogenesis imperfecta type 1 and Marfan's syndrome. When there were not enough family members to help localize the gene by traditional linkage methods, or the individual did not fit a known syndrome that would allow population studies to be conducted, the mutations in these cases could not be identified. With the new sequencing capacity it will be possible to sequence extremely large proportions of the genome (such as, for example, all exons) in a single sequencing run. Such cases may be studied again, and it is highly likely that new disease genes affecting bone fragility will be identified, not just of relevance to these extreme phenotypes but also to control of BMD in the general population.
Two further influences on human variation that have yet to be addressed significantly in osteoporosis include copy number variation (CNV) and gene-gene interaction. CNV is known to be common throughout the genome and is likely to influence gene expression. High-throughput, accurate genotyping methods for CNV are still in development, but array-based methods show promise. Gene-gene interaction is known from mouse models to influence skeletal development significantly [55] and is thus likely also to contribute to human skeletal development. All genome-wide association studies to date have been single-marker studies, but it is likely that once sufficient cases have been screened, more complex genetic models will be tested.
Mouse genetics to date has contributed much to what we know about the genetic epidemiology of bone fragility and associated phenotypes, such as BMD and bone microarchitecture. Hypothesis-free gene mapping of bone fragility genes has made slow progress though. Congenic approaches, investigating the genetic causes of differences in bone parameters between inbred mouse strains, has yielded some success in the identification of Alox12, implicating the lipoxygenase system in osteoporosis [56]. However, the inbred nature of the mice restricts the mapping resolution that can be obtained, and most established linkages with bone parameters have not resulted in identification of the causative gene. An alternate approach is ENU mutagenesis, in which male mice are treated with the alkylating agent ethinyl-nitrosourea, causing point mutations in sperm DNA. Offspring of these mice carry these mutations. By screening thousands of offspring of mutagenized mice, mice with phenotypes generally caused by monogenic point mutations caused by the ENU can be identified. These monogenic variants are much easier to map than congenic genes, and because the mutations concerned are not as severe as knock-out or knock-in methods, the models are more physiological. This approach is being used by a number of groups worldwide to create new mouse models of osteoporosis.
Conclusion
This is a time of great excitement in the world of genetics generally, and in osteoporosis genetics specifically. The publication of the Wellcome Trust Case Control Consortium (less than 12 months ago at the time of writing) [57] was not only an enormous leap forward in identifying that genes that underlie complex genetic disorders such as inflammatory bowel disease, ankylosing spondylitis, and type 1 diabetes. It also provided proof that the approach adopted was going to work for other complex quantitative traits such as osteoporosis. The success of early genome-wide association studies in osteoporosis supports this position. Already, these studies have identified novel pathways that contribute to control of BMD and bone fragility with possible therapeutic targets. The possibility of genetic prognostic tests, adding to existing predictive information from BMD, is likely to become a reality within the next decade. Hopefully, the frustrations of the past few decades have taught the genetics community that careful phenotyping, sophisticated study design, adequately powered cohorts, and collaboration are key elements to successful gene identification. We have finished with the beginning and can now see ways and means to achieve a successful future.
Abbreviations
BMD: bone mineral density; CNV: copy number variation; ENU: ethinyl-nitrosourea; LRP5: lipoprotein-related receptor protein 5; SNP: single nucleotide polymorphism.
Competing interests
The authors declare that they have no competing interests.
References
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Hormone Res. 2000;54(suppl 1):58–63. doi: 10.1159/000063449. [DOI] [PubMed] [Google Scholar]
- Chang KP, Center JR, Nguyen TV, Eisman JA. Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 2004;19:532–536. doi: 10.1359/JBMR.040109. [DOI] [PubMed] [Google Scholar]
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, Jonsson B, Oden A, Johansson H, De Laet C. The burden of hospitalised fractures in Sweden. Osteoporos Int. 2005;16:222–228. doi: 10.1007/s00198-004-1686-2. [DOI] [PubMed] [Google Scholar]
- Slemenda CW, Turner CH, Peacock M, Christian JC, Sorbel J, Hui SL, Johnston CC. The genetics of proximal femur geometry, distribution of bone mass and bone mineral density. Osteoporos Int. 1996;6:178–182. doi: 10.1007/BF01623944. [DOI] [PubMed] [Google Scholar]
- Arden NK, Baker J, Hogg C, Baan K, Spector TD. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. J Bone Miner Res. 1996;11:530–534. doi: 10.1002/jbmr.5650110414. [DOI] [PubMed] [Google Scholar]
- Koller DL, Liu G, Econs MJ, Hui SL, Morin PA, Joslyn G, Rodriguez LA, Conneally PM, Christian JC, Johnston CC, Jr, Foroud T, Peacock M. Genome screen for quantitative trait loci underlying normal variation in femoral structure. J Bone Miner Res. 2001;16:985–991. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- Flicker L, Faulkner KG, Hopper JL, Green RM, Kaymacki B, Nowson CA, Young D, Wark JD. Determinants of hip axis length in women aged 10–89 years: a twin study. Bone. 1996;18:41–45. doi: 10.1016/8756-3282(95)00418-1. [DOI] [PubMed] [Google Scholar]
- Deng HW, Mahaney MC, Williams JT, Li J, Conway T, Davies KM, Li JL, Deng H, Recker RR. Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet Epidemiol. 2002;22:12–25. doi: 10.1002/gepi.1040. [DOI] [PubMed] [Google Scholar]
- Slemenda CW, Christian JC, Williams CJ, Norton JA, Johnston CC., Jr Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res. 1991;6:561–567. doi: 10.1002/jbmr.5650060606. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass, and fat mass: same genes or same environments? Am J Epidemiol. 1998;147:3–16. doi: 10.1093/oxfordjournals.aje.a009362. [DOI] [PubMed] [Google Scholar]
- Harris M, Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Genetic and environmental correlations between bone formation and bone mineral density: a twin study. Bone. 1998;22:141–145. doi: 10.1016/S8756-3282(97)00252-4. [DOI] [PubMed] [Google Scholar]
- Pocock N, Eisman J, Hopper J, Yeates M, Sambrook P, Eberl S. Genetic determinants of bone mass in adults. A twin study. J Clin Invest. 1987;80:706–710. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen R, Jouanny P, Guillemin F, Kuntz C, Pourel J, Siest G. Segregation analysis and variance components analysis of bone mineral density in healthy families. J Bone Miner Res. 1995;10:2017–2022. doi: 10.1002/jbmr.5650101223. [DOI] [PubMed] [Google Scholar]
- Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8:1–9. doi: 10.1002/jbmr.5650080102. [DOI] [PubMed] [Google Scholar]
- Duncan E, Cardon L, Sinsheimer J, Wass J, Brown M. Site and gender specificity of inheritance of bone mineral density. J Bone Miner Res. 2003;18:1531–1538. doi: 10.1359/jbmr.2003.18.8.1531. [DOI] [PubMed] [Google Scholar]
- Deng HW, Livshits G, Yakovenko K, Xu FH, Conway T, Davies KM, Deng H, Recker RR. Evidence for a major gene for bone mineral density/content in human pedigrees identified via probands with extreme bone mineral density. Ann Hum Genet. 2002;66:61–74. doi: 10.1017/S0003480001008958. [DOI] [PubMed] [Google Scholar]
- Livshits G, Deng HW, Nguyen TV, Yakovenko K, Recker RR, Eisman JA. Genetics of bone mineral density: evidence for a major pleiotropic effect from an intercontinental study. J Bone Miner Res. 2004;19:914–923. doi: 10.1359/JBMR.040132. [DOI] [PubMed] [Google Scholar]
- Cardon L, Garner C, Bennett S, Mackay I, Edwards R, Cornish J, Hegde M, Murray M, Reid I, Cundy T. Evidence for a major gene for bone mineral density in idiopathic osteoporotic families. J Bone Miner Res. 2000;15:1132–1137. doi: 10.1359/jbmr.2000.15.6.1132. [DOI] [PubMed] [Google Scholar]
- Ginsburg E, Skaric-Juric T, Kobyliansky E, Karasik D, Malkin I, Rudan P. Evidence on major gene control of cortical index in pedigree data from Middle Dalmatia, Croatia. Am J Hum Biol. 2001;13:398–408. doi: 10.1002/ajhb.1064. [DOI] [PubMed] [Google Scholar]
- Cohen Z, Kalichman L, Kobyliansky E, Malkin I, Almog E, Livshits G. Cortical index and size of hand bones: segregation analysis and linkage with the 11q12-13 segment. Med Sci Monit. 2003;9:MT13–MT20. [PubMed] [Google Scholar]
- Deng HW, Xu FH, Huang QY, Shen H, Deng H, Conway T, Liu YJ, Liu YZ, Li JL, Zhang HT, Davies KM, Recker RR. A whole-genome linkage scan suggests several genomic regions potentially containing quantitative trait loci for osteoporosis. J Clin Endocrinol Metab. 2002;87:5151–5159. doi: 10.1210/jc.2002-020474. [DOI] [PubMed] [Google Scholar]
- Pelat C, Van Pottelbergh I, Cohen-Solal M, Ostertag A, Kaufman JM, Martinez M, de Vernejoul MC. Complex segregation analysis accounting for GxE of bone mineral density in European pedigrees selected through a male proband with low BMD. Ann Hum Genet. 2007;71:29–42. doi: 10.1111/j.1469-1809.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Fox KM, Cummings SR, Powell-Threets K, Stone K. Family history and risk of osteoporotic fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1998;8:557–562. doi: 10.1007/s001980050099. [DOI] [PubMed] [Google Scholar]
- Deng HW, Chen WM, Recker S, Stegman MR, Li JL, Davies KM, Zhou Y, Deng H, Heaney R, Recker RR. Genetic determination of Colles' fracture and differential bone mass in women with and without Colles' fracture. J Bone Miner Res. 2000;15:1243–1252. doi: 10.1359/jbmr.2000.15.7.1243. [DOI] [PubMed] [Google Scholar]
- Kannus P, Palvanen M, Kaprio J, Parkkari J, Koskenvuo M. Genetic factors and osteoporotic fractures in elderly people: prospective 25 year follow up of a nationwide cohort of elderly Finnish twins. BMJ. 1999;319:1334–1337. doi: 10.1136/bmj.319.7221.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor A, Snieder H, Spector TD. Genetic factors and osteoporotic fractures in elderly people. Twin data support genetic contribution to risk of fracture. BMJ. 2000;320:1669–1670. doi: 10.1136/bmj.320.7250.1669. author reply 1670-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew T, Antioniades L, Scurrah KJ, Macgregor AJ, Spector TD. Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J Bone Miner Res. 2005;20:67–74. doi: 10.1359/JBMR.041015. [DOI] [PubMed] [Google Scholar]
- Michaelsson K, Melhus H, Ferm H, Ahlbom A, Pedersen NL. Genetic liability to fractures in the elderly. Arch Intern Med. 2005;165:1825–1830. doi: 10.1001/archinte.165.16.1825. [DOI] [PubMed] [Google Scholar]
- Keen RW, Hart DJ, Arden NK, Doyle DV, Spector TD. Family history of appendicular fracture and risk of osteoporosis: a population-based study. Osteoporos Int. 1999;10:161–166. doi: 10.1007/s001980050211. [DOI] [PubMed] [Google Scholar]
- Seeman E, Hopper JL, Bach LA, Cooper ME, Parkinson E, McKay J, Jerums G. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med. 1989;320:554–558. doi: 10.1056/NEJM198903023200903. [DOI] [PubMed] [Google Scholar]
- Seeman E, Tsalamandris C, Formica C, Hopper JL, McKay J. Reduced femoral neck bone density in the daughters of women with hip fractures: the role of low peak bone density in the pathogenesis of osteoporosis. J Bone Miner Res. 1994;9:739–743. doi: 10.1002/jbmr.5650090520. [DOI] [PubMed] [Google Scholar]
- Liu YZ, Liu YJ, Recker RR, Deng HW. Molecular studies of identification of genes for osteoporosis: the 2002 update. J Endocrinol. 2003;177:147–196. doi: 10.1677/joe.0.1770147. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/S0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden L, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick M, Wu D, Insogna K, Lifton R. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, Rizzoli R, Antonarakis SE. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet. 2004;74:866–875. doi: 10.1086/420771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay MA, Woon PY, Zhang Y, Miles LJ, Duncan EL, Ralston SH, Compston JE, Cooper C, Keen R, Langdahl BL, MacLelland A, O'Riordan J, Pols HA, Reid DM, Uitterlinden AG, Wass JA, Brown MA. Influence of LRP5 polymorphisms on normal variation in BMD. J Bone Miner Res. 2004;19:1619–1627. doi: 10.1359/JBMR.040704. [DOI] [PubMed] [Google Scholar]
- Koh JM, Jung MH, Hong JS, Park HJ, Chang JS, Shin HD, Kim SY, Kim GS. Association between bone mineral density and LDL receptor-related protein 5 gene polymorphisms in young Korean men. J Korean Med Sci. 2004;19:407–412. doi: 10.3346/jkms.2004.19.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi T, Furuta I, Watanabe Y, Tsukamoto K, Tomita H, Tsujihata M, Ohta T, Kishino T, Matsumoto N, Minakami H, Niikawa N, Yoshiura K. LRP5, low density lipoprotein receptor related protein 5, is a determinant for bone mineral density. J Hum Genet. 2004;49:80–86. doi: 10.1007/s10038-003-0111-6. [DOI] [PubMed] [Google Scholar]
- Zhang ZL, Qin YJ, He JW, Huang QR, Li M, Hu YQ, Liu YJ. Association of polymorphisms in low-density lipoprotein receptor-related protein 5 gene with bone mineral density in postmenopausal Chinese women. Acta Pharmacol Sin. 2005;26:1111–1116. doi: 10.1111/j.1745-7254.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- Bollerslev J, Wilson SG, Dick IM, Islam FM, Ueland T, Palmer L, Devine A, Prince RL. LRP5 gene polymorphisms predict bone mass and incident fractures in elderly Australian women. Bone. 2005;36:599–606. doi: 10.1016/j.bone.2005.01.006. [DOI] [PubMed] [Google Scholar]
- van Meurs JB, Rivadeneira F, Jhamai M, Hugens W, Hofman A, van Leeuwen JP, Pols HA, Uitterlinden AG. Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res. 2006;21:141–150. doi: 10.1359/JBMR.050904. [DOI] [PubMed] [Google Scholar]
- Koay MA, Tobias JH, Leary SH, Steer CD, Brown MA. The effect of LRP5 polymorphisms on bone mineral density is apparent in childhood. Calcif Tissue Int. 2007;81:1–9. doi: 10.1007/s00223-007-9024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims AM, Shephard N, Carter K, Doan T, Dowling A, Duncan EL, Eisman J, Jones G, Nicholson G, Prince R, Seeman E, Thomas G, Wass JA, Brown MA. Genetic analyses in a sample of individuals with high or low bone density demonstrates association with multiple Wnt pathway genes. J Bone Miner Res. 2007;23:499–506. doi: 10.1359/jbmr.071113. [DOI] [PubMed] [Google Scholar]
- vvan Meurs JB, Trikalinos TA, Ralston SH, Balcells S, Brandi ML, Brixen K, Kiel DP, Langdahl BL, Lips P, Ljunggren O, Lorenc R, Obermayer-Pietsch B, Ohlsson C, Pettersson U, Reid DM, Rousseau F, Scollen S, Van Hul W, Agueda L, Akesson K, Benevolenskaya LI, Ferrari SL, Hallmans G, Hofman A, Husted LB, Kruk M, Kaptoge S, Karasik D, Karlsson MK, Lorentzon M, et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:1277–1290. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD. Bone mineral density, osteoporosis, and osteoporotic fractures: a genomewide association study. Lancet. 2008;371:1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, Brown A, Gardner JC, Galas D, Schatzman RC, Beighton P, Papapoulos S, Hamersma H, Brunkow ME. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitterlinden AG, Arp PP, Paeper BW, Charmley P, Proll S, Rivadeneira F, Fang Y, van Meurs JB, Britschgi TB, Latham JA, Schatzman RC, Pols HA, Brunkow ME. Polymorphisms in the sclerosteosis/van Buchem disease gene (SOST) region are associated with bone-mineral density in elderly whites. Am J Hum Genet. 2004;75:1032–1045. doi: 10.1086/426458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel DP, Demissie S, Dupuis J, Lunetta KL, Murabito JM, Karasik D. Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet. 2007;8(suppl 1):S14. doi: 10.1186/1471-2350-8-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Center JR, Nguyen TV, Bagger Y, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K. Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008;358:2355–2365. doi: 10.1056/NEJMoa0801197. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Ralston SH, Bennett ST, Brandi ML, Grinberg D, Karassa FB, Langdahl B, van Meurs JB, Mosekilde L, Scollen S, Albagha OM, Bustamante M, Carey AH, Dunning AM, Enjuanes A, van Leeuwen JP, Mavilia C, Masi L, McGuigan FE, Nogues X, Pols HA, Reid DM, Schuit SC, Sherlock RE, Uitterlinden AG, GENOMOS Study Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA. 2004;292:2105–2114. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- Vilarino-Guell C, Brown MA. Bigger is better, but it's not just size that counts: the estrogen receptor gene and osteoporosis. BoneKEy-Osteovision. 2005;2:14–20. [Google Scholar]
- Koller DL, Liu L, Alam I, Sun Q, Econs MJ, Foroud T, Turner CH. Epistatic effects contribute to variation in bone density in Fischer 344 × Lewis F2 rats. J Bone Miner Res. 2008;23:41–47. doi: 10.1359/jbmr.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science. 2004;303:229–232. doi: 10.1126/science.1090985. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]


