Abstract
Severe malaria is associated with decreased nitric oxide (NO) production and low plasma concentrations of l-arginine, the substrate for NO synthase. Supplementation with l-arginine has the potential to improve NO bioavailability and outcomes. We developed a pharmacokinetic model for l-arginine in moderately severe malaria to explore the concentration-time profile and identify important covariates. In doses of 3, 6, or 12 g, l-arginine was infused over 30 min to 30 adults with moderately severe malaria, and plasma concentrations were measured at 8 to 11 time points. Patients who had not received l-arginine were also assessed and included in the model. The data were analyzed using a population approach with NONMEM software. A two-compartment linear model with first-order elimination best described the data, with a clearance of 44 liters/h (coefficient of variation [CV] = 52%) and a volume of distribution of 24 liters (CV = 19%). The natural time course of l-arginine recovery was described empirically by a second-order polynomial with a time to half recovery of 26 h. The half-life of exogenous l-arginine was reduced in patients with malaria compared with that for healthy adults. Weight and ethnicity were significant covariates for clearance. MATLAB simulations of dosing schedules for use in future studies predicted that 12 g given over 6, 8, or 12 h will provide concentrations above the Km of endothelial cell CAT-1 transporters in 90%, 75%, and 60% of patients, respectively.
The treatment of severe malaria currently relies on antimalarial drugs and supportive treatments, but the early case fatality rate remains high (8). Adjunctive therapies targeting underlying pathogenic processes early in the treatment of severe malaria may reduce mortality further, but to date none have proven efficacious (10). l-Arginine has been proposed as a potential adjunctive therapy for severe malaria because of its ability to increase nitric oxide (NO) production in endothelial and other cells (16, 34).
We have previously described impaired production of NO (1, 34); low plasma concentrations of its precursor, l-arginine (16); and impaired NO-dependent endothelial function in cases of severe malaria (34). Endothelial dysfunction is a measure of endothelial activation and may play a role in the pathogenesis of severe malaria by increasing the adhesion of parasitized erythrocytes to the endothelium and thereby worsening microcirculatory obstruction and oxygen delivery (9). NO downregulates endothelial inflammation (7) and reduces the cytoadherence of parasitized erythrocytes in vitro (20, 23). Endothelial NO production is dependent on the intracellular movement of extracellular l-arginine by cationic amino acid transporter protein-1 (CAT-1) (35). Estimates for the half-saturating concentration (Km) of extracellular l-arginine for CAT-1 are 100 to 150 μmol/liter (32), within the estimated range of the Km of extracellular l-arginine for intracellular NO production (73 to 150 μmol/liter) (11, 14). In severe malaria, plasma l-arginine concentrations are below this Km, likely contributing to the decreased NO production and endothelial dysfunction found in severe disease. Hypoargininemia also results in NO synthase production of reactive oxygen species instead of NO, resulting in an increase in oxidative stress (24, 30). By infusing l-arginine in patients with moderately severe malaria, we were able to significantly improve endothelial function (34). Previous pharmacokinetic studies of the exogenous administration of l-arginine have been conducted primarily with healthy adult individuals (4, 5), with no data on pharmacokinetic parameters in patients with acute infections such as malaria.
We undertook a prospective observational study to evaluate the natural time course of the recovery of l-arginine concentrations in parallel with a single, ascending-dose study of l-arginine infusion in adults with moderately severe malaria. A population pharmacokinetic model was developed, and simulations were carried out to assess the effectiveness of various regimens of l-arginine infusion to maintain plasma concentrations above the Km of the endothelial cell CAT transporters.
MATERIALS AND METHODS
Study site and subjects.
The study was conducted at Mitra Masyarakat Hospital in Timika, Papua, Indonesia, an area with unstable transmission of malaria and high rates of hospitalization from both falciparum and vivax malaria (15, 21). Ethical approval was obtained from the Health Research Ethics Committees of the National Institute of Health Research and Development, Indonesia, and Menzies School of Health Research, Australia. Written informed consent was obtained from patients or attending relatives in Indonesian or a local language when necessary.
Adults older than 18 years with moderately severe falciparum malaria were enrolled at the emergency department or outpatient clinic (34). Moderately severe falciparum malaria was defined as a fever or history of a fever in the past 48 h, with >1,000 asexual Plasmodium falciparum parasites/μl of blood (a threshold for clinical falciparum malaria in Papua [26]) in patients with no other identified etiology who required inpatient parenteral therapy but did not exhibit the warning signs or criteria for severe malaria as defined by the World Health Organization (31). The exclusion criteria were being pregnant or breastfeeding, having been treated with parenteral antimalarials for >18 h prior to admission, and/or having mixed P. falciparum/Plasmodium vivax infections. Patients were also excluded if significant comorbidities (including diabetes; known cardiac, renal or hepatic disease; a concurrent infection[s]; concurrent use of any medication; a hemoglobin count of <6 g/dl; and/or a systolic blood pressure of <100 mm Hg) or biochemical abnormalities (a baseline venous bicarbonate level of <20 mmol/liter, potassium level of ≥4.2 mmol/liter, glucose level of <3 mmol/liter, or chloride level of >106 mmol/liter) were identified. In those receiving l-arginine, an allergy to l-arginine was an additional exclusion criterion.
Study design.
Eligible subjects were enrolled, as described previously, into one of two groups: an intervention arm receiving intravenous l-arginine or an observational arm in which the subjects received a similar volume of saline (34). Enrollment was nonrandomized, with all subjects receiving standard antimalarial therapy with intravenous quinine (34). There were no significant differences in the baseline demographic or clinical characteristics between those receiving saline or l-arginine (34). In the intervention group, three groups of 10 different patients were given 3 g (17 mmol), 6 g (34 mmol) or 12 g (68 mmol) of l-arginine diluted in normal saline to a concentration of 10% or less by an infusion pump over 30 min via an intravenous cannula in the antecubital fossa. All patients gave a standardized medical history and underwent serial physical examinations. For the observational study, venous blood was collected into tubes containing lithium heparin upon recruitment and at regular intervals until discharge. For patients receiving l-arginine, venous blood was collected before and immediately at the end of the infusion and repeated at approximately 5, 20, 30, 60, and 120 min and 4, 8, and 24 h after the end of the infusion. Hemoglobin and white blood cell counts were measured by Coulter counter, and routine biochemistry and acid base parameters were analyzed with a bedside biochemical analyzer (i-Stat Corp., Windsor, NJ). Parasite counts were determined by Giemsa-stained thick and thin fields and were cross-checked by an experienced microscopist.
Determination of plasma concentrations of l-arginine.
Plasma was separated by centrifugation within 30 min of collection and stored at −70°C. Amino acids were extracted from 50 μl of plasma after the addition of 50 μl of an internal standard (norleucine) and 200 μl of cold ethanol. Deproteinized plasma was derivatized with AccQFluor reagent (Waters Corp., Milford, MA), and amino acids were measured by high-performance liquid chromatography (Shimadzu, Kyoto, Japan), using a method modified from that of van Wandelen and Cohen (28). By this method, the percent coefficient of variation (CV%) for l-arginine was 4.6 at 77 μM, with a lower limit of detection of 2.5 μM (0.53 mg/liter).
Population pharmacokinetic modeling.
Data were analyzed using the first-order conditional estimation (FOCE) method with interaction in NONMEM (version 5, level 1.1) with the G77 FORTRAN compiler (3). An evaluation of model suitability was conducted with standard goodness-of-fit criteria such as measurement of objective function, parameter estimates, between-subject variability (BSV) and diagnostic plots (12). The standard three-stage population analysis approach was used to identify covariates (18). Model selection was based on three further criteria. (i) Models had to have statistical significance corresponding to a drop in NONMEM objective function between successive models of 3.84 units (χ2 test, P < 0.05), with a one-parameter difference between models. (ii) The parameter estimates were required to be physiologically plausible (e.g., a nonnegative value for clearance or a central volume less than the plasma volume). This criterion was also applied to between-subject variance, where extremely small or large values were considered indicators of overparameterization of the model. (iii) Models had to have stability, which was assessed by altering the initial parameter estimates and/or changing the number of significant digits. Neither should affect the final parameter values if the model is stable. For covariate analysis, additional criteria were also considered: (i) clinical significance (defined as a change in parameter values of >20% over the range of the usual values of the covariate); (ii) a biologically plausible relationship; and (iii) a reduction in random BSV.
Model development. Structural models.
Compartmental models were parameterized in terms of volume of distribution (V) and clearance (CL). For the natural recovery of l-arginine, a model was constructed to allow for the effect of the infection on the turnover of l-arginine concentrations. The assumptions were that the effects of the infection occurred 48 h prior to admission, resulting in a decrease in l-arginine concentration until antimalarial treatment was started. This corresponded to the duration of symptoms before patients sought medical help.
Dosing simulations.
Stochastic simulations were performed using MATLAB (version 6.5, release 13) to identify suitable dosing regimens for l-arginine that maintained the concentrations above the Km of the CAT transporter, set as 150 μmol/liter. This was performed by generating the pharmacokinetic profiles of 1,000 virtual subjects under different dosing regimens and assessing the proportion of patients that had concentrations above the Km value.
RESULTS
A total of 78 patients were enrolled, 48 in the observational group and 30 in the group receiving l-arginine. The patient characteristics are shown in Table 1.
TABLE 1.
Baseline characteristics of patients
| Characteristic | Saline infusion group | l-Arginine infusion group |
|---|---|---|
| Total no. of subjects | 48 | 30 |
| Mean age [yr (range)] | 28 (18-56) | 28 (18-54) |
| No. (%) of males | 32 (67) | 20 (67) |
| Mean wt [kg (range)] | 58 (43-77) | 58 (42-70) |
| No. (%) of Papuan highlanders | 37 (77) | 23 (77) |
| No. (%) of current smokers | 19 (40) | 11 (37) |
| No. (%) of ex-smokers | 7 (14) | 4 (13) |
| Median no. of days of fever before presentation (IQR)a | 2 (1-5) | 2 (1-4) |
| Mean systolic blood pressure [mm Hg (range)] | 114 (88-152) | 109 (90-138) |
| No. (%) hypertensive on enrollment | 2 | 0 |
| Mean pulse rate [beats/minute (range)] | 86 (56-118) | 80 (54-116) |
| Mean respiratory rate [breaths/minute (range)] | 25 (14-42) | 24 (18-32) |
| Mean temp [°C (range)] | 36.5 (34.1-39.8) | 37 (34.8-40.2) |
| Mean white blood cell count [103 μl−1 (range)] | 5.9 (2.6-10.8) | 6.2 (2.3-11.7) |
| Mean hemoglobin count [g/dl (95% CI)] | 12.8 (7.1-16.7) | 12.3 (7.5-17) |
| mean l-arginine plasma concn [μmol/liter (95% CI)] | 42 (37-45) | 37 (33-43) |
| Mean lactate concn [mmol/liter (95% CI)] | 1.29 (1.1-1.50) | 1.5 (1.2-1.8) |
| Geometric mean parasite density [μl−1 (range)] | 13,297 (850-127,350) | 17,221 (890-281,864) |
IQR, interquartile range.
Safety of l-arginine infusions.
l-Arginine infusions were well tolerated. No new symptoms developed during infusion except for mild pain at the infusion site, with no evidence of inflammation, in two patients. There were no statistically or clinically significant effects on pulse rate or respiratory rate before, during, or after the infusion of any dose of l-arginine, with continuous electrocardiography readings remaining normal throughout (33). The mean systolic and diastolic blood pressures did not change significantly immediately following the 3 g and 6 g arginine infusions. Patients given 12 g had a transient, statistically but not clinically significant mean decrease in systolic (5 mm Hg; 95% confidence interval [CI], 3 to 8 mm Hg; P = 0.01) and diastolic (5 mm Hg; 95% CI, 0.5 to 9 mm Hg; P = 0.03) blood pressures measured immediately at the end of the infusion, which returned to baseline thereafter. Changes in glucose or electrolyte (potassium, phosphate, bicarbonate, pH, chloride) measurements following infusion were not clinically important. The full safety profile following l-arginine infusion has been reported separately (33).
Population pharmacokinetics. l-Arginine concentrations pre- and postinfusion.
Mean baseline l-arginine concentrations in plasma were 42 μmol/liter (95% CI, 37 to 45) in the observational group and 37 μmol/liter (95% CI, 33 to 43) in those who were to receive l-arginine (P = 0.32). Mean peak l-arginine concentrations after the end of the infusions of 3, 6, and 12 g rose to 288 μmol/liter (95% CI, 172 to 405), 809 μmol/liter (95% CI, 592 to 1,027), and 1,310 μmol/liter (95% CI, 911 to 1,709), respectively. Conversely, there was no significant change in those patients who received saline infusions.
Natural recovery of l-arginine.
A turnover model was used to describe the increase in l-arginine over time in individuals who did not receive exogenous l-arginine. This model was defined by the following equations.
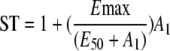 |
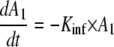 |
 |
In this model, ST is the theoretical effect of the infective process on the elimination of arginine, A1 represents the infective process, Kinf is the first-order rate constant of the decline in the infective process, Emax is the maximum fraction that the infective process can increase the turnover of l-arginine, E50 is the amount at which this process is at 50% of its maximum, Kin is the zero-order rate of production of l-arginine, Kout is the first-order rate constant of the loss of arginine, and A2 is the amount of l-arginine. An instantaneous infective process initiated 2 days before the patient was enrolled in the study was assumed. The time chosen was similar to the actual duration of the symptoms recorded (median, 2 days; range, 1 to 14 days). The infective process then triggered an increased turnover of l-arginine, probably via increased catabolism from a variety of metabolic pathways, including arginase, arginine:glycine amidinotransferase, and NO synthase (19). The model allowed for l-arginine to be constantly produced from a large pool not affected by the process and which then returned to preinfective amounts once the infective process abated. However, the model was sensitive to initial estimates. An empirical second-order polynomial was used as the final model.
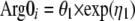 |
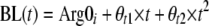 |
where Arg0i represents the ith patient's baseline value of arginine, ηi is the difference between an individual's value of Arg0 and the average value in the population, θ1 is the average value of Arg0 in the population, θt1 and θt2 are the coefficients in the polynomial linking arginine recovery to time, exp represents the exponential, BL(t) is the baseline value of arginine concentration at time t, and t is time and is initiated at 2 days prior to presentation (approximately the start of symptoms). Using this model, the time to half recovery was 26 h, and the baseline value of l-arginine was 33 μmol/liter with BSV of 43%.
Pharmacokinetics of l-arginine after infusion.
For patients who received l-arginine, the data were best described by a two-compartment linear model with first-order elimination and log-normal BSV for the clearance and central volume compartment. The error model was a combined proportional and additive residual variance model. The model allowed for the baseline concentrations and natural recovery of l-arginine using the second-order polynomial model described for the natural recovery. The concentration-time profiles for the observed and predicted l-arginine values and the weighted residual plot are shown in Fig. 1 and 2A, respectively. The half-life at α phase (t1/2α) was 15 min, and the t1/2β was 3.75 h, with this phase contributing to 40% of the area under the concentration-time curve. By use of estimates obtained from simulations assuming a log-normal distribution, the CV% of t1/2α was 84% and that of t1/2β was 39%.
FIG. 1.
Concentration-time profiles (A to D) and logs of the concentration-time profiles (E to H) of l-arginine before, during, and up to 24 h after infusion of saline (A and E) or doses of 3 g (B and F), 6 g (C and G), and 12 g (D and H) of l-arginine. The times of the l-arginine infusions were from 0 to 0.5 h.
FIG. 2.
Weighted residual plots for the baseline two-compartment first-order elimination model (A) and for the final covariate model (B).
Incorporation of weight as a covariate for CL and the addition of an ethnic group (Papuan versus non-Papuan) improved the model fit significantly, and both were therefore included in the final model. The final parameter estimates for the covariate model are summarized in Table 2, and the weighted residuals are displayed in Fig. 2B. Inclusion of sex and leukocyte and parasite counts as covariates for clearance and the weight/ethnic group to the central volume compartment did not improve the model fit. The residual error did not change between the full and base pharmacokinetic models, but the BSV on CL decreased by 62%.
TABLE 2.
Parameter estimates for final covariate model
| Parameter | Value | SE (% CV) |
|---|---|---|
| Exogenous l-arginine | ||
| CL (liter h−1)b | 31 | 17 |
| V1 (liter)c | 27 | 13 |
| V2 (liter)e | 21 | 39 |
| Q (liter h−1)d | 74 | 18 |
| θ8 (allometric constant for CL) | 2.46 | 23 |
| θ4 (fractional effect of ethnicity) | 1.84 | 17 |
| θ10 (allometric constant for V1) | 0.781 | 71 |
| ωCL (% CV)f | 32 | 30 |
| ωV1 (% CV)g | 19 | 68 |
| Endogenous l-arginine | ||
| Arg0a (μmol/liter) | 33 | 10 |
| θt1a (μmol/liter/h) | 0.0612 | 78 |
| θt2a (μmol/liter/h2) | −0.000139 | 84 |
| ωArg0 (% CV) | 43 | 43 |
| Residual error | ||
| σaddh (μmol/liter) | ≈0 | 22 |
| σpropi (CV) | 28.6 |
For endogenous l-arginine recovery after falciparum malaria infection, where θ t1 and θ t2 are the first- and second-order coefficients of the polynomial.
CL, clearance in first compartment.
V1, volume of distribution in first compartment.
Q, clearance in second compartment.
V2, volume of distribution in second compartment.
ωCL, between-subject variance for clearance in first compartment.
ωV1, between-subject variance for volume of distribution in first compartment.
σadd, standard deviation of the additive component of the residual error.
σprop, standard deviation of the proportional component of the residual error.
Dosing simulations.
Using the baseline model, random simulations were performed with sampling of 1,000 virtual subjects from the distribution for CL, the volume of the central compartment, and baseline l-arginine concentrations. The Km of the CAT transporters responsible for the intracellular transport of l-arginine was assumed to be 150 μmol/liter (26 mg/liter). Each virtual subject was dosed with one of the following dosage regimens: (i) 12 g over 1 h; (ii) 12 g by continuous infusion over 6, 8, and 12 h; or (iii) intermittent infusions of 3 g, 3 g, and 6 g given over 30 min at 4 h intervals, 6 g and 6 g given over 30 min at 4 h intervals, and 6 g and 6 g given over 2 h at 4-h intervals. Concentration-time courses were calculated 12 to 16 h postdosing for the various regimens.
Each regimen was assessed for the percentage of patients above the Km at the end of the infusions. Regimen one provided concentrations above the Km for 50% of patients at 2 h and 25% at 3 h after infusion. The long-duration infusions used in regimen two maintained concentrations just above Km for a time period about equal to the duration of the infusion. With 12 g of l-arginine, 90%, 75%, and 60% of individuals achieved the target Km when infused over 6, 8, and 12 h, respectively. For the multiple-dose regimens, a dosage of 6 g given over 2 h and repeated 4 h later provided sufficient time above the Km and attained peak concentrations equivalent to 12 g, constant-infusion dosing.
DISCUSSION
In this study, the population pharmacokinetic parameters of endogenous and exogenous l-arginine were estimated for patients with moderately severe malaria. Increased oxidation without a compensatory increase in the de novo synthesis of endogenous l-arginine has been described for sepsis (2, 29). This work led investigators to postulate that l-arginine becomes a conditionally essential amino acid during acute inflammatory states (2) and therefore may be required as part of standard nutritional therapy in severely ill patients with infections or burns. l-Arginine infusion in adults with sepsis results in only very transient hemodynamic changes (17). These findings suggest that rapid turnover of l-arginine occurs in patients with acute inflammatory states, although there are no pharmacokinetic data available for ill subjects. Previously published studies of l-arginine pharmacokinetics have been done with healthy volunteers (4, 22, 25, 27), in whom l-arginine metabolism is likely to differ significantly from that in acutely ill individuals.
Early l-arginine pharmacokinetic studies (22, 27) were limited by short sampling periods and insensitive enzymatic and photometric assays with high coefficients of variation. More-recent studies have examined the pharmacokinetics after infusions of 6 g and 30 g of l-arginine over 30 min into healthy volunteers (4, 13, 25). After a 6 g infusion, the mean peak l-arginine concentration in plasma of 822 μmol/liter (4) was comparable to the mean peak concentration of 809 μmol/liter for patients with malaria. In contrast, doses of 30 g of l-arginine in healthy volunteers achieved mean plasma concentrations of 6,223 to 7,978 μmol/liter (4, 13, 25), higher than the peak of 1,310 μmol/liter we observed in patients with malaria. The results of one of the volunteer studies were analyzed with a standard two-stage method and reported dose-dependent pharmacokinetics, with a decreasing half-life and volume of distribution with the higher dose (4). The authors suggested that this observation was due to the renal threshold of l-arginine being exceeded with the larger dose. However, in our study there was no evidence for nonlinearity at the dosages used. In healthy individuals, the elimination half-life ranged from 40 to 60 min (4), while in our study the t1/2α and t1/2β were 15 min and 3.75 h, respectively. Possible explanations for the different findings include the short sampling times and the use of noncompartmental analysis in the previous study. This would serve to limit the previous study analyses to the consideration of a single exponential decay model with a half-life that would be expected to lie approximately between our two half-lives. Previous studies with healthy volunteers did not identify covariates. In contrast, we found that weight and ethnic group influenced the pharmacokinetics of l-arginine in patients with moderately severe malaria.
To our knowledge, this is the first study that has explored the pharmacokinetics of the natural recovery as well as the administration of exogenous l-arginine in patients with falciparum malaria or, indeed, any acute inflammatory disease state. Inflammatory processes are likely to affect l-arginine homeostasis in patients with malaria. Studies with critically ill children have shown an increase in the oxidation of l-arginine (2), with no change in flux compared to that for healthy adults, and in contrast to that for adults with sepsis, who had a decreased flux (29). In these studies, NO production accounted for 6% of l-arginine consumption in acutely ill children, compared to approximately 2 to 3% in septic adults and 4 to 5% in healthy adult controls. These studies suggest that most of the plasma l-arginine is metabolized by other enzymatic pathways, including those of arginase and arginine:glycine amidinotransferase, which produce ornithine and creatine, respectively (32). The increased plasma arginase activity we noted with malaria (34) is likely to contribute to increased catabolism and the shorter half-life of l-arginine we found with acute malaria. The differences in l-arginine metabolism suggest that understanding the pharmacokinetics of l-arginine during acute malaria is crucial to modeling the time course of drug exposure and developing a dosing strategy which may differ from that for healthy individuals.
In patients with severe malaria, the eventual target group, we believe the window for l-arginine therapy would be within the first 24 to 48 h, when the patients are the most acutely unwell and before the natural recovery from hypoargininemia and endothelial dysfunction. This is also the time window in which the use of artesunate, the most rapidly parasiticidal drug yet available, has been unable to reduce early fatality in cases of severe malaria (8). In patients with severe malaria, l-arginine would be given as a continuous infusion for the time period required to increase the l-arginine concentration and increase NO production. With this background, the other major goal of the study was to simulate the concentration-time profile of various l-arginine dosage regimens using the pharmacokinetics model developed. Sustained production of NO by both immune (6) and endothelial cells (35) is dependent on the transport of extracellular l-arginine into cells by CAT with a Km of 70 to 150 μmol/liter, and we used 150 μmol/liter as the target concentration. The simulations demonstrated that regimens of continuous infusion of 12 g over 6, 8, and 12 h would exceed the Km in 90%, 75%, and 60% of patients at the end of the infusion, but because of the short half-life, such concentrations would be maintained only for the duration of the infusion. However, simulations of multiple-dosing regimens also provide other options which would provide satisfactory l-arginine concentration-time exposures. In the absence of a combined pharmacokinetic/pharmacodynamic model, we do not know the optimal dosage regimen.
In conclusion, a study of a single, ascending-dose l-arginine infusion in adults with moderately severe malaria was conducted, and the dosing regimen was found to be safe, with no clinically significant adverse effects. The pharmacokinetic parameters estimated were significantly different from those of previous studies with healthy volunteers. Simulations of various dosing regimens using these parameters to maintain l-arginine concentrations above the Km of the CAT were done. These regimens will be assessed in future trials with patients with severe malaria to determine the safety and efficacy of l-arginine as an adjunctive therapy.
Acknowledgments
We thank Christabelle Darcy, Ferryanto Chalfein, Kim Piera, Prayoga Roesmini, Yoshi Elvi, Betsy Hill, Govert Waramori, and Sri Rahayu for technical and logistical assistance; David Celermajer, Don Granger, Brice Weinberg, and Bert Lopansri for advice; Marlini Malisan and Margaretha Ferre for nursing assistance; Mitra Masyarakat Hospital staff for clinical support; and Lembaga Pengembangan Masyarakat Amungme Kamoro, Jeanne Rini, Julie Simpson, and Paulus Sugiarto for support. The members of the Data Safety Monitoring Committee were Peter Morris, Nani Sukasediati, Paulus Sugiarto, Paul Kelly, and Stephen Halpin.
The study was funded by the National Health and Medical Research Council of Australia (NHMRC 283321 and 290208 and a Practitioner Fellowship to N.M.A.), the Wellcome Trust (grant GR071614MA), and the Tudor Foundation. R.N.P. is supported by a Wellcome Trust Career Development Award (074637).
N.M.A. is named as an inventor in a U.S. patent for the use of l-arginine as a treatment for severe malaria but has transferred all rights to his institutional malaria research collaborations. This patent was issued for U.S. rights only, and no rights are being sought in other countries.
Footnotes
Published ahead of print on 6 October 2008.
REFERENCES
- 1.Anstey, N. M., J. B. Weinberg, M. Y. Hassanali, E. D. Mwaikambo, D. Manyenga, M. A. Misukonis, D. R. Arnelle, D. Hollis, M. I. McDonald, and D. L. Granger. 1996. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argaman, Z., V. R. Young, N. Noviski, L. Castillo-Rosas, X. M. Lu, D. Zurakowski, M. Cooper, C. Davison, J. F. Tharakan, A. Ajami, and L. Castillo. 2003. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit. Care Med. 31:591-597. [DOI] [PubMed] [Google Scholar]
- 3.Beal, S. L., and L. Sheiner. 1998. NONMEM user's guide, parts I to VII. NONMEM Project Group, San Francisco, CA.
- 4.Bode-Böger, S. M., R. H. Böger, A. Galland, D. Tsikas, and J. C. Frolich. 1998. L-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship. Br. J. Clin. Pharmacol. 46:489-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böger, R. H., and S. M. Bode-Böger. 2001. The clinical pharmacology of L-arginine. Annu. Rev. Pharmacol. Toxicol. 41:79-99. [DOI] [PubMed] [Google Scholar]
- 6.Closs, E. I., A. Simon, N. Vekony, and A. Rotmann. 2004. Plasma membrane transporters for arginine. J. Nutr. 134:2752S-2759S; discussion, 2765S-2767S. [DOI] [PubMed] [Google Scholar]
- 7.De Caterina, R., P. Libby, H. B. Peng, V. J. Thannickal, T. B. Rajavashisth, M. A. Gimbrone, Jr., W. S. Shin, and J. K. Liao. 1995. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Investig. 96:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dondorp, A., F. Nosten, K. Stepniewska, N. Day, and N. White. 2005. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366:717-725. [DOI] [PubMed] [Google Scholar]
- 9.Dondorp, A. M., E. Pongponratn, and N. J. White. 2004. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 89:309-317. [DOI] [PubMed] [Google Scholar]
- 10.Golenser, J., J. McQuillan, L. Hee, A. J. Mitchell, and N. H. Hunt. 2006. Conventional and experimental treatment of cerebral malaria. Int. J. Parasitol. 36:583-593. [DOI] [PubMed] [Google Scholar]
- 11.Granger, D. L., J. B. Hibbs, Jr., J. R. Perfect, and D. T. Durack. 1990. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J. Clin. Investig. 85:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, B., and S. B. Duffull. 2003. Development of a dosing strategy for enoxaparin in obese patients. Br. J. Clin. Pharmacol. 56:96-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hishikawa, K., T. Nakaki, M. Tsuda, H. Esumi, H. Ohshima, H. Suzuki, T. Saruta, and R. Kato. 1992. Effect of systemic L-arginine administration on hemodynamics and nitric oxide release in man. Jpn. Heart J. 33:41-48. [DOI] [PubMed] [Google Scholar]
- 14.Iyengar, R., D. J. Stuehr, and M. A. Marletta. 1987. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc. Natl. Acad. Sci. USA 84:6369-6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karyana, M., L. Burdarm, S. Yeung, E. Kenangalem, N. Wariker, R. Maristela, K. Gde Umana, R. Vemuri, M. J. Okoseray, P. M. Penttinen, P. Ebsworth, P. Sugiarto, N. M. Anstey, E. Tjitra, and R. N. Price. 2008. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malaria J. 7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopansri, B. K., N. M. Anstey, J. B. Weinberg, G. J. Stoddard, M. R. Hobbs, M. C. Levesque, E. D. Mwaikambo, and D. L. Granger. 2003. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet 361:676-678. [DOI] [PubMed] [Google Scholar]
- 17.Lorente, J. A., L. Landin, R. De Pablo, E. Renes, and D. Liste. 1993. L-arginine pathway in the sepsis syndrome. Crit. Care Med. 21:1287-1295. [DOI] [PubMed] [Google Scholar]
- 18.Mandema, J. W., D. Verotta, and L. B. Sheiner. 1992. Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J. Pharmacokinet. Biopharm. 20:511-528. [DOI] [PubMed] [Google Scholar]
- 19.Morris, S. M., Jr. 2004. Enzymes of arginine metabolism. J. Nutr. 134:2743S-2747S. [DOI] [PubMed] [Google Scholar]
- 20.Pino, P., I. Vouldoukis, N. Dugas, M. Conti, J. Nitcheu, B. Traore, M. Danis, B. Dugas, and D. Mazier. 2004. Induction of the CD23/nitric oxide pathway in endothelial cells downregulates ICAM-1 expression and decreases cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell. Microbiol. 6:839-848. [DOI] [PubMed] [Google Scholar]
- 21.Ratcliff, A., H. Siswantoro, E. Kenangalem, R. Maristela, R. M. Wuwung, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet 369:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts, I., and I. M. Smith. 1984. A simple method for the measurement of arginine in serum. Ann. Clin. Biochem. 21:515-518. [DOI] [PubMed] [Google Scholar]
- 23.Serirom, S., W. H. Raharjo, K. Chotivanich, S. Loareesuwan, P. Kubes, and M. Ho. 2003. Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am. J. Pathol. 162:1651-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuehr, D., S. Pou, and G. M. Rosen. 2001. Oxygen reduction by nitric-oxide synthases. J. Biol. Chem. 276:14533-14536. [DOI] [PubMed] [Google Scholar]
- 25.Tangphao, O., M. Grossmann, S. Chalon, B. B. Hoffman, and T. F. Blaschke. 1999. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br. J. Clin. Pharmacol. 47:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjitra, E., S. Suprianto, B. J. Currie, P. S. Morris, J. R. Saunders, and N. M. Anstey. 2001. Therapy of uncomplicated falciparum malaria: a randomized trial comparing artesunate plus sulfadoxine-pyrimethamine versus sulfadoxine-pyrimethamine alone in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 65:309-317. [DOI] [PubMed] [Google Scholar]
- 27.van Haeften, T. W., and C. H. Konings. 1989. Arginine pharmacokinetics in humans assessed with an enzymatic assay adapted to a centrifugal analyzer. Clin. Chem. 35:1024-1026. [PubMed] [Google Scholar]
- 28.van Wandelen, C., and S. A. Cohen. 1997. Using quaternary high-performance liquid chromatography eluent systems for separating 6-aminoquinolyl-N-hydroxysuccinimidylcarbamate-derivatized amino acid mixtures. J. Chromatog. A 763:11-22. [Google Scholar]
- 29.Villalpando, S., J. Gopal, A. Balasubramanyam, V. P. Bandi, K. Guntupalli, and F. Jahoor. 2006. In vivo arginine production and intravascular nitric oxide synthesis in hypotensive sepsis. Am. J. Clin. Nutr. 84:197-203. [DOI] [PubMed] [Google Scholar]
- 30.Wang, W., S. Wang, L. Yan, P. Madara, A. Del Pilar Cintron, R. A. Wesley, and R. L. Danner. 2000. Superoxide production and reactive oxygen species signaling by endothelial nitric-oxide synthase. J. Biol. Chem. 275:16899-16903. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 32.Wu, G., and S. M. Morris, Jr. 1998. Arginine metabolism: nitric oxide and beyond. Biochem. J. 336:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeo, T. W., D. A. Lampah, R. Gitawati, E. Tjitra, E. Kenangalem, D. L. Granger, J. B. Weinberg, B. K. Lopansri, R. N. Price, D. S. Celermajer, S. B. Duffull, and N. M. Anstey. 2008. Safety profile of L-arginine infusion in moderately severe falciparum malaria. PLoS ONE 3:e2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeo, T. W., D. A. Lampah, R. Gitawati, E. Tjitra, E. Kenangalem, Y. R. McNeil, C. J. Darcy, D. L. Granger, J. B. Weinberg, B. K. Lopansri, R. N. Price, S. B. Duffull, D. S. Celermajer, and N. M. Anstey. 2007. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J. Exp. Med. 204:2693-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zani, B. G., and H. G. Bohlen. 2005. Transport of extracellular l-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am. J. Physiol. Heart Circ. Physiol. 289:H1381-H1390. [DOI] [PubMed] [Google Scholar]




