Abstract
Pseudomonas aeruginosa offers substantial therapeutic challenges due to its high intrinsic resistance to many antibiotics and its propensity to develop mutational and/or adaptive resistance. The PA14 comprehensive mutant library was screened for mutants exhibiting either two- to eightfold increased susceptibilities (revealing genes involved in intrinsic resistance) or decreased susceptibilities (mutational resistance) to the fluoroquinolone ciprofloxacin. Thirty-five and 79 mutants with increased and decreased susceptibilities, respectively, were identified, as confirmed by broth dilution.
Pseudomonas aeruginosa is a major cause of hospital infections and is the pathogen most commonly associated with mortality in cystic fibrosis patients. Infections with this organism are very difficult to treat due to its high intrinsic antibiotic resistance. Fluoroquinolones, such as ciprofloxacin, are broad-spectrum antimicrobials that target the bacterial enzymes DNA gyrase and topoisomerase IV (6) and are clinically used to treat chronic P. aeruginosa infections in cystic fibrosis patients (5, 9). The massive use of fluoroquinolones has led to increased quinolone resistance in P. aeruginosa (18) as well as multidrug resistance, a serious problem in the clinic. Certain mutations that lead to ciprofloxacin supersusceptibility (e.g., lon [1]) or resistance (e.g., mutations in the genes for the target topoisomerase enzymes or the upregulation of efflux pump expression [10]) are already known. In addition, subinhibitory concentrations of fluoroquinolones play an important role in the development of resistance, in that P. aeruginosa cultures pretreated with subinhibitory concentrations of ciprofloxacin develop an adaptive resistance phenotype (1).
It has been proposed that antibiotics rarely have a simple mechanism of action. Microarray studies indicate that they lead to the upregulation of dozens to hundreds of genes when they are used at concentrations at or near the MIC (3, 4, 14). It has been suggested that some of these genes are involved in target inhibition, others are involved with the induction of cellular stress pathways, and some are involved with defensive measures that the bacterium takes to resist the actions of antibiotics. In this view of antibiotic action, it seems likely that there should be many more genes that are involved in increased or decreased susceptibility in bacteria than have previously been supposed and that some of these might have clinical relevance.
Therefore, in the present study we screened a comprehensive PA14 transposon mutant library (13) for mutants that showed either increased susceptibility (reflecting intrinsic resistance) or decreased susceptibility (reflecting mutational resistance) to ciprofloxacin as a survey to reveal the extent of the ciprofloxacin resistome. Freshly diluted overnight cultures were used to inoculate approximately 3 × 104 cells per spot on to Mueller-Hinton agar plates containing ciprofloxacin at 0.025 μg/ml (half the MIC for the parent strain) to screen for increased susceptibility and at 0.2 μg/ml to screen for decreased susceptibility. The mutants identified by this approach were confirmed to show a changed susceptibility by determination of the MICs (17), and changes as small as twofold were taken into account. We acknowledge that twofold changes in MICs are generally considered within the error of the standard assessment protocols. However, only results for those mutants for which we could consistently confirm changes in at least three independent measurements are shown. Growth was measured in liquid medium at 37°C by using a Tecan Spectrafluor Plus apparatus by measuring the absorbance at 620 nm every 18 min for 24 h under shaking conditions.
The first ciprofloxacin screen at 0.025 μg/ml yielded a total of 62 mutants with an inability to grow in the plate assay, leading to 28 confirmed mutants with an increase in susceptibility (Table 1). An additional seven mutants were identified by specifically measuring the MIC of ciprofloxacin for mutants with mutations in the same operon as that for the mutants that appeared in the screen, for a total of 35 mutants (Fig. 1; Table 1). Mutants with mutations located in an operon might exhibit polar effects.
TABLE 1.
Changes in susceptibility of confirmed P. aeruginosa mutants showing increased susceptibility
| PA no.a | Gene name | Gene description | Gene class(es)b | Fold increased susceptibility | Growth ratec | Regulation by ciprofloxacind |
|---|---|---|---|---|---|---|
| PA0334 | Putative MFS transporter | MP, TSM | 2 | ++ | ||
| PA0336 | ygdP | Dinucleoside polyphosphate hydrolase | NB | 2 | ++ | ↑*# |
| PA0337 | ptsP | Phosphoenolpyruvate-protein phosphotransferase | TSM | 2 | ++ | |
| PA0338 | Hypothetical protein | HYP, M/A | 2 | ++ | ↑*# | |
| PA0425 | mexA | RND multidrug efflux membrane fusion protein | TSM, AB | 2 | ++ | |
| PA0426 | mexB | RND multidrug efflux transporter | TSM, AB | 2 | ++ | ↑* |
| PA0427 | oprM | Major intrinsic multiple-antibiotic-resistance efflux outer membrane protein OprM | AB, MP, TSM | 2 | ++ | ↑# |
| PA0702 | Hypothetical protein | MP, HYP | 2 | ++ | ||
| PA0703 | Probable major facilitator superfamily transporter | MP, TSM | 2 | ++ | ||
| PA0966 | ruvA | Holliday junction DNA helicase | DRR | 2 | (++) | |
| PA1098 | fleS | Two-component sensor | TCRS | 2 | (++) | |
| PA1375 | pdxB | Erythronate-4-phosphate dehydrogenase | CCC, others | 2 | + | |
| PA1588 | sucC | Succinyl coenzyme A synthetase beta subunit | EM | 2 | + | |
| PA1611 | Putative sensor/response regulator hybrid | TR, TCRS | 4 | + | ↑* | |
| PA1667 | Hypothetical protein | HYP | 2 | + | ||
| PA1777 | oprF | Major porin and structural outer membrane porin | MP, TSM | 2 | + | ↓# |
| PA1800 | tig | Trigger factor | CD, HSP | 2 | ++ | ↓# |
| PA1801 | clpP | ATP-dependent Clp protease proteolytic subunit | HSP | 2 | + | |
| PA1802 | clpX | ATP-dependent Clp protease ATP-binding subunit | HSP | 2e | ++ | |
| PA1803 | lon | Lon protease | A/P, TM | 4 | ++ | |
| PA2432 | Putative transcriptional regulator | TR, others | 2 | ++ | ||
| PA2549 | Hypothetical protein | MP, HYP | 2 | + | ||
| PA2615 | ftsK | Cell division/stress response protein | CD | 8 | + | |
| PA3516 | Probable lyase | PE | 2 | ++ | ||
| PA3517 | Putative lyase | CCC | 2 | ++ | ||
| PA3738 | xerD | Integrase/recombinase xerD | DRR | 4 | + | |
| PA4459 | Hypothetical protein | HYP | 2 | ++ | ↑# | |
| PA4667 | Hypothetical protein | HYP | 2 | ++ | ||
| PA4685 | Hypothetical protein | HYP | 2 | + | ↑* | |
| PA4781 | Putative two-component response regulator | TR, TCRS | 2 | + | ||
| PA5253 | algP | Alginate regulatory protein AlgP | TR | 2 | ++ | |
| PA5280 | sss | Site-specific recombinase | DRR | 4 | + | |
| PA5345 | recG | ATP-dependent DNA helicase RecG | DRR | 4 | ++ | |
| PA5366 | pstB | Phosphate ABC transporter, ATP-binding protein | MP, TSM | 2 | + | |
| PA5375 | betT1 | Choline/carnitine/betaine transporter family protein | MP, TSM | 2 | ++ | ↓*# |
Note that these are the original assignments for these gene mutations and were not confirmed again in this study, although the mutants were used as obtained with minimal subculture. We could also confirm 10 mutations in operons or an overlap between libraries. The mutants with the underlined mutations were tested after the initial screen due to the presence of mutations in an operonic relationship with genes that, when they were altered, influenced susceptibility to ciprofloxacin.
A/P, adaptation, protection; TSM, transport of small molecules; TM, translation, posttranslational modification; PE, putative enzyme; TR, transcriptional regulator; HYP, hypothetical protein; MP, membrane protein; EM, energy metabolism; DRR, DNA replication recombination; CCC, carbon compound metabolism; CD, cell division; NB, nucleotide biosynthesis and metabolism; M/A, motility and attachment; AB, antibiotic resistance and susceptibility; TCRS, two-component regulatory systems; HSP, chaperones and heat shock proteins.
++, normal growth rate compared to that of the wild type; (++), normal growth rate compared to that of the wild type but reduced yield; +, reduced growth rate compared to that of the wild type.
Data are from previous reports (1, 2). All P. aeruginosa genes were differentially expressed by treatment with 0.3× MIC (*) and 1× MIC (#) of ciprofloxacin. The direction of the arrow indicates the trend of expression relative to that by untreated cells.
Change in susceptibility observed after 48 h.
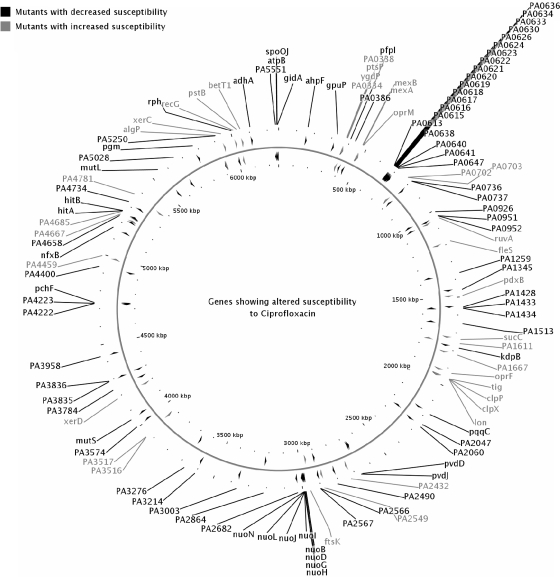
FIG. 1.
Distribution of mutations around the genome. The genome image was generated by using CGView (16).
The majority of these mutants demonstrated only twofold changes in susceptibility, although a mutant with a mutation in ftsK was eightfold more susceptible. Noticeable among the ciprofloxacin mutants with increased susceptibility was the number of mutants with mutations that were involved in DNA replication and repair, such as the Holliday junction helicase ruvA, the ATP-dependent RNA helicase recG, the recombinase xerD, and the site-specific recombinase sss. In addition, we observed the major intrinsic multidrug efflux pump mexAB-oprM, the recently identified lon mutant (1), and three other genes (namely, tig, clpP, and clpX) in the same operon. clpP and clpX encode ATP-dependent proteases like Lon. Not surprisingly, a few mutants (e.g., sss, xerD, ftsK, and ruvA mutants) showed somewhat slower growth than the wild-type strain PA14. However, all of these mutants remained more susceptible even after 48 h, while several of the other slower-growing mutants tested were not more susceptible to ciprofloxacin, indicating that slow growth did not cause increased ciprofloxacin susceptibility per se.
The constellation of genes involved in intrinsic resistance is demonstrated in Fig. 1, which indicates that these genes were spread throughout the chromosome. Although the high-throughput nature of this screen did not permit us to complement each mutant, we were able to confirm the increased susceptible phenotype with several independent isolates from the PAO1 lux mutant library (12) and also confirmed the existence of multiple mutants with mutations in five operons. Interestingly, as predicted, we were able to demonstrate that 10 of the genes giving rise to an increase in susceptibility upon transposon mutation were among those differentially expressed in response to ciprofloxacin on our previous microarrays (2). Even though the mutant with a mutation in clpP showed only a twofold altered susceptibility, it could be successfully complemented with the cloned gene. We also successfully complemented with the cloned gene the mutant that had a fleS mutation that showed a stable twofold increase in susceptibility to ciprofloxacin and norfloxacin compared to that of the wild-type strain PAO1 and for which the killing rate by ciprofloxacin was increased compared to that for the wild-type strain PAO1. FleS is known to be involved in the regulation of flagellum biosynthesis (15); and we were also able to complement a major defect in swarming motility, an ability to swarm somewhat on swimming medium, and an observed 60% decrease in static biofilm formation compared to that by wild-type strain PAO1 (data not shown).
Screening of the library for mutants that showed at least a twofold decrease in susceptibility to ciprofloxacin identified 46 mutants. A further 13 mutants with mutations in adjacent genes in operons and 20 mutants with phage-related mutations were included, for a total of 79 mutants with decreased susceptibility (Fig. 1; Table 2). It is worth noting that such a high-throughput approach is applicable only for genes for which the complete loss of the protein is practical (i.e., nonessential genes). Indeed, nearly all mutants with decreased susceptibility tested were able to grow as well as the wild type (Table 2). We were able to identify previously known genes such as the mexCD-oprJ efflux regulator nfxB, mutators mutS and mutL, and the phage-related mutations and observed multiple mutations in nine operons, including the nuoD NADH dehydrogenase operon. We also observed mutations in several iron transport genes, consistent with recent views suggesting roles for free radicals in antibiotic killing (7, 11). Among the genes giving rise to decreased susceptibility upon transposon mutation, we found 32 that were differentially expressed in response to ciprofloxacin (2). It should be noted that while the library was quite comprehensive, it was not complete; e.g., a mutation in the mexS gene that regulates the MexEF-OprN efflux operon was not available.
TABLE 2.
Changes in susceptibility of confirmed P. aeruginosa mutants showing decreased susceptibility
| PA no.a | Gene name | Gene description | Gene class(es)b | Fold decreased susceptibility | Growth ratec | Regulated by ciprofloxacind |
|---|---|---|---|---|---|---|
| PA0140 | ahpF | Alkyl hydroperoxide reductase subunit F | A/P | 4 | ++ | |
| PA0287 | Putative sodium:solute symporter | TSM | 2 | ++ | ||
| PA0355 | pfpI | Protease | TM | 2e | ++ | |
| PA0386 | Putative oxygen-independent coproporphyrinogen III oxidase | PE | 2 | ++ | ||
| PA0613e | Conserved hypothetical protein | HYP, PR | 2 | ++ | ↑*# | |
| PA0615 | Conserved hypothetical protein | HYP, PR | 2 | ++ | ↑*# | |
| PA0616 | Hypothetical protein | HYP | 2 | ++ | ↑*# | |
| PR | ||||||
| PA0617 | Putative base plate assembly protein W | PR | 2e | ++ | ↑*# | |
| PA0618 | Putative phage base plate assembly protein | PR | 2 | ++ | ↑*# | |
| PA0619 | Putative phage tail protein | PR | 2 | ++ | ↑*# | |
| PA0620 | Putative tail fiber protein | PR | 2 | ++ | ↑*# | |
| PA0621 | Putative tail fiber assembly protein | PR | 2 | ++ | ↑*# | |
| PA0622 | Putative phage tail sheath protein | PR | 1.5e | ++ | ↑*# | |
| PA0623 | Putative phage tail tube protein | PR | 2 | ++ | ↑*# | |
| PA0624 | Conserved hypothetical protein | PR | 2 | ++ | ↑*# | |
| PA0626 | Putative tail formation protein | PR | 2 | ++ | ↑*# | |
| PA0630 | Hypothetical protein | PR | 2 | ++ | ↑*# | |
| PA0633 | Putative major tail protein V | PR | 2 | ++ | ↑*# | |
| PA0634 | Hypothetical protein | PR | 2 | ++ | ↑*# | |
| PA0636 | Putative tail length determination protein | PR | 2 | ++ | ↑*# | |
| PA0638 | Putative minor tail protein L | PR | 2 | ++ | ↑# | |
| PA0640 | Putative phage tail assembly protein | PR | 1.5 | ++ | ↑*# | |
| PA0641 | Putative phage-related protein, tail component | PR | 2-4 | ++ | ↑*# | |
| PA0647 | Conserved hypothetical protein | PR | 2 | ++ | ↑*# | |
| PA0736-PA0737 | Hypothetical protein | HYP | 2 | ++ | ↑*# | |
| PA0926 | Hypothetical protein | HYP | 4 | ++ | ||
| PA0951-PA0952 | Hypothetical protein | HYP | 4 | ++ | ||
| PA1259 | Conserved hypothetical protein | HYP | 2e | ++ | ||
| PA1345 | gshB | Glutathione synthase | HYP | 2 | ++ | |
| PA1428 | Hypothetical protein | HYP | 2 | ++ | ||
| PA1433 | Conserved hypothetical protein | MP | 2e | ++ | ↑# | |
| PA1434 | Putative periplasmic protein | HYP | 2 | ++ | ||
| PA1513 | Hypothetical protein | MP, HYP | 2 | ++ | ||
| PA1634 | kdpB | Potassium-transporting ATPase, B chain | TSM | 2e | ++ | |
| PA1987 | pqqC | Pyrroloquinoline biosynthesis protein C | BC | 2 | ++ | |
| PA2047 | AraC family transcriptional regulator | TR | 8 | ++ | ||
| PA2060 | Putative permease of ABC transporter | TSM | 2e | ++ | ↑* | |
| PA2399 | pvdD | Pyoverdine synthetase D | SF | 2 | ++ | |
| A/P | ||||||
| PA2400 | pvdJ | Pyoverdine synthesis protein | A/P | 2e | ++ | |
| PA2490 | Conserved hypothetical protein | HYP | 4 | ++ | ||
| PA2566-PA2567 | Hypothetical protein | HYP | 2 | ++ | ||
| PA2638 | nuoB | NADH dehydrogenase I chain B | EM | 2e | ++ | |
| PA2639 | nuoD | NADH dehydrogenase I chains C and D | EM | 2e | ++ | |
| PA2642 | nuoG | NADH dehydrogenase I chain G | EM | 2e | ++ | ↓# |
| PA2643 | nuoH | NADH dehydrogenase I chain H | EM | 2 | ++ | |
| PA2644 | nuoI | NADH dehydrogenase I chain I | EM | 2 | ++ | |
| PA2645 | nuoJ | NADH dehydrogenase I chain H | EM | 2 | ++ | |
| PA2647 | nuoL | NADH dehydrogenase I chain L | EM | 2e | ++ | |
| PA2649 | nuoN | NADH dehydrogenase I chain N | EM | 2e | ++ | ↑* |
| PA2682 | Putative dienelactone hydrolase | PE | 2e | ++ | ||
| PA2864 | Hypothetical protein | HYP | 2 | ++ | ||
| PA3003 | Conserved hypothetical protein | HYP | 2 | ++ | ||
| PA3214 | Hypothetical protein | HYP | 2 | ++ | ||
| PA3276 | Hypothetical protein | HYP | 2e | ++ | ↓* | |
| PA3574 | TetR family transcriptional regulator | TR | 2 | ++ | ||
| PA3620 | mutS | DNA mismatch repair protein MutS | DRR | 4 | ++ | |
| PA3784 | Conserved hypothetical protein | HYP | 2e | ++ | ↑# | |
| PA3835-PA3836 | Hypothetical protein | HYP | 2 | ++ | ||
| PA3958 | Possible nuclease or phosphatase | HYP | 2e | ++ | ↑*# | |
| PA4222 | Putative ATP-binding component of ABC transporter | TSM | 4 | (++) | ||
| PA4223 | Putative ATP-binding component of ABC transporter | MP, TSM | 4 | (++) | ||
| PA4225 | pchF | Pyochelin synthetase | SF, TSM | 2 | ++ | |
| PA4400 | Probable pyrophosphohydrolase | DRR | 4 | ++ | ||
| PA4600 | nfxB | Transcriptional regulatory protein | TR | 4 | ++ | |
| PA4658 | Conserved hypothetical protein | HYP | 2e | ++ | ||
| PA4687 | hitA | Ferric iron-binding periplasmic protein | TSM | 2 | ++ | |
| PA4688 | hitB | Iron (III) transport system permease HitB | MP, TSM | 2e | ++ | ↑*# |
| PA4734 | Conserved hypothetical protein | HYP | 2 | ++ | ||
| PA4946 | mutL | DNA mismatch repair protein MutL | DRR | 4 | ++ | ↑# |
| PA5028 | Hypothetical protein | HYP | 4 | ++ | ||
| PA5131 | pgm | Phosphoglycerate mutase | CCC | 2 | ++ | |
| PA5250 | Putative integral membrane transport protein | MP, HYP | 2e | ++ | ||
| PA5334 | rph | RNase PH | T | 2e | ++ | |
| PA5427 | adhA | Alcohol dehydrogenase, zinc containing | EM, CCC | 4 | ++ | ↓# |
| PA5551 | Hypothetical | HYP | 2 | ++ | ||
| PA5560 | atpB | ATP synthase A chain | EM | 2 | ++ | ↑# |
| PA5562 | spoOJ | Chromosome partitioning protein Spo0J | CD | 2e | ++ | |
| PA5565 | gidA | Glucose-inhibited division protein A | CD | 2 | + | ↑# |
| PA14_ 46620 | Pyridine nucleotide-disulfide oxidoreductase | PE | 2-4 | ++ |
Note that these are the original assignments for these gene mutations and were not confirmed again in this study, although the mutants were used as obtained with minimal subculture. We could also confirm 39 mutations in operons or an overlap between libraries. The mutants with the underlined mutations were tested after the initial screen due to the presence of mutations in an operonic relationship with genes that, when they were altered, influenced susceptibility to ciprofloxacin.
A/P, adaptation, protection; TSM, transport of small molecules; TM, translation; posttranslational modification; PE, putative enzyme; TR, transcriptional regulator; HYP, hypothetical protein; PR, related to phage; MP, membrane protein; BC, biosynthesis of cofactors; SF, secreted factors; EM, energy metabolism; DRR, DNA replication recombination; CCC, carbon compound metabolism; T, transcription; CD, cell division.
++, normal growth rate compared to the wild type; (++), normal growth rate compared to that of the wild type but reduced yield; +, reduced growth rate compared to that of the wild type.
Data are from previous reports (1, 2). All P. aeruginosa genes were differentially expressed by treatment with 0.3× MIC (*) and 1× MIC (#) of ciprofloxacin. The direction of the arrow indicates the trend of expression relative to that for untreated cells.
Change in susceptibility observed after 48 h.
The resistome comprises all genes that, when they are mutated, give rise to altered susceptibility. The present survey indicates for the first time that the resistome for ciprofloxacin in P. aeruginosa is very large; i.e., it comprises more than 100 genes. It is important to note that while we have not demonstrated that these mechanisms are clinically relevant, they do indicate the enormous gene pool that can influence susceptibility to this antibiotic class. Where this may become important is in understanding two complex clinical phenomena, namely, MIC creep (in which the background level of intrinsic resistance to a given antibiotic in a population of clinical isolates rises over time) and adaptive resistance (in which the level of resistance is affected by environmental factors, such as growth in vivo or exposure to antibiotics at sub-MIC). MIC creep has previously been supposed to represent the accumulation of mutations over time (5, 8) and differs from obvious clinical resistance, which is caused by breakthrough mutations (e.g., efflux pump overexpression mutations) that cause very large changes in susceptibility. Adaptive resistance has also been proposed to represent a complex phenomenon in which multiple genes that influence gene expression can combine to induce resistance. Indeed, no fewer than 43 of the genes that gave rise to altered ciprofloxacin susceptibility were included in the list of those that are differentially expressed in P. aeruginosa in the presence of ciprofloxacin (2), which is known to promote adaptive resistance to itself. By performing a broad survey, as described here, we have provided much food for thought, and it will be essential in future studies to follow up these observations with detailed studies to determine if these candidate mutations are indeed relevant to clinically meaningful antibiotic resistance.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research as well as the Canadian Cystic Fibrosis Foundation (CCFF). E.B.M.B. was a recipient of a scholarship from CCFF, and B.K.K. was a recipient of a CIHR-UBC TRID scholarship. I.W. thanks the Juergen Manchot Foundation and the Mucoviszidose e.V., Bonn (German Cystic Fibrosis Association), for financial support. R.E.W.H. holds a Canada Research Chair.
Footnotes
Published ahead of print on 29 September 2008.
REFERENCES
- 1.Brazas, M. D., E. B. M. Breidenstein, J. Overhage, and R. E. W. Hancock. 2007. Role of Lon, an ATP-dependent protease homolog, in resistance of Pseudomonas aeruginosa to ciprofloxacin. Antimicrob. Agents Chemother. 51:4276-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brazas, M. D., and R. E. W. Hancock. 2005. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3222-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazas, M. D., and R. E. W. Hancock. 2005. Using microarray gene signatures to elucidate mechanisms of antibiotic action and resistance. Drug Discov. Today 10:1245-1252. [DOI] [PubMed] [Google Scholar]
- 4.Cirz, R. T., B. M. O'Neill, J. A. Hammond, S.R. Head, and F. E. Romesberg. 2006. Defining the Pseudomonas aeruginosa SOS response and its role on the global response to the antibiotic ciprofloxacin. J. Bacteriol. 188:7101-7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimatatac, E. L., M. M. Alejandria, C. Montalban, C. Pineda, C. Ang, and R. L. Delino. 2003. Clinical outcomes and costs of care of antibiotic resistant Pseudomonas aeruginosa infections. Phil. J. Microbiol. Infect. Dis. 32:159-167. [Google Scholar]
- 6.Drlica, K., and X. Xhao. 1997. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61:377-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer, D. J., M. A. Kohanski, B. Hayete, and J. J. Collins. 2007. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson, M. I., E. M. Scott, and P. S. Collier. 1991. Development of resistance to ciprofloxacin in nutrient-rich and nutrient-limited growth conditions in vitro by Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 35:2649-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 10.Hooper, D. C. 1999. Mechanisms of fluoroquinolone resistance. Drug Resist. Updat. 2:38-55. [DOI] [PubMed] [Google Scholar]
- 11.Kohanski, M. A., D. J. Dwyer, B. Hayete, C.A. Lawrence, and J. J. Collins. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797-810. [DOI] [PubMed] [Google Scholar]
- 12.Lewenza, S., R. K. Falsafi, G. Winsor, W.J. Gooderham, J. B. McPhee, F. S. L. Brinkman, and R. E. W. Hancock. 2005. Construction of a mini-Tn5-luxCDABE mutant library in Pseudomonas aeruginosa PAO1: a tool for identifying differentially regulated genes. Genome Res. 15:583-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati, N. T., J. M. Urbach, S. Miyata, D. G. Lee, E. Drenkard, G. Wu, J. Villanueva, T. Wei, and F. M. Ausubel. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. USA 103:2833-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linares, J. F., I. Gustafsson, F. Baquero, and J. L. Martinez. 2008. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA 103:19484-19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchings, B. W., E. C. Almira, S. Lory, and R. Ramphal. 1995. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect. Immun. 63:4868-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stothard, P., and D. S. Wishart. 2005. Circular genome visualization and exploration using CGView. Bioinformatics 21:537-539. [DOI] [PubMed] [Google Scholar]
- 17.Wiegand, I., K. Hilpert, and R. E. W. Hancock. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protocols 3:163-175. [DOI] [PubMed] [Google Scholar]
- 18.Wu, Y. L., E. M. Scott, A.L. Po, and V. N. Tariq. 1999. Development of resistance and cross-resistance in Pseudomonas aeruginosa exposed to subinhibitory antibiotic concentrations. APMIS 107:585-592. [PubMed] [Google Scholar]