Abstract
New drugs are needed for treatment of Toxoplasma gondii infections. We tested derivatives of principles found in Isatis indigotica for in vitro efficacy against T. gondii infection. Indirubin-3′-oxime analogs showed modest micromolar activity, while tryptanthrin derivatives displayed 50% inhibitory doses in the low nanomolar range. Tryptanthrins have potential as anti-Toxoplasma infection therapeutics.
Toxoplasma gondii is an apicomplexan parasite with worldwide distribution. T. gondii infections in humans range from overwhelming infection in neonates and immunocompromised individuals to inapparent infections in immunocompetent hosts. Current medications for the prevention and treatment of Toxoplasma infection are hampered by toxicity and limited efficacy.
Our approach to address this drug deficit was to examine natural and synthetic derivatives of principles found in the Chinese medicinal herb Isatis indigotica for the ability to disrupt the in vitro growth of T. gondii. Our primary focus centered around analogs of the components indirubin 1a (Table 1) and tryptanthrin 2a (Table 2) (7). Derivatives of these compounds have been shown to possess activity against several protozoan pathogens. Specifically, both classes of compounds have been shown to inhibit some strains of Leishmania spp. (3, 4; E. Xingi, V. Myrianthopoulos, D. Smirlis, S. Bisti, P. Magiatis, E. Mikros, L. Skaltsounis, and K. Soteriadou, presented at the 3rd COST B22 Annual Congress, Drug Discovery and Development for Parasitic Diseases, Athens, Greece, 2006). In addition, tryptanthrin derivatives have been reported to inhibit Trypanosoma brucei (11) and another apicomplexan parasite, Plasmodium falciparum (2). To our knowledge, there are no reports of the activities of these compounds as inhibitors of T. gondii. Therefore, we examined the ability of derivatives of indirubin and tryptanthrin to inhibit the growth of T. gondii in cell culture.
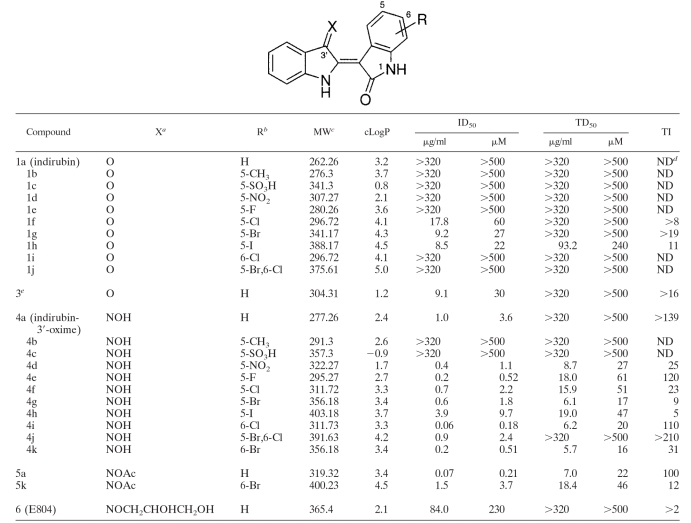
TABLE 1.
In vitro inhibition of T. gondii by indirubin and synthetic derivatives
X, substitution at position X.
R, substitution at position R.
MW, molecular weight.
ND, not determined. TI ≤ 1.
Substitution at position 1-N-acetyl.
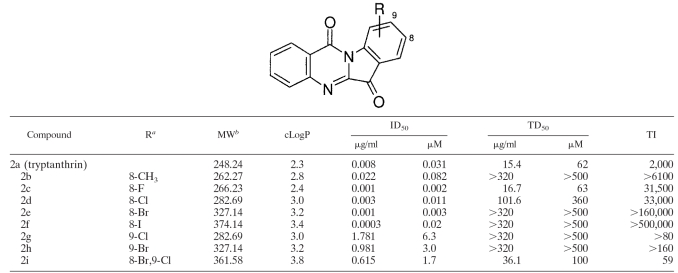
TABLE 2.
In vitro inhibition of T. gondii by tryptanthrin and synthetic derivatives
R, substitution at position R.
MW, molecular weight.
Indirubin-5-sulfonic acid 1c was obtained from Alexis Biochemicals, San Diego, CA. Indirubin-3′-oxime-5-sulfonic acid 4c and indirubin derivative E804 6 (9) were purchased from Calbiochem, Darmstadt, Germany. Trimethoprim and indirubin-3′-oxime 4a were obtained from Sigma Chemical Co. (St. Louis, MO). All other indirubin and tryptanthrin derivatives were synthesized by standard methods (1, 12). The analogs of indirubin 1a were prepared by condensation of 3-acetoxy indole with the appropriate isatin derivatives in the presence of sodium carbonate. Treatment of 1a with hydroxylamine hydrochloride in pyridine gave the 3′-oxime analogs 4 (4a to 4k). The 1-N-acetyl derivative 3 and 3′-acetoxy-oxime compounds 5 (5a and 5k) were prepared by treatment of compound 1a or 4a with acetic anhydride. The analogs of tryptanthrin 2a were synthesized from isatoic anhydride and the corresponding isatin derivatives in refluxing toluene with triethylamine as cosolvent.
Compounds were tested for in vitro efficacy against T. gondii tachyzoites, using methods which have been described in detail in previous publications (5). Briefly, test and control drugs (320 to 0.32 μg/ml) were added to normal human fibroblasts (ATCC, Manassas, VA) plated in 96-well plates. Directly following this, 50 tachyzoites of a β-galactosidase (β-Gal)-producing strain of T. gondii, RH (ATCC 50839), was added to 6/8 wells in each column. After 4 days at 37°C in 5% CO2, the β-Gal substrate chlorophenol red-β-d-galactopyranoside was added to these same wells for 1 additional day. Cytotoxicity was measured in the remaining 2/8 wells using a commercially available cell viability reagent (One Solution; Promega). Color reactions in all wells were read in a Vmax Microplate reader (Molecular Devices, CA). The 50% inhibitory dose (ID50) and the 50% toxic dose (TD50) were calculated by applying the Reed and Muench formula (10) using a minimum of 12 determinations for the ID50 and 6 determinations for the TD50. The therapeutic index (TI) was calculated by the equation TI = TD50/ID50. Stock solutions of compounds were prepared in 50% or 100% dimethyl sulfoxide.
For each compound, the cLogP value was calculated using ChemDraw version 11.0 software (Cambridgesoft, Cambridge, MA).
In our assay, indirubin 1a and six of its derivatives (analogs 1b to 1e and 1i to 1j) were inactive against T. gondii (Table 1). Only the 5-halogen-substituted analogs 1f to 1h displayed some potency (ID50 of >20 μM) but less than that of the control drug trimethoprim. The introduction of 1-N-acetyl group (compound 3) yielded increased efficacy (ID50 = 30 μM) compared to that of the parent compound 1a. In the 3′-oxime series of compounds 4, the 6-chloro 4i, the 5-fluoro 4e, and the 6-bromo 4k substituted analogs proved to be the most effective inhibitors (ID50 = 0.18 to 0.52 μM) but also showed substantial cytotoxicity. Of note, halogen substituents larger than fluorine in the 5 position demonstrated both lower efficacy and selectivity. Thus, the 5-iodo 4h (ID50 = 9.7 μM) was determined to be the least potent and least selective (TI = 5) of the 5-halogen-substituted 3′-oxime derivatives. The di-substituted 5-bromo-6-chloro derivative 4j was the most selective indirubin-3′-oxime (TI, >210). The 3′-acetoxy-oxime derivative 5a was more than 17-fold more potent than the corresponding 3′-oxime 4a, though the cytotoxicity was somewhat increased by this structural modification. The ether derivative 6 was 64-fold less potent than 4a.
Tryptanthrin 2a and five of its derivatives, 2b to 2f, displayed the highest TI of all the compounds tested (Table 2 and Fig. 1). The 8-halogen derivatives 2c to 2f had the lowest ID50 values, with a range of 1 to 11 nM, and, with the exception of the 8-fluoro 2c, exhibited less cytotoxicity than parent compound 2a. The 8-iodo 2f was 30-fold more potent than the parent compound 2a. Interestingly the 9-halogen derivatives 2g and 2h, as well as the 8,9-di-halogen compound 2i, showed >150-fold less activity against T. gondii than the 8-halogen derivatives. The cLogP values of the compounds are shown in Table 1 and 2. All of the compounds meet the standard of being “drug-like,” according to criteria proposed by Lipinski et al. (8).
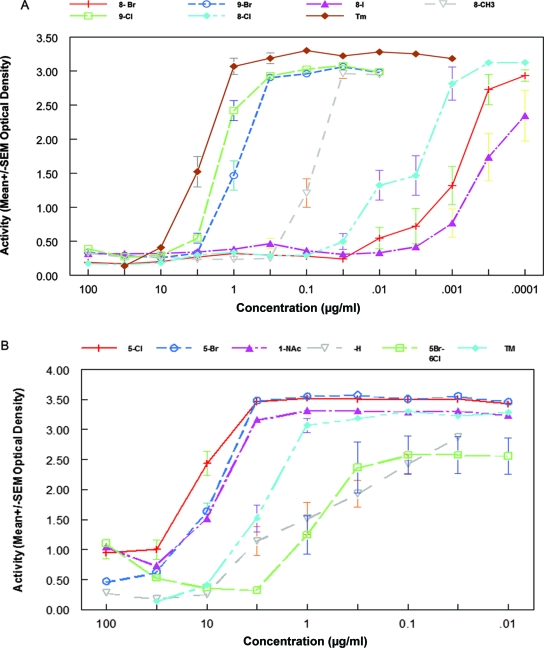
FIG. 1.
Inhibitory activity of selected tryptanthrin and indirubin compounds. The values on the y axis represent the amount of β-Gal activity measured following 5 days of cultivation of 50 tachyzoites of a β-Gal-producing strain of T. gondii in human foreskin fibroblast cells inoculated along with the indicated concentration of the compound. The full chemical structures are shown in Tables 1 and 2. The values in panel A indicate the activity of the tryptanthrin derivatives, and the values depicted in panel B indicate the activity of the indirubin derivatives. The effect of trimethoprim (Tm) is shown for comparison. None of the indicated compounds demonstrated cytotoxicity at the concentrations tested. Compounds with ID50 values of more than 100 μg/ml are not depicted.
Indirubin compounds have been shown to inhibit Leishmania mexicana by the inhibition of CRK3 cyclin-dependent kinase (4). However, the effect of these compounds on the replication of T. gondii has not been previously described. Recently, a number of cell cycle-regulated kinases have been identified in T. gondii which are distinct from those found in mammalian cells (6). Additional studies should be directed at further delineation of these kinases as targets of the indirubin derivatives.
The mechanisms of action of the tryptanthrin group of compounds have not been fully elucidated. Our data indicate that structure-activity relationships of this class of T. gondii inhibitors are well in line with observations previously reported. Bhattacharjee et al. (2) found that halogenated forms of tryptanthrin are more potent inhibitors of Plasmodium malaria than the parent compound. We also found that halogenated forms of tryptanthrin are potent inhibitors of T. gondii, with the highest degree of inhibitory activity being measured in the 8-iodine derivative. In addition, derivatives 2c to 2e reportedly inhibit T. brucei but at more than 1,000-fold the ID50 value for T. gondii (11). It is also of note that the halogenated forms of tryptanthrin showed less cytotoxicity in human cells than the parent compound. The halogenation of these compounds, thus, both increases anti-T. gondii activity and decreases cytotoxic effects on human cells, with a resulting substantial increase in the therapeutic index.
In summary, the tryptanthrin derivatives presented in this paper represent a new class of compounds with substantial activity against in vitro growth of T. gondii. As these studies were performed using in vitro system assays and nonaqueous solvents, they should be verified in more physiological systems such as ones employing animal models.
Acknowledgments
This work was supported by funding from the Stanley Medical Research Institute.
Footnotes
Published ahead of print on 29 September 2008.
REFERENCES
- 1.Beauchard, A., Y. Ferandin, S. Frere, O. Lozach, M. Blairvacq, L. Meijer, V. Thiery, and T. Besson. 2006. Synthesis of novel 5-substituted indirubins as protein kinases inhibitors. Bioorg. Med. Chem. 14:6434-6443. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee, A. K., M. G. Hartell, D. A. Nichols, R. P. Hicks, B. Stanton, J. E. van Hamont, and W. K. Milhous. 2004. Structure-activity relationship study of antimalarial indolo[2,1-b]quinazoline-6,12-diones (tryptanthrins). Three dimensional pharmacophore modeling and identification of new antimalarial candidates. Eur. J. Med. Chem. 39:59-67. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee, A. K., D. J. Skanchy, B. Jennings, T. H. Hudson, J. J. Brendle, and K. A. Werbovetz. 2002. Analysis of stereoelectronic properties, mechanism of action and pharmacophore of synthetic indolo[2,1-b]quinazoline-6,12-dione derivatives in relation to antileishmanial activity using quantum chemical, cyclic voltammetry and 3-D-QSAR CATALYST procedures. Bioorg. Med. Chem. 10:1979-1989. [DOI] [PubMed] [Google Scholar]
- 4.Grant, K. M., M. H. Dunion, V. Yardley, A. L. Skaltsounis, D. Marko, G. Eisenbrand, S. L. Croft, L. Meijer, and J. C. Mottram. 2004. Inhibitors of Leishmania mexicana CRK3 cyclin-dependent kinase: chemical library screen and antileishmanial activity. Antimicrob. Agents Chemother. 48:3033-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones-Brando, L., E. F. Torrey, and R. Yolken. 2003. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr. Res. 62:237-244. [DOI] [PubMed] [Google Scholar]
- 6.Khan, F., J. Z. Tang, C. L. Qin, and K. Kim. 2002. Cyclin-dependent kinase TPK2 is a critical cell cycle regulator in Toxoplasma gondii. Mol. Microbiol. 45:321-332. [DOI] [PubMed] [Google Scholar]
- 7.Liau, B. C., T. T. Jong, M. R. Lee, and S. S. Chen. 2007. LC-APCI-MS method for detection and analysis of tryptanthrin, indigo, and indirubin in Daqingye and Banlangen. J. Pharm. Biomed. Anal. 43:346-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipinski, C. A., F. Lombardo, B. W. Dominy, and P. J. Feeney. 1997. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23:3-25. [DOI] [PubMed] [Google Scholar]
- 9.Nam, S., R. Buettner, J. Turkson, D. Kim, J. Q. Cheng, S. Muehlbeyer, F. Hippe, S. Vatter, K. H. Merz, G. Eisenbrand, and R. Jove. 2005. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc. Natl. Acad. Sci. USA 102:5998-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-499. [Google Scholar]
- 11.Scovill, J., E. Blank, M. Konnick, E. Nenortas, and T. Shapiro. 2002. Antitrypanosomal activities of tryptanthrins. Antimicrob. Agents Chemother. 46:882-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma, V. M., P. Prasanna, K. V. Adi Seshu, B. Renuka, C. V. Laxman Rao, G. Sunil Kumar, C. P. Narasimhulu, P. Aravind Babu, R. C. Puranik, D. Subramanyam, A. Venkateswarlu, S. Rajagopal, K. B. S. Kumar, C. S. Rao, N. V. S. R. Mamidi, D. S. Deevi, R. Ajaykumar, and R. Rajagopalan. 2002. Novel indolo[2,1-b]quinazoline analogues as cytostatic agents: synthesis, biological evaluation and structure-activity relationship. Bioorg. Med. Chem. Lett. 12:2303-2307. [DOI] [PubMed] [Google Scholar]