Abstract
In this study, we investigated whether the ability of Eph receptor signaling to mediate cell repulsion is antagonized by fibroblast growth factor receptor (FGFR) activation that can promote cell invasion. We find that activation of FGFR1 in EphB2-expressing cells prevents segregation, repulsion, and collapse responses to ephrinB1 ligand. FGFR1 activation leads to increased phosphorylation of unstimulated EphB2, which we show is caused by down-regulation of the leukocyte common antigen–related tyrosine phosphatase receptor that dephosphorylates EphB2. In addition, FGFR1 signaling inhibits further phosphorylation of EphB2 upon stimulation with ephrinB1, and we show that this involves a requirement for the mitogen-activated protein kinase (MAPK) pathway. In the absence of activated FGFR1, EphB2 activates the MAPK pathway, which in turn promotes EphB2 activation in a positive feedback loop. However, after FGFR1 activation, the induction of Sprouty genes inhibits the MAPK pathway downstream of EphB2 and decreases cell repulsion and segregation. These findings reveal a novel feedback loop that promotes EphB2 activation and cell repulsion that is blocked by transcriptional targets of FGFR1.
Introduction
The control of cell movement is essential for the establishment and maintenance of tissue organization during embryogenesis. For example, mixing of cell populations that have distinct regional or tissue identity is prevented by inhibition of cell migration across borders (Steinberg and Takeichi, 1994; Irvine and Rauskolb, 2001; Pasini and Wilkinson, 2002). Furthermore, some tissues are assembled by the guidance of actively migrating cells and neuronal growth cones to specific destinations in which extracellular cues encountered along the migration route control the direction of movement. Typically, this guidance involves multiple signals, some of which attract cells toward a destination, whereas others are repulsive and prevent cells from entering inappropriate territory (Tessier-Lavigne and Goodman, 1996). The use of multiple cues raises the question of how diverse signals act together to regulate cell migration. Such integration can occur by convergence of downstream pathways, for example on central components of cytoskeletal regulation, and/or by interactions between distinct receptors that modulate each others' activity (Huber et al., 2003).
Eph receptor tyrosine kinases and ephrins have roles in the guidance of migrating cells and neuronal growth cones and in restricting intermingling between adjacent tissue domains (Kullander and Klein, 2002; Poliakov et al., 2004; Pasquale, 2005). In vertebrates, Eph receptors and ephrins comprise two families of membrane-bound molecules that are divided into two classes: in general, EphA receptors bind the glycosyl phosphatidyl inositol–anchored ephrinA proteins, and EphB receptors bind the transmembrane ephrinB proteins (Gale et al., 1996). Upon binding, Eph receptors and ephrins become clustered, and both components transduce signals, in the case of Eph receptors and ephrinB proteins in part via phosphorylation of conserved tyrosine residues (Holland et al., 1996; Kullander and Klein, 2002; Palmer et al., 2002; Pasquale, 2005).
Functional studies have implicated Eph receptors and ephrins in the guidance of migrating cells and axons in which activation leads to repulsion responses that inhibit entry into ligand-expressing territory (Flanagan and Vanderhaeghen, 1998; Kullander and Klein, 2002; Poliakov et al., 2004). However, in other contexts, Eph–ephrin interactions can lead to increased axon outgrowth or cell migration (Santiago and Erickson, 2002; Hansen et al., 2004). The biochemical mechanisms underlying these distinct cell responses are not known, but in in vitro assays it has been found that low densities of ephrin promote outgrowth and integrin-mediated adhesion, whereas high densities trigger repulsion and de-adhesion (Huynh-Do et al., 1999; Hansen et al., 2004). Thus, the cell response appears to depend on the degree of receptor activation.
Several lines of evidence raise the possibility that there is antagonism between the Eph–ephrin system and other receptor tyrosine kinases in the control of cell migration. FGF receptors (FGFRs) promote axon outgrowth (McFarlane et al., 1996) and cell migration (Webb et al., 1997; Montell, 1999; Sun et al., 1999; Kubota and Ito, 2000), which could oppose the restriction of cell migration by Eph–ephrin signaling. Furthermore, FGFR and many other receptor tyrosine kinases activate the MAPK pathway, whereas Eph receptors can have antagonistic effects on cell behavior by inhibiting MAPK pathway activation (Elowe et al., 2001; Miao et al., 2001; Kim et al., 2002; Miller et al., 2003; Picco et al., 2007). Direct cross talk can occur in which activation of FGFR1 leads to phosphorylation of EphA4 (Yokote et al., 2005) and ephrinB1 (Chong et al., 2000) independently of activation by ephrin and Eph ligands, respectively. In the case of EphA4, this cross-activation promotes cell proliferation (Yokote et al., 2005), whereas FGFR1 antagonizes the ability of ephrinB1 to cause cell de-adhesion (Chong et al., 2000) and enable the migration of cells to the eye field (Moore et al., 2004).
Therefore, we set out to test whether FGFR activation affects the segregation of cell populations by Eph receptors and ephrins. We report that activation of FGFR1 in EphB2-expressing cells inhibits repulsion and segregation responses to ephrinB1. This change in cell response is caused by inhibition of a positive feedback loop that promotes high level EphB2 activation required for cell repulsion.
Results
Effect of FGFR1 on sorting and repulsion responses of EphB2 cells
Previous studies have used transient overexpression assays in embryo cells to show that interactions between Eph receptors and ephrins can restrict intermingling and segregate cell populations (Mellitzer et al., 1999; Tanaka et al., 2003). However, this approach has the disadvantage that overexpression and the presence of endogenous ligands lead to a high baseline activation of receptor. To establish a more amenable assay, we used HEK293 cells that express low levels of endogenous EphB and ephrinB proteins and made stable lines that express ephrinB1 or EphB2 and have little receptor autoactivation. To enable activation of FGFR in EphB2 cells independently of endogenous FGFs, we established cell lines that coexpress inducible FGFR (iFGFR), an inducibly activated fusion protein of membrane-anchored FGFR1 cytoplasmic domain and a peptide sequence that is dimerized by the addition of a small molecule, AP20187 (Welm et al., 2002; Pownall et al., 2003). Membrane-targeted GFP is coexpressed in specific cell lines so they can be identified in cell-mixing assays. These cell lines are referred to henceforth by the combination of exogenous genes that they are expressing, and unless otherwise stated, iFGFR has been activated with dimerizing compound.
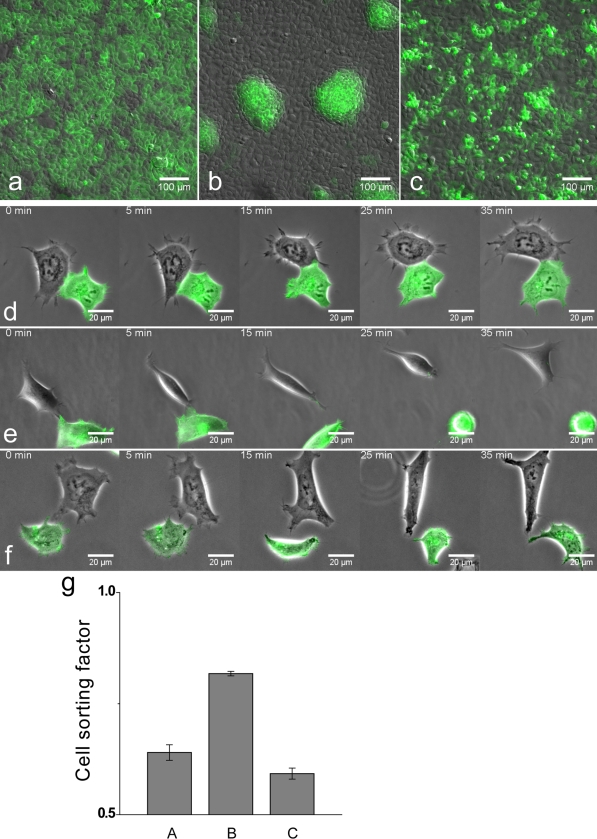
To test whether EphB2–ephrinB1 interactions can mediate cell segregation in this system, we performed coculture assays in which a mixture of two cell populations is plated out at moderate density such that cells can freely migrate until they become confluent over a 48-h period. We found that although EphB2+GFP cells remain intermingled with EphB2 cells (Fig. 1, a and g), EphB2+GFP cells segregate from ephrinB1 cells to form large aggregates with sharp interfaces between the two cell populations (Fig. 1, b and g). In contrast, there is little segregation of EphB2+iFGFR+GFP cells and ephrinB1 cells (Fig. 1, c and g).
Figure 1.
Effect of FGFR on EphB2 cell segregation and repulsion. Stable HEK293 cell lines were generated that express EphB2, EphB2+GFP, EphB2+iFGFR+GFP, or ephrinB1. Cell behavior was studied after mixing different combinations of these cell lines with GFP-expressing and -nonexpressing cells visualized by fluoresence and relief-contrast microscopy. (a–c) Cell segregation assays in which cells are plated at moderate density and incubated for 48 h, during which time they achieve confluence. (a) EphB2+GFP cells remain intermingled with EphB2 cells. (b) EphB2+GFP cells segregate from ephrinB1 cells. (c) EphB2+iFGFR +GFP cells fail to segregate from ephrinB1 cells. (d–f) Time-lapse videos of typical behaviors of EphB2-expressing cells after interaction with ephrinB1 cells. (d) An EphB2+GFP cell is not repelled and remains in contact with an EphB2 cell. (e) Upon touching an ephrinB1 cell, an EphB2+GFP cell rapidly retracts and rounds up for >35 min (n = 10/10). (f) After interaction with an ephrinB1 cell, an EphB2+iFGFR+GFP cell retracts but does not round up and reestablishes cell processes by 25 min (n = 8/8). (g) Quantitation of the results of cell segregation assays (a–c) was performed by the nearest neighbor method (Mochizuki et al., 1998). For a random distribution of two populations, on average half of the contacts of any cell type are with cells of the same type. The proportion of contacts between like cells increases for segregated populations. Because of clonal growth, cells are not randomly distributed in control assays. Error bars indicate SEM (n = 4).
To study individual cell behavior, we plated out mixtures of EphB2 cells and ephrinB1 cells at low density and captured videos of their interactions. In preliminary experiments, we found substantial variability in the cell responses. We reasoned that this might be because of variation in how many contacts EphB2 cells have made with ephrinB1 cells because repeated activation and endocytosis of receptor (Marston et al., 2003; Zimmer et al., 2003) decreases the steady-state level of cell surface EphB2 (unpublished data). Therefore, we performed time-lapse analysis of the first contact between cells after plating. We found that although EphB2 cells were not repelled and often remained in contact upon interaction with other EphB2 cells (Fig. 1 d and Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200807151/DC1), there was a rapid and sustained collapse of cell processes and rounding up of EphB2 cells that have touched ephrinB1 cells (Fig. 1 e and Video 2). In contrast, upon interaction with ephrinB1 cells, EphB2+iFGFR1 cells had only a transient collapse response that did not lead to rounding up (Fig. 1 f and Video 3).
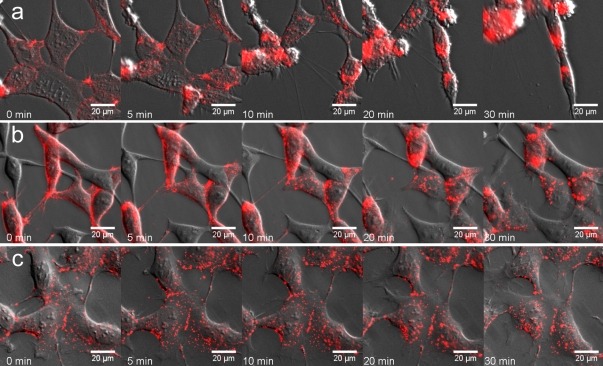
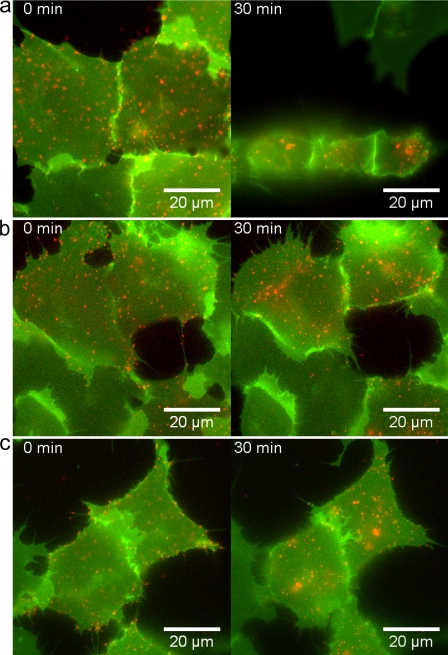
It is possible that the decreased cell repulsion after FGFR activation is secondary to changes in cell–cell interactions (e.g., binding required for Eph receptor activation). Therefore, we analyzed the effect of iFGFR in collapse assays in which EphB2 is activated by soluble ephrinB1-Fc rather than by ephrinB1 cells. We concurrently visualized receptor endocytosis by prelabeling cell surface EphB2 with Cy3-labeled anti-EphB2 antibody; this antibody alone does not induce the collapse of EphB2 cells (Fig. 2 c). We found that activation of EphB2 with ephrinB1-Fc led to rapid retraction of processes and cell rounding accompanied by translocation of cell surface EphB2 into cytoplasmic vesicles within 5 min (Fig. 2 a and Video 4, available at http://www.jcb.org/cgi/content/full/jcb.200807151/DC1). In contrast, EphB2+iFGFR1 cells did not collapse or round up even after 60 min, although the rate of ephrinB1-Fc endocytosis into these cells was similar to that in EphB2 cells (Fig. 2 b and Video 5). Collectively, the results of these assays show that FGFR1 activation in EphB2 cells leads to decreased segregation from ephrinB1 cells and inhibits cell repulsion and collapse responses of EphB2 cells to ephrinB1.
Figure 2.
Effect of FGFR on the collapse response of EphB2 cells. EphB2 or EphB2+iFGFR cells were incubated at 4°C with Cy3-labeled anti-EphB2 antibody to label cell surface EphB2. (a and b) The cells were incubated with culture medium at 37°C containing 1 μg/ml ephrinB1-Fc, and time-lapse images were collected. (a) More than 90% of EphB2 cells undergo rapid collapse with concurrent endocytosis of cell surface EphB2. (b) More than 95% of EphB2+iFGFR cells do not collapse, whereas endocytosis of EphB2 occurs with similar kinetics as for EphB2 cells. (c) EphB2 cells incubated with anti-EphB2 antibody in the absence of ephrinB1-Fc do not collapse.
FGFR1 activation leads to increased baseline EphB2 phosphorylation
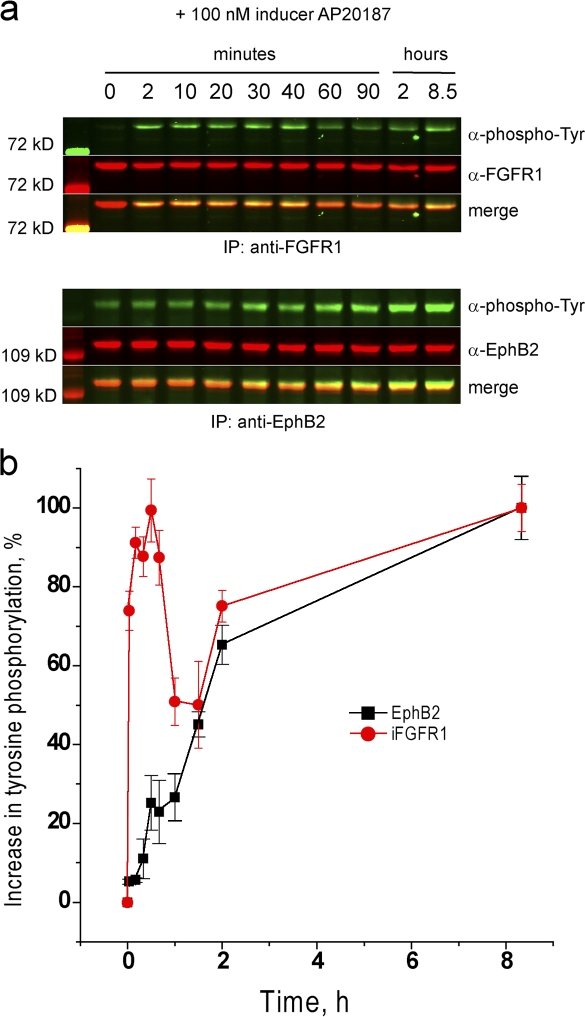
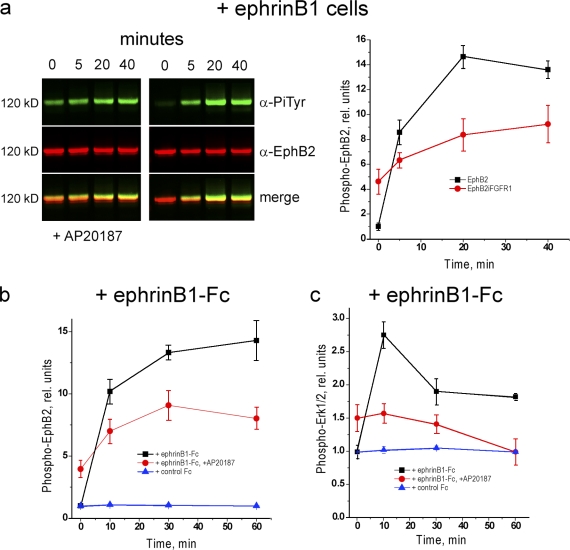
FGFR activation could inhibit cell repulsion responses to EphB2 activation by one or both of two mechanisms: by inhibiting EphB2 activation or by antagonizing downstream pathways required for cell repulsion. In initial experiments, we analyzed the effect of FGFR1 activation on EphB2 phosphorylation before the addition of ephrinB1. To our surprise, we found that activation of FGFR1 leads to a progressive increase in the level of ephrin-independent EphB2 phosphorylation (Fig. 3).
Figure 3.
Effect of iFGFR activation on baseline phosphorylation of EphB2. EphB2+iFGFR cells were incubated with AP20187 to activate iFGFR, and samples were collected at intervals for immunoprecipitation and Western blot analysis to detect tyrosine phosphorylation of iFGFR (a, top) and of EphB2 (a, bottom). The time course of phosphorylation is shown in b. Phosphorylation of iFGFR rapidly increases within minutes, and baseline (ephrin independent) phosphorylation of EphB2 progressively increases over an 8-h period. Error bars indicate range (n = 3). Tyr, tyrosine.
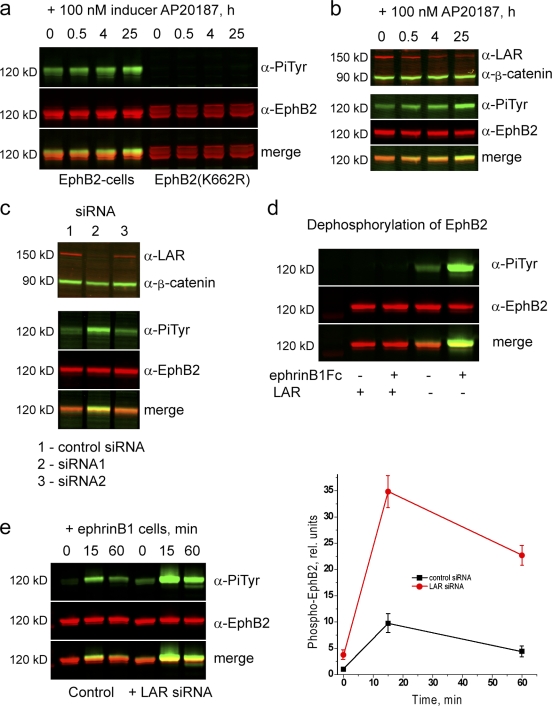
One potential mechanism underlying the increase in ephrin-independent phosphorylation is that FGFR1 directly or indirectly phosphorylates EphB2 perhaps via a physical interaction. However, the increase in baseline EphB2 phosphorylation is slow in comparison with FGFR1 activation (Fig. 3 b), and EphB2 and activated FGFR1 do not coimmunoprecipitate (not depicted), which is inconsistent with a direct coupling between their activation. Furthermore, a kinase-inactive EphB2 mutant is not phosphorylated in the presence of activated FGFR1 (Fig. 4 a), suggesting that the increase in baseline phosphorylation involves EphB2 autoactivation.
Figure 4.
Role of LAR in dephosphorylation of EphB2. Immunoprecipitations and Western blot analyses were performed to analyze mechanisms that may affect the level of EphB2 phosphorylation. (a) Time course of the effect of iFGFR activation on the phosphorylation of EphB2 (left) or kinase-inactive EphB2 (K662R; right). Kinase-inactive EphB2 is not phosphorylated after iFGFR activation. (b) After iFGFR activation, the expression level of the receptor tyrosine phosphatase LAR progressively decreases (top), concurrent with the increase in baseline EphB2 phosphorylation (bottom). (c) siRNA-mediated knockdown of LAR in EphB2 cells (lane 2) leads to increased baseline phosphorylation of EphB2 in comparison with control siRNA (lane 1). An siRNA that causes less of a decrease in LAR has a smaller effect on EphB2 phosphorylation (lane 3). (d) Immunoprecipitated EphB2 (with or without prior activation by ephrinB1) was incubated with or without the cytoplasmic domain of LAR before Western blot analysis. There is a major decrease in EphB2 phosphorylation after incubation with LAR (left) compared with controls not incubated with LAR (right). (e) The time course of ephrinB1-induced phosphorylation of EphB2 was determined with (left) or without (right) prior knockdown of LAR. The results are shown quantitated in the graph on the right. Knockdown of LAR leads to a major increase in the ephrinB1-induced phosphorylation of EphB2. Error bars indicate range (n = 3). PiTyr, phosphorylated tyrosine.
Clues to a potential mechanism came from a study showing that the leukocyte common antigen–related (LAR) receptor tyrosine phosphatase is down-regulated after FGFR1 activation in NBT-II carcinoma cells (Billottet et al., 2004) and a study showing that Eph receptor and LAR homologues act synergistically in morphogenesis in Caenorhabditis elegans (Harrington et al., 2002). Therefore, we tested whether LAR dephosphorylates EphB2. We first analyzed whether FGFR1 activation affects LAR expression in HEK293 cells and found that there was a progressive down-regulation of LAR protein, which is concurrent with the increase in EphB2 phosphorylation (Fig. 4 b). Because there was no down-regulation of LAR mRNA (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200807151/DC1), the decrease in LAR protein may occur through posttranslational mechanisms (e.g., proteolytic cleavage as occurs after EGF receptor activation; Ruhe et al., 2006). Second, we performed siRNA-mediated knockdowns to inhibit LAR expression and found that these lead to an increase in the baseline (Fig. 4 c) and ephrinB1-activated (Fig. 4 e) phosphorylation of EphB2. Finally, we tested whether LAR phosphatase acts directly on EphB2 by coincubation of the cytoplasmic domain of LAR with immunoprecipitated phospho-EphB2 followed by Western blot analysis. We found that LAR dephosphorylates EphB2 in vitro (Fig. 4 d). Collectively, these findings suggest that LAR dephosphorylates EphB2 and that down-regulation of LAR expression by activated FGFR1 underlies the increase in the baseline phosphorylation of EphB2.
FGFR1 inhibits ephrin-mediated activation of EphB2
We analyzed whether the presence of activated FGFR1 affects the phosphorylation of EphB2 upon interaction with ephrinB1 cells. In contrast to the effect on baseline EphB2 phosphorylation, ephrin-induced phosphorylation is inhibited in the presence of activated FGFR1; after activation by ephrinB1 cells or ephrinB1-Fc, there is a less than twofold further increase in EphB2 phosphorylation that reaches a maximal level of ∼50% of that achieved in the absence of FGFR1 (Fig. 5, a and b).
Figure 5.
Effect of FGFR on ephrin-induced activation of EphB2 and MAPK. Immunoprecipitation and Western blots analyses were performed to determine the effect of prior iFGFR activation on the time course of ephrinB1-induced phosphorylation of EphB2 and activation of the MAPK pathway. (a) After iFGFR activation, there is a lower maximal phosphorylation of EphB2 (left) after incubation with ephrinB1 cells compared with in the absence of iFGFR (right); quantitation is shown in the graph on the right. (b) Analogous experiments were performed by activating EphB2 with soluble ephrinB1-Fc; in controls, Fc alone does not activate EphB2. Similar results are obtained as for activation by ephrinB1 cells, arguing against the possibility that the effect of iFGFR on EphB2 activation is secondary to altered cell–cell contacts. (c) Detection of ERK phosphorylation reveals that EphB2 activates the MAPK pathway (black line), but after iFGFR activation, EphB2 instead inhibits the MAPK pathway (red line). For Western blots, see Fig. 7. Error bars indicate range (n = 3). PiTyr, phosphorylated tyrosine.
It was possible that the inhibition of activation by ephrinB1 is a consequence of the increased baseline phosphorylation of EphB2 that could, for example, promote receptor endocytosis so that less is available at the cell surface for interaction with ephrin. However, there is a major increase in ephrinB1-induced EphB2 phosphorylation after LAR knockdown (Fig. 4 e). Therefore, the inhibitory effect of FGFR1 on ephrin-induced EphB2 activation is not caused by a prior increase in EphB2 autoactivation; on the contrary, the inhibitory mechanism dominates over the increase in EphB2 phosphorylation that would otherwise occur because of decreased LAR.
A MAPK feedback loop promotes EphB2 activation
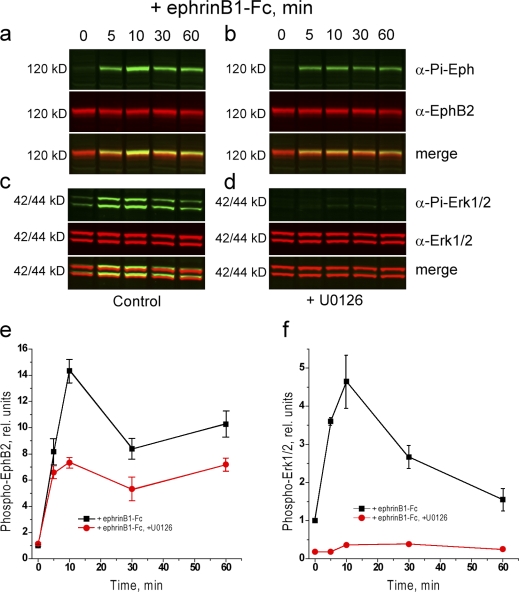
Our findings can be explained by a model in which inhibition of the MAPK pathway by EphB2 is required for repulsion (Elowe et al., 2001; Tong et al., 2003), and the antagonistic effect of FGFR1 is caused by activation of MAPK. However, we unexpectedly found that in the absence of prior activation of FGFR1, EphB2 activates the MAPK pathway (Fig. 5 c and see Fig. 7, a and c). In contrast, after FGFR1 activation, there is a decrease in MAPK phosphorylation upon activation of EphB2 by ephrinB1 (Fig. 5 c). These findings suggest that EphB2-mediated cell repulsion does not require inhibition of MAPK. On the contrary, cell repulsion and segregation occurs in EphB2 cells in which EphB2 activates MAPK but not in EphB2+iFGFR cells in which EphB2 inhibits MAPK. This raised the possibility that MAPK activation is required for EphB2-mediated cell repulsion. To test this, we analyzed the effect of blocking the MAPK pathway on the response of EphB2 cells. We found that incubation of cells with U0126, an inhibitor of MAPK/extracellular signal-regulated kinase (ERK) kinase (MEK), inhibits the collapse of EphB2 cells that normally occurs upon activation with soluble ephrinB1-Fc (Fig. 6, a and b), similar to the inhibitory effect of FGFR1 activation (Fig. 6 c).
Figure 7.
Effect of blocking MAPK on EphB2 activation. Immunoprecipitation and Western blot analyses were performed to determine the effect on inhibition of the MAPK pathway with U0126 on the ephrinB1-induced activation of EphB2. The level of EphB2 activation is decreased in the presence of 20 μM U0126 (a and b; quantitation shown in e). The activation of MAPK by EphB2 and blockade by U0126 are shown in c and d; quantitation is shown in f. Error bars indicate range (n = 3). Pi, phosphorylated.
Figure 6.
Effect on blocking MAPK on cell response to EphB2 activation. (a–c) Collapse assays were performed to determine the effect of ephrinB1-Fc on EphB2+GFP cells in the absence (a) or presence (b) of blocking ERK activation with 20 μM U0126 or after iFGFR activation (c). The cells were incubated at 4°C with Qdot605-labeled anti-EphB2 antibodies to label cell surface EphB2. The cells were incubated with culture medium at 37°C containing 1 μg/ml ephrinB1-Fc, and time-lapse images were collected. Control EphB2 cells undergo collapse after the addition of ephrinB1-Fc but do not collapse when the MAPK pathway is blocked or after activation of iFGFR (right).
By analogy with other receptors (Campbell and Holt, 2003; Piper et al., 2006), the activation of MAPK by EphB2 could have a direct role in cell collapse responses. An alternative possibility that MAPK increases EphB2 activation seemed inconsistent with our finding that FGFR1 (which activates MAPK) inhibits ephrinB1-induced EphB2 phosphorylation. Nevertheless, we tested this possibility and found that blocking of MEK activation with U0126 (Fig. 7, a, b, and e) or MEK inhibitor 1 (not depicted) leads to a decrease in ephrinB1-induced phosphorylation of EphB2. Similarly, we found that U0126 inhibits the activation of EphB2 in transfected HeLa cells (unpublished data), indicating that this relationship with MAPK occurs in other cell types. The finding that EphB2 activates MAPK, which in turn is required to achieve high levels of EphB2 phosphorylation, suggests that a positive feedback loop mediated by MAPK promotes EphB2 activation.
Effect of FGFR target genes on EphB2-mediated cell repulsion
Our findings present an apparent paradox that activation of FGFR1 (which activates MAPK) has the same effect on EphB2 activation and cell repulsion responses as the inhibition of MAPK. One potential explanation is that FGFR activation induces the expression of feedback antagonists of the MAPK pathway, including Sprouty genes (Casci et al., 1999; Kim and Bar-Sagi, 2004; Mason et al., 2006), which could inhibit MAPK activation by EphB2. We analyzed the expression of Sprouty family members and found that Sprouty2 and Sprouty4 are induced upon iFGFR activation (Fig. S1).
To test whether Sprouty2 or Sprouty4 alter cell responses and MAPK activation by EphB2, we transfected Sprouty expression constructs into EphB2 cells and incubated these with ephrinB1-Fc. We concurrently visualized the dynamics of receptor endocytosis by prelabeling cell surface EphB2 with Qdot605-labeled anti-EphB2 antibody. We found that neither the collapse response of EphB2 cells to ephrinB1 nor the activation of MAPK by EphB2 was altered significantly by the expression of Sprouty genes (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200807151/DC1). However, MAPK activation by FGFR1 was also not affected by Sprouty2 or Sprouty4 (Fig. S2). Several previous studies have similarly found that in some cell types, Sprouty proteins do not inhibit the MAPK pathway, suggesting that this activity of Sprouty is context dependent (Egan et al., 2002; Wong et al., 2002; Rubin et al., 2003; Choi et al., 2006). Therefore, we considered the possibility that other transcriptional targets of FGFR are required for Sprouty to inhibit MAPK activation and thus decrease EphB2-mediated cell repulsion.
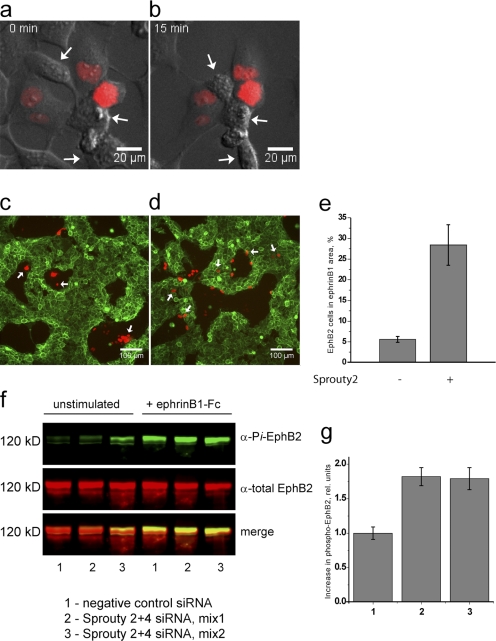
In the absence of iFGFR activator, EphB2+iFGFR cells express a low level of FGFR1 target genes such as Sprouty2 (Fig. S1), which is presumably caused by a low amount of autoactivation, but still respond to ephrinB1 in collapse (Fig. 8, a and b) and segregation assays (Fig. 8 c). Therefore, we tested whether the collapse and segregation responses of these cells to ephrinB1 are altered by overexpression of Sprouty2. We found that the collapse response is inhibited in Sprouty-expressing cells (coexpressing red fluorescent protein [RFP] reporter) but still occurs in nontransfected cells (Fig. 8, a and b). To assess the effect on cell segregation, we mixed ephrinB1 cells with EphB2+iFGFR cells, some of which are transfected with control (RFP) or Sprouty2+RFP expression vector. We found that although all control-transfected EphB2 cells segregated (Fig. 8 c), many Sprouty2-transfected EphB2 cells failed to segregate or were located at the interface with ephrinB1 cells (Fig. 8 d). These findings show that Sprouty2 inhibits cell repulsion and segregation responses to ephrinB1 in the context of prolonged low level FGFR1 activation.
Figure 8.
Effect of Sprouty2 expression of cell responses to EphB2 activation. (a–d) EphB2+iFGFR cells in the absence of iFGFR activator were used to analyze the effect of overexpressing Sprouty2 on collapse (a and b) and segregation (c and d) responses to ephrinB1 (see the Results for an explanation of why EphB2-iFGFR cells were used). (a and b) In collapse assays, EphB2+iFGFR cells were transfected with vector coexpressing Sprouty2 and nuclear-targeted RFP. After the addition of ephrinB1-Fc, >90% of nontransfected cells undergo collapse (arrows), whereas only 27% of Sprouty2/RFP-expressing cells undergo collapse. (c) In control cell segregation assays, EphB2+iFGFR cells were transfected with vector expressing RFP and were mixed with ephrinB1+GFP cells. All RFP-expressing cells cosegregate with nontransfected EphB2 cells. (d and e) Cell segregation assays were performed with EphB2+iFGFR cells transfected with Sprouty2+RFP. Many Sprouty2/RFP-expressing cells remain in or are at the interface of ephrinB2+GFP territory and have thus failed to cosegregate with nontransfected EphB2 cells (quantified by counting the number of cells in e). (f and g) The role of FGFR-induced Sprouty genes in the inhibition of EphB2 activation was analyzed by siRNA knockdown. f shows Western blots, and g shows the quantitations of the increase in phospho-EphB2 normalized to the negative control siRNA. Knockdown with two independent sets of Sprouty2 and Sprouty4 siRNAs leads to an increase in ephrinB1-induced EphB2 phosphorylation in EphB2+iFGFR cells (with iFGFR activator) compared with control siRNA. (e and g) Error bars indicate SEM (n = 4). Pi, phosphorylated.
A model in which Sprouty expression downstream of FGFR inhibits the activation of EphB2 predicts that knockdown of Sprouty will alleviate the effect of FGFR activation on EphB2 phosphorylation. To test this, we performed knockdowns of Sprouty2 plus Sprouty4 in activated EphB2+iFGFR cells and determined the amount of ephrinB1-induced phosphorylation of EphB2. We found that knockdowns of Sprouty2 plus Sprouty4 with two sets of siRNAs each led to an ∼1.8-fold increase in EphB2 phosphorylation compared with the amount in the presence of control siRNA (Fig. 8, f and g).
Discussion
The control of cell migration requires the integration of diverse signals that promote attraction or repulsion. Because FGFR activation can increase the migration and invasiveness of cells during development and tumor metastasis, we tested whether FGFR activation affects the ability of Eph–ephrin signaling to restrict the intermingling of cells across boundaries. We found that activation of FGFR1 in EphB2-expressing cells inhibits the segregation of EphB2 cells from ephrinB1 cells and decreases the repulsion response of EphB2 cells to ephrinB1.
Such antagonism between different receptors can be mediated by cross-inhibition of receptor activity and/or by receptors having opposite effects on a downstream signal transduction pathway. We found that the activation of EphB2 by ephrinB1 is decreased in the presence of activated FGFR1, reaching a maximal level 30–50% of that achieved in the absence of FGFR. A potential significance of this finding is suggested by studies showing that high levels of Eph receptor activation are required for de-adhesion or growth cone repulsion, whereas at lower levels of activation there is increased integrin-mediated cell adhesion or migration of growth cones (Huynh-Do et al., 1999; Hansen et al., 2004). Consistent with this, a decrease in Eph receptor activation because of cis-inhibition by coexpressed ephrin (Hornberger et al., 1999; Carvalho et al., 2006) or truncated Eph receptor (Holmberg et al., 2000) is associated with decreased growth cone repulsion or increased cell adhesion. Therefore, FGFR activation may decrease the repulsion of EphB2 cells by ephrinB1 at least in part by inhibition of EphB2 activation.
We found that FGFR activation also leads to a slow increase in the baseline phosphorylation of EphB2 in the absence of ephrinB1 and show that this is caused by down-regulation of the receptor tyrosine phosphatase LAR, which dephosphorylates EphB2 and limits the amount of activation by ephrin. Similarly, the receptor tyrosine phosphatase Ptpro has been found to dephosphorylate Eph receptors (Shintani et al., 2006). These receptor phosphatases may counteract the autoactivation of Eph receptors to maintain a low baseline phosphorylation and limit the amount and duration of Eph receptor phosphorylation after activation by ephrin.
Role of MAPK in cell responses to EphB2 activation
Our experiments raise the question of how FGFR activation leads to a decrease in EphB2 activation by ephrinB1. A potential explanation came from our finding that EphB2 activates the Ras–MAPK pathway and that this pathway in turn is required for high levels of EphB2 activation and for a cell collapse response to clustered ephrinB1. The effects of FGFR on sorting and collapse responses of EphB2 cells to ephrinB1 are mimicked by the expression of a transcriptional target of FGFR signal transduction, Sprouty2, that is a feedback antagonist of the Ras–MAPK pathway. Furthermore, we find that knockdown of Sprouty2 plus Sprouty4 alleviates the inhibitory effect of FGFR on EphB2 activation. These findings suggest that a MAPK-dependent positive feedback loop increases the amount of EphB2 activation. One potential model is that MAPK activity promotes repulsion responses of EphB2 cells to ephrinB1 solely by increasing EphB2 activation via this feedback loop. It is also possible that the MAPK pathway is required for cell repulsion independently of its effect on EphB2 activation, and indeed, MAPK is involved in growth cone repulsion by Slit (Campbell and Holt, 2003; Piper et al., 2006).
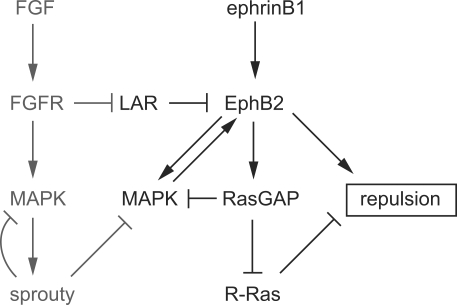
Previous studies have shown that binding of Ras–GTPase-activating protein (GAP) to EphB2 is essential for a neurite repulsion response in differentiated NG108 cells (Elowe et al., 2001; Tong et al., 2003). Because RasGAP inhibits the MAPK pathway, these findings suggested that the inhibition of MAPK is required for neurite repulsion, whereas in our assays, inhibition of MAPK leads to a loss of cell repulsion. One possible explanation for the difference is that there is a distinct role of MAPK in repulsion responses of HEK293 cell processes compared with neurites. Indeed, in some contexts MAPK is required for growth cone attraction and promotes cell migration (Perron and Bixby, 1999; Sahai et al., 2001; Campbell and Holt, 2003; Huang et al., 2004), and thus inhibition of MAPK may be required for repulsion. Alternatively, it may not be the ability of RasGAP to inhibit MAPK that is required for neurite repulsion downstream of EphB2 but rather that RasGAP inhibits R-Ras, a promoter of cell adhesion and migration (Dail et al., 2006). Collectively, these observations suggest a model in which a cell repulsion response to EphB2 is enabled by a MAPK-dependent positive feedback on receptor activation, which then acts through pathways that include the inhibition of R-Ras by RasGAP (Fig. 9). A need to sufficiently inhibit R-Ras could contribute to the requirement for a threshold level of Eph receptor activation to achieve cell repulsion (Dail et al., 2006). Because RasGAP inhibits MAPK, competing positive and negative loops may modulate the dynamics of EphB2 and MAPK activation (Fig. 9). Our finding that after FGFR activation EphB2 inhibits rather than activates the MAPK pathway can be explained by a shift toward the inhibitory pathway caused by the presence of Sprouty. A difficulty with this model is that even after induction of Sprouty2, FGFR activation leads to a net increase in MAPK activity that would be predicted to promote EphB2 activation. A possible explanation is that FGFR and EphB2 do not appear to interact and thus may activate MAPK in different subcellular compartments, whereas Sprouty2 can inhibit MAPK downstream of EphB2. In contrast, FGFR and EphA4, which cross-activate each other, do form a complex (Yokote et al., 2005). Therefore, we propose that positive feedback requires MAPK activation local to EphB2. However, Eph receptors can inhibit MAPK downstream of other receptors (Miao et al., 2001; Kim et al., 2002; Picco et al., 2007), and it will therefore be important to analyze the spatial localization of MAPK activation and inhibition.
Figure 9.
Model of relationship between EphB2, FGFR, MAPK, and cell repulsion. The diagram depicts relationships deduced in the current study integrated with the results of other studies described in the Discussion. Baseline activation of EphB2 is kept low by LAR and other receptor PTPs, which also limit the amount of ephrin-induced phosphorylation. LAR is down-regulated by FGFR1 activation. In the absence of FGFR1 activation, there is a high level of activation of EphB2 by ephrinB1 involving a positive feedback loop mediated by the MAPK pathway; this positive loop is limited by the inhibition of MAPK by RasGAP. EphB2 activation triggers multiple pathways that promote cell repulsion, one of which involves the inhibition of R-Ras by RasGAP. After induction of Sprouty genes downstream of FGFR, there is inhibition of the positive MAPK loop downstream of EphB2. This shifts the response to inhibition of MAPK by EphB2 and a lower level of EphB2 activation and cell repulsion.
A question that now needs to be addressed is the mechanism of positive feedback of the MAPK pathway on EphB2 activation. Studies of other receptors suggest many candidate mechanisms, including increased translation, trafficking or recycling that maintains the level of cell surface receptor, or modulation of a tyrosine phosphatase or kinase that affects receptor phosphorylation (Biscardi et al., 1999; Ekman et al., 2002; Ming et al., 2002; Agazie and Hayman, 2003; Campbell and Holt, 2003; Haj et al., 2003; Marsh et al., 2003; Reynolds et al., 2003; Qin et al., 2005; Piper et al., 2006; Stetak et al., 2006).
Effect of FGFR activation and Sprouty2 on cell responses to EphB2
We found that in EphB2 cells, overexpressed Sprouty2 does not inhibit MAPK activation downstream of EphB2 (or FGFR), and this accounts for its lack of effect on cell responses to ephrinB1. Several studies have shown that in some situations, Sprouty proteins can even promote rather than inhibit MAPK activation (Egan et al., 2002; Wong et al., 2002; Rubin et al., 2003) and that the positive or negative effect of Sprouty can depend on the cell differentiation status (Choi et al., 2006). The ability of Sprouty2 to inhibit the MAPK pathway may therefore require unidentified cofactors or substrates that have restricted expression. We found that Sprouty2 did inhibit ephrinB1-induced collapse and cell sorting when expressed in EphB2+iFGFR cells in the absence of iFGFR activator. Because there is low level induction of FGFR target genes in these cells, this finding suggests that one or more of these targets is required to enable Sprouty2 to inhibit the MAPK pathway.
Potential role of feedback control of EphB2 activation
Our findings raise the question of the role of a positive feedback loop in EphB2 activation. Modeling and experimental analysis of signal transduction pathways has shown that positive feedback loops lead to bistability in which a progressively increasing amount of input leads to a sharp transition from a low to high level of output (Santos et al., 2007). It can be envisaged that such a switch would ensure that once activation is sufficient to counteract the effect of receptor phosphatases and initiate positive feedback, this would rapidly lead to maximal EphB2 activation and the appropriate cell response. Our data suggest that EphB2 both activates and inhibits MAPK and that the balance shifts toward inhibition and lack of positive feedback if other MAPK inhibitors, such as Sprouty, are present. This context-dependent relationship can explain why different studies have found that EphB2 can inhibit (Elowe et al., 2001) or activate (Zisch et al., 2000) the MAPK pathway. Achieving the appropriate relationship is likely to be functionally important because inhibition or activation of the MAPK pathway by Eph receptors has been implicated in control of the differentiation, migration, and proliferation of cells (Miao et al., 2001; Vindis et al., 2003; Aoki et al., 2004; Corrigan et al., 2005; Picco et al., 2007).
Materials and methods
Antibodies and reagents
Anti-EphB2 antibodies and recombinant ephrinB1-Fc chimera were obtained from R&D Systems, and antiphospho-Eph receptor antiserum was obtained from C. Nobes (University of Bristol, Bristol, England, UK). Antidiphosphorylated ERK1/2 antibody, anti-ERK1/2 antiserum, anti–β-catenin antiserum, anti-Flag M2 antibody, basic FGF, and fibronectin were obtained from Sigma-Aldrich, anti-HA antibody was obtained from Roche, antiphosphotyrosine antibody was obtained from Millipore, and anti-FRBP12 antibody was obtained from Thermo Fisher Scientific. Secondary donkey anti–mouse, anti–rabbit, and anti–goat antibodies conjugated to IRDye700 and IRDye800 were obtained from Rockland Immunologicals, Cy3-conjugated AffiniPure F(ab′)2 fragment donkey anti–goat IgG and AffiniPure Fab fragment rabbit anti–goat IgG were obtained from Jackson ImmunoResearch Laboratories, and Qdot605 Antibody Conjugation kit was obtained from Tebu-Bio. AP20187 reagent to dimerize iFGFR was supplied by ARIAD Pharmaceuticals. MEK inhibitor 1, U0126, and recombinant catalytic domain D1 of LAR were obtained from EMD.
Plasmid constructs
Constructs were provided as follows: human Sprouty4 from C.J.M. de Vries (Academic Medical Center, Amsterdam, Netherlands); human Sprouty2 from G.R. Guy (Institute of Molecular and Cell Biology, Singapore); H2B-mRFP from S. Megason and S. Fraser (California Institute of Technology, Pasadena, CA); iFGFR from E. Pownall (University of York, York, England, UK); EphB2 K662R mutant from R. Klein (Max Planck Institute of Neurobiology, Martinsried, Germany); and mouse EphB2 and ephrinB1 as described previously (Mellitzer et al., 1999).
Cell culture and cell behavior assays
HEK293 cells were cultured and visualized during time-lapse experiments at 37°C with 5% CO2 in DME supplemented with 10% FCS, glutamine, and antibiotics. Stable HEK293 cell lines expressing EphB2, EphB2 plus membrane-targeted GFP (EphB2+GFP), EphB2 plus iFGFR1 (EphB2+iFGFR1), EphB2 plus iFGFR1 and membrane-anchored GFP (EphB2+iFGFR1+GFP), and ephrinB1 were generated using selection with G418 and/or hygromycin. Dissociated cell suspensions were made using Accutase (PAA Laboratories) for cell segregation assays or enzyme-free cell dissociation buffer (Invitrogen) for time-lapse studies. After washing with culture medium, two cell lines were mixed in equal proportion and plated onto a fibronectin-coated coverglass system (chambered 1.0 borosilicate; Lab-Tek). Cells were visualized using an RT live-imaging workstation (Deltavision; Applied Precision, LLC) on a microscope (IX-70; Olympus) with a charge-coupled device camera (MicroMax 1300 YHS; Roper Scientific) in a heated environmental chamber (37°C; 5% CO2). For cell segregation assays, the cultures were plated at 65,000 cells/cm2 and grown for 3–4 d until confluence. Images of segregated cells were taken with a 10×/0.4 NA objective (Olympus) and GFP filter set (Chroma Technology Corp.). Quantitation of segregation was performed by the nearest neighbor method (Mochizuki et al., 1998). For time-lapse studies of cell interactions, cells were plated at 20,000 cells/cm2, and initial cell–cell contacts were visualized using fluorescence (GFP filter set) and phase-contrast optics with a 40×/0.6 NA objective (Olympus).
For collapse assays, EphB2-expressing cells were plated 24 h before the experiment at 50,000 cells/cm2 and prelabeled with a 1:1 molar mixture of goat anti-EphB2 antibodies and Cy3-conjugated anti–goat IgG or Qdot605-conjugated anti–goat Fab fragments for 10 min at 4°C. After extensive washing, warm culture medium including 1 μg/ml ephrinB1-Fc chimera was added, and time-lapse images of cell behavior were captured using a 40×/0.6 NA objective.
To activate iFGFR, 100 nM AP20187 was included in the cell culture medium during segregation assays or added 24 h before carrying out time-lapse and collapse assays. To study effects of Sprouty genes, transient transfection of cells with Sprouty2, Sprouty4, and H2B-mRFP cDNA was performed using Fugene 6 transfection reagent (Roche). Time-lapse and still images were collected using Softworx acquisition software (Applied Precision, LLC). Videos of cell behavior were created using ImageJ software (National Institutes of Health).
siRNA knockdowns
Knockdown of LAR–protein tyrosine phosphatase (PTP) or of Sprouty expression in HEK293 cells was performed by using Silencer siRNA oligonucleotides against human PTP receptor type F (LAR-PTP) or Sprouty2 and Sprouty4 from Applied Biosystems. In each experiment, a nontargeting siRNA was used as control. Transfection of siRNAs into cells was performed for 24 h with X-tremeGENE siRNA Transfection Reagent (Roche) according to the manufacturer's recommendations. The extent of knockdown after 48–72 h was analyzed by using antibodies or RT-PCR.
Phosphorylation analysis
EphB2-expressing cells grown on culture dishes were stimulated with a suspension of ephrinB1-expressing cells or with 1 μg/ml of soluble ephrinB1-Fc chimera and/or 100 nM AP20187 to activate iFGFR. Phosphorylation of EphB2, ERK1/2, and iFGFR1 in cell lysates or direct Western blotting was detected simultaneously by using phosphospecific antibodies. Levels of phosphorylation were normalized using antibodies for total protein.
In vitro dephosphorylation assay
EphB2-expressing HEK293 cells were left untreated or were stimulated with 1 μg/ml ephrinB1-Fc for 30 min, and EphB2 was immunoprecipitated from cell lysates (as described in the previous paragraph). After extensive washes, half of each immunoprecipitate was incubated with ∼5 U of the soluble catalytic domain of LAR in dephosphorylation buffer (20 mM Hepes, pH 7.4, 10 mM dithiothreitol, 150 mM NaCl, and 2 mM EDTA) for 1 h at 37°C. The other half of the immunoprecipitates was incubated in the absence of LAR. The reaction was stopped by adding sample buffer, proteins were separated by SDS-PAGE, and the tyrosine phosphorylation state of the proteins was analyzed by Western blotting.
Quantitative Western blot assays
Cells were chilled on ice and lysed in cell lysis buffer (1% NP-40, 20 mM Hepes, pH 7.4, 100 mM NaCl, 10 mM Na4P2O7, 1 mM CaCl2, 1 mM MgCl2, Halt protease inhibitor cocktail [Thermo Fisher Scientific], and phosphatase inhibitor cocktail set II [EMD]) for 10 min at 4°C. Cell lysates were centrifuged at 16,000 g for 10 min at 4°C. Protein concentration in cell lysates was measured using bicinchoninic acid protein assay (Thermo Fisher Scientific). After equalizing protein concentration with lysis buffer, lysates were immunoprecipitated or run directly on SDS-PAGE. Immunoprecipitation was performed with antibodies prebound to protein G Sepharose 4 Fast Flow (GE Healthcare) for 1–2 h at 4°C, which was washed as follows: lysis buffer, twice in lysis buffer containing 0.85 M NaCl, lysis buffer, and 10 mM Hepes, pH 7.0, all containing 2 mM sodium orthovanadate. Proteins bound to protein G Sepharose were eluted with 2× SDS sample buffer for 10 min at 70°C and run on SDS-PAGE. Transfer of proteins from gels on Immobilon-FL membranes (Millipore) was performed using XCell II Blot module (Invitrogen). Detection and quantification were performed using the Infrared Imaging System protocol (Odyssey; LI-COR Biosciences). The membranes were stained simultaneously with pairs of primary and secondary antibodies conjugated to infrared fluorescent dyes IR700 and IR800. After staining, the membranes were scanned using 700- and 800-nm channels on an imager (Odyssey), and the intensity of staining was determined using the median background method.
Online supplemental material
Fig. S1 shows the expression of Sprouty2, Sprouty4, and LAR-PTP mRNA in EphB2 cells and in EphB2+iFGFR cells in the absence or presence of AP20187. Fig. S2 shows the lack of effect of Sprouty2 and Sprouty4 on EphB2 and MAPK activation in EphB2 cells. Videos 1–3 show the effect of FGFR on EphB2 cell repulsion. Video 1 is a negative control showing the interaction between an EphB2+GFP cell and an EphB2 cell corresponding to Fig. 1 d. Video 2 shows the interaction between an EphB2+GFP cell and an ephrinB1 cell corresponding to still images in Fig. 1 e. Video 3 shows the interaction between an EphB2+iFGFR+GFP cell and an ephrinB1 cell corresponding to still images in Fig. 1 f. Videos 4 and 5 show the effect of FGFR on EphB2 cell collapse. Video 4 shows the collapse response of EphB2 cells to ephrinB1-Fc corresponding to still images in Fig. 2 a. Video 5 shows the collapse response of EphB2+iFGFR cells to ephrinB1-Fc corresponding to still images in Fig. 2 b. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200807151/DC1.
Acknowledgments
We thank A. Gould, J.-P. Vincent, Q. Xu, S. Gerety, R. Gonzalez-Quevedo, and F. Prin for helpful comments on the manuscript and C. Nobes, E. Pownall, R. Klein, S. Fraser, S. Megason, C. de Vries, G. Guy, and ARIAD Pharmaceuticals for constructs and reagents.
M.L. Cotrina's present address is Dept. of Neurosurgery, Division of Glial Disease and Therapeutics, University of Rochester Medical Center, Rochester, NY 14642.
A. Pasini's present address is Laboratoire de Genetique et Physiologie du Developpement, Institute de Biologie du Developpement de Marseille, F-13288 Marseille, Cedex 09, France.
Abbreviations used in this paper: ERK, extracellular signal-regulated kinase; FGFR, FGF receptor; GAP, GTPase-activating protein; iFGFR, inducible FGFR; LAR, leukocyte common antigen related; MEK, MAPK/ERK kinase; PTP, protein tyrosine phosphatase.
References
- Agazie, Y.M., and M.J. Hayman. 2003. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol. Cell. Biol. 23:7875–7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, M., T. Yamashita, and M. Tohyama. 2004. EphA receptors direct the differentiation of mammalian neural precursor cells through a mitogen-activated protein kinase-dependent pathway. J. Biol. Chem. 279:32643–32650. [DOI] [PubMed] [Google Scholar]
- Billottet, C., N. Elkhatib, J.P. Thiery, and J. Jouanneau. 2004. Targets of fibroblast growth factor 1 (FGF-1) and FGF-2 signaling involved in the invasive and tumorigenic behavior of carcinoma cells. Mol. Biol. Cell. 15:4725–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscardi, J.S., M.C. Maa, D.A. Tice, M.E. Cox, T.H. Leu, and S.J. Parsons. 1999. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 274:8335–8343. [DOI] [PubMed] [Google Scholar]
- Campbell, D.S., and C.E. Holt. 2003. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 37:939–952. [DOI] [PubMed] [Google Scholar]
- Carvalho, R.F., M. Beutler, K.J. Marler, B. Knoll, E. Becker-Barroso, R. Heintzmann, T. Ng, and U. Drescher. 2006. Silencing of EphA3 through a cis interaction with ephrinA5. Nat. Neurosci. 9:322–330. [DOI] [PubMed] [Google Scholar]
- Casci, T., J. Vinos, and M. Freeman. 1999. Sprouty, an intracellular inhibitor of Ras signaling. Cell. 96:655–665. [DOI] [PubMed] [Google Scholar]
- Choi, H., S.Y. Cho, R.H. Schwartz, and K. Choi. 2006. Dual effects of Sprouty1 on TCR signaling depending on the differentiation state of the T cell. J. Immunol. 176:6034–6045. [DOI] [PubMed] [Google Scholar]
- Chong, L.D., E.K. Park, E. Latimer, R. Friesel, and I.O. Daar. 2000. Fibroblast growth factor receptor-mediated rescue of x-ephrinB1induced cell dissociation in Xenopus embryos. Mol. Cell. Biol. 20:724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan, C., R. Subramanian, and M.A. Miller. 2005. Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development. 132:5225–5237. [DOI] [PubMed] [Google Scholar]
- Dail, M., M. Richter, P. Godement, and E.B. Pasquale. 2006. Eph receptors inactivate R-Ras through different mechanisms to achieve cell repulsion. J. Cell Sci. 119:1244–1254. [DOI] [PubMed] [Google Scholar]
- Egan, J.E., A.B. Hall, B.A. Yatsula, and D. Bar-Sagi. 2002. The bimodal regulation of epidermal growth factor signaling by human Sprouty proteins. Proc. Natl. Acad. Sci. USA. 99:6041–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman, S., A. Kallin, U. Engstrom, C.H. Heldin, and L. Ronnstrand. 2002. SHP-2 is involved in heterodimer specific loss of phosphorylation of Tyr771 in the PDGF beta-receptor. Oncogene. 21:1870–1875. [DOI] [PubMed] [Google Scholar]
- Elowe, S., S.J. Holland, S. Kulkarni, and T. Pawson. 2001. Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol. Cell. Biol. 21:7429–7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan, J.G., and P. Vanderhaeghen. 1998. The ephrins and Eph receptors in neural development. Ann. Rev. Neurosci. 21:309–345. [DOI] [PubMed] [Google Scholar]
- Gale, N.W., S.J. Holland, D.M. Valenzuela, A. Flenniken, L. Pan, T.E. Ryan, M. Henkemeyer, K. Strebhardt, H. Hirai, D.G. Wilkinson, et al. 1996. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 17:9–19. [DOI] [PubMed] [Google Scholar]
- Haj, F.G., B. Markova, L.D. Klaman, F.D. Bohmer, and B.G. Neel. 2003. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J. Biol. Chem. 278:739–744. [DOI] [PubMed] [Google Scholar]
- Hansen, M.J., G.E. Dallal, and J.G. Flanagan. 2004. Retinal axon response to ephrin-As shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron. 42:717–730. [DOI] [PubMed] [Google Scholar]
- Harrington, R.J., M.J. Gutch, M.O. Hengartner, N.K. Tonks, and A.D. Chisholm. 2002. The C. elegans LAR-like receptor tyrosine phosphatase PTP-3 and the VAB-1 Eph receptor tyrosine kinase have partly redundant functions in morphogenesis. Development. 129:2141–2153. [DOI] [PubMed] [Google Scholar]
- Holland, S.J., N.W. Gale, G. Mbamalu, G.D. Yancopoulos, M. Henkemeyer, and T. Pawson. 1996. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 383:722–725. [DOI] [PubMed] [Google Scholar]
- Holmberg, J., D.L. Clarke, and J. Frisen. 2000. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 408:203–206. [DOI] [PubMed] [Google Scholar]
- Hornberger, M.R., D. Dutting, T. Ciossek, T. Yamada, C. Handwerker, S. Lang, F. Weth, J. Huf, R. Wessel, C. Logan, et al. 1999. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron. 22:731–742. [DOI] [PubMed] [Google Scholar]
- Huang, C., K. Jacobson, and M.D. Schaller. 2004. MAP kinases and cell migration. J. Cell Sci. 117:4619–4628. [DOI] [PubMed] [Google Scholar]
- Huber, A.B., A.L. Kolodkin, D.D. Ginty, and J.F. Cloutier. 2003. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 26:509–563. [DOI] [PubMed] [Google Scholar]
- Huynh-Do, U., E. Stein, A.A. Lane, H. Liu, D.P. Cerretti, and T.O. Daniel. 1999. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J. 18:2165–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine, K.D., and C. Rauskolb. 2001. Boundaries in development: formation and function. Annu. Rev. Cell Dev. Biol. 17:189–214. [DOI] [PubMed] [Google Scholar]
- Kim, H.J., and D. Bar-Sagi. 2004. Modulation of signalling by Sprouty: a developing story. Nat. Rev. Mol. Cell Biol. 5:441–450. [DOI] [PubMed] [Google Scholar]
- Kim, I., Y.S. Ryu, H.J. Kwak, S.Y. Ahn, J.L. Oh, G.D. Yancopoulos, N.W. Gale, and G.Y. Koh. 2002. EphB ligand, ephrinB2, suppresses the VEGF- and angiopoietin 1-induced Ras/mitogen-activated protein kinase pathway in venous endothelial cells. FASEB J. 16:1126–1128. [DOI] [PubMed] [Google Scholar]
- Kubota, Y., and K. Ito. 2000. Chemotactic migration of mesencephalic neural crest cells in the mouse. Dev. Dyn. 217:170–179. [DOI] [PubMed] [Google Scholar]
- Kullander, K., and R. Klein. 2002. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 3:475–486. [DOI] [PubMed] [Google Scholar]
- Marsh, H.N., C.I. Dubreuil, C. Quevedo, A. Lee, M. Majdan, G.S. Walsh, S. Hausdorff, F.A. Said, O. Zoueva, M. Kozlowski, et al. 2003. SHP-1 negatively regulates neuronal survival by functioning as a TrkA phosphatase. J. Cell Biol. 163:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston, D.J., S. Dickinson, and C.D. Nobes. 2003. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat. Cell Biol. 5:879–888. [DOI] [PubMed] [Google Scholar]
- Mason, J.M., D.J. Morrison, M.A. Basson, and J.D. Licht. 2006. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 16:45–54. [DOI] [PubMed] [Google Scholar]
- McFarlane, S., E. Cornel, E. Amaya, and C.E. Holt. 1996. Inhibition of FGF receptor activity in retinal ganglion cell axons causes errors in target recognition. Neuron. 17:245–254. [DOI] [PubMed] [Google Scholar]
- Mellitzer, G., Q. Xu, and D.G. Wilkinson. 1999. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 400:77–81. [DOI] [PubMed] [Google Scholar]
- Miao, H., B.R. Wei, D.M. Peehl, Q. Li, T. Alexandrou, J.R. Schelling, J.S. Rhim, J.R. Sedor, E. Burnett, and B. Wang. 2001. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell Biol. 3:527–530. [DOI] [PubMed] [Google Scholar]
- Miller, M.A., P.J. Ruest, M. Kosinski, S.K. Hanks, and D. Greenstein. 2003. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 17:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming, G.L., S.T. Wong, J. Henley, X.B. Yuan, H.J. Song, N.C. Spitzer, and M.M. Poo. 2002. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 417:411–418. [DOI] [PubMed] [Google Scholar]
- Mochizuki, A., N. Wada, H. Ide, and Y. Iwasa. 1998. Cell-cell adhesion in limb-formation, estimated from photographs of cell sorting experiments based on a spatial stochastic model. Dev. Dyn. 211:204–214. [DOI] [PubMed] [Google Scholar]
- Montell, D.J. 1999. The genetics of cell migration in Drosophila melanogaster and Caenorhabditis elegans development. Development. 126:3035–3046. [DOI] [PubMed] [Google Scholar]
- Moore, K.B., K. Mood, I.O. Daar, and S.A. Moody. 2004. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev. Cell. 6:55–67. [DOI] [PubMed] [Google Scholar]
- Palmer, A., M. Zimmer, K.S. Erdmann, V. Eulenburg, A. Porthin, R. Heumann, U. Deutsch, and R. Klein. 2002. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol. Cell. 9:725–737. [DOI] [PubMed] [Google Scholar]
- Pasini, A., and D.G. Wilkinson. 2002. Stabilizing the regionalisation of the developing vertebrate central nervous system. Bioessays. 24:427–438. [DOI] [PubMed] [Google Scholar]
- Pasquale, E.B. 2005. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 6:462–475. [DOI] [PubMed] [Google Scholar]
- Perron, J.C., and J.L. Bixby. 1999. Distinct neurite outgrowth signaling pathways converge on ERK activation. Mol. Cell. Neurosci. 13:362–378. [DOI] [PubMed] [Google Scholar]
- Picco, V., C. Hudson, and H. Yasuo. 2007. Ephrin-Eph signalling drives the asymmetric division of notochord/neural precursors in Ciona embryos. Development. 134:1491–1497. [DOI] [PubMed] [Google Scholar]
- Piper, M., R. Anderson, A. Dwivedy, C. Weinl, F. van Horck, K.M. Leung, E. Cogill, and C. Holt. 2006. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 49:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov, A., M. Cotrina, and D.G. Wilkinson. 2004. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 7:465–480. [DOI] [PubMed] [Google Scholar]
- Pownall, M.E., B.E. Welm, K.W. Freeman, D.M. Spencer, J.M. Rosen, and H.V. Isaacs. 2003. An inducible system for the study of FGF signalling in early amphibian development. Dev. Biol. 256:89–99. [DOI] [PubMed] [Google Scholar]
- Qin, Y., Y. Zhu, J.P. Baumgart, R.L. Stornetta, K. Seidenman, V. Mack, L. van Aelst, and J.J. Zhu. 2005. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 19:2000–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, A.R., C. Tischer, P.J. Verveer, O. Rocks, and P.I. Bastiaens. 2003. EGFR activation coupled to inhibition of tyrosine phosphatases causes lateral signal propagation. Nat. Cell Biol. 5:447–453. [DOI] [PubMed] [Google Scholar]
- Rubin, C., V. Litvak, H. Medvedovsky, Y. Zwang, S. Lev, and Y. Yarden. 2003. Sprouty fine-tunes EGF signaling through interlinked positive and negative feedback loops. Curr. Biol. 13:297–307. [DOI] [PubMed] [Google Scholar]
- Ruhe, J.E., S. Streit, S. Hart, and A. Ullrich. 2006. EGFR signaling leads to downregulation of PTP-LAR via TACE-mediated proteolytic processing. Cell. Signal. 18:1515–1527. [DOI] [PubMed] [Google Scholar]
- Sahai, E., M.F. Olson, and C.J. Marshall. 2001. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 20:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago, A., and C.A. Erickson. 2002. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development. 129:3621–3632. [DOI] [PubMed] [Google Scholar]
- Santos, S.D., P.J. Verveer, and P.I. Bastiaens. 2007. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol. 9:324–330. [DOI] [PubMed] [Google Scholar]
- Shintani, T., M. Ihara, H. Sakuta, H. Takahashi, I. Watakabe, and M. Noda. 2006. Eph receptors are negatively controlled by protein tyrosine phosphatase receptor type O. Nat. Neurosci. 9:761–769. [DOI] [PubMed] [Google Scholar]
- Steinberg, M.S., and M. Takeichi. 1994. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci. USA. 91:206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetak, A., E.F. Hoier, A. Croce, G. Cassata, P.P. Di Fiore, and A. Hajnal. 2006. Cell fate-specific regulation of EGF receptor trafficking during Caenorhabditis elegans vulval development. EMBO J. 25:2347–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., E.N. Meyers, M. Lewandoski, and G.R. Martin. 1999. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13:1834–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, M., T. Kamo, S. Ota, and H. Sugimura. 2003. Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J. 22:847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., and C.S. Goodman. 1996. The molecular biology of axon guidance. Science. 274:1123–1133. [DOI] [PubMed] [Google Scholar]
- Tong, J., S. Elowe, P. Nash, and T. Pawson. 2003. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J. Biol. Chem. 278:6111–6119. [DOI] [PubMed] [Google Scholar]
- Vindis, C., D.P. Cerretti, T.O. Daniel, and U. Huynh-Do. 2003. EphB1 recruits c-Src and p52Shc to activate MAPK/ERK and promote chemotaxis. J. Cell Biol. 162:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, S.E., K.K. Lee, M.K. Tang, and D.A. Ede. 1997. Fibroblast growth factors 2 and 4 stimulate migration of mouse embryonic limb myogenic cells. Dev. Dyn. 209:206–216. [DOI] [PubMed] [Google Scholar]
- Welm, B.E., K.W. Freeman, M. Chen, A. Contreras, D.M. Spencer, and J.M. Rosen. 2002. Inducible dimerization of FGFR1: development of a mouse model to analyze progressive transformation of the mammary gland. J. Cell Biol. 157:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, E.S., C.W. Fong, J. Lim, P. Yusoff, B.C. Low, W.Y. Langdon, and G.R. Guy. 2002. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J. 21:4796–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokote, H., K. Fujita, X. Jing, T. Sawada, S. Liang, L. Yao, X. Yan, Y. Zhang, J. Schlessinger, and K. Sakaguchi. 2005. Trans-activation of EphA4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc. Natl. Acad. Sci. USA. 102:18866–18871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer, M., A. Palmer, J. Kohler, and R. Klein. 2003. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat. Cell Biol. 5:869–878. [DOI] [PubMed] [Google Scholar]
- Zisch, A.H., C. Pazzagli, A.L. Freeman, M. Schneller, M. Hadman, J.W. Smith, E. Ruoslahti, and E.B. Pasquale. 2000. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene. 19:177–187. [DOI] [PubMed] [Google Scholar]