Abstract
Transient exposure of immature animals during the brain growth spurt period to ethanol triggers neuroapoptosis in the developing brain. Here we report that lithium, when administered in a single, well-tolerated dose to infant mice, suppresses spontaneous neuroaoptosis that occurs naturally in the developing brain, and prevents ethanol from triggering neuroapoptosis. To explore lithium's mechanism of action, we focused on kinase signaling systems (ERK, Akt, JNK) that are believed to play a regulatory role in cell survival, and found that very rapidly after ethanol administration there is a suppression of ERK phosphorylation, and that lithium stimulates ERK phosphorylation and prevents ethanol from suppressing this phosphorylation process. Ethanol also suppressed pAKT, but lithium did not counteract this effect. We also found that ethanol activates the JNK system, but this cannot explain the neurotoxic action of ethanol, because JNK activation did not occur in the same neuronal populations that are killed by ethanol.
INTRODUCTION
Transient exposure of infant rodents to several classes of drugs, including those that block NMDA glutamate receptors, those that activate GABAA receptors, and ethanol (which has both NMDA antagonist and GABAmimetic properties), triggers widespread neurodegeneration in the developing brain (Ikonomidou et al., 1999, 2000; Olney et al., 2002a; Bittigau et al., 2002; Jevtovic-Todorovic et al., 2003; Wozniak et al., 2004). The cell death process triggered by these drugs displays all of the classical ultrastructural characteristics of apoptosis (Olney et al., 2002; Dikranian et al., 2001, 2005) and is mediated by the Bax-dependent mitochondrial intrinsic pathway involving cytochrome-c release and activation of caspases 9 and 3 (Olney et al., 2002b; Young et al., 2003, 2005). The window of vulnerability to these agents coincides with the developmental period of rapid synaptogenesis (Ikonomidou et al., 1999, 2000), also known as the brain growth spurt period, which in mice and rats occurs primarily during the first 2 weeks after birth, but in humans extends from about mid-gestation to several years after birth (Dobbing and Sands, 1979).
It was recently reported (Zhong et al., 2006) that lithium suppresses ethanol-induced neuroapoptosis in the infant mouse brain, and we have observed (Creeley et al., 2007) that lithium suppresses the programmed cell death process that occurs naturally in the developing brain. These findings are consistent with one another, in that ethanol-induced neuroapoptosis is thought to be an abnormal augmentation of the neuroapoptosis process that occurs naturally during development. Using in vitro methods, Zhong et al. (2006) explored the hypothesis that lithium's protective action might be explained by an effect on the glycogen synthase kinase-3β (GSK3β) system, but they found no evidence that either ethanol or lithium significantly affected this system. The present study was undertaken to further explore the potential of lithium to protect against ethanol-induced neuroapoptosis, and to examine specific kinase signaling systems (ERK, Akt, JNK) that are believed to play a regulatory role in cell survival, for their potential involvement in mediating the apoptogenic action of ethanol and/or the protective action of lithium.
Materials and Methods
Animals and drugs
All animal procedures were conducted in accordance with guidelines developed by the National Academy of Science and were approved by the Washington University Animal Care Committee. In all experiments, postnatal day 6 (P6) C57BL6 mice weighing from 2.5 to 3.5 g were used.
To study histopathological changes following exposure to ethanol and/or lithium, experimental animals received lithium carbonate intraperitoneally (ip) together with ethanol, which was administered subcutaneously (sc). Lithium carbonate (Sigma Chemical, St. Louis, MO, USA) was prepared in distilled water (acidified with hydrochloric acid and subsequently neutralized with sodium hydroxide) and administered at either 3 or 6 mEq/kg. Ethanol was mixed in distilled water as a 20% (v/v) solution and administered at a dose of 2.5 g/kg. This dose of ethanol has been shown to trigger a robust neuroapoptosis response within 3 – 4 h in infant mice (Young and Olney, 2006). Control animals received the same doses of ethanol alone, lithium alone or saline. Twelve litters were used and for testing any drug or drug combination the control and experimental pups were drawn randomly from the same litters. Throughout all experiments the control and experimental pups were maintained at a constant ambient temperature (30°C) in containers separate from the maternal cage. Six hours after initiation of drug exposure, the pups were deeply anesthetized with pentobarbital and perfused with fixative (4% paraformaldehyde in Tris buffer) through the left cardiac ventricle to allow their brains to be evaluated for histopathological changes.
Following perfusion fixation, the brains were removed from the skull and immersed in the perfusion fixative overnight in the refrigerator, then were bisected sagittally, sectioned serially by vibratome in the sagittal plane and stained immunohistochemically with antibodies to activated caspase-3 (AC3) by methods described below. AC3 immunohistochemistry was used because this method robustly and reliably stains neurons in early stages of cell death before they decompose into unrecognizable (and uncountable) entities. In prior studies we have tested the reliability of AC3 immunostaining for detecting apoptotic cell death by comparing it with TUNEL and silver staining, both of which are markers for cell death, and found consistently that the pattern of neuronal degeneration revealed by AC3 immunostaining following neuroapoptogenic drug treatment is exactly reproduced by each of these other two methods (Ikonomidou et al., 1999, 2000; Olney et al., 2002a, b). In addition, we have confirmed cell death by electron microscopy, and determined that the dying cells have ultrastructural characteristics of neurons and display pathomorphological changes characteristic of apoptosis (Ikonomidou et al., 1999; Jevtovic-Todorovic et al., 2003; Olney et al., 2002b; Dikranian et al., 2001, 2005).
To explore mechanisms that might account for ethanol's apoptogenic action or lithium's neuroprotective action, we focused on kinase signaling systems (ERK, Akt, JNK) that are believed to play a regulatory role in cell survival. These signaling systems are thought to exert either a pro- or anti- apoptotic effect by triggering protein-protein interactions that alter the homeostatic balance between Bax and BclXL. To explore the role of kinase pathways, we administered a single sc dose of saline or ethanol and/or ip dose of lithium to P7 infant mice and performed western blot measurement of phosphorylated and non-phosphorylated ERK and Akt. We sought evidence in the caudate/putamen for rapid alterations in these kinase systems (within ≤ 2 hrs) because AC3 evidence for neuroapoptosis is readily detected in this brain region within 3 to 4 hrs after ethanol administration. We also performed IHC staining for phospho-c-Jun, the substrate of activated JNK, at time intervals from 30 min to 5 hrs following administration of ethanol to P7 infant mice. For this purpose, the brains were perfused with fixative consisting of paraformaldehyde 4% in Tris buffer.
Immunohistochemistry
Vibratome sections (70 µm thick) were washed in 0.01M phosphate-buffered saline (PBS), quenched for 10 min in a solution of methanol containing 3% hydrogen peroxide, then incubated for 1 hr in blocking solution (2% BSA/0.2% milk/0.1 % Triton X-100 in PBS), followed by incubation overnight in primary antiserum against AC3 or phospho-c-Jun antiserum (both obtained from Cell Signaling Technology, Beverly, Massachusetts) diluted 1:1000 in blocking solution. The sections were then incubated for 1 hr in secondary antibody (goat anti-rabbit 1:200 in blocking solution), and reacted in the dark with ABC reagents (standard Vectastain ABC Elite Kit, Vector Labs, Burlingame, CA) for 1 hour. The sections were then washed 3 times with PBS, and incubated with VIP reagent (Vector VIP substrate kit for peroxidase, Vector Labs, Burlingame, CA) to develop a purple color.
Quantitative cell counts
Quantitative counts were performed on serial vibratome sections (70µm) stained by the AC3 method and chosen using systematic random sampling in accordance with principles of unbiased stereology (Gundersen et al., 1988). AC3-positive profiles were counted in 6 brain regions (cerebral cortex, caudate-putamen, thalamus, superior colliculus, inferior colliculus, cerebellum). Every eighth section containing a given region of interest was selected and these sections were imaged and quantitatively evaluated with the help of a stereology system consisting of the following components: Stereo Investigator (MicroBrightField, Inc, Colchester, VT) on a Pentium III PC, connected to a Prior Optiscan motorized stage (ES103 XYZ system, Prior Scientific Inc, Rockland, MA) mounted on a Nikon Labophot-2 microscope. AC3-positive profiles were included in the counts if they had visible dendrites and/or if the cell body was ≥ 8 µm in diameter. The population estimator function of Stereo Investigator was used to mark each profile while it was counted to ensure that no profile would be missed or counted twice. The boundaries of the area of interest were traced into the PC and from the tracings Stereo Investigator calculated the area in each section from which counts were obtained. The total area from which counts were obtained from multiple sections (n ≥ 5) and multiple regions (n = 6) was calculated and multiplied by 70µm (thickness of each section) to yield a value for the total volume from which counts were obtained for a given brain. A mean (± SEM) density value (number of AC3-positive profiles per mm3) for all brains representing a given treatment condition was calculated by dividing the total counts by the total volume from which counts were obtained for that treatment condition. The counts were performed in a blinded manner in which the treatment condition was unknown to the person doing the counting.
Western Blot
After various durations of drug exposure, the animals were decapitated, the brains placed on ice, and the caudate-putamen region dissected free and homogenized in cell lysis buffer (Cell Signaling Technology, Beverly, Massachusetts). Cytosolic lysates containing equal amounts of protein (40µg) were resolved by SDS-PAGE and transferred to PVDF. Blots were blocked in 3% milk in 0.125% Tween 20 for 1 h and then incubated overnight at 4°C with antibodies against phospho-ERK1 or 2, phospho-Akt, or the non-phosphorylated isoforms of ERK 1&2 and Akt (Cell Signaling Technology, Beverly, Massachusetts) using eIF4E as internal standard. After washing 3 times, the membrane was incubated with 1:1666 secondary antibody with horseradish peroxidase in blocking solution for 1 h. The signal was developed with Pierce SuperSignal West Dura Extended Duration Substrate (Pierce, Rockford, IL) and captured and quantified with a FujiFilm LAS 3000 Imaging station.
Statistical analysis
Two types of data, stereological counts of AC3-positive profiles and Western blot determinations, were analyzed for statistical significance using Prism (GraphPad Software, Inc.). For experiments involving quantification of AC3-positive profiles, each treatment group was comprised of at least 6 animals. For Western blot experiments involving quantification, the means were determined from at least nine independent experiments. For all experimental conditions tested the experimental group was matched with a saline control group drawn from the same litters. One way ANOVA and Bonferroni's multiple comparisons test were used to analyze data pertaining to AC3-positive profile counts, and Western blot data were analyzed by a one sample t test.
Results
Lithium suppression of spontaneous neuroapoptosis
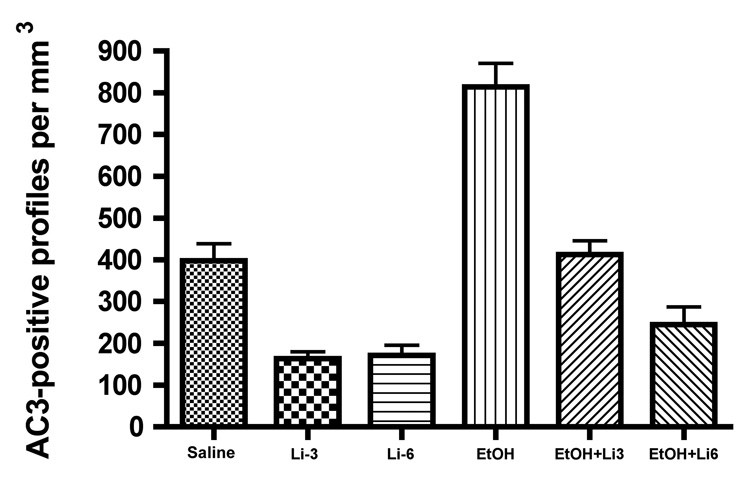
Quantitative counts of AC3-positive profiles in 6 different brain regions revealed that lithium, at either 3 or 6 mEq/kg, reduced the mean (± SEM) density of stained profiles (number per mm3) to about 40% of that in the brains of littermate controls, signifying that lithium suppressed the process by which neurons naturally undergo apoptosis in the developing mouse brain (Fig 1). Following either dose of lithium, the suppression was significant at a level of p < 0.001 (Bonferroni's post hoc test).
Figure 1.
Lithium (Li) suppression of spontaneous neuroapoptosis and protection against neuroapoptosis induced by ethanol (EtOH). Li at either 3 or 6 mEq/kg suppressed spontaneous neuroapoptosis, reducing it to a level about 40% of the saline control level, and EtOH caused a significant increase in neuroapoptosis compared to littermate saline controls. These treatment effects were highly significant by one way ANOVA [F(5, 64) = 34.8, P < 0.0001], and both of these treatment effects were significant at a level of p < 0.001 by Bonferroni post hoc analysis. Li completely eliminated the neuroapoptosis response to EtOH, reducing it to a level not significantly different from the saline control level if the lithium dose was 3mEq/kg, and reducing it below the saline control level if the dose of lithium was 6 mEq/kg. In fact, the combination of EtOH + Li 6mEq/kg resulted in a suppressed rate of neuroapoptosis that was not significantly different from that induced by Li in the absence of EtOH.
Lithium prevention of ethanol-induced neuroapoptosis
The density of AC3-stained profiles in the brains of mice that received ethanol was significantly increased compared to littermate controls, a difference significant at a level of p < 0.001. Lithium at 3 mEq/kg counteracted ethanol's proapoptotic action, causing the density of AC3-stained profiles to remain at a level not significantly different from littermate controls. Lithium at 6 mEq/kg, when administered together with ethanol, counteracted ethanol's action completely, and additionally reduced the AC3-positive profile density to a level not significantly different from the suppressed level induced by lithium 6 mEq/kg in the absence of ethanol. The degree to which lithium suppressed spontaneous neuroapoptosis, and the degree to which it protected against ethanol-induced neuroapoptosis, was similar in each brain region, signifying that lithium suppresses the apoptosis mechanism very effectively throughout the brain. The histological appearance of AC3 staining in the caudate/putamen following treatment with saline, lithium, ethanol or ethanol + lithium is illustrated in Fig. 2.
Figure 2.
Photomicrographs showing the appearance of AC3-stained profiles in the caudate-putamen region of the P6 mouse brain following treatment with saline (A), Li 6 mEq/kg (B), ethanol (C) or Li 6 mEq/kg + ethanol (D). Li by itself (B) substantially suppresses the rate of neuroapoptosis compared to the saline control (A). The rate of neuroapoptosis is substantially increased by ethanol (C), and when Li is administered together with ethanol (D), the rate remains suppressed to a level comparable to that associated with Li alone (B) (Magnification ×100).
Effects of ethanol and/or lithium on ERK and Akt
Western blot analysis revealed that in the caudate/putamen of infant mouse brain, ethanol caused a marked and very rapid (within less than 30 min) suppression of phosphorylated ERK 1 and 2 (extracellular signal-regulated protein kinases) (Fig 3). We then determined that lithium by itself promotes phosphorylation of ERK 1/2, and potently prevents ethanol from suppressing ERK 1/2 phosphorylation (Fig 4). Ethanol suppressed phosphorylation of Akt to a moderate, but significant degree. Lithium by itself did not promote phosphorylation of Akt and when administered with ethanol it did not counteract ethanol's suppression of Akt phosphorylation. In the 2 h period during which the phosphorylated forms of ERK1/2 and Akt showed changes in response to ethanol and/or lithium, there was no change in total ERK1/2 or total Akt, indicating that the effect of these agents is at the post-translational modification level and not at the gene expression level.
Figure 3.
Western blots depicting the time course of ethanol suppression of pERK 1 and 2 in caudate/putamen of infant mouse brain. Protein elF4E is used as internal loading control. A high dose of ethanol (5 g/kg) was used in these experiments because we wanted to produce a strong enough response so that it could be readily detected as early as it occurs. Suppression of both ERK 1 and 2 is already evident at 15 min and becomes progressively more pronounced at 30 and 60 min following ethanol administration.
Figure 4.
Western blot evaluation of changes in phosphorylated and total ERK 1/2 and Akt two hours after administration of ethanol (EtOH)(2.5 g/kg) and/or lithium (Li)(6 mEq/kg). Representative blots for each protein under each treatment condition are shown in Fig. A. The blots were read in a FujiFilm LAS 3000 imaging station and the values normalized to eIF4E protein as an internal control. The values for the experimental conditions in each experiment were further normalized as a percentage of the internal saline control for that experiment. The means (± SEM) derived from 9 or more animals exposed to each experimental condition are given in Fig. B as a percent of the saline control (dashed line). For statistical analysis, a one sample t test was used in which the mean for each experimental condition was compared to a theoretical mean of 100%. The amount of total ERK 1/2 and total Akt remained unchanged following EtOH and/or Li treatment. Li caused a significant increase in pERK1 and pERK2 (p = 0.013 and 0.001 respectively); but no change in pAkt. EtOH caused a marked suppression in pERK1 and pERK2 (p < 0.0001 and < 0.002, respectively) and coadministration of Li prevented this suppressant effect, leaving pERK1 and 2 unchanged (not significantly different) from control levels. Ethanol moderately suppressed pAkt (p < 0.03) compared to saline controls, but Li did not counteract this suppressant effect.
Effects of ethanol on the JNK system
To study the JNK (c-Jun N-terminal Kinase) system we performed immunohistochemical staining for phospho-c-Jun, the substrate of activated JNK. We found increased staining of cellular profiles for phospho-c-Jun beginning 2 h after ethanol administration in several brain regions, including the olfactory bulb, caudate-putamen, hippocampus, thalamus, cerebral cortex and inferior colliculus, but this was deemed unrelated to the neuroapoptosis process because the pattern of this staining did not co-localize, either by region or cell type, with the pattern of AC3 staining (marker for neuroapoptosis). For example, in the olfactory bulb, AC3-positive neurons were localized to the granule cell zone whereas phospho-c-Jun staining was localized to the mitral cell layer, and in the caudate-putamen AC3-staining was intensely positive in many profiles distributed widely throughout this region, and phospho-c-Jun staining was not convincingly positive in more than a few profiles anywhere in this region. The discordant staining patterns for AC3 and phospho-c-Jun in the olfactory bulb and caudate-putamen are illustrated in Fig 5.
Figure 5.
Immunohistochemical staining for phospho-c-Jun or activated caspase 3 (AC3) in the infant mouse olfactory bulb or caudate nucleus 3 hours following ethanol treatment. In the olfactory bulb, many cell bodies in the mitral cell layer (MCL) are positive for phospho-c-Jun and very few are positive for AC3. The opposite is true for cell bodies in the granule cell zone (GCZ) where AC3 profiles are relatively abundant but only a rare profile is positive for phospho-c-Jun. In the caudate nucleus occasional profiles in scattered distribution are faintly positive for phospho-c-Jun, and abundant profiles distributed throughout this region are strongly positive for AC3. Thus, the staining patterns for these two markers bear no resemblance to one another at either a regional or cellular level. Phospho-c-Jun is present in increased concentration in some cells (e.g. mitral cells of the olfactory bulb) but these are not the same cells that are undergoing apoptosis, as indicated by AC3 positivity.
Lithium blood concentrations
In human patients receiving lithium chronically for treatment of bipolar disorder, blood levels are monitored at 12 h following the last dose of lithium and the dose is adjusted to maintain blood levels in the range of 0.8 to 1.2 mEq/L. Our neuroprotection data suggest that Li can provide almost complete protection against neuroapoptosis when administered at 3 mEq/kg, and the 6 mEq/kg dose is in excess of the required dose. Therefore, we assayed Li plasma levels, using ion-coupled mass spectrometry, in 7 day old infant mice at 1, 3, 6, 12 and 24 h following administration of Li at 3 mEq/kg, and found the values to be 1.9, 1.8, 1.5, 1.2 and 0.7 mEq/L, respectively. Thus, the values at 12 h are at the upper end and at 24 h at the lower end of the range considered therapeutic for adult humans. We also obtained plasma Li levels following Li administration to adult mice and found that the values at 1 hr were approximately one third as high as in infants, and at 12 h Li was almost totally cleared from the blood. Similar findings have been reported by Messiha (1976) following Li administration to adult mice. This suggests that Li is cleared from the blood much more rapidly in adult compared to infant mice. We are not aware of any studies addressing whether a similar differential exists for adult versus infant humans.
Discussion
Our findings document that in the in vivo mouse brain lithium exerts a strong neuroprotective effect against the apoptogenic action of ethanol. Lithium conferred essentially complete protection in each of 6 brain regions where ethanol is known to trigger neuroapoptosis. The neuroprotective action of lithium is rapid in onset and the effect is relatively long-lasting, long enough so that a single clinically relevant dose conferred protection over a 6 h interval during which abundant neuroapoptosis would have otherwise occurred.
The finding that lithium confers relatively complete protection against the apoptogenic action of ethanol and also suppresses spontaneous neuroapoptosis, suggests that the drug-induced and spontaneous phenomena are the same process with respect to the final pathway through which the apoptosis trigger is tripped. Although the initiating mechanisms are not identical, the data suggest that at some point in the intracellular signaling pathways there is a convergence into a single pathway where lithium can exert a counteractive influence that arrests further propagation of the apoptosis signal. Our findings identify phosphorylation of ERK 1 and 2 as a step in the signaling process where the actions of ethanol and those of lithium impinge with opposing actions, and the action of lithium is strong enough to prevail. Our ERK findings are consistent with prior reports that ethanol suppresses ERK phosphorylation in the in vivo cerebral cortex and hippocampus of 5 day-old rats (Davis et al., 1999; Chandler and Sutton, 2005).
The efficacy of lithium in protecting against ethanol-induced neuroapoptosis raises the important question whether lithium might also protect against the neuroapoptogenic actions of anesthetic drugs. Recently we have explored this possibility and found that lithium does protect against neuroapoptosis induced by ketamine, propofol or isoflurane (Olney et al., 2007). Not only does this recommend lithium as a potential anti-apoptotic neuroprotective agent, but it suggests the important possibility that other agents, acting by a similar mechanism, can be developed that can stabilize apoptosis pathways in the developing brain and totally prevent unwanted apoptotic processes.
Lithium has long been used as a mood stabilizer in the treatment of manic-depressive (bipolar) disorder, and more recently has been reported to have neuroprotective properties (Chuang et al., 2004) relevant to adult neurological disorders such as Alzheimer's disease (Noble et al., 2005), Huntington's disease (Wei et al., 2001) and ischemic stroke (Ren et al., 2003). Mechanisms underlying lithium's mood stabilizing and neuroprotective properties in the adult brain remain to be elucidated, but putative candidates include inhibition of glycogen synthase kinase-3β (GSK3β) (Noble et al., 2005; Sasaki et al., 2006; Hongisto et al., 2003), stimulation of heat shock protein-70 (Ren et al., 2003), inhibition of Ca influx through the NMDA glutamate receptor (Chuang et al., 2004), and activation of the ERK signaling pathway (Einat et al., 2003). However, these postulated mechanisms are based on evidence generated in the adult nervous system or in the petri dish. To the best of our knowledge, there are no prior studies demonstrating in the in vivo developing brain that lithium stimulates ERK phosphorylation or that increased phosphorylation of ERK is protective against drug-induced developmental neuroapoptosis.
The neuroapoptosis literature is massive, but models for inducing apoptotic neurodegeneration in the in vivo developing nervous system are scarce, the two best characterized examples being the phenomenon under investigation herein, and degeneration of sympathetic neurons induced by trophic factor deprivation, as originally described by Levi-Montalcini (1969) and others (Sabatini et al., 1965). As Dikranian et al (2001, 2005) recently pointed out, these two phenomena have identical ultrastructural characteristics and both meet all of the morphological criteria originally stipulated by Wyllie et al (1980) for recognizing apoptosis. Neuroapoptosis induced by trophic factor deprivation has been studied extensively and reportedly involves activation of the JNK intracellular signaling pathway (Putcha et al., 2002). Our experiments provide evidence for activation of the JNK pathway by ethanol, but we deemed this to be irrelevant to the neuroapoptogenic action of ethanol because it did not occur in the neuronal populations that undergo apoptosis following ethanol treatment.
Recently, Zhong et al (27) reported that lithium protects against ethanol-induced neuroapoptosis and, based on in vitro data, they concluded that the Akt/GSK3β system plays no role in lithium's protective action. Our findings are confirmatory of Zhong et al. with respect to lithium protecting against ethanol neurotoxicity. Our finding that ethanol moderately but significantly suppresses Akt phosphorylation is contrary to their conclusion that ethanol has no effect on this pathway. A possible explanation for these conflicting observations is that our studies were performed in vivo and theirs were performed in vitro. This may be an important difference in that they used cerebellar granule cell cultures as their in vitro model, and we have observed (Dikranian et al., 2005) in the in vivo infant mouse cerebellum that granule cells are not sensitive to ethanol-induced neuroapoptosis. Therefore, findings in this in vitro system are not necessarily relevant to the in vivo phenomenon. A more important difference between our findings and theirs is with repect to their conclusion that the locus of lithium's action is downstream at the level of caspases 9 and 3. We agree that ethanol-induced neuroapoptosis involves activation of caspases 9 and 3 (Olney et al., 2002b; Young et al., 2005), and one would expect that activation of these caspases would be prevented by an anti-apoptotic action of lithium anywhere upstream of these caspases. Our evidence in vivo places the interaction of ethanol and lithium upstream at the level of the ERK 1/2 pathway. This is an important distinction in that an upstream action on ERK 1/2 will prevent ethanol from inducing either cell injury or cell death, whereas blocking caspase activation downstream is not likely to prevent cell injury, and there is evidence (Young et al., 2005) that it also may fail to prevent cell death. Zhong et al. did not evaluate the effects of lithium on the ERK1/2 signaling system.
While our findings point to the ERK pathway as a major intersection where lithium and ethanol have opposing actions, this is very likely not a full explanation either for ethanol's apoptotic action or lithium's protective action. There is strong evidence that apoptosis can be triggered through kinase signaling systems, but it may require a combined impingement on two or more kinase systems to efficiently induce apoptosis. For example, Marushige and Marushige (1999) found that activation of JNK can trigger apoptosis if the Akt pathway is simultaneously suppressed, but JNK activation alone was ineffective. Similary, Akt suppression alone was ineffective, but became markedly effective if the ERK pathway was simultaneously suppressed. This conceptual framework provides an excellent explanation for the apoptotic action of ethanol, in that ethanol suppressed the Akt pathway (moderately) and the ERK pathway markedly. However, the Akt pathway apparently does not contribute to lithium's protective action in that lithium did not counteract ethanol's suppressant effect on pAkt.
Lithium is an FDA-approved drug for use in the treatment of bipolar affective disorder and has been used extensively off-label for several other neuropsychiatric disorders. However, it has not been approved nor proposed for uses targeting the developing brain. The risk of lithium causing harmful side effects depends on the dose, duration of treatment and age of the patient at the time of treatment. Lithium is known to have weak teratogenic effects in humans, but this has been described only after chronic exposure of the fetus during the first trimester of pregnancy (Gille and Bannigan, 2006). There are no known toxic effects associated with exposure of either immature animals or humans to a single clinically relevant dose of lithium in late gestation or early childhood. However, before lithium could be recommended as an antidote against drug-induced developmental neuroapoptosis, a more complete safety evaluation is needed, and a major issue that must be carefully investigated is whether lithium's ability to suppress spontaneous developmental neuroapoptosis has any lasting consequences. Moreover, while lithium appears to be very effective in preventing ethanol-induced neuroapoptosis, using lithium during pregnancy to prevent alcohol from damaging the fetal brain may not be the best solution. Mothers who have a drinking habit would very likely misuse lithium in pursuit of that habit. Developing effective programs for ensuring abstinence from ethanol during pregnancy is the most sound approach for achieving this very important goal.
AKNOWLEDGMENTS
Supported in part by NIH grants DA 05072, HD 37100, T32 MH 14677 and T32 DA 07261.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proceedings Nat. Acad Sciences. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM. Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit. Rev. Neurobiol. 2004;16:83–90. doi: 10.1615/critrevneurobiol.v16.i12.90. [DOI] [PubMed] [Google Scholar]
- Creeley CE, Young C, Olney JW. Lithium suppresses spontaneous neuroapoptosis in the developing mouse brain. Program No.169.14. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2007. [Google Scholar]
- Dikranian K, Ishimaru MJ, Tenkova T, Labruyere J, Qin YQ, Ikonomidou C, Olney JW. Apoptosis in the in vivo mammalian forebrain. Neurobiol Dis. 2001;8:359–379. doi: 10.1006/nbdi.2001.0411. [DOI] [PubMed] [Google Scholar]
- Dikranian K, Qin YQ, Labruyere J, Nemmers B, Olney JW. Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Dev. Brain Res. 2005;155:1–13. doi: 10.1016/j.devbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J. Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Current Pharm. Des. 2006;12:1531–1541. doi: 10.2174/138161206776389804. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenerg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hongisto V, Smeds N, Brecht S, Herdegen T, Courtney MJ, Coffey ET. Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol. Cell Biol. 2003;23:6027–6036. doi: 10.1128/MCB.23.17.6027-6036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova T, Stevoska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and the fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R, Caramia D, Angeletti U. Alteration of the fine structure of nucleoli in sympathetic neurons following NGF-antiserum treatment. Brain Res. 1969;12:54–73. doi: 10.1016/0006-8993(69)90055-9. [DOI] [PubMed] [Google Scholar]
- Marushige K, Marushige Y. Changes in the mitogen-activated protein kinase and phosphatidylinositol-3-kinase/Akt signaling associated with the induction of apoptosis. Anticancer Res. 1999;19:3865–3871. [PubMed] [Google Scholar]
- Messiha FS. Distribution and retention of exogenously administered alkali metal ions in the mouse brain. Arch. Int. Pharmacodyn. Ther. 1976;219:87–96. [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Dev. Brain Res. 2002a;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D’Sa C, Roth KA. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol. Dis. 2002b;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- Olney JW, Sraiko M, Johnson SA, Cattano D, Young C. A single dose of lithium prevents developmental neuroapoptosis induced by ethanol or anesthetic drugs; Am Soc Anesthesiol Annual Meeting 2007; 2007. Abstr. #A2117 pub online at www.asaabstracts.com. [Google Scholar]
- Putcha GV, Harris CA, Moulder KL, Easton RM, Thompson CB, Johnson EM., Jr Intrinsic and extrinsic pathway signaling during neuronal apoptosis: lessons from the analysis of mutant mice. J. Cell Biol. 2002;157:441–453. doi: 10.1083/jcb.200110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Senatorov VV, Chen RW, Chuang DM. Post-insult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc. Natl. Acad. Sci. USA. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini MT, De Iraldi A, De Robertis E. Early effect of antiserum (AS) against the nerve growth factor (NGF) on the structure of sympathetic neurons. J. Exp. Neurol. 1965;12:70–383. doi: 10.1016/0014-4886(65)90079-8. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Han F, Shioda N, Moriguchi S, Kasahara J, Ishiguro K, Fukunaga K. Lithium-induced activation of Akt and CaM kinase II contributes to its neuroprotective action in a rat microsphere embolism model. Brain Res. 2006;1108:98–106. doi: 10.1016/j.brainres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Int. Rev. Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Young C, Olney JW. Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiol. Dis. 2006;22:548–554. doi: 10.1016/j.nbd.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Young C, Klocke B, Tenkova T, Choi J, Labruyere J, Qin Q, Holtzman DM, Roth KA, Olney JW. Ethanol-Induced Neuronal Apoptosis in the in vivo Developing Mouse Brain is BAX Dependent. Cell Death Differentiation. 2003;10:1148-115. doi: 10.1038/sj.cdd.4401277. [DOI] [PubMed] [Google Scholar]
- Young C, Roth KA, Klocke BJ, West T, Holtzman DM, Labruyere J, Qin YQ, Dikranian K, Olney JW. Role of caspase-3 in ethanol-induced developmental neurodegeneration. Neurobiol. Dis. 2005;20:608–614. doi: 10.1016/j.nbd.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Wei H, Qin ZH, Senatorov VV, Wei W, Wang Y, Qian Y, Chuang DM. Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington's disease. Neuroscience. 2001;106:603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Zhong J, Yang X, Yao W, Lee W. Lithium protects ethanol-induced neuronal apoptosis. Biochem. Biophys. Res. Commun. 2006;350:905–910. doi: 10.1016/j.bbrc.2006.09.138. [DOI] [PubMed] [Google Scholar]