Abstract
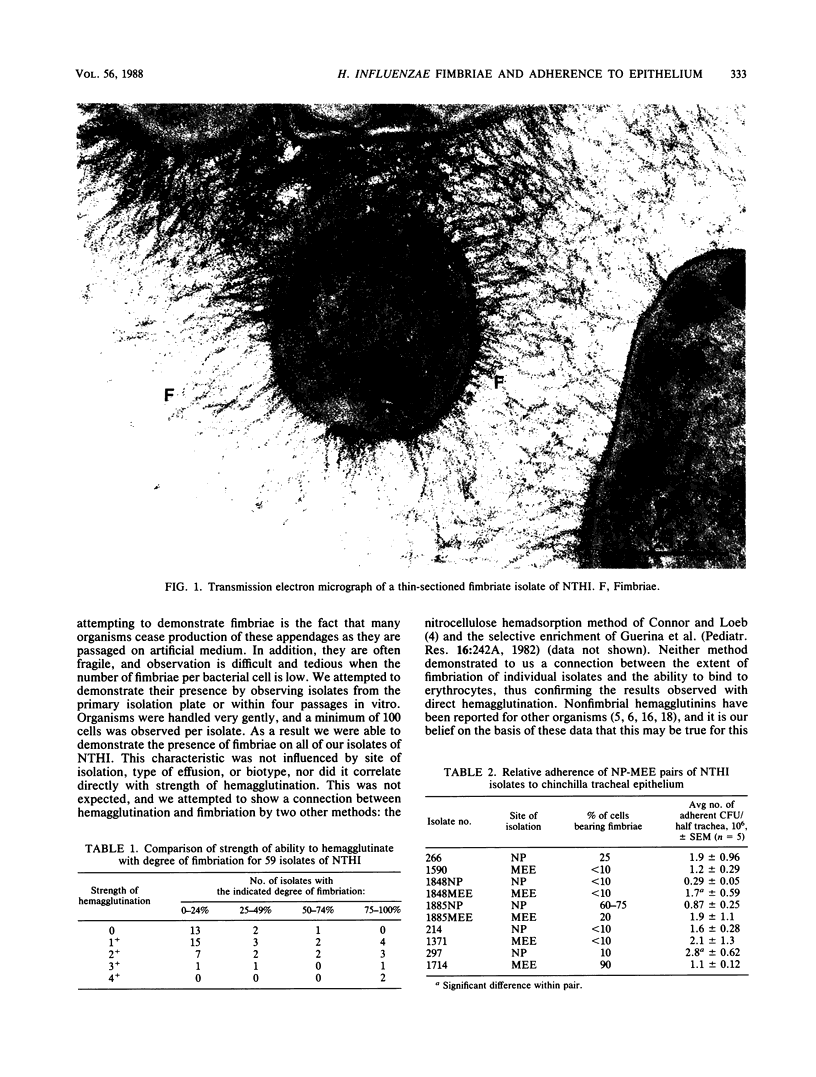
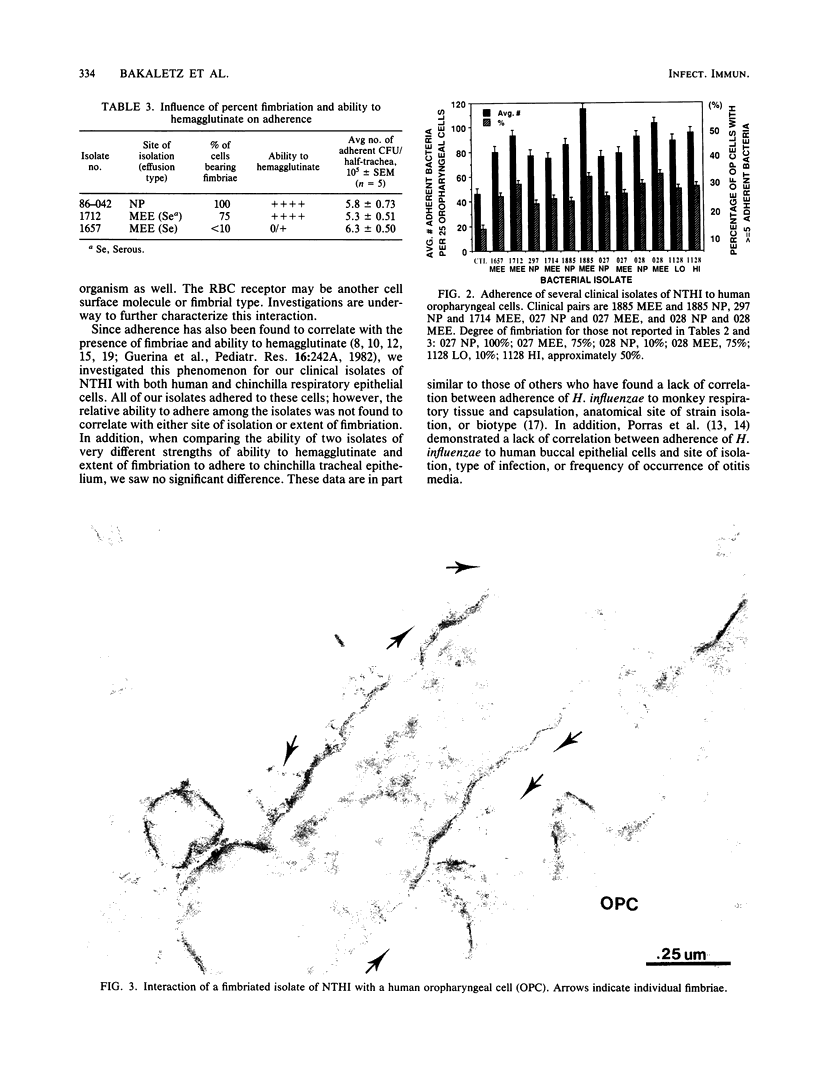
To date, we have examined nearly 60 clinical isolates of nontypable Haemophilus influenzae (26 nasopharyngeal, 33 from middle ear effusions) and have found that 100% were fimbriated. The percentage of cells bearing fimbriae within each isolate varied from less than 10 to 100%, with fimbriae being either peritrichous or bipolar in distribution. Fimbriae were approximately 2.4 to 3.6 nm in width; however, there was a high degree of variability in both length and number of fimbriae per individual bacterial cell among these isolates. All isolates tested adhered to both human oropharyngeal cells and chinchilla tracheal epithelium regardless of the degree to which the particular isolate was fimbriate. The level or degree of fimbriation did not correlate with either site of isolation, biotype, strength of hemagglutination reaction, or type of effusion present in the ear. These appendages appear to be quite different from those described for type b H. influenzae in which the ability to adhere and strength of ability to hemagglutinate correlated strongly with degree of fimbriation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Shero M., Dudas K. C., Stack R. R., Klohs W., LaScolea L. J., Murphy T. F., Mylotte J. M. Fimbriation of Haemophilus species isolated from the respiratory tract of adults. J Infect Dis. 1984 Jul;150(1):40–43. doi: 10.1093/infdis/150.1.40. [DOI] [PubMed] [Google Scholar]
- Bakaletz L. O., Rheins M. S. A whole-organ perfusion model of Bordetella pertussis adherence to mouse tracheal epithelium. In Vitro Cell Dev Biol. 1985 Jun;21(6):314–320. doi: 10.1007/BF02691578. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Hoglund L. E. Mycoplasma pneumoniae infection of intact guinea pig tracheas cultured in a unique matrix-embed/perfusion system. In Vitro. 1981 Oct;17(10):847–858. doi: 10.1007/BF02618279. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Porter T. N., Schaeffer A. J., Duncan J. L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985 Nov;50(2):370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe R. M., Mason E. O., Jr, Kaplan S. L., Umstead C. L., Yow M. D., Feigin R. D. Adherence of Haemophilus influenzae to buccal epithelial cells. Infect Immun. 1982 Jan;35(1):166–172. doi: 10.1128/iai.35.1.166-172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason E. O., Jr, Kaplan S. L., Wiedermann B. L., Norrod E. P., Stenback W. A. Frequency and properties of naturally occurring adherent piliated strains of Haemophilus influenzae type b. Infect Immun. 1985 Jul;49(1):98–103. doi: 10.1128/iai.49.1.98-103.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E., To C. C., Brinton C. C. Immunization of suckling pigs against enteric enterotoxigenic Escherichia coli infection by vaccinating dams with purified pili. Infect Immun. 1978 Jul;21(1):269–274. doi: 10.1128/iai.21.1.269-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Volpel K., Loh B. A., Speert D. P. Fimbriae (pili): molecular basis of Pseudomonas aeruginosa adherence. Clin Invest Med. 1986;9(2):113–118. [PubMed] [Google Scholar]
- Porras O., Dillon H. C., Jr, Gray B. M., Svanborg-Edén C. Lack of correlation of in vitro adherence of Haemophilus influenzae to epithelial cells with frequent occurrence of otitis media. Pediatr Infect Dis J. 1987 Jan;6(1):41–45. doi: 10.1097/00006454-198701000-00011. [DOI] [PubMed] [Google Scholar]
- Porras O., Svanborg-Edén C., Lagergård T., Hanson L. A. Method for testing adherence of Haemophilus influenzae to human buccal epithelial cells. Eur J Clin Microbiol. 1985 Jun;4(3):310–315. doi: 10.1007/BF02013659. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Klemm P., Korhonen T. K. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-D-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1234–1242. doi: 10.1128/jb.168.3.1234-1242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Jacobs R. F., Haas J. E., Smith A. L. Adherence of Haemophilus influenzae to monkey respiratory tissue in organ culture. J Gen Microbiol. 1984 Jun;130(6):1437–1447. doi: 10.1099/00221287-130-6-1437. [DOI] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Role of antibody to leukocytosis-promoting factor hemagglutinin and to filamentous hemagglutinin in immunity to pertussis. Infect Immun. 1981 Mar;31(3):1223–1231. doi: 10.1128/iai.31.3.1223-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., Mendelman P. M., Haas J. E., Schoenborn M. A., Mack K. D., Smith A. L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984 Dec;46(3):787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]