Abstract
Communication between cell surface proteins and the nucleus is integral to many cellular adaptations. In the case of ion channels in excitable cells, the dynamics of signaling to the nucleus are particularly important because the natural stimulus, surface membrane depolarization, is rapidly pulsatile. To better understand excitation–transcription coupling we characterized the dependence of cAMP response element–binding protein phosphorylation, a critical step in neuronal plasticity, on the level and duration of membrane depolarization. We find that signaling strength is steeply dependent on depolarization, with sensitivity far greater than hitherto recognized. In contrast, graded blockade of the Ca2+ channel pore has a remarkably mild effect, although some Ca2+ entry is absolutely required. Our data indicate that Ca2+/CaM-dependent protein kinase II acting near the channel couples local Ca2+ rises to signal transduction, encoding the frequency of Ca2+ channel openings rather than integrated Ca2+ flux—a form of digital logic.
Introduction
In excitable cells, voltage-dependent Ca2+ channels perform the important task of coupling membrane depolarization to diverse biological responses, including muscle contraction, secretion, and gene expression (Hille, 2001). Much is known about relationships between channel activation and biological output in excitation–contraction (E-C) and excitation–secretion (E-S) coupling. For example, E-C and E-S coupling both occur close to the channel, on a millisecond time scale. Yet, although coupling to muscle contraction and neurotransmitter release share the generic feature of a steeply cooperative relationship between Ca2+ channel activation and functional response, their dependence on Ca2+ flux differs (for reviews see Schneider, 1994; Schneggenburger and Neher, 2005). Skeletal E-C coupling shows little or no dependence on Ca2+ influx (Armstrong et al., 1972; Schneider and Chandler, 1973; Rios and Brum, 1987; Beam and Franzini-Armstrong, 1997; Franzini-Armstrong and Protasi, 1997), whereas E-S coupling is exquisitely sensitive to the magnitude of Ca2+ entry (Dodge and Rahamimoff, 1967; Llinas et al., 1981; Augustine et al., 1985; Bollmann et al., 2000; Schneggenburger and Neher, 2000; Sudhof, 2004).
Unlike E-C and E-S coupling, excitation–transcription (E-T) coupling has not been so quantitatively examined, possibly because Ca2+ channel activation and the final event are widely separated spatially, temporally, and methodologically. First, induction of gene transcription often involves local signaling near the plasma membrane, but culminates in responses in the nucleus, up to tens of micrometers away. Second, brief cell depolarization may drive gene expression minutes to hours later. Third, the initiating event in E-T coupling is electrophysiological but the final outcome is a biochemical response, typically studied in populations of cells. In neurons, the most extensively studied example of E-T coupling is signaling to the transcription factor cAMP response element–binding protein (CREB) via phosphorylation at Ser133, which is critical for CRE-mediated gene expression and many adaptive changes in neurons (Lonze and Ginty, 2002; Carlezon et al., 2005). L-type Ca2+ channels play an advantaged role in such signaling (Murphy et al., 1991; West et al., 2002; Deisseroth et al., 2003; Dolmetsch, 2003). The advantage arises because L-type channels have private access to local Ca2+-dependent signaling machinery (Deisseroth et al., 1996; Dolmetsch et al., 2001; Weick et al., 2003; Zhang et al., 2005). Thus, key initial events in E-T coupling may occur near the channel, just as in E-C and E-S coupling.
Considerable uncertainty surrounds functional and molecular aspects of downstream events that mediate signaling from activated L-type channels to the nucleus. CaM basally tethered to L-type channels (Zuhlke et al., 1999) seems essential for MAPK signaling to CREB in response to prolonged depolarization (Dolmetsch et al., 2001). However, whether L-type channels enlist this resident CaM to signal to CREB after brief depolarization is unclear, as are the nature of rapid coupling between depolarization and CREB phosphorylation and the identity of downstream molecular mechanisms.
In addressing unanswered questions, it is worthwhile to note classical studies on E-C and E-S coupling, some dating back half a century (Hodgkin and Horowicz, 1960; Katz and Miledi, 1967; Armstrong et al., 1972; Chapman and Tunstall, 1981; Llinas et al., 1981; Augustine et al., 1985; for reviews see Schneider, 1994; Augustine, 2001; Schneggenburger and Neher, 2005). Such studies addressed generic questions that remain for E-T coupling. How local are the signaling events immediately downstream of Ca2+ channel activation? How rapidly is the biochemical sensor engaged? What is the stimulus-response input–output relationship? How steeply does the coupling depend on depolarization, channel gating, and Ca2+ influx? We approached these fundamental issues using a method to assess signaling strength in terms of the dynamics of the Ser133-phospho-CREB (pCREB) response. Our findings revealed that E-T coupling depends on Ca2+ channel activation in a steeply cooperative manner, following a nearly cubic power law, so that small voltage changes produce large changes in signal strength. This steepness arises from changes in L-type channel open probability (Po), not simply total Ca2+ influx per se, indicating that incoming Ca2+ drives a local sensor to near saturation. Several lines of evidence implicated Ca2+/CaM-dependent protein kinase II (CaMKII) as the integrator of channel activity that signals to CREB. Like CREB phosphorylation, local CaMKII activity requires L-type channel gating, yet is only mildly dependent on the magnitude of Ca2+ flux. This coupling mechanism provides a form of digital logic wherein depolarizations leading to CREB phosphorylation are encoded in the frequency of Ca2+ channel openings rather than the integrated Ca2+ flux.
Results
Signaling to nuclear CREB in rat superior cervical ganglion (SCG) neurons
Rat SCG cultures are widely used to study many aspects of neuronal function, including Ca2+ channel properties and intracellular Ca2+ dynamics (Adams and Brown, 1973; Mains and Patterson, 1973; Hille, 1994). We previously found that depolarizing SCG neurons results in L-type Ca2+ channel–dependent phosphorylation of CREB and CRE-mediated gene expression (Wheeler and Cooper, 2001; Wheeler et al., 2006). Because SCG cultures contain a homogenous population of neurons that do not form functional synapses (O'Lague et al., 1978), raising extracellular [K+] produces a stable and reproducible depolarization (Wheeler et al., 2006), in essence providing a high-throughput voltage clamp. Thus, we could relate biochemical responses (signaling to nuclear CREB), averaged over many neurons, with electrophysiological and Ca2+ imaging measurements obtained from individual neurons.
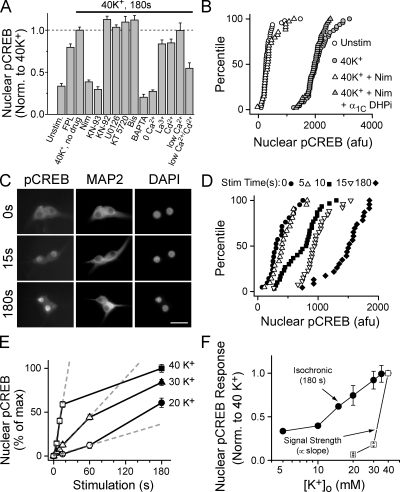
Basic features of signaling in SCG neurons are illustrated in Fig. 1 (A and B). Depolarization with 40 mM K+ for 3 min led to nuclear pCREB in every neuron (Fig. 1 B). This signaling required Ca2+ influx, as it was not seen with a Ca2+-free solution or after preloading with the Ca2+ chelator BAPTA (Fig. 1 A). The L-type channel antagonist nimodipine blocked signaling to CREB (Fig. 1 A), and signaling was rescued by adenoviral-mediated gene transfer of a nimodipine-insensitive L-type channel (Fig. 1 B). FPL 64176, which specifically increases the Po of L-type channels (Kunze and Rampe, 1992; Liu et al., 2003) drove signaling even in the absence of depolarization (i.e., in 5 mM K+), consistent with the idea that signaling to CREB is highly sensitive to activation of L-type channels. CREB phosphorylation by 40 mM K+ was unaffected by blocking CaV2 (N and P/Q type) channels (unpublished data), the majority class in SCG neurons (Hirning et al., 1988). With regard to biochemical pathways, the Ca2+/CaM-dependent kinase (CaMK) inhibitor KN93 (but not its inactive congener KN92) blocked signaling, whereas inhibitors of MEK, PKA, and PKC (U0126, KT5720, and bisindolylmaleimide I, respectively) were without effect (Fig. 1 A).
Figure 1.
Strength of signaling to CREB in SCG neurons: dependence on activation of L-type Ca2+ channels and a CaM kinase pathway. (A) Nuclear pCREB levels after a 3-min, 40-mM K+ or FPL 64176 stimulation. Drugs (KN-93, CaMK inhibitor; KN-92, inactive congener of KN-93; U0126, MEK inhibitor; KT 5720, PKA inhibitor; Bis [bisindolylmaleimide I], PKC inhibitor) were added 1 min before stimulation. Some cultures were preloaded with 100 μM BAPTA-AM for 30 min at 37°C, stimulated in 0 mM of extracellular Ca2+ and EGTA (0 Ca2+) or 100 μM Ca2+ and no EGTA (low Ca2+), and/or stimulated in 200 μM La3+ or Cd2+ after preincubation for 1 s. Data are from two or more experiments of >40 neurons each. (B) Cumulative histograms of pCREB levels in individual neurons from a representative experiment. Cells stimulated for 3 min as indicated. Infecting cells with adenovirus expressing dihydropyridine-insensitive L-type Ca2+ channel (DHPi) rescued signaling to CREB. (C–E) Data from a representative experiment. Cells were either mock stimulated in 5-mM K+ Tyrode's (t = 0) or stimulated with solutions containing 20, 30, or 40 mM K+ for the time indicated, placed back in 5 mM K+ solution, and then fixed at 3 min. (C) 5–d in vitro SCG neurons stained for pCREB and MAP2; nuclei counterstained with DAPI. Bar, 50 μm. (D) Cumulative histograms of pCREB levels from individual neurons. (E) Mean pCREB levels plotted against stimulation time; [K+] as indicated. Dashed lines are linear fits of initial data points; slope used as index of CREB signal strength. (F) Isochronic nuclear pCREB levels measured at 3 min (•); CREB signal strength (□), measured as in E. Isochronic data from three independent experiments, except the 10- and 15-mM K+ data are the mean of >15 cells from a single duplicate experiment. Error bars represent SEM.
With respect to Ca2+ channel flux, CREB phosphorylation was only mildly reduced by the Ca2+ flux inhibitors La3+ (200 μM, P < 0.01) and Cd2+ (200 μM, P < 0.05), despite blocking the majority of Ca2+ current. Further, reducing external Ca2+ to 100 μM failed to decrease the pCREB response (P > 0.9). However, when combined, Cd2+ and low Ca2+ dramatically reduced signaling (P < 0.005), which is consistent with competition between Ca2+ and Cd2+ for a high affinity pore binding site (Lansman et al., 1986; Chow, 1991; Yang et al., 1993; Ellinor et al., 1995). These data pointed toward an unexpected conclusion: although some Ca2+ flux is absolutely required for signaling, large reduction in Ca2+ entry only marginally affects signaling. These experiments focused on the steady-state response of pCREB after a prolonged stimulation. To gain insight into underlying mechanisms, we redirected our experiments to focus on the dynamics of signaling.
The relationship between pCREB and stimulus time as a readout of signal strength
Much as monitoring the rate of a biochemical reaction provides an index of enzymatic activity, tracking the development of pCREB provides a measure of relative signal strength in response to a given depolarization. We found that varying stimulation time resulted in graded levels of pCREB. Stimulation with 40 mM K+ for a few seconds, followed by an appropriate delay, resulted in a weak but clearly detectable pCREB signal in individual neurons (Fig. 1, C and D; and Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200805048/DC1; see Materials and methods); however, stimulation for 10 s or more largely saturated the pCREB response. In contrast, weaker stimuli (e.g., 20–30 mM K+) drove much slower signaling, although pCREB eventually approached the level attained with 40 mM K+ (Fig. 1 E). To estimate the strength of signaling, we determined the per-unit-time effect of a given stimulus by measuring the initial slope of the time-dependent increase in pCREB (Fig. 1 E, dashed gray lines). Defined in this way, CREB signal strength was a steep function of external [K+] (Fig. 1 F, open squares). Because of rapid saturation, measuring isochronic pCREB levels generated by prolonged depolarization (e.g., 3 min; Fig. 1 F, closed circles) did not reflect the true signaling strength. In contrast, tracking the initial slope of the increase in pCREB revealed a very dramatic change in output with small gradations in input, demonstrating the inherent sensitivity of the signaling system.
CREB signal strength is a steep function of membrane potential and Ca2+ channel function
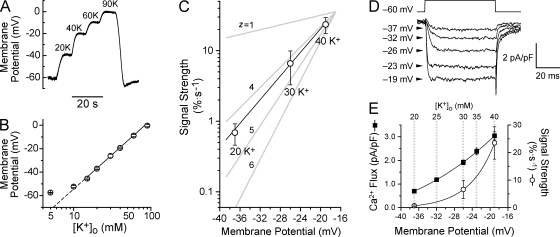
Because CREB signaling was steeply dependent on [K+], we related signal strength to both depolarization and channel activity. We measured membrane voltage in various K+ concentrations, using external solutions identical to those in the pCREB experiments. The resting potential in 5 mM K+ was approximately −60 mV (Fig. 2, A and B), which is consistent with previous studies (O'Lague et al., 1974; Wheeler et al., 2006). Exposure to 20, 30, and 40 mM K+ depolarized the cells to −37, −26, and −19 mV, respectively, following the Nernst relationship (Fig. 2 B). Plotting CREB signal strength against voltage (Fig. 2 C) revealed a steep relationship of 5.6 mV per e-fold change.
Figure 2.
CREB signal strength is a steep function of membrane potential and channel activity. (A) SCG neuron under current clamp in TTX. In 4 mM K+, the cell rested at −60 mV. Exposure to [K+] as indicated caused stepwise depolarizations. (B) Membrane potential plotted against [K+]o. Slope of linear fit is 58.1 mV per 10-fold change in [K+]o. (C) CREB signal strength, measured as in Fig. 1 E and in Materials and methods (five to eight experiments), plotted against voltage. 100% is the full change in pCREB from baseline to maximal levels. Linear fit shows that signal strength is steeply voltage dependent, as if governed by a gating particle with valence (z) between 4 and 5. (D) Voltage-clamp recordings with 2-mM Ca2+ Tyrode's as bathing solution. Pulse depolarizations to the voltages indicated. Traces are the mean of ten consecutive sweeps at each voltage. (E) Ca2+ flux (▪; as in D) and signal strength (○) plotted versus membrane potential. Solid lines are single exponential fits. Dashed lines indicate the voltages generated by each [K+]o (n = 5–8 experiments). Error bars represent SEM.
To relate CREB signal strength to channel activity, we measured Ca2+ currents in 2 mM Ca2+, at voltages corresponding to each particular [K+]. The mild depolarizations to the voltages indicated in Fig. 2 D elicited small but resolvable Ca2+currents with very little inactivation. Over the voltage range we examined (−37 to −19 mV), Ca2+ flux essentially follows the foot of a Boltzmann curve. Accordingly, Ca2+ flux displayed an exponential dependence on depolarization (Fig. 2 E), with an e-fold increase per 24.0 mV. This is milder than expected for a single species of Ca2+ channel, likely reflecting contributions of multiple L-type channels, including CaV1.3 (α1D) (Lin et al., 1996), which activates at more negative potentials than CaV1.2 (α1C) (Xu and Lipscombe, 2001).
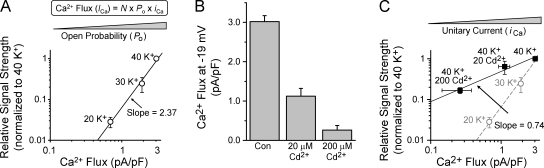
Ca2+ channel Po controls CREB signal strength
To examine further the relationship between CREB signal strength and channel activity, we plotted signal strength against total Ca2+ flux for stimulations with 20, 30, and 40 mM K+ (Fig. 3 A). On a log–log scale, the data were fit by a straight line with a slope of 2.37, indicating that CREB signal strength was proportional to [Ca2+ flux]2.37. When CREB signal strength was plotted against the nimodipine-sensitive portion of the flux, the steep relationship remained (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200805048/DC1), confirming that signal strength is steeply dependent on changes in L-type channel activity. The integrated Ca2+ flux through an individual channel is the product of channel Po and unitary Ca2+ flux (iCa). Voltage changes in the range we studied (–37 to −19 mV) will have little effect on iCa; thus, Po underlies the steeply increasing signal strength. However, the question remained whether signal strength is determined simply by total Ca2+ flux by itself or by underlying changes in channel gating.
Figure 3.
CREB signal strength is steeply dependent on channel Po but not Ca2+ flux per se. (A) Log–log plot of signal strength versus Ca2+ flux for the [K+]o indicated. Solid line shows the linear fit of the data. (B) Whole-cell Ca2+ flux (as in Fig. 2 D), in the presence of 0, 20, or 200 μM Cd2+. (C) CREB signal strength for 40 K+ with 0, 20, or 200 μM Cd2+ measured as described in Fig. 1 and Materials and methods; n = 3–6 experiments. The solid line is a linear fit of the Cd2+ data. For comparison, the dashed line and gray data points are reproduced from A. Error bars represent SEM.
Unlike depolarization, which acts on Po, Cd2+ affects iCa. In Ca2+, Cd2+ blocks and unblocks the channel pore on a microsecond scale, thereby decreasing integrated Ca2+ flux through the open channel (Lansman et al., 1986; Chow, 1991). This property is shared by high voltage–activated Ca2+ channels such as L- and N-type channels (Wakamori et al., 1998; Sather and McCleskey, 2003), which are the main Ca2+ channels expressed in cultured SCG neurons (Hirning et al., 1988). Cd2+ potently blocked whole-cell Ca2+ flux at −19 mV (Fig. 3 B) without affecting depolarization (unpublished data). We measured signaling to CREB in response to 40 mM K+ (−19 mV) with or without Cd2+ (added 1 s before stimulation and maintained throughout). Despite sharply reducing Ca2+ flux, Cd2+ had a surprisingly mild effect on CREB signal strength (Fig. 3 C); the regression line had a slope of 0.74, much shallower than the slope of 2.37 found for gradations in [K+]. This disparity is underscored by considering stimulation with 40 mM K+ in 200 μM Cd2+, which resulted in much less Ca2+ flux than stimulation with 20 mM K+ (P < 10−7), yet drove much stronger signaling (P < 0.005). Thus, the trickle of Ca2+ flux that remains in the presence of Cd2+ is sufficient to engage signaling to CREB. In contrast, when L-type channels were completely prevented from opening with nimodipine, signaling to CREB was virtually eliminated (Fig. 1 A). Together, these findings demonstrate that the relationship between depolarization and CREB signal strength is not a simple function of total Ca2+ flux, but is closely tied to voltage-dependent changes in Po.
We also manipulated iCa by lowering [Ca2+]o and obtained data similar to the Cd2+ results (Fig. 1 A). However, we consider the Cd2+ experiments to be more reliable because changing [Ca2+]o alters membrane surface potential (Hille, 2001) and evokes signaling through the Ca2+-sensing receptor (Vizard et al., 2008), effects that could complicate the interpretation.
Bulk intracellular [Ca2+] does not control signal strength
The steep dependence of signaling on Po but not iCa led us to examine how CREB signaling could be highly sensitive to the activation state of the channel, but not the size of the Ca2+ flux. An obvious candidate mechanism is conformational coupling between L-type Ca2+ channels and ryanodine receptors (RyRs), as in skeletal muscle E-C coupling (Beam and Franzini-Armstrong, 1997; Franzini-Armstrong and Protasi, 1997; De Crescenzo et al., 2006; for review see Schneider, 1994). However, the requirement for extracellular Ca2+ (Fig. 1 A) ruled out a strictly skeletal-like mechanism. In addition, blocking Ca2+-induced Ca2+ release from RyRs, a key step in cardiac E-C coupling (Stern, 1992; Cannell et al., 1995; Lopez-Lopez et al., 1995; Guatimosim et al., 2002; Bers and Guo, 2005), did not affect signaling to CREB (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200805048/DC1).
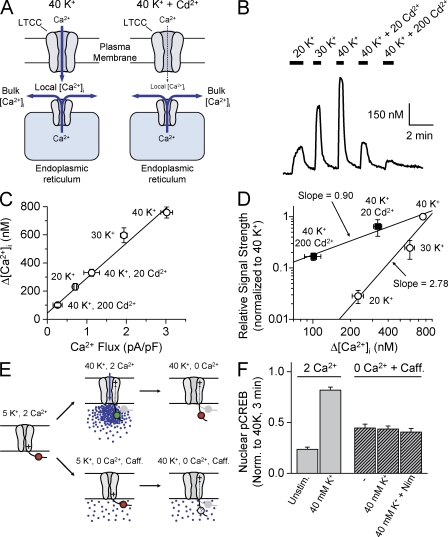
We next considered other mechanisms of Ca2+ release from internal stores, e.g., IP3-mediated Ca2+ release (Berridge, 1998). For example, a rise in local Ca2+ in the narrow space between the plasma membrane and the store membrane (Henkart, 1980; Lencesova et al., 2004) can trigger Ca2+ release from stores to elevate bulk [Ca2+]i (Cannell et al., 1995; Lopez-Lopez et al., 1995; Wang et al., 2001; Guatimosim et al., 2002). In this scenario, even if Ca2+ flux were reduced with Cd2+, the local Ca2+ rise might still trigger Ca2+ release from stores, and signal strength would simply be a function of bulk [Ca2+]i (Fig. 4 A).
Figure 4.
Bulk [Ca2+]i is neither necessary nor sufficient to dictate CREB signal strength. (A) Despite Cd2+ effects on Ca2+ flux, release from intracellular stores might maintain bulk [Ca2+]. (B) Fura-2 Ca2+ imaging experiment. Various [K+]o with and without Cd2+ were applied as indicated. (C) Increased [Ca2+]i plotted against Ca2+ flux (from Fig. 3). Δ[Ca2+]i data from 10–23 cells and three to five experiments. Linear fit correlation coefficient equals 0.995 (P < 0.0005). (D) Log–log plot of CREB signal strength versus Δ[Ca2+]i for the indicated conditions. Solid lines are linear fits through the data points collected with varying [K+]o or [Cd2+]. (E) Possible gating-driven conformational changes working in concert with elevated bulk Ca2+. (top) Hypothetical conformational change repositions a local signaling determinant (circle). When combined with high local Ca2+ elevations, strong signaling to the nucleus is engaged (green). In the Ca2+-free solution (0 Ca2+), the conformational change remains, but the signaling determinant is not activated (red). (bottom) Can conformational change contribute to signaling in conjunction with increases in bulk Ca2+. (F) Nuclear pCREB levels from cells stimulated for 10 s in 2 mM Ca2+ (high local Ca2+) or for 15 s in the presence of caffeine with 0 Ca2+ (bulk Ca2+). Error bars represent SEM.
We measured the relationship between signal strength and bulk [Ca2+]i elevations (measured with the Ca2+ indicator Fura-2; Fig. 4 B). We found a linear relationship between [Ca2+]i rises and membrane Ca2+ flux (Fig. 4 C). Accordingly, when signal strength was plotted against the [Ca2+]i rise, we observed two distinct relationships (Fig. 4 D), with an approximately threefold difference in slopes on a log–log plot, very similar to disparate relationships we observed with Ca2+ flux. Thus, CREB signal strength cannot be solely determined by changes in bulk [Ca2+]i.
As an additional check of the mild dependence on iCa, we performed similar experiments with La3+, which has long been used to reduce open channel flux (Hagiwara and Takahashi, 1967; Lansman et al., 1986). Like Cd2+, La3+ potently blocked the Ca2+ rise but had little effect on signal strength (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200805048/DC1).
CREB signal strength is determined by Ca2+ acting near the channel
Our results indicated that bulk [Ca2+]i is not sufficient to determine signal strength. However, [Ca2+]i may be necessary, signaling in conjunction with L-type channels that act as voltage detectors independent of ion flux (Murata et al., 2005; Hegle et al., 2006; Kaczmarek, 2006). A joint requirement for elevated [Ca2+]i and L-type channel conformational change would neatly explain both the privileged role of L-type channels and the steep dependence on Po.
To test this, we independently manipulated [Ca2+]i and L-type channel conformation (Fig. 4 E). 10 mM caffeine raised [Ca2+]i by ∼250 nM (Fig. S2); this rise was greater than achieved with 40 mM K+/200 μM Cd2+, which produced robust signaling to CREB (Fig. 4 D). In principle, such a rise in [Ca2+]i meets the putative requirement for bulk Ca2+ elevation; indeed, caffeine produced a modest phosphorylation of CREB, even in Ca2+-free solution (Fig. 4 F). In the key test, we looked for an additional effect of a conformational change, produced by depolarizing with a 40-mM K+, Ca2+-free solution. Under these conditions, either with or without nimodipine to block channel gating, depolarization was without additional effect beyond caffeine alone (Fig. 4 F). In contrast, Ca2+ delivered by surface membrane influx evoked a strong pCREB response (Fig. 4 F, left). These results indicate that pairing voltage-dependent conformational changes with rises in bulk [Ca2+] failed to enhance signaling to CREB. Thus, bulk Ca2+ is neither necessary nor sufficient to control CREB signal strength in response to mild depolarization.
CaMKII autophosphorylation at the cell surface correlates with CREB signal strength
Our findings suggested that signaling is mediated by a local Ca2+ sensor that is weakly sensitive to changes in iCa yet highly sensitive to channel Po. Because closed dwell time (but not open dwell time) is strongly voltage dependent, depolarization changes the channel's duty cycle by increasing the frequency of channel openings and that of transient [Ca2+]i rises near the channel. Thus, a plausible candidate sensor could be positioned close to L-type channels and responsive to the frequency of local [Ca2+]i elevations. This led us to consider CaMKII. Although not generally considered important in signaling to CREB (but see Takeda et al., 2007), it is a logical candidate. Because CaMKII is tethered to L-type channels (Hudmon et al., 2005), it is strategically positioned to respond to local [Ca2+]i transients. Furthermore, autophosphorylation and persistent activation of CaMKII increase steeply with the frequency of Ca2+ pulses (De Koninck and Schulman, 1998).
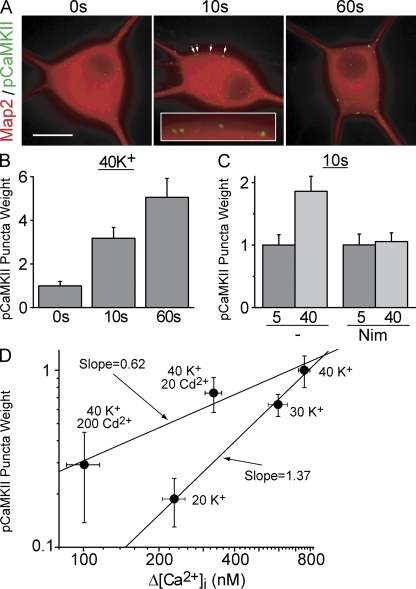
We first asked whether the same stimuli that drive rapid signaling to CREB can activate CaMKII. Brief exposure to 40 mM K+, followed by immediate fixation, resulted in the formation of pCaMKII immunoreactive puncta near the cell surface (Fig. 5 A), which is consistent with local CaMKII activation. To quantify the extent of activation, we identified pCaMKII puncta by an objective criterion (see Materials and methods) and determined their integrated puncta weight for individual cells. CaMKII activation was rapid, with detectable pCaMKII puncta after a 2.5-s stimulation (not depicted), and increasingly stronger activation at 10 and 60 s (Fig. 5, A and B). As with signaling to CREB, pCaMKII puncta formation required L-type channel activity (Fig. 5 C). Importantly, grading iCa had a much milder effect on CaMKII activation than changing Po (Fig. 5 D): the ratio of log–log slopes was 2–3, highly reminiscent of signaling to pCREB itself (compare with Fig. 3 C and Fig. 4 D). Together, these data support the hypothesis that local changes in CaMKII activation help couple L-type channel activation to the nuclear response.
Figure 5.
L-type channel activity produces phosphoCaMKII puncta near the cell surface. (A) SCG neurons stimulated with 40 mM K+ for 0, 10, or 60 s and immediately fixed and stained for Map2 (red) and pCaMKII (green). Arrows point to pCaMKII puncta (magnified in the inset). Background pCaMKII staining was subtracted to highlight punctate staining (see Materials and methods). Bar, 20 μm (B) pCaMKII puncta weight from experiments as in A. (C) Nimodipine blocks pCaMKII puncta formation. (D) pCaMKII puncta weight from cells treated for 60 s as shown on a log–log plot against the rise in bulk Ca2+ (Δ[Ca2+]i are taken from Fig. 4). For all, n = 3 independent experiments. Error bars represent SEM.
CaMKII is essential for L-type channel signaling to CREB
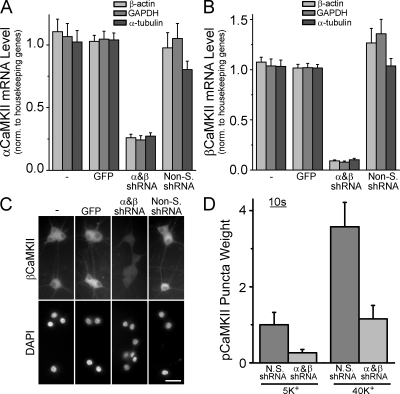
To directly test the role of CaMKII in signaling to CREB, we knocked down the expression of CaMKII using lentiviruses expressing short hairpin RNAs (shRNAs) targeting both α and βCaMKII, the main CaMKII isoforms in neurons. Knocking down α or β individually resulted in reciprocal mRNA regulation (unpublished data), as previously shown at the protein level (Thiagarajan et al., 2002). Therefore, we knocked down both subunits concurrently and obtained a 75 and 90% decrease in α and βCaMKII mRNA, respectively (Fig. 6, A and B). This reduction was relative to neurons that were uninfected or infected with viruses expressing GFP alone or a nonsilencing shRNA. We verified knockdown at the protein level by immunostaining βCaMKII (Fig. 6 C; the β isoform was chosen because of its robust staining compared with α). Importantly, CaMKII knockdown dramatically reduced pCaMKII puncta formation upon K+ stimulation (Fig. 6 D).
Figure 6.
Knockdown of α and βCaMKII eliminates the formation of pCaMKII puncta. αCaMKII (A) and βCaMKII (B) mRNA levels in SCG neurons assessed by quantitative RT-PCR. Some cultures infected with lentiviruses expressing GFP alone, shRNAs against α and βCaMKII, or nonsilencing control shRNAs. Data normalized against three housekeeping genes and plotted relative to the GFP only (empty vector) control (see Materials and methods). n = 3–4 experiments done in triplicate. (C) SCG neurons infected as in A and B and stained with an antibody against βCaMKII; nuclei were counterstained with DAPI. Bar, 50 μm. (D) pCaMKII puncta weight from cells infected with α and βCaMKII or nonsilencing control lentiviruses, stimulated for 10 s with 40 mM K+ and immediately fixed. Data from two independent experiments done in duplicate (10–15 neurons each). Error bars represent SEM.
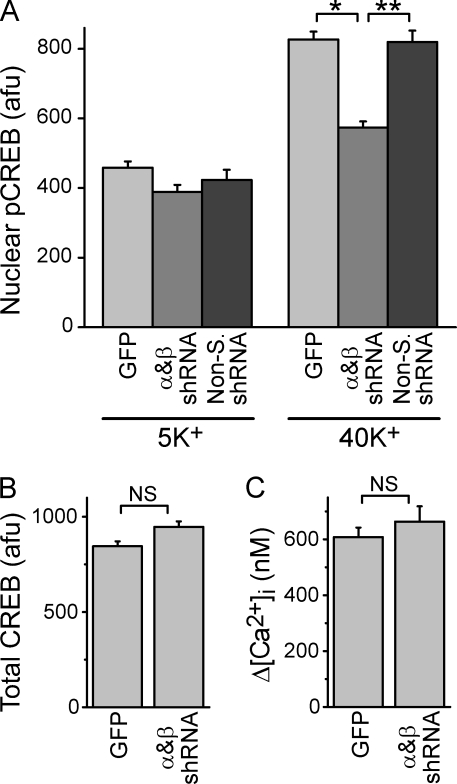
To test whether CaMKII linked L-type channel activity to CREB signaling, we examined pCREB formation after CaMKII knockdown; to avoid saturating the pCREB response, cells were stimulated with 40 mM K+ for 2.5 s. When we knocked down both α and βCaMKII, signaling to the nucleus was reduced by >65% (Fig. 7 A), despite normal CREB protein levels (Fig. 7 B) and L-type channel function (Fig. 7 C). Collectively, these data indicated that CaMKII acts downstream of channel activation and Ca2+ influx to couple L-type channels to rapid nuclear pCREB formation.
Figure 7.
Knockdown of α and βCaMKII inhibits L-type channel signaling to CREB. (A) pCREB levels from neurons stimulated for 2.5 s with 5- or 40-mM K+ Tyrode's and transferred to a 5 mM K+ solution for 45 s before fixation. Cultures were infected with GFP alone, α and βCaMKII, or nonsilencing lentiviruses. Data are from three to six independent experiments (15–30 cells from duplicate coverslips in each). *, P < 5 × 10−17; **, P < 5 × 10−10. (B) Total nuclear CREB levels in neurons infected with GFP alone or α and βCaMKII lentiviruses. Data from three independent experiments, two done in parallel with pCREB experiments in A (46–87 neurons). (C) Peak Ca2+ elevations (Δ[Ca2+]i) from sister cultures to those in B. Data are from seven separate experiments (31–49 cells). Error bars represent SEM.
Discussion
E-T coupling is important for a wide range of excitable cells. However, unlike other forms of excitation–response coupling, many features of E-T coupling have remained unclear. We characterized the basic properties of E-T coupling by relating L-type Ca2+ channel activity to the strength of signaling to CREB. We found that signal strength is steeply dependent on Ca2+ channel activity, obeying a nearly third power relationship, such that small changes in voltage produced large changes in the nucleus. Surprisingly, when we kept voltage fixed and manipulated Ca2+ entry by grading single channel flux, the relationship between Ca2+ current and CREB signal strength was far shallower, with an exponent less than one. This dichotomy underscores the conclusion that L-type channel signaling to CREB is not simply dictated by global [Ca2+]i, nor by integrated Ca2+ flux, but is responsive to the pattern of channel opening and closing. We hypothesized that Ca2+ entry via L-type channels drives a local sensor to saturation, thereby dampening the impact of grading open channel Ca2+ flux, in essence, digitizing local Ca2+ signals arising from channel opening events. This led us to consider CaMKII as an on-the-spot integrator of L-type channel activity in E-T coupling. Direct experimental tests provided compelling evidence that CaMKII indeed acts as a local transducer of the frequency of L-type channel openings in rapid signaling to the nucleus.
Features of the signaling mechanism: bulk or local Ca2+?
Functional analysis provided constraints on the molecular nature of L-type channel–pCREB coupling. We asked how, for a given total Ca2+ flux, signaling could be so much stronger with a high Po and small iCa than with low Po and large iCa. We tested various scenarios in which signal strength was dictated not simply by the size of the integrated Ca2+ influx but by channel gating. We found that the underlying mechanism must sense local Ca2+ transients near the channel, thus allowing brief and rapid fluctuations in local [Ca2+] to control signal strength. Three results weighed against bulk Ca2+ changes as the key determinant in signaling. First, involvement of bulk [Ca2+] increases generated by locally triggered RyRs were excluded because preventing RyR activity did not affect signal strength (Fig. S3). Second, similar bulk [Ca2+] elevations produced by either high Po /small iCa or low Po /large iCa produced very different outcomes. A bulk Ca2+ sensor distant from the channel could not provide such a distinction because of the blurring effects of Ca2+ diffusion from the channel's mouth. Third, we excluded the idea that bulk [Ca2+] is necessary for signaling, acting in concert with a readout of channel conformation. Conformational changes of L-type channels (without Ca2+ flux) had no effect on signaling when coupled with rises in bulk [Ca2+]. These data indicate that, with the relatively mild depolarizations studied here, rises in bulk [Ca2+] are neither necessary nor sufficient for rapid signaling to CREB.
If not by raising bulk Ca2+, how do L-type channels communicate with the nucleus? A remaining possibility is that the relevant Ca2+ signaling takes place in a domain near the point of entry (Chad and Eckert, 1984; Simon and Llinas, 1985; Augustine et al., 2003). At the inner mouth of the pore, [Ca2+] must increase in proportion to unitary flux to drive continual diffusion of Ca2+ away from the entry site (Klingauf and Neher, 1997). How could a sensor of this Ca2+ account for the steep dependence of signaling on Po, but only mild dependence on iCa? In a working model that emphasizes nanodomain signaling (Fig. 8 A), we propose that Ca2+ saturates a local sensor during each individual channel opening. As the membrane depolarizes and Po increases, the channel's duty cycle shifts in favor of more openings, resulting in a steep increase in signal strength. In contrast, Cd2+ and La3+ sharply decreased iCa yet only mildly reduced signal strength. This is because the local Ca2+ rise due to a single open channel largely saturates the Ca2+ sensor so that decreasing flux has little effect on sensor activation. According to this scenario, local saturation transforms fluctuating Ca2+ signals into a digital format; depolarization encodes as the frequency of a series of binary pulses of Ca2+ sensor activity. The usefulness of digital encoding at the front end of a signaling pathway hinges on the existence of a decoder of pulse frequency, hence our consideration of CaMKII.
Figure 8.
Model of local CaM saturation and frequency-dependent CaMKII activation. Schematic of the effects of three different types of stimulation on single channel kinetics and subsequent local effects on [Ca2+], Ca2+/CaM levels, and CaMKII activity that lead to CREB phosphorylation in the nucleus. (A) Individual L-type channels in the open state. Ca2+ flows through the channel (red arrow) and results in a plume of Ca2+ near the pore (red/yellow gradient). Pore block with Cd2+ markedly decreases flux (dashed red arrow), resulting in a proportionately lower [Ca2+] in the plume. Local CaM molecules (dots) are either in the apo-CaM form (gray) or Ca2+ bound (green). A local CaM target (presumably CaMKII) is depicted as a blue square. (B) Schematized record of L-type channels opening briefly and intermittently during depolarization to give rise to unitary current (black). Closed intervals are abbreviated by increasing depolarization. Open channel current amplitude is decreased by Cd2+. Local Ca2+ transients (red) mirror single channel currents, rising and falling quickly. During channel opening, the local Ca2+ levels saturate the local pool of apo-CaM, resulting in large elevations in Ca2+/CaM even when Ca2+ transients are diminished by Cd2+. Thus, transient elevations of Ca2+/CaM, shown as solid green bars, match the channel openings in frequency but are not greatly affected by the magnitude of the single channel current. (C) Local Ca2+/CaM pulses each have an incremental effect on local CaMKII activity (blue trace). With mild depolarization, pulses arrive at a low frequency and CaMKII activation decays between pulses. Upon stronger depolarization, interpulse intervals shorten and decay of activation is slowed by intersubunit phosphorylation, resulting in greater accumulation of CaMKII activation. This holds even when iCa is decreased with Cd2+. (D) Schematic representation of data on CREB signal strength. The seemingly paradoxical finding that potent block of Ca2+ flux (red) with Cd2+ has a relatively weak effect on signal strength (blue) can be explained by local CaM saturation, allowing for potent local CaMKII activation even when Ca2+ flux is greatly decreased.
Recruitment of CaMKII as a steeply nonlinear reporter of channel activity
CaMKII has received little attention as a player in E-T coupling, but recent findings (Takeda et al., 2007) made it a plausible candidate. CaMKII tethers directly to L-type channels (Hudmon et al., 2005) and is thus well positioned to respond to Ca2+ sensors near the channel mouth. Furthermore, the multimeric structure of CaMKII supports intersubunit phosphorylation, and thereby confers a steep dependence on the frequency of Ca2+/CaM pulses (De Koninck and Schulman, 1998; Dupont et al., 2003). Frequency-dependent activation of CaMKII is known to be important for Ca2+-dependent facilitation of L-type channels themselves (Xiao et al., 1994; Yuan and Bers, 1994; Zuhlke et al., 1999; Hudmon et al., 2005).
Testing CaMKII led to four lines of evidence to support its participation in rapid signaling to the nucleus. First, the general CaMK inhibitor KN-93 blocked L-type channel-dependent signaling to pCREB. Second, upon L-type channel activation, pCaMKII puncta formed at the cell surface. Third, decreasing CaMKII levels reduced both the weight of pCaMKII puncta and the strength of CREB signaling. Fourth, pCaMKII formation was much more sensitive to changing Po than iCa, thus accounting for the disparate effects on signaling to CREB. Collectively, these data provide compelling evidence that local CaMKII activation is critical in decoding Ca2+ transients generated by L-type channel openings. In line with local Ca2+ sensing, pCaMKII puncta appeared at or near the cell surface but did not translocate to the nucleus. This focuses attention on targets of CaMKII as likely participants in downstream signaling. CaM kinases are often able to act on each other as kinase kinases (Sugita et al., 1994; Soderling, 1999; Sakagami et al., 2005; Chow and Means, 2007), raising the possibility that CaMKII might lead to activation of a kinase like CaMKIδ, which along with CaM (Deisseroth et al., 1998; Sakagami et al., 2005) translocates to the nucleus upon depolarization (Sakagami et al., 2005).
The strong evidence for CaMKII raises the notion that CaM might be the saturating Ca2+ sensor proposed in our model (Fig. 8, green). What features of CaM would allow it to play such a role? We first considered that CaM binds the IQ motif within the C terminus of L-type channels (Zuhlke et al., 1999). IQ-bound CaM has been implicated in slow signaling to pCREB that relies on MAPK (Dolmetsch et al., 2001). However, the rapid signaling studied here is unlikely to involve this tethered CaM molecule. First, the IQ-bound CaM is tightly bound to the channel (Erickson et al., 2001; Pitt et al., 2001) and cannot simultaneously bind the channel and activate target enzymes. Second, a single tethered CaM would be insufficient to trigger persistent activation of CaMKII, which has ≥12 Ca2+/CaM binding sites and requires binding of multiple CaMs to drive autophosphorylation (Hanson and Schulman, 1992). More likely, CaMKII is activated by the large pool of ∼25 mobile apo-CaM molecules that reside within 40 nm of the channel mouth (Mori et al., 2004). The millimolar peak levels of [Ca2+]i attained when channels are open will far exceed CaM's EC50 of 5–10 μM (Burgoyne, 2007), thereby saturating the local cadre of apo-CaM.
With mild depolarization, the frequency of L-type channel openings would be low enough to allow Ca2+ to dissociate from CaM and/or for Ca2+/CaM to diffuse away from its target between openings. Thus, pulsatile Ca2+ entry will be converted to brief, pulse-like surges of Ca2+/CaM, with the same temporal pattern as the channel openings (Fig. 8 B, middle). Even when Ca2+ influx is sharply reduced (e.g., by Cd2+ block), the local Ca2+ rise would still be sufficient to activate most of the local CaM molecules (Fig. 8 A, right), hence the shallow dependence on the open channel Ca2+ flux (Fig. 8 B, right). In contrast, varying the level of depolarization would sharply alter the frequency of channel openings, in turn strongly affecting the pattern of local Ca2+/CaM rises and thus the extent to which CaMKII is activated and autophosphorylated, which is in line with its well described frequency dependence (De Koninck and Schulman, 1998; Dupont et al., 2003). With mild depolarization, unitary openings and Ca2+/CaM pulses are infrequent, CaM largely dissociates from CaMKII during the interpulse interval, and CaMKII activity is low. With stronger depolarization, unitary openings come in rapid succession, leaving less time for CaM dissociation, resulting in greater temporal summation and more CaMKII activation. When adjacent subunits bind Ca2+/CaM, they reciprocally prolong their activated state through autophosphorylation and CaM trapping, a steeply frequency-dependent process. Further, because decreasing Ca2+ flux through open channels has little effect on Ca2+/CaM elevations, local CaMKII activation is largely dictated by the frequency of channel openings and not the magnitude of the open channel Ca2+ flux, thus accounting for the dichotomous dependences of pCaMKII on Po and iCa (Fig. 5 D). Finding that pCaMKII is less steeply voltage dependent than pCREB may indicate that CREB signaling is mediated by the cooperative action of multiple L-type channel–CaMKII complexes.
A remaining issue is whether there is any role for a voltage-dependent conformational change. We ruled out a critical role of conformational changes working in conjunction with bulk Ca2+, but did not exclude a conformational change working in coordination with local Ca2+ signaling. The latter scenario gains credence from experiments where immobilizing the Ca2+ channel's C terminus prevented CREB phosphorylation but spared Ca2+ influx (Kobrinsky et al., 2003). A depolarization-driven conformational change in the L-type channel could support kinase activation or allow the activated kinase to access its target (Artalejo et al., 1992). Such possibilities have already been proposed for voltage-dependent facilitation (Hoshi et al., 1984; Pietrobon and Hess, 1990; Artalejo et al., 1992), another CaMKII-dependent process (Xiao et al., 1994; Lee et al., 2006).
Further structural information on the L-type channel–CaMKII interaction will help clarify whether a direct interaction is necessary for signaling to CREB. CaMKII interactions with the CaV1.2 (α1C) L-type channel are supported by binding determinants within multiple cytoplasmic loops; SCG neurons express multiple CaMKII isoforms and multiple L-type isoforms (CaV1.2 and CaV1.3). A first step will be to determine whether structural determinants of αCaMKII binding to CaV1.2 (Hudmon et al., 2005) generalize to other kinase and channel isoforms.
Comparisons with E-C and E-S coupling
The steep voltage dependence of E-T coupling was comparable to that found in classical studies of E-C and E-S coupling (Hodgkin and Horowicz, 1960; Katz and Miledi, 1967; Chapman and Tunstall, 1981; Table I). In the case of contraction, skeletal E-C coupling is independent of Ca2+ flux (Armstrong et al., 1972), whereas E-T coupling requires at least minimal Ca2+ influx (Greenberg et al., 1986; Morgan and Curran, 1986; Fig. 1 A), indicating divergent mechanisms. E-T coupling is more comparable to cardiac E-C coupling, wherein Ca2+ flux through L-type channels is necessary to activate RyR (Bers, 2002), and RyR activity is proportional to the square of the local [Ca2+] (Santana et al., 1996). Interestingly, the “gain” of L-type channel coupling to RyRs decreases at stronger depolarizations, possibly reflecting some kind of saturation (Altamirano and Bers, 2007), akin to what we infer here. However, in cardiac E-C coupling, RyRs are absolutely required, and gradations in iCa or Po have largely similar effects on RyR function (Altamirano and Bers, 2007). Thus, E-T coupling is mechanistically different from both cardiac and skeletal E-C coupling.
Table I. Features of excitation–response coupling.
| Coupling
|
||||
|---|---|---|---|---|
| E-S | Cardiac E-C | Skeletal E-C | E-T | |
| Input–output speed | ms | ms | ms | s–min |
| Relationship to voltage (mV/e-fold change) | 4.3a | 6.2b | 3.1c | 5.6 |
| Requires Ca2+ flux through membrane Ca2+ channels | Yes | Yes | No | Yes |
| Voltage dependence above and beyond Ca2+ flux | No | No | Yes | Yes |
| Locally mediated signaling | Yes | Yes | Yes | Yes |
| Requires Ca2+ release from stores | No | Yes | Yes | No |
| Target of Ca2+ or conformational coupling | synaptotagmin | RyRs | RyRs | CaM/CaMKII |
Results in bold are from the present study.
Our finding that CREB signal strength follows [Ca2+ flux]2–3 is reminiscent of E-S coupling, where the corresponding exponent is 3–5 (Dodge and Rahamimoff, 1967; Augustine et al., 1985; Bollmann et al., 2000; Schneggenburger and Neher, 2000; for review see Augustine, 2001). Importantly, the steep Ca2+ dependence of secretion, caused by multiple Ca2+ ions acting on synaptotagmin Ca2+ sensors (Sudhof, 2004), is maintained even when iCa is graded (Wu and Saggau, 1994; Mintz et al., 1995; Shahrezaei et al., 2006). In this respect, E-S coupling contrasts sharply with E-T coupling, in which grading Ca2+ flux with Cd2+ reveals a distinct, shallow relationship.
L-type channel signaling from a broader functional perspective
We focused on steady depolarization over a relatively negative voltage range as a way of defining the limiting voltage dependence of E-T coupling (Almers, 1978). Under these conditions L-type channels dominate signaling, an advantage for mechanistic studies. For this purpose, using action potentials would be interesting, but also complicated, as they sweep rapidly through a wide range of voltages and drive activation of multiple Ca2+ channel types. In fact, CaV2 channels recruited by strong depolarization also signal to CREB (Wheeler, D.G., C.F. Barrett, and R.W. Tsien. 2007. Society for Neuroscience Annual Meeting. Abstr. 784.20), accounting for the ability of non–L-type channels to contribute to signaling to CREB with repetitive spiking (Brosenitsch and Katz, 2001; Zhao et al., 2007). Interestingly, signaling by CaV2 channels is dependent on bulk [Ca2+] (compare with Hardingham et al., 2001), in striking contrast to the L-type channel–dedicated, local Ca2+/CaMKII-dependent mechanism described here. The importance of L-type Ca2+ channels may be further enhanced by an atypical gating in which, after a delay, some of the channels open upon repolarization after a previous strong depolarization (Pietrobon and Hess, 1990; Kavalali and Plummer, 1994; Koschak et al., 2007). Such gating would not be triggered by the stimuli we used, but might become significant with repetitive action potentials as stimuli.
Our results have functional implications for CRE-dependent gene expression, which requires long lasting CREB phosphorylation (Bito et al., 1996). Under physiological conditions, neurons undergo bouts of depolarizing activity (e.g., bursts of spikes, synaptic depolarizations, upstates, and/or tonic depolarization triggered by G protein–coupled receptors). Although individual units of physiological activity may be too brief to induce gene expression, a series of such bouts, each with an incremental effect on pCREB, will enhance and prolong CREB phosphorylation, thus engaging transcription. Thus, our findings on the voltage and time dependence of CREB phosphorylation shed light on the dynamics of signaling by single bouts of depolarization and give insight into the underlying mechanisms.
Materials and methods
Cell culture
SCG neurons were dissected from postnatal day 0–1 Sprague-Dawley rats and incubated for 30 min at 37°C in a solution containing 15 mg/ml trypsin (Sigma-Aldrich). Cells were then washed, dissociated by trituration with a fire-polished, siliconized Pasteur pipette, and plated on laminin/poly-l-lysine–coated glass coverslips (BD) in 24-well plates. The cultures were maintained in 5% CO2 at 37°C in L-15 medium (Invitrogen) supplemented with sodium bicarbonate, penicillin/streptomycin, glucose, 10% FBS, 25 ng/ml of nerve growth factor, and a vitamin mix (Hawrot and Patterson, 1979). Cultures were fed 1 d after plating by replacing half the medium with medium containing 5 μM of cytosine arabinoside and were subsequently fed every third day. In all experiments, neurons were used 4–6 d after plating.
Adenovirus production and infection
SCG neurons were infected with an adenovirus expressing a dihydropyridine-insensitive L-type (α1C,77) channel driven by the synapsin I promoter as previously described (Wheeler and Cooper, 2001; Wheeler et al., 2006). Near 100% infection efficiency was achieved by adding ∼50 infectious particles per microliter to the growth medium (1.5 ml in 1 well of a 24-well plate) 1 d after plating.
Stimulation and immunocytochemistry
We induced CREB phosphorylation in two different ways. To measure isochronic pCREB levels (Fig. 1, A and B), cells were stimulated at room temperature with the indicated high [K+] solution for 3 min, and then immediately fixed. To measure signal strength we used a ballistic protocol wherein cells were stimulated with high [K+] for the indicated time and then placed in control (5 mM K+) solution for a given period of time before fixation. In Fig. 1 (C–F), the stimulation time plus the delay before fixation totaled 3 min, allowing direct comparison with the data in Fig. 1 (A and B). A series of control experiments with various [K+] and stimulus durations revealed that a 45-s delay in control solution provides maximal phosphorylation of CREB; beyond this time, pCREB levels decline, reaching baseline levels within a few minutes. Therefore, in all experiments measuring CREB signal strength, except those in Fig. 1, the delay between stimulation and fixation was set at 45 s (see Fig. S1).
Control solution (5-mM K+ Tyrode's) consisted of 150 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM Hepes, and 10 mM glucose, pH 7.3. When stimulating with elevated [K+], Na+ was reduced to maintain osmolarity. The Ca2+-free solution was prepared by replacing the CaCl2 with MgCl2 and adding 0.5 mM EGTA. Before stimulation, cells were pretreated with 0.5 μM tetrodotoxin (TTX), which remained present during stimulation. In experiments with Cd2+ or La3+, the ions were applied only at the time of stimulation or 1 s before.
Cells were fixed in ice-cold 4% paraformaldehyde in phosphate buffer supplemented with 4% (wt/vol) sucrose. Fixed cells were then permeabilized with Triton X-100, blocked with 6% normal goat serum, and incubated overnight at 4°C in primary antibodies: rabbit anti-pCREB (1:133 dilution; Cell Signaling Technology); mouse anti-MAP2 antibody (HM-2; 1:1,000; Sigma-Aldrich); rabbit anti-pCaMKII against Thr286 of αCaMKII or Thr287 of βCaMKII (1:10,000; Cell Signaling Technology); mouse anti-βCaMKII (1:200; Invitrogen). The next day, cells were washed with PBS, incubated at room temperature for 45 min in a 1:200 dilution of anti-rabbit–Alexa488 and/or anti-mouse–Alexa568 (Invitrogen), washed with PBS, and mounted using VECTASHIELD mounting medium with DAPI (Vector Laboratories). The cells were imaged through a 40× (1.3 NA) oil objective on an epifluorescent microscope (Axioplan) equipped with a digital camera (AxioCam) using AxioVision 3.1 (all from Carl Zeiss, Inc.).
Electrophysiology
Whole-cell voltage- and current-clamp recordings were performed at room temperature (20–22°C) using a patch-clamp amplifier (Axopatch 200B; MDS Analytical Technology). Patch pipettes were pulled in a puller (P-97; Sutter Instrument Co.) from borosilicate glass capillaries and heat polished before use with a microforge (MF-9; Narishige). Pipette resistance was ∼2–4 MΩ when filled with internal solution. For current-clamp recordings, the bath solution was the same Tyrode's solution used for CREB phosphorylation experiments, containing 2 mM Ca2+, and the pipette contained 122 mM of potassium gluconate, 9 mM NaCl, 1.8 mM MgCl2, 0.9 mM EGTA, 5 mM ATP, 0.5 mM GTP, 9 mM Hepes, and 14 mM phosphocreatine, pH 7.3; for some recordings, 0.5 μM TTX was included in the bath solution to block action potentials. For recording whole-cell Ca2+ currents in voltage clamp, again the bath solution was the same 2-mM Ca2+ Tyrode's solution used in pCREB experiments, supplemented with 1 mM TEA and 0.5 μM TTX, and the pipette contained 122 mM Cs-Asp, 10 mM Hepes, 10 mM EGTA, 5 mM MgCl2, 4 mM ATP, and 0.4 mM GTP, pH 7.5. The traces shown are the mean of 10 consecutive sweeps.
Bath solution exchanges were performed via gravity-fed perfusion, with complete volume exchange in <5 s. Series resistance was compensated electronically by ≥90%, and membrane capacitance was corrected online; residual linear capacitive and leak currents were subtracted by the –P/4 method. Data were passed through a 4-pole low-pass Bessel filter, digitized using a Digidata 1320A (MDS Analytical Technology), and stored on a personal computer for offline analysis. Currents were filtered at 1–2 kHz and digitized at 2–20 kHz. Pulse depolarizations were applied at 10-s intervals.
Ca2+ imaging
SCG neurons were loaded for 30–60 min with 2 μM Fura-2 AM (Invitrogen) and 0.02% Pluronic F-127 (Invitrogen) in conditioned SCG growth medium in a 37°C/5% CO2 incubator. Fura-2 fluorescence, measured at 0.33 Hz, was elicited by excitation at 340 and 380 nm, detected at 510 nm, and imaged with a charge coupled device camera (C4742-95 Orca; Hamamatsu Photonics). The 340/380 ratio was quantified using regions of interest outlining the entire cell body. After background subtraction, ratios were converted to [Ca2+]i based on an empirical fit of ratios obtained from a Fura-2 Ca2+ imaging calibration kit (Invitrogen). Bath solution exchanges were performed via gravity-fed perfusion, with 50% volume exchange in <2 s. When measuring the effect of grading single channel current, Cd2+ was applied 30 s before the K+ challenge. In experiments with La3+, there was no La3+ preincubation. The solutions used were the same as described above for pCREB stimulation, and all solutions contained 0.5 μM TTX.
Lentiviral-mediated knockdown of αCaMKII and βCaMKII
The self-inactivating bicistronic lentiviral transfer vector construct pLVTHM and the second generation lentivirus packaging and envelope plasmids were provided by D. Trono (University of Geneva, Geneva, Switzerland) (Wiznerowicz and Trono, 2003).The pLVTHM lentiviral vector carries the EF1α promoter to drive GFP and the H1 RNA polymerase III promoter to permit the expression of an shRNA for RNA interference (Wiznerowicz and Trono, 2003). Two complementary DNA oligonucleotides (Operon Biotechnologies, Inc.) were annealed to produce a double-stranded DNA fragment encoding a 19-nucleotide sense strand (uppercase), 9-nucleotide loop (lowercase italicized), and 19-nucleotide antisense strand (uppercase) of the αCaMKII, βCaMKII, or nonsilencing sequence. The sequence of the αCaMKII shRNA is as follows: 5′-cgcgtccccGAATGATGGCGTGAAGGAAttcaagagaTTCCTTCACGCCATCATTCtttttggaaat-3′ (sense) and 5′-cgatttccaaaaaGAATGATGGCGTGAAGGAAtctcttgaaTTCCTTCACGCCATCATTCgggga-3′ (antisense). The αCaMKII target sequence corresponds to bases 1010–1028 of the αCaMKII mRNA (GenBank/EMBL/DDBJ accession no. NM_012920). The βCaMKII shRNA (5′-cgcgtccccGAGTATGCAGCTAAGATCAttcaagagaTGATCTTAGCTGCATACTCtttttggaaat-3′ [sense] and 5′-cgatttccaaaaaGAGTATGCAGCTAAGATCAtctcttgaaTGATCTTAGCTGCATACTCgggga [antisense]) targets bases 197–215 of the βCaMKII mRNA (GenBank/EMBL/DDBJ accession no. NM_021739). We also used an shRNA with a nonsilencing target sequence: 5′-cgcgtccccTCGCTTGGGCGAGAGTAAGttcaagagaCTTACTCTCGCCCAAGCGAtttttggaaat-3′ (sense) and 5′-cgatttccaaaaaTCGCTTGGGCGAGAGTAAGtctcttgaaCTTACTCTCGCCCAAGCGAgggga-3′ (antisense).
The duplex DNAs of shRNA-αCaMKII, shRNA-βCaMKII, and shRNA-nonsilencing were cloned into the MluI and ClaI sites of the pLVTHM vector. After confirming the ability of each shRNA to knock down their intended targets in HEK293T cells overexpressing wild-type αCaMKII or βCaMKII, viral particles were produced by transfection (Lipofectamine 2000; Invitrogen) of 293T cells plated in 100-mm dishes in DME plus 10% FBS. Subconfluent 293T cells were cotransfected with 10 μg of lentiviral vector containing the transgene, 7.5 μg of the packaging plasmid psPAX2, and 3 μg of the envelope plasmid pMD2.g. After 16 h, the medium was changed. The supernatant was collected 24 h later (∼10 ml per dish) and cleared of cell debris by filtering through a 0.45-μm filter. The viral particles were concentrated by ultracentrifugation of the filtrate at 70,000 g for 2 h at 4°C using a rotor (SW28; Beckman Coulter). The viral pellet was then resuspended in sterile PBS, aliquoted, and stored at −80°C. Lentivirus particles (0.15–0.5 μl of viral stock diluted in 20 μl PBS per coverslip) were added to SCG cultures containing 500 μl of medium the day after plating. 24 h later, the cultures were fed with 1 ml of medium and used 3 d later, when GFP could be detected in ∼100% of the neurons.
Real-time PCR
4 d after infection, the medium was replaced with the RNA stabilization reagent RNALater (QIAGEN) and the cells were stored at 4°C until use. Total RNA was isolated from cultured cells using the RNeasy Micro kit (QIAGEN) and reverse transcribed into cDNA using a QuantiTect RT-PCR kit (QIAGEN), as per the manufacturer's instructions. Each sample was from one 12-mm coverslip containing ∼50–100 neurons. Real-time PCR studies were performed with DyNAmo HS SYBR Green Master Mix (Finnzymes) using the DNA engine Opticon 2 (Bio-Rad Laboratories) through 45 PCR cycles (94°C for 10 s, 60°C for 30 s, and 72°C for 30 s). In each experiment, three separate coverslips were used per condition and each cDNA sample, equivalent to RNA from one well of cultured cells (24-well plate), was run in duplicate for the target genes (αCaMKII and βCaMKII) and the housekeeping genes (β-actin, α-tubulin, and GAPDH). Specificity of amplicons was determined by melting curve analysis, gel electrophoresis, and DNA sequencing. Primer pair sequences were as follows: αCaMKII (GenBank/EMBL/DDBJ accession no. NM_012920), forward primer 5′-CTCTGAGAGCACCAACACCA-3′ (nucleotides 1031–1050) and reverse primer 5′-CCATTGCTTATGGCTTCGAT-3′ (1113–1132); βCaMKII (M16112), forward primer 5′-GTCCACCGCGGCCTC-3′ (1040–1054) and reverse primer 5′-TTTTGGTGCTGTTTGTCTGGG-3′ (1123–1143); β-actin (NM_03144), forward primer 5′-AGGCCCCTCTGAACCCTAAG-3′ (401–420) and reverse primer 5′-CCAGAGGCATACAGGGACAAC-3′ (498–519); α-tubulin (GenBank/EMBL/DDBJ accession no. NM_001007004), forward primer 5′-GAGATCCGAAATGGCCCGTAC-3′ (263–283) and reverse primer 5′-GCCAATGGTGTAGTGACCACG-3′ (347–367); GAPDH (GenBank/EMBL/DDBJ accession no. NM_017008), forward primer 5′-AACCTGCCAAGTATGATGACATCA-3′(822–845) and reverse primer 5′-TGTTGAAGTCACAGGAGACAACCT-3′ (909–932). All primers were synthesized by Operon Biotechnologies, Inc.
The relative mRNA levels were calculated by normalization of cycle threshold (C(t)) values of the target gene (αCaMKII and βCaMKII) to each of the reference genes (β-actin, α-tubulin, and GAPDH) using the comparative C(t) (ΔΔC(t)) method, where ΔC(t) values were calculated from the following three equations: (1) ΔC(t)treatment = C(t)target − C(t)reference; (2) ΔC(t)control = C(t)target − C(t)reference; and (3) ΔΔC(t) = ΔC(t)treatment − ΔC(t)control.
Data are reported as the mean ratio of fold change in the mRNA of interest corrected for each reference gene (2-ΔΔC(t)). The control group was always the empty vector condition, thus the ratio of 1. Real-time PCR experiments were analyzed using one-way analysis of variance with Student-Newman-Keuls Multiple Comparisons post hoc (p-values) tests. Differences between means were considered a priori as significant at P < 0.05.
Data analysis
Electrophysiology data were acquired and analyzed using Clampex 8.2 and Clampfit 8.2, respectively (MDS Analytical Technologies). The exponential fits in Fig. 2 E were calculated using the equation y = y0 + A × e(x/k), where y is Ca2+ flux or pCREB signal strength, y0 is the pedestal, A is amplitude, and k is given in mV/e-fold change in y. Unless otherwise indicated, in electrophysiology and Fura-2 imaging experiments, sample size represents the number of individual cells recorded or analyzed. Data are presented as mean ± SEM. When not stated, p-values are from Students' t tests.
Image analysis
We quantified pCREB staining using Axiovision LE 4.2 (Carl Zeiss, Inc.). The nuclear marker, DAPI, and an antibody against the neuron-specific protein MAP2 were used to delineate neuronal nuclei, which were used as regions of interest. Mean pixel intensity in the pCREB channel was measured using these regions of interest and the “off-cell” background measured near the cell was subtracted. To determine CREB signal strength, in individual experiments the mean pCREB level in control (unstimulated) cells was considered as baseline and set to 0% and the pCREB level after a 3-min, 40-mM K+ stimulation was set to maximum (i.e., 100%). The slope of the initial relationship between stimulation time and pCREB level in a given experiment was used as a measure of CREB signal strength, units being percentage per second. Where indicated, signal strength under each condition was normalized to the strength in 40 mM K+ to correct for slight culture-to-culture variations.
To measure pCaMKII puncta, we captured 8-bit epifluorescent images and subtracted background staining using the rolling ball method (Sternberg, 1983) in ImageJ (National Institutes of Health; for theoretical analysis of a similar approach, see Mager et al., 2007). After subtraction, we thresholded images such that only those pixels 5 units (0–255 scale) above background remained. We then classified puncta as regions with a minimum of four adjacent suprathreshold pixels. For each punctum, the product of the punctum size and mean pixel intensity (above background) was used as a read-out of the magnitude of the local pCaMKII response or the punctum weight. The sum of all puncta on an individual cell gave its pCaMKII puncta weight.
Online supplemental material
Fig. S1 shows that two protocols for measuring CREB signal strength differ strongly in absolute terms but yield similar estimates of the steep dependence on [K+]o and hence membrane potential. Fig. S2 shows that CREB signal strength is steeply dependent on L-type channel activity. Fig. S3 shows that RyRs do not account for the importance of Po in determining CREB signal strength. Fig. S4 shows that La3+-mediated inhibition of single channel flux potently blocks rises in [Ca2+]i but has very little effect on CREB signal strength. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200805048/DC1.
Acknowledgments
We thank Richard Lewis, Paul De Koninck, Andy Hudmon, and A. Pejmun Haghighi for helpful comments and discussions.
This study was supported by awards from the Zaffaroni Foundation and the Tobacco-Related Disease Research Program (D.G. Wheeler) and by research grants from the National Institute of General Medical Sciences and National Institute of Neurological Disorders and Stroke (R.W. Tsien).
P. Safa's present address is MDS Analytical Technologies, Sunnyvale, CA 94089.
C.F. Barrett's present address is Dept. of Neurology and Dept. of Human Genetics, Leiden University Medical Centre, 2300 RC Leiden, Netherlands.
Abbreviations used in this paper: CaMKII, Ca2+/CaM-dependent protein kinase II; CREB, cAMP response element–binding protein; E-C, excitation–contraction; E-S, excitation–secretion; E-T, excitation–transcription; iCa, unitary Ca2+ flux; pCREB, Ser133-phospho-CREB; Po, open probability; RyR, ryanodine receptor; SCG, superior cervical ganglion; shRNA, short hairpin RNA; TTX, tetrodotoxin.
References
- Adams, P.R., and D.A. Brown. 1973. Action of -aminobutyric acid (GABA) on rat sympathetic ganglion cells. Br. J. Pharmacol. 47:639P–640P. [PMC free article] [PubMed] [Google Scholar]
- Almers, W. 1978. Gating currents and charge movements in excitable membranes. Rev. Physiol. Biochem. Pharmacol. 82:96–190. [DOI] [PubMed] [Google Scholar]
- Altamirano, J., and D.M. Bers. 2007. Voltage dependence of cardiac excitation-contraction coupling: unitary Ca2+ current amplitude and open channel probability. Circ. Res. 101:590–597. [DOI] [PubMed] [Google Scholar]
- Armstrong, C.M., F.M. Bezanilla, and P. Horowicz. 1972. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N'-tetracetic acid. Biochim. Biophys. Acta. 267:605–608. [DOI] [PubMed] [Google Scholar]
- Artalejo, C.R., S. Rossie, R.L. Perlman, and A.P. Fox. 1992. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 358:63–66. [DOI] [PubMed] [Google Scholar]
- Augustine, G.J. 2001. How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 11:320–326. [DOI] [PubMed] [Google Scholar]
- Augustine, G.J., M.P. Charlton, and S.J. Smith. 1985. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J. Physiol. 367:163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine, G.J., F. Santamaria, and K. Tanaka. 2003. Local calcium signaling in neurons. Neuron. 40:331–346. [DOI] [PubMed] [Google Scholar]
- Beam, K.G., and C. Franzini-Armstrong. 1997. Functional and structural approaches to the study of excitation-contraction coupling. Methods Cell Biol. 52:283–306. [DOI] [PubMed] [Google Scholar]
- Berridge, M.J. 1998. Neuronal calcium signaling. Neuron. 21:13–26. [DOI] [PubMed] [Google Scholar]
- Bers, D.M. 2002. Cardiac excitation-contraction coupling. Nature. 415:198–205. [DOI] [PubMed] [Google Scholar]
- Bers, D.M., and T. Guo. 2005. Calcium signaling in cardiac ventricular myocytes. Ann. NY Acad. Sci. 1047:86–98. [DOI] [PubMed] [Google Scholar]
- Bito, H., K. Deisseroth, and R.W. Tsien. 1996. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 87:1203–1214. [DOI] [PubMed] [Google Scholar]
- Bollmann, J.H., B. Sakmann, and J.G. Borst. 2000. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 289:953–957. [DOI] [PubMed] [Google Scholar]
- Brosenitsch, T.A., and D.M. Katz. 2001. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J. Neurosci. 21:2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne, R.D. 2007. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat. Rev. Neurosci. 8:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell, M.B., H. Cheng, and W.J. Lederer. 1995. The control of calcium release in heart muscle. Science. 268:1045–1049. [DOI] [PubMed] [Google Scholar]
- Carlezon, W.A. Jr., R.S. Duman, and E.J. Nestler. 2005. The many faces of CREB. Trends Neurosci. 28:436–445. [DOI] [PubMed] [Google Scholar]
- Chad, J.E., and R. Eckert. 1984. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys. J. 45:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, R.A., and J. Tunstall. 1981. The tension-depolarization relationship of frog atrial trabeculae as determined by potassium contractures. J. Physiol. 310:97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, F., and A. Means. 2007. The calcium/calmodulin-dependent protein kinase cascades. In Calcium: A Matter of Life or death. Vol. 41. J. Krebs and M. Michalak, editors. Elselvier B.V., Amsterdam. 345–364.
- Chow, R.H. 1991. Cadmium block of squid calcium currents. Macroscopic data and a kinetic model. J. Gen. Physiol. 98:751–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crescenzo, V., K.E. Fogarty, R. Zhuge, R.A. Tuft, L.M. Lifshitz, J. Carmichael, K.D. Bellve, S.P. Baker, S. Zissimopoulos, F.A. Lai, et al. 2006. Dihydropyridine receptors and type 1 ryanodine receptors constitute the molecular machinery for voltage-induced Ca2+ release in nerve terminals. J. Neurosci. 26:7565–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck, P., and H. Schulman. 1998. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 279:227–230. [DOI] [PubMed] [Google Scholar]
- Deisseroth, K., H. Bito, and R.W. Tsien. 1996. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 16:89–101. [DOI] [PubMed] [Google Scholar]
- Deisseroth, K., E.K. Heist, and R.W. Tsien. 1998. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 392:198–202. [DOI] [PubMed] [Google Scholar]
- Deisseroth, K., P.G. Mermelstein, H. Xia, and R.W. Tsien. 2003. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr. Opin. Neurobiol. 13:354–365. [DOI] [PubMed] [Google Scholar]
- Dodge, F.A. Jr., and R. Rahamimoff. 1967. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 193:419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch, R. 2003. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci. STKE. 2003:PE4. [DOI] [PubMed] [Google Scholar]
- Dolmetsch, R.E., U. Pajvani, K. Fife, J.M. Spotts, and M.E. Greenberg. 2001. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 294:333–339. [DOI] [PubMed] [Google Scholar]
- Dupont, G., G. Houart, and P. De Koninck. 2003. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium. 34:485–497. [DOI] [PubMed] [Google Scholar]
- Ellinor, P.T., J. Yang, W.A. Sather, J.F. Zhang, and R.W. Tsien. 1995. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 15:1121–1132. [DOI] [PubMed] [Google Scholar]
- Erickson, M.G., B.A. Alseikhan, B.Z. Peterson, and D.T. Yue. 2001. Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron. 31:973–985. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong, C., and F. Protasi. 1997. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 77:699–729. [DOI] [PubMed] [Google Scholar]
- Greenberg, M.E., E.B. Ziff, and L.A. Greene. 1986. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 234:80–83. [DOI] [PubMed] [Google Scholar]
- Guatimosim, S., K. Dilly, L.F. Santana, M. Saleet Jafri, E.A. Sobie, and W.J. Lederer. 2002. Local Ca(2+) signaling and EC coupling in heart: Ca(2+) sparks and the regulation of the [Ca(2+)](i) transient. J. Mol. Cell. Cardiol. 34:941–950. [DOI] [PubMed] [Google Scholar]
- Hagiwara, S., and K. Takahashi. 1967. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J. Gen. Physiol. 50:583–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, P.I., and H. Schulman. 1992. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu. Rev. Biochem. 61:559–601. [DOI] [PubMed] [Google Scholar]
- Hardingham, G.E., F.J. Arnold, and H. Bading. 2001. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 4:261–267. [DOI] [PubMed] [Google Scholar]
- Hawrot, E., and P.H. Patterson. 1979. Long-term culture of dissociated sympathetic neurons. Methods Enzymol. 58:574–584. [DOI] [PubMed] [Google Scholar]
- Hegle, A.P., D.D. Marble, and G.F. Wilson. 2006. A voltage-driven switch for ion-independent signaling by ether-a-go-go K+ channels. Proc. Natl. Acad. Sci. USA. 103:2886–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart, M. 1980. Identification and function of intracellular calcium stores in axons and cell bodies of neurons. Fed. Proc. 39:2783–2789. [PubMed] [Google Scholar]
- Hille, B. 1994. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 17:531–536. [DOI] [PubMed] [Google Scholar]
- Hille, B. 2001. Ion Channels of Excitable Membranes. Sinauer, Sunderland, MA. 814 pp.
- Hirning, L.D., A.P. Fox, E.W. McCleskey, B.M. Olivera, S.A. Thayer, R.J. Miller, and R.W. Tsien. 1988. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 239:57–61. [DOI] [PubMed] [Google Scholar]
- Hodgkin, A.L., and P. Horowicz. 1960. Potassium contractures in single muscle fibres. J. Physiol. 153:386–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi, T., J. Rothlein, and S.J. Smith. 1984. Facilitation of Ca2+-channel currents in bovine adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 81:5871–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon, A., H. Schulman, J. Kim, J.M. Maltez, R.W. Tsien, and G.S. Pitt. 2005. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J. Cell Biol. 171:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek, L.K. 2006. Non-conducting functions of voltage-gated ion channels. Nat. Rev. Neurosci. 7:761–771. [DOI] [PubMed] [Google Scholar]
- Katz, B., and R. Miledi. 1967. A study of synaptic transmission in the absence of nerve impulses. J. Physiol. 192:407–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali, E.T., and M.R. Plummer. 1994. Selective potentiation of a novel calcium channel in rat hippocampal neurones. J. Physiol. 480:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf, J., and E. Neher. 1997. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys. J. 72:674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrinsky, E., E. Schwartz, D.R. Abernethy, and N.M. Soldatov. 2003. Voltage-gated mobility of the Ca2+ channel cytoplasmic tails and its regulatory role. J. Biol. Chem. 278:5021–5028. [DOI] [PubMed] [Google Scholar]
- Koschak, A., G.J. Obermair, F. Pivotto, M.J. Sinnegger-Brauns, J. Striessnig, and D. Pietrobon. 2007. Molecular nature of anomalous L-type calcium channels in mouse cerebellar granule cells. J. Neurosci. 27:3855–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze, D.L., and D. Rampe. 1992. Characterization of the effects of a new Ca2+ channel activator, FPL 64176, in GH3 cells. Mol. Pharmacol. 42:666–670. [PubMed] [Google Scholar]
- Lansman, J.B., P. Hess, and R.W. Tsien. 1986. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 88:321–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T.S., R. Karl, S. Moosmang, P. Lenhardt, N. Klugbauer, F. Hofmann, T. Kleppisch, and A. Welling. 2006. Calmodulin kinase II is involved in voltage-dependent facilitation of the L-type Cav1.2 calcium channel: identification of the phosphorylation sites. J. Biol. Chem. 281:25560–25567. [DOI] [PubMed] [Google Scholar]
- Lencesova, L., A. O'Neill, W.G. Resneck, R.J. Bloch, and M.P. Blaustein. 2004. Plasma membrane-cytoskeleton-endoplasmic reticulum complexes in neurons and astrocytes. J. Biol. Chem. 279:2885–2893. [DOI] [PubMed] [Google Scholar]
- Lin, Z., C. Harris, and D. Lipscombe. 1996. The molecular identity of Ca channel alpha 1-subunits expressed in rat sympathetic neurons. J. Mol. Neurosci. 7:257–267. [DOI] [PubMed] [Google Scholar]
- Liu, L., P.K. Gonzalez, C.F. Barrett, and A.R. Rittenhouse. 2003. The calcium channel ligand FPL 64176 enhances L-type but inhibits N-type neuronal calcium currents. Neuropharmacology. 45:281–292. [DOI] [PubMed] [Google Scholar]
- Llinas, R., I.Z. Steinberg, and K. Walton. 1981. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys. J. 33:323–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze, B.E., and D.D. Ginty. 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 35:605–623. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez, J.R., P.S. Shacklock, C.W. Balke, and W.G. Wier. 1995. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 268:1042–1045. [DOI] [PubMed] [Google Scholar]
- Mager, D.E., E. Kobrinsky, A. Masoudieh, A. Maltsev, D.R. Abernethy, and N.M. Soldatov. 2007. Analysis of functional signaling domains from fluorescence imaging and the two-dimensional continuous wavelet transform. Biophys. J. 93:2900–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains, R.E., and P.H. Patterson. 1973. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J. Cell Biol. 59:329–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz, I.M., B.L. Sabatini, and W.G. Regehr. 1995. Calcium control of transmitter release at a cerebellar synapse. Neuron. 15:675–688. [DOI] [PubMed] [Google Scholar]
- Morgan, J.I., and T. Curran. 1986. Role of ion flux in the control of c-fos expression. Nature. 322:552–555. [DOI] [PubMed] [Google Scholar]
- Mori, M.X., M.G. Erickson, and D.T. Yue. 2004. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 304:432–435. [DOI] [PubMed] [Google Scholar]
- Murata, Y., H. Iwasaki, M. Sasaki, K. Inaba, and Y. Okamura. 2005. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 435:1239–1243. [DOI] [PubMed] [Google Scholar]
- Murphy, T.H., P.F. Worley, and J.M. Baraban. 1991. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 7:625–635. [DOI] [PubMed] [Google Scholar]
- O'Lague, P.H., K. Obata, P. Claude, E.J. Furshpan, and D.D. Potter. 1974. Evidence for cholinergic synapses between dissociated rat sympathetic neurons in cell culture. Proc. Natl. Acad. Sci. USA. 71:3602–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Lague, P.H., E.J. Furshpan, and D.D. Potter. 1978. Studies on rat sympathetic neurons developing in cell culture. II. Synaptic mechanisms. Dev. Biol. 67:404–423. [DOI] [PubMed] [Google Scholar]
- Pietrobon, D., and P. Hess. 1990. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 346:651–655. [DOI] [PubMed] [Google Scholar]
- Pitt, G.S., R.D. Zuhlke, A. Hudmon, H. Schulman, H. Reuter, and R.W. Tsien. 2001. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J. Biol. Chem. 276:30794–30802. [DOI] [PubMed] [Google Scholar]
- Rios, E., and G. Brum. 1987. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 325:717–720. [DOI] [PubMed] [Google Scholar]
- Sakagami, H., A. Kamata, H. Nishimura, J. Kasahara, Y. Owada, Y. Takeuchi, M. Watanabe, K. Fukunaga, and H. Kondo. 2005. Prominent expression and activity-dependent nuclear translocation of Ca2+/calmodulin-dependent protein kinase Idelta in hippocampal neurons. Eur. J. Neurosci. 22:2697–2707. [DOI] [PubMed] [Google Scholar]
- Santana, L.F., H. Cheng, A.M. Gomez, M.B. Cannell, and W.J. Lederer. 1996. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ. Res. 78:166–171. [DOI] [PubMed] [Google Scholar]
- Sather, W.A., and E.W. McCleskey. 2003. Permeation and selectivity in calcium channels. Annu. Rev. Physiol. 65:133–159. [DOI] [PubMed] [Google Scholar]
- Schneggenburger, R., and E. Neher. 2000. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 406:889–893. [DOI] [PubMed] [Google Scholar]
- Schneggenburger, R., and E. Neher. 2005. Presynaptic calcium and control of vesicle fusion. Curr. Opin. Neurobiol. 15:266–274. [DOI] [PubMed] [Google Scholar]
- Schneider, M.F. 1994. Control of calcium release in functioning skeletal muscle fibers. Annu. Rev. Physiol. 56:463–484. [DOI] [PubMed] [Google Scholar]
- Schneider, M.F., and W.K. Chandler. 1973. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 242:244–246. [DOI] [PubMed] [Google Scholar]
- Shahrezaei, V., A. Cao, and K.R. Delaney. 2006. Ca2+ from one or two channels controls fusion of a single vesicle at the frog neuromuscular junction. J. Neurosci. 26:13240–13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, S.M., and R.R. Llinas. 1985. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys. J. 48:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling, T.R. 1999. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 24:232–236. [DOI] [PubMed] [Google Scholar]
- Stern, M.D. 1992. Theory of excitation-contraction coupling in cardiac muscle. Biophys. J. 63:497–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg, S. 1983. Biomedical image processing. Computer. 16:22–34. [Google Scholar]
- Sudhof, T.C. 2004. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27:509–547. [DOI] [PubMed] [Google Scholar]
- Sugita, R., H. Mochizuki, T. Ito, H. Yokokura, R. Kobayashi, and H. Hidaka. 1994. Ca2+/calmodulin-dependent protein kinase kinase cascade. Biochem. Biophys. Res. Commun. 203:694–701. [DOI] [PubMed] [Google Scholar]
- Takeda, H., Y. Kitaoka, Y. Hayashi, T. Kumai, Y. Munemasa, H. Fujino, S. Kobayashi, and S. Ueno. 2007. Calcium/calmodulin-dependent protein kinase II regulates the phosphorylation of CREB in NMDA-induced retinal neurotoxicity. Brain Res. 1184:306–315. [DOI] [PubMed] [Google Scholar]
- Thiagarajan, T.C., E.S. Piedras-Renteria, and R.W. Tsien. 2002. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 36:1103–1114. [DOI] [PubMed] [Google Scholar]
- Vizard, T.N., G.W. O'Keeffe, H. Gutierrez, C.H. Kos, D. Riccardi, and A.M. Davies. 2008. Regulation of axonal and dendritic growth by the extracellular calcium-sensing receptor. Nat. Neurosci. 11:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori, M., M. Strobeck, T. Niidome, T. Teramoto, K. Imoto, and Y. Mori. 1998. Functional characterization of ion permeation pathway in the N-type Ca2+ channel. J. Neurophysiol. 79:622–634. [DOI] [PubMed] [Google Scholar]
- Wang, S.Q., L.S. Song, E.G. Lakatta, and H. Cheng. 2001. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 410:592–596. [DOI] [PubMed] [Google Scholar]
- Weick, J.P., R.D. Groth, A.L. Isaksen, and P.G. Mermelstein. 2003. Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J. Neurosci. 23:3446–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, A.E., E.C. Griffith, and M.E. Greenberg. 2002. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 3:921–931. [DOI] [PubMed] [Google Scholar]
- Wheeler, D.G., and E. Cooper. 2001. Depolarization strongly induces human cytomegalovirus major immediate-early promoter/enhancer activity in neurons. J. Biol. Chem. 276:31978–31985. [DOI] [PubMed] [Google Scholar]
- Wheeler, D.G., C.F. Barrett, and R.W. Tsien. 2006. L-type calcium channel ligands block nicotine-induced signaling to CREB by inhibiting nicotinic receptors. Neuropharmacology. 51:27–36. [DOI] [PubMed] [Google Scholar]
- Wiznerowicz, M., and D. Trono. 2003. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 77:8957–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L.G., and P. Saggau. 1994. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J. Neurosci. 14:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, R.P., H. Cheng, W.J. Lederer, T. Suzuki, and E.G. Lakatta. 1994. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc. Natl. Acad. Sci. USA. 91:9659–9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W., and D. Lipscombe. 2001. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 21:5944–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., P.T. Ellinor, W.A. Sather, J.F. Zhang, and R.W. Tsien. 1993. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 366:158–161. [DOI] [PubMed] [Google Scholar]
- Yuan, W., and D.M. Bers. 1994. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am. J. Physiol. 267:H982–H993. [DOI] [PubMed] [Google Scholar]
- Zhang, H., A. Maximov, Y. Fu, F. Xu, T.S. Tang, T. Tkatch, D.J. Surmeier, and I. Bezprozvanny. 2005. Association of CaV1.3 L-type calcium channels with Shank. J. Neurosci. 25:1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, R., L. Liu, and A.R. Rittenhouse. 2007. Ca2+ influx through both L- and N-type Ca2+ channels increases c-fos expression by electrical stimulation of sympathetic neurons. Eur. J. Neurosci. 25:1127–1135. [DOI] [PubMed] [Google Scholar]
- Zuhlke, R.D., G.S. Pitt, K. Deisseroth, R.W. Tsien, and H. Reuter. 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 399:159–162. [DOI] [PubMed] [Google Scholar]