Abstract
Doripenem, a 1β-methylcarbapenem, is a broad-spectrum antibiotic approved for the treatment of complicated urinary tract and complicated intra-abdominal infections. An indication for hospital-acquired pneumonia including ventilator-associated pneumonia is pending. The current study examined the activity of doripenem against recent clinical isolates for the purposes of its ongoing clinical development and future longitudinal analysis. Doripenem and comparators were tested against 12,581 U.S. clinical isolates collected between 2005 and 2006 including isolates of Staphylococcus aureus, coagulase-negative staphylococci, Streptococcus pneumoniae, Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter spp. MICs (μg/ml) were established by broth microdilution. By MIC90, doripenem was comparable to imipenem and meropenem in activity against S. aureus (methicillin susceptible, 0.06; resistant, 8) and S. pneumoniae (penicillin susceptible, ≤0.015; resistant, 1). Against ceftazidime-susceptible Enterobacteriaceae, the MIC90 of doripenem (0.12) was comparable to that of meropenem (0.12) and superior to that of imipenem (2), though susceptibility of isolates exceeded 99% for all evaluated carbapenems. The activity of doripenem was not notably altered against ceftazidime-nonsusceptible or extended-spectrum β-lactamase screen-positive Enterobacteriaceae. Doripenem was the most potent carbapenem tested against P. aeruginosa (MIC90/% susceptibility [%S]: ceftazidime susceptible = 2/92%S, nonsusceptible = 16/61%S; imipenem susceptible = 1/98.5%S, nonsusceptible = 8/56%S). Against imipenem-susceptible Acinetobacter spp., doripenem (MIC90 = 2, 89.1%S) was twice as active by MIC90 as were imipenem and meropenem. Overall, doripenem potency was comparable to those of meropenem and imipenem against gram-positive cocci and doripenem was equal or superior in activity to meropenem and imipenem against Enterobacteriaceae, including β-lactam-nonsusceptible isolates. Doripenem was the most active carbapenem tested against P. aeruginosa regardless of β-lactam resistance.
Gram-negative bacilli play a significant role in the most prevalent types of nosocomial infections including hospital-acquired pneumonia, ventilator-associated pneumonia, urinary tract infections, and intra-abdominal infections (7, 31, 32). Although substantial attention has been given to the development of agents to combat drug-resistant gram-positive cocci, pursuit of antimicrobials for use in infections caused by gram-negative bacilli has not been as extensive (20). This trend combined with the continued emergence and spread of gram-negative bacilli resistant to β-lactam agents (9, 23, 28) has resulted in a growing need for new agents active against the resistant gram-negative pathogens encountered in hospitals.
The stability of carbapenems to a wide variety of β-lactamases produced by gram-negative bacilli (4, 18), combined with their relatively low toxicity potential, makes this class of antibiotics good candidates for clinical development and use against hospital-associated gram-negative bacterial infections. Doripenem, an investigational 1β-methylcarbapenem (11), is one such compound currently under development for the treatment of complicated urinary tract infections, complicated intra-abdominal infections, and nosocomial pneumonia including ventilator-associated pneumonia. Previous surveillance studies indicate that doripenem is active in vitro against bacteria commonly associated with these indications (1, 6, 8, 13, 14, 35, 36) and is effective in vivo in murine models of bacteremia and pulmonary infection and a rat intrauterine infection model (22, 35).
Doripenem is an agent that primarily will be used to treat infections caused by gram-negative bacteria resistant to a variety of antimicrobial agents. As such, it also will be used in an environment where there is high selective pressure for the emergence and spread of resistance. Therefore, this study was undertaken to establish the current baseline activity of doripenem against the spectrum of bacterial species, and their accompanying resistance phenotypes, for which it will be used. The analysis and findings of this surveillance initiative that involved recent clinical isolates obtained from institutions across the United States will serve as a reference for monitoring changes in doripenem activity should they occur during clinical development and use.
MATERIALS AND METHODS
Organism collection and identification.
From November 2005 to June 2006 a total of 12,581 nonconsecutive and nonduplicate clinically significant isolates were collected from 44 hospitals across the United States, with the exception of Streptococcus pneumoniae, which was collected from 157 hospitals. No selection criteria were applied during collection with the exception of staphylococci, where an approximately 70% bias toward methicillin-resistant isolates was requested. These institutions represented all nine U.S. Bureau of the Census regions. Isolates were limited to one per patient, and Amies transport swabs (Copan Diagnostics Inc., Corona, CA) from overnight cultures of the bacterial isolates were shipped to a central laboratory (Eurofins Medinet, Inc., Herndon, VA) for testing. Upon receipt, isolates were plated and subcultured onto appropriate media, after which the reported identifications of isolates were confirmed by standard clinical methods (24). Pure cultures of the isolates were frozen and stored at −70°C. Isolates received included 1,814 of Staphylococcus aureus, 642 of coagulase-negative staphylococci (CoNS), 3,932 of S. pneumoniae, 1,772 of Escherichia coli, 1,227 of Klebsiella pneumoniae, 636 of Proteus mirabilis, 508 of Enterobacter cloacae, 514 of Citrobacter spp., 372 of Serratia marcescens, 875 of Pseudomonas aeruginosa, and 289 of Acinetobacter spp. These included 28% urinary tract isolates, 28% respiratory tract isolates, 18% bloodstream isolates, 14% wound isolates, and 3% skin isolates with the remainder (approximately 9%) of either other or unknown origin.
Antimicrobial testing.
All isolates were tested by broth microdilution according to the guidelines set forth in standard M7-A7 by the Clinical and Laboratory Standards Institute (CLSI [formerly NCCLS], Wayne, PA) (2). Overnight cultures grown from isolated colonies on the abovementioned plates were adjusted to the appropriate optical density (2) and were used to inoculate Sensititre microdilution panels (Trek Diagnostics, Westlake, OH) for susceptibility testing. The antimicrobial agents tested varied by species; each comparator tested by species is listed in Tables 1 to 3. Drug ranges were selected to allow for quality control in accordance with CLSI standards (3) for all relevant organism and drug combinations evaluated in the study, and the quality of the panels was assessed continually during testing. MICs were interpreted as susceptible, intermediate, or resistant according to CLSI M100-S17 criteria (3), where applicable. FDA interpretive criteria were applied to doripenem results (susceptible, ≤0.5 μg/ml for Enterobacteriaceae, ≤1 μg/ml for Acinetobacter spp., and 2 μg/ml for P. aeruginosa). Results were examined to ensure that reported MICs were within acceptable standards set by CLSI (3) based on the comparator agent used and the following ATCC quality control strains: ATCC 25922 (E. coli), ATCC 27853 (P. aeruginosa), ATCC 29212 (Enterococcus faecalis), ATCC 29213 (S. aureus), ATCC 35218 (E. coli), ATCC 49619 (S. pneumoniae), and ATCC 700603 (K. pneumoniae). Extended-spectrum β-lactamase (ESBL) screen-positive isolates of E. coli, K. pneumoniae, and P. mirabilis were identified in accordance with CLSI criteria (3) as isolates that had cefotaxime or ceftazidime MICs of ≥1 μg/ml that decreased ≥3 twofold dilutions when tested in conjunction with clavulanic acid. Derepressed AmpC screen-positive isolates of Enterobacter spp. and Citrobacter spp. were identified essentially as described by Livermore and Brown (21) and Potz et al. (30) as isolates which were resistant to ceftazidime, cefotaxime, and ceftriaxone but not resistant to cefepime and carbapenems.
TABLE 1.
In vitro activities of doripenem and comparators against gram-positive organisms
| Organism and phenotypea (n) | Doripenem
|
Imipenem
|
Meropenem
|
Ertapenem
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml)
|
%S | MIC (μg/ml)
|
%S | MIC (μg/ml)
|
%S | MIC (μg/ml)
|
%S | |||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |||||
| S. aureus | ||||||||||||
| MS (505) | 0.03 | 0.06 | —b | 0.03 | 0.03 | 100.0 | 0.06 | 0.12 | 100.0 | 0.25 | 0.5 | 99.4 |
| MR (1,309) | 0.5 | 8 | —c | 0.25 | 8 | —c | 1 | 8 | —c | 2 | 32 | —c |
| CoNS | ||||||||||||
| MS (169) | 0.03 | 0.06 | —b | ≤0.015 | 0.03 | 99.4 | 0.06 | 0.12 | 99.4 | 0.25 | 0.5 | 99.4 |
| MR (473) | 1 | 8 | —c | 0.25 | 16 | —c | 2 | 16 | —c | 8 | >32 | —c |
| S. pneumoniae | ||||||||||||
| PEN S (2,474) | ≤0.015 | ≤0.015 | —b | ≤0.015 | ≤0.015 | 99.9 | ≤0.015 | ≤0.015 | 100.0 | 0.03 | 0.03 | 100.0 |
| PEN I (887) | 0.03 | 0.5 | —b | 0.03 | 0.25 | 84.4 | 0.06 | 0.5 | 83.9 | 0.12 | 1 | 94.5 |
| PEN R (571) | 0.5 | 1 | —b | 0.5 | 1 | 2.1 | 1 | 1 | 2.3 | 2 | 2 | 39.6 |
MS, methicillin susceptible; MR, methicillin resistant; PEN S, PEN I, and PEN R, penicillin susceptible, intermediate, and resistant, respectively.
CLSI and FDA breakpoints unavailable for interpretation of susceptible, intermediate, and/or resistant.
According to CLSI standards, for methicillin-resistant staphylococci, results for carbapenems should be reported as resistant or not be reported.
TABLE 3.
In vitro activities of doripenem and comparators against P. aeruginosa and Acinetobacter spp.a
| Organism, antimicrobial agent, and phenotype (n) | MIC (μg/ml)
|
%S | %I | %R | |||
|---|---|---|---|---|---|---|---|
| Range | Mode | MIC50 | MIC90 | ||||
| P. aeruginosa | |||||||
| Doripenemb | |||||||
| All (875) | 0.03->32 | 0.12 | 0.25 | 4 | 88.3 | ||
| CAZ S (765) | 0.03->32 | 0.12 | 0.25 | 2 | 92.3 | ||
| CAZ NS (110) | 0.12-32 | 0.5 | 1 | 16 | 60.9 | ||
| IPM S (668) | 0.03-32 | 0.12 | 0.25 | 1 | 98.5 | ||
| IPM NS (207) | 0.12->32 | 4 | 2 | 16 | 55.6 | ||
| IPM | |||||||
| All | 0.25->32 | 2 | 4 | 32 | 76.3 | 7.7 | 16.0 |
| CAZ S | 0.25->32 | 2 | 4 | 16 | 79.3 | 7.6 | 13.1 |
| CAZ NS | 0.5->32 | 4 | 4 | >32 | 55.5 | 8.2 | 36.4 |
| IPM S | 0.25-4 | 2 | 2 | 4 | 100.0 | 0.0 | 0.0 |
| IPM NS | 8->32 | 8 | 16 | >32 | 0.0 | 32.4 | 67.6 |
| Meropenem | |||||||
| All | 0.03->32 | 0.5 | 1 | 16 | 85.3 | 4.6 | 10.2 |
| CAZ S | 0.03->32 | 0.5 | 1 | 8 | 88.8 | 4.7 | 6.5 |
| CAZ NS | 0.12->32 | 0.5 | 4 | 32 | 60.9 | 3.6 | 35.5 |
| IPM S | 0.03-32 | 0.5 | 0.5 | 2 | 98.5 | 0.7 | 0.7 |
| IPM NS | 0.25->32 | 16 | 8 | 32 | 42.5 | 16.9 | 40.6 |
| Cefepime | |||||||
| CAZ S | 0.03->32 | 4 | 2 | 8 | 95.2 | 3.8 | 1.0 |
| CAZ NS | 4->32 | 16 | 16 | 32 | 25.5 | 48.2 | 26.4 |
| IPM S | 0.03->32 | 4 | 2 | 8 | 90.7 | 7.0 | 2.2 |
| IPM NS | 0.25->32 | 4 | 8 | 32 | 72.5 | 16.9 | 10.6 |
| CAZ | |||||||
| CAZ S | ≤0.03-8 | 2 | 2 | 4 | 100.0 | 0.0 | 0.0 |
| CAZ NS | 16->32 | >32 | 32 | >32 | 0.0 | 23.6 | 76.4 |
| IPM S | ≤0.03->32 | 2 | 2 | 8 | 90.9 | 2.2 | 6.9 |
| IPM NS | 0.5->32 | 2 | 4 | >32 | 76.3 | 5.3 | 18.4 |
| Piperacillin-tazobactam | |||||||
| CAZ S | ≤0.25->128 | 8 | 16 | 64 | 96.3 | 0.0 | 3.7 |
| CAZ NS | 1->128 | >128 | >128 | >128 | 17.3 | 0.0 | 82.7 |
| IPM S | ≤0.25->128 | 8 | 16 | 64 | 90.6 | 0.0 | 9.4 |
| IPM NS | 1->128 | 16 | 16 | >128 | 72.9 | 0.0 | 27.1 |
| Acinetobacter spp. | |||||||
| Doripenemb | |||||||
| All (289) | ≤0.015->32 | 0.25 | 0.5 | 32 | 73.4 | ||
| IPM S (238) | ≤0.015-8 | 0.25 | 0.25 | 2 | 89.1 | ||
| IPM NS (51) | 8->32 | >32 | 32 | >32 | 0.0 | ||
| IPM | |||||||
| All | ≤0.015->32 | 0.5 | 1 | >32 | 82.4 | 0.7 | 17.0 |
| IPM S | ≤0.015-4 | 0.5 | 0.5 | 4 | 100.0 | 0.0 | 0.0 |
| IPM NS | 8->32 | >32 | >32 | >32 | 0.0 | 3.9 | 96.1 |
| Meropenem | |||||||
| All | ≤0.015->32 | 2 | 1 | >32 | 80.6 | 1.4 | 18.0 |
| IPM S | ≤0.015-16 | 2 | 1 | 4 | 97.9 | 1.7 | 0.4 |
| IPM NS | 16->32 | >32 | >32 | >32 | 0.0 | 0.0 | 100.0 |
| Cefepime | |||||||
| IPM S | 0.03->32 | 1 | 4 | 32 | 73.1 | 11.8 | 15.1 |
| IPM NS | 8->32 | >32 | 32 | >32 | 2.0 | 33.3 | 64.7 |
| CAZ | |||||||
| IPM S | 0.06->32 | 4 | 4 | >32 | 66.8 | 5.5 | 27.7 |
| IPM NS | 2->32 | >32 | >32 | >32 | 15.7 | 0.0 | 84.3 |
| Piperacillin-tazobactam | |||||||
| IPM S | ≤0.25->128 | >128 | 16 | >128 | 52.1 | 18.1 | 29.8 |
| IPM NS | 64->128 | >128 | >128 | >128 | 0.0 | 3.9 | 96.1 |
CAZ, ceftazidime; IPM, imipenem; S, susceptible; NS, nonsusceptible; I, intermediate; R, resistant.
FDA interpretive criteria used for doripenem test results: ≤2 μg/ml for susceptibility against P. aeruginosa and ≤1 μg/ml for susceptibility against Acinetobacter baumannii; nonsusceptible interpretation not available.
RESULTS
Gram-positive cocci.
The activities of doripenem and other carbapenems against gram-positive cocci are presented in Table 1. This level of activity was within 1 doubling dilution of that of imipenem (MIC90 = 0.03 μg/ml) and was twofold and eightfold higher than those of meropenem (MIC90 = 0.12 μg/ml) and ertapenem (MIC90 = 0.5 μg/ml), respectively. MICs were elevated against methicillin-resistant isolates for all evaluated carbapenems.
Similar results were observed for doripenem against CoNS, where doripenem had a MIC90 of 0.06 μg/ml against methicillin-susceptible CoNS, which was within 1 doubling dilution of that of imipenem (MIC90 = 0.03 μg/ml), twofold lower than that of meropenem (MIC90 = 0.12 μg/ml), and eightfold greater than that of ertapenem (MIC90 = 0.5 μg/ml).
Doripenem, meropenem, and imipenem were equal in activity against penicillin-susceptible isolates of S. pneumoniae (MIC90s ≤ 0.015 μg/ml). Based on MIC90 analysis, this level of activity was superior to those of all other β-lactams tested (data not shown). MICs of doripenem and the other carbapenems increased severalfold among penicillin-intermediate and penicillin-resistant populations.
Enterobacteriaceae.
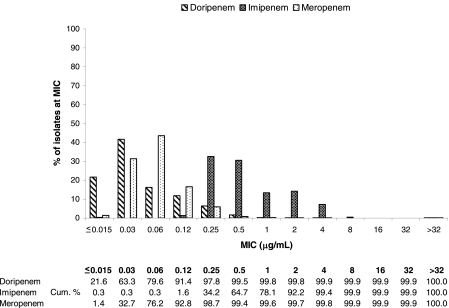
The activities of doripenem and comparator β-lactams against cephalosporin-susceptible and -resistant enteric bacilli are presented in Table 2. Against ceftazidime-susceptible Enterobacteriaceae, doripenem had a MIC90 of 0.12 μg/ml, which was the same as that of meropenem (MIC90 = 0.12 μg/ml) and severalfold lower than that of imipenem (MIC90 = 2 μg/ml). A difference in activity of doripenem relative to imipenem and/or meropenem was noted against enteric bacilli at lower concentrations based on MIC distribution (Fig. 1). For Enterobacteriaceae overall, 63.3% of the isolates were inhibited by doripenem at concentrations of ≤0.03 μg/ml whereas meropenem inhibited 32.7% of isolates over the same concentration range. In contrast, a concentration of 0.5 μg/ml of imipenem was required to inhibit 64.7% of the tested Enterobacteriaceae isolates. Nonetheless, this difference in activity profile did not translate to any differences in susceptibility, as by current interpretive criteria, the tested Enterobacteriaceae were >99% susceptible (%S) to doripenem, imipenem, and meropenem.
TABLE 2.
In vitro activities of doripenem and comparators against Enterobacteriaceaea
| Organism, antimicrobial agent, and phenotype (n) | MIC (μg/ml)
|
%S | %I | %R | |||
|---|---|---|---|---|---|---|---|
| Range | Mode | MIC50 | MIC90 | ||||
| All Enterobacteriaceae | |||||||
| Doripenemb | |||||||
| All (5,029) | ≤0.015->32 | 0.03 | 0.03 | 0.12 | 99.5 | ||
| CAZ S (4,728) | ≤0.015->32 | 0.03 | 0.03 | 0.12 | 99.7 | ||
| CAZ NS (301) | ≤0.015-32 | 0.06 | 0.06 | 0.25 | 96.7 | ||
| Imipenem | |||||||
| All | ≤0.015->32 | 0.25 | 0.5 | 2 | 99.4 | 0.4 | 0.1 |
| CAZ S | ≤0.015->32 | 0.25 | 0.5 | 2 | 99.6 | 0.3 | 0.1 |
| CAZ NS | 0.12-32 | 1 | 1 | 2 | 97.3 | 1.7 | 1.0 |
| Meropenem | |||||||
| All | ≤0.015->32 | 0.06 | 0.06 | 0.12 | 99.8 | 0.0 | 0.1 |
| CAZ S | ≤0.015->32 | 0.06 | 0.06 | 0.12 | 99.9 | 0.0 | 0.1 |
| CAZ NS | ≤0.015->32 | 0.06 | 0.12 | 0.5 | 98.0 | 0.7 | 1.3 |
| Cefepime | |||||||
| CAZ S | ≤0.015->32 | 0.03 | 0.03 | 0.12 | 99.8 | 0.0 | 0.1 |
| CAZ NS | 0.06->32 | 4 | 2 | 32 | 82.4 | 5.6 | 12.0 |
| CAZ | |||||||
| CAZ S | ≤0.03-8 | 0.12 | 0.12 | 0.5 | 100.0 | 0.0 | 0.0 |
| CAZ NS | 16->32 | >32 | >32 | >32 | 0.0 | 7.6 | 92.4 |
| Ceftriaxone | |||||||
| CAZ S | ≤0.015->64 | 0.03 | 0.03 | 0.25 | 99.3 | 0.4 | 0.3 |
| CAZ NS | 0.25->64 | >64 | >64 | >64 | 7.6 | 26.9 | 65.4 |
| Piperacillin-tazobactam | |||||||
| CAZ S | ≤0.25->128 | 4 | 4 | 8 | 95.8 | 2.3 | 1.9 |
| CAZ NS | 0.5->128 | >128 | 128 | >128 | 15.6 | 20.6 | 63.8 |
| E. coli | |||||||
| Doripenem | |||||||
| All (1,772) | ≤0.015-1 | ≤0.015 | ≤0.015 | 0.03 | 99.8 | ||
| Non-ESBL (1,742) | ≤0.015-1 | ≤0.015 | ≤0.015 | 0.03 | 99.8 | ||
| ESBL (30) | ≤0.015-0.06 | 0.03 | 0.03 | 0.06 | 100.0 | ||
| Imipenem | |||||||
| All | ≤0.015-4 | 0.25 | 0.25 | 0.5 | 100.0 | 0.0 | 0.0 |
| Non-ESBL | ≤0.015-4 | 0.25 | 0.25 | 0.5 | 100.0 | 0.0 | 0.0 |
| ESBL | 0.25-2 | 0.5 | 0.5 | 1 | 100.0 | 0.0 | 0.0 |
| Meropenem | |||||||
| All | ≤0.015-2 | 0.03 | 0.03 | 0.06 | 100.0 | 0.0 | 0.0 |
| Non-ESBL | ≤0.015-2 | 0.03 | 0.03 | 0.06 | 100.0 | 0.0 | 0.0 |
| ESBL | ≤0.015-0.12 | 0.06 | 0.06 | 0.06 | 100.0 | 0.0 | 0.0 |
| Cefepime | |||||||
| Non-ESBL | ≤0.015-32 | 0.03 | 0.03 | 0.06 | 99.8 | 0.1 | 0.1 |
| ESBL | ≤0.015->32 | >32 | 32 | >32 | 40.0 | 3.3 | 56.7 |
| CAZ | |||||||
| Non-ESBL | ≤0.03->32 | 0.12 | 0.12 | 0.25 | 98.6 | 0.5 | 0.9 |
| ESBL | ≤0.03->32 | >32 | 32 | >32 | 33.3 | 10.0 | 56.7 |
| Ceftriaxone | |||||||
| Non-ESBL | ≤0.015->64 | 0.03 | 0.03 | 0.06 | 98.7 | 1.1 | 0.2 |
| ESBL | ≤0.015->64 | >64 | >64 | >64 | 20.0 | 13.3 | 66.7 |
| Piperacillin-tazobactam | |||||||
| Non-ESBL | ≤0.25->128 | 4 | 4 | 8 | 93.6 | 3.2 | 3.2 |
| ESBL | 2->128 | >128 | 16 | >128 | 53.3 | 16.7 | 30.0 |
| K. pneumoniae | |||||||
| Doripenem | |||||||
| All (1,227) | ≤0.015-32 | 0.03 | 0.03 | 0.06 | 99.6 | ||
| Non-ESBL (1,183) | ≤0.015-32 | 0.03 | 0.03 | 0.06 | 99.7 | ||
| ESBL (44) | ≤0.015-8 | 0.03 | 0.03 | 0.12 | 95.5 | ||
| Imipenem | |||||||
| All | ≤0.015-32 | 0.5 | 0.5 | 1 | 99.7 | 0.2 | 0.2 |
| Non-ESBL | ≤0.015-32 | 0.5 | 0.5 | 1 | 99.8 | 0.1 | 0.1 |
| ESBL | 0.12-16 | 0.5 | 0.5 | 1 | 95.5 | 2.3 | 2.3 |
| Meropenem | |||||||
| All | ≤0.015->32 | 0.06 | 0.06 | 0.06 | 99.7 | 0.0 | 0.3 |
| Non-ESBL | ≤0.015->32 | 0.06 | 0.06 | 0.06 | 99.8 | 0.0 | 0.2 |
| ESBL | 0.03-32 | 0.06 | 0.06 | 0.25 | 95.5 | 0.0 | 4.5 |
| Cefepime | |||||||
| Non-ESBL | ≤0.015->32 | 0.03 | 0.03 | 0.06 | 99.7 | 0.2 | 0.2 |
| ESBL | 0.5->32 | >32 | 8 | >32 | 68.2 | 2.3 | 29.5 |
| CAZ | |||||||
| Non-ESBL | ≤0.03->32 | 0.12 | 0.12 | 0.5 | 99.2 | 0.3 | 0.6 |
| ESBL | 0.5->32 | >32 | >32 | >32 | 4.5 | 0.0 | 95.5 |
| Ceftriaxone | |||||||
| Non-ESBL | ≤0.015->64 | 0.03 | 0.03 | 0.12 | 99.3 | 0.3 | 0.3 |
| ESBL | 4->64 | >64 | 64 | >64 | 9.1 | 18.2 | 72.7 |
| Piperacillin-tazobactam | |||||||
| Non-ESBL | ≤0.25->128 | 4 | 4 | 16 | 95.9 | 2.4 | 1.7 |
| ESBL | 4->128 | >128 | >128 | >128 | 11.4 | 11.4 | 77.3 |
| P. mirabilis | |||||||
| Doripenem | |||||||
| All (636) | ≤0.015-2 | 0.12 | 0.12 | 0.5 | 98.9 | ||
| Non-ESBL (634) | ≤0.015-2 | 0.25 | 0.12 | 0.5 | 98.9 | ||
| ESBL (2) | 0.06-0.12 | NA | NA | NA | 100.0 | ||
| Imipenem | |||||||
| All | ≤0.015->32 | 4 | 2 | 4 | 97.3 | 2.5 | 0.2 |
| Non-ESBL | ≤0.015->32 | 4 | 2 | 4 | 97.3 | 2.5 | 0.2 |
| ESBL | 0.5-1 | NA | NA | NA | 100.0 | 0.0 | 0.0 |
| Meropenem | |||||||
| All | ≤0.015-2 | 0.12 | 0.12 | 0.25 | 100.0 | 0.0 | 0.0 |
| Non-ESBL | ≤0.015-2 | 0.12 | 0.12 | 0.25 | 100.0 | 0.0 | 0.0 |
| ESBL | 0.12-0.12 | NA | NA | NA | 100.0 | 0.0 | 0.0 |
| Cefepime | |||||||
| Non-ESBL | ≤0.015-32 | 0.03 | 0.03 | 0.06 | 99.7 | 0.2 | 0.2 |
| ESBL | 1-2 | NA | NA | NA | 100.0 | 0.0 | 0.0 |
| CAZ | |||||||
| Non-ESBL | ≤0.03-32 | ≤0.03 | ≤0.03 | 0.06 | 99.5 | 0.2 | 0.3 |
| ESBL | 8-16 | NA | NA | NA | 50.0 | 50.0 | 0.0 |
| Ceftriaxone | |||||||
| Non-ESBL | ≤0.015-32 | ≤0.015 | ≤0.015 | ≤0.015 | 99.7 | 0.3 | 0.0 |
| ESBL | 0.06-1 | NA | NA | NA | 100.0 | 0.0 | 0.0 |
| Piperacillin-tazobactam | |||||||
| Non-ESBL | ≤0.25->128 | 0.5 | 0.5 | 2 | 99.4 | 0.3 | 0.3 |
| ESBL | 0.5-1 | NA | NA | NA | 100.0 | 0.0 | 0.0 |
| E. cloacae | |||||||
| Doripenem | |||||||
| All (508) | ≤0.015-4 | 0.03 | 0.03 | 0.12 | 99.2 | ||
| Nonderep. AmpC (405) | ≤0.015-4 | 0.03 | 0.03 | 0.06 | 99.3 | ||
| Derep. AmpC (103) | 0.03-2 | 0.12 | 0.12 | 0.25 | 99.0 | ||
| Imipenem | |||||||
| All | 0.25-8 | 1 | 1 | 2 | 99.6 | 0.4 | 0.0 |
| Nonderep. AmpC | 0.25-8 | 1 | 1 | 2 | 99.5 | 0.5 | 0.0 |
| Derep. AmpC | 0.25-4 | 1 | 1 | 2 | 100.0 | 0.0 | 0.0 |
| Meropenem | |||||||
| All | ≤0.015-8 | 0.06 | 0.06 | 0.25 | 99.6 | 0.4 | 0.0 |
| Nonderep. AmpC | 0.03-8 | 0.06 | 0.06 | 0.12 | 99.5 | 0.5 | 0.0 |
| Derep. AmpC | ≤0.015-4 | 0.25 | 0.25 | 0.5 | 100.0 | 0.0 | 0.0 |
| Cefepime | |||||||
| Nonderep. AmpC | ≤0.015->32 | 0.03 | 0.06 | 0.25 | 97.5 | 0.7 | 1.7 |
| Derep. AmpC | 0.12-16 | 4 | 4 | 8 | 93.2 | 6.8 | 0.0 |
| CAZ | |||||||
| Nonderep. AmpC | 0.06->32 | 0.25 | 0.25 | 2 | 93.6 | 0.7 | 5.7 |
| Derep. AmpC | 8->32 | >32 | >32 | >32 | 1.0 | 1.9 | 97.1 |
| Ceftriaxone | |||||||
| Nonderep. AmpC | ≤0.015->64 | 0.12 | 0.25 | 4 | 91.9 | 4.7 | 3.5 |
| Derep. AmpC | 16->64 | >64 | >64 | >64 | 0.0 | 6.8 | 93.2 |
| Piperacillin-tazobactam | |||||||
| Nonderep. AmpC | ≤0.25->128 | 4 | 4 | 16 | 93.3 | 3.2 | 3.5 |
| Derep. AmpC | 16->128 | >128 | >128 | >128 | 5.8 | 11.7 | 82.5 |
| Citrobacter spp. | |||||||
| Doripenem | |||||||
| All (514) | ≤0.015-1 | 0.03 | 0.03 | 0.06 | 99.8 | ||
| Nonderep. AmpC (459) | ≤0.015-1 | 0.03 | 0.03 | 0.06 | 99.8 | ||
| Derep. AmpC (55) | ≤0.015-0.12 | 0.06 | 0.06 | 0.06 | 100.0 | ||
| Imipenem | |||||||
| All | ≤0.015-16 | 0.5 | 1 | 2 | 99.8 | 0.0 | 0.2 |
| Nonderep. AmpC | ≤0.015-16 | 0.5 | 0.5 | 2 | 99.8 | 0.0 | 0.2 |
| Derep. AmpC | 0.5-4 | 1 | 1 | 2 | 100.0 | 0.0 | 0.0 |
| Meropenem | |||||||
| All | ≤0.015-4 | 0.06 | 0.06 | 0.12 | 100.0 | 0.0 | 0.0 |
| Nonderep. AmpC | ≤0.015-4 | 0.06 | 0.06 | 0.06 | 100.0 | 0.0 | 0.0 |
| Derep. AmpC | 0.03-0.25 | 0.12 | 0.12 | 0.25 | 100.0 | 0.0 | 0.0 |
| Cefepime | |||||||
| Nonderep. AmpC | ≤0.015->32 | 0.03 | 0.03 | 0.06 | 99.6 | 0.2 | 0.2 |
| Derep. AmpC | 0.06-4 | 1 | 1 | 2 | 100.0 | 0.0 | 0.0 |
| CAZ | |||||||
| Nonderep. AmpC | ≤0.03->32 | 0.12 | 0.25 | 1 | 98.3 | 0.4 | 1.3 |
| Derep. AmpC | 32->32 | >32 | >32 | >32 | 0.0 | 0.0 | 100.0 |
| Ceftriaxone | |||||||
| Nonderep. AmpC | ≤0.015->64 | 0.06 | 0.12 | 0.5 | 97.8 | 1.1 | 1.1 |
| Derep. AmpC | 8->64 | 32 | 64 | >64 | 1.8 | 43.6 | 54.5 |
| Piperacillin-tazobactam | |||||||
| Nonderep. AmpC | ≤0.25->128 | 4 | 4 | 16 | 93.7 | 3.7 | 2.6 |
| Derep. AmpC | 16->128 | >128 | 128 | >128 | 3.6 | 29.1 | 67.3 |
| S. marcescens | |||||||
| Doripenem | |||||||
| All (372) | 0.03->32 | 0.12 | 0.12 | 0.25 | 98.7 | ||
| CAZ S (362) | 0.03->32 | 0.12 | 0.12 | 0.25 | 98.6 | ||
| CAZ NS (10) | 0.06-0.5 | 0.25 | 0.12 | 0.25 | 100.0 | ||
| Imipenem | |||||||
| All | ≤0.015->32 | 2 | 2 | 4 | 98.9 | 0.3 | 0.8 |
| CAZ S | ≤0.015->32 | 2 | 2 | 4 | 98.9 | 0.3 | 0.8 |
| CAZ NS | 1-4 | 2 | 2 | 4 | 100.0 | 0.0 | 0.0 |
| Meropenem | |||||||
| All | ≤0.015->32 | 0.12 | 0.12 | 0.25 | 99.2 | 0.0 | 0.8 |
| CAZ S | ≤0.015->32 | 0.12 | 0.12 | 0.25 | 99.2 | 0.0 | 0.8 |
| CAZ NS | 0.06-0.25 | 0.25 | 0.25 | 0.25 | 100.0 | 0.0 | 0.0 |
| Cefepime | |||||||
| CAZ S | ≤0.015-4 | 0.06 | 0.06 | 0.25 | 100.0 | 0.0 | 0.0 |
| CAZ NS | 1->32 | 2 | 4 | 16 | 80.0 | 10.0 | 10.0 |
| CAZ | |||||||
| CAZ S | ≤0.03-4 | 0.12 | 0.12 | 0.5 | 100.0 | 0.0 | 0.0 |
| CAZ NS | 32->32 | >32 | >32 | >32 | 0.0 | 0.0 | 100.0 |
| Ceftriaxone | |||||||
| CAZ S | ≤0.015->64 | 0.25 | 0.25 | 1 | 98.1 | 1.4 | 0.6 |
| CAZ NS | 8->64 | >64 | 64 | >64 | 10.0 | 30.0 | 60.0 |
| Piperacillin-tazobactam | |||||||
| CAZ S | ≤0.25->128 | 4 | 4 | 16 | 93.6 | 4.4 | 1.9 |
| CAZ NS | 2->128 | 128 | 64 | >128 | 20.0 | 30.0 | 50.0 |
Abbreviations: CAZ, ceftazidime; S, susceptible; NS, nonsusceptible; ESBL, extended-spectrum β-lactamases; Derep. AmpC, derepressed AmpC β-lactamase; Nonderep. AmpC, nonderepressed AmpC β-lactamase; I, intermediate; R, resistant; NA, not applicable (<10 isolates).
FDA interpretive criteria used for doripenem test results: ≤0.5 μg/ml for susceptible; nonsusceptible interpretation not available.
FIG. 1.
MIC distributions and cumulative susceptibilities of doripenem and comparator MICs against Enterobacteriaceae (n = 5,029).
The level of doripenem activity against Enterobacteriaceae was essentially maintained against populations not susceptible to broad-spectrum cephalosporins (i.e., nonsusceptible to ceftazidime). The MIC90 of doripenem (0.25 μg/ml) against ceftazidime-nonsusceptible enteric bacteria was within 2 doubling dilutions of those observed with ceftazidime-susceptible isolates, and this pattern was maintained for each enteric species tested (Table 2). Based on MIC90 data, doripenem was twice as potent as meropenem (0.25 μg/ml versus 0.5 μg/ml) and eightfold more potent than imipenem (2 μg/ml) against ceftazidime-nonsusceptible Enterobacteriaceae. This pattern of activity was consistent across several species including E. coli, K. pneumoniae, E. cloacae, and Citrobacter spp.
Against ESBL screen-positive isolates of E. coli (n = 29), K. pneumoniae (n = 44), and P. mirabilis (n = 2), doripenem maintained the same level of activity as was observed against Enterobacteriaceae overall (95.5%S [K. pneumoniae] to 100%S [E. coli and P. mirabilis]; MIC90s = 0.12 μg/ml for E. coli and K. pneumoniae) (Table 2). Against ESBL isolates, doripenem was more active than meropenem at concentrations of ≤0.03 μg/ml, where 63% of isolates were inhibited by doripenem relative to the 17% that were inhibited by meropenem (data not shown). Both doripenem and meropenem were more potent against ESBL isolates overall than was imipenem (MIC90 = 1 μg/ml); however, regardless of the difference in potency among the evaluated carbapenems, all were highly active against ESBL isolates. The activity of doripenem was also evaluated against isolates of E. cloacae and Citrobacter spp. suspected to have derepressed AmpC expression (derepressed AmpC isolates) based on their β-lactam susceptibility profiles (Table 2). These isolates were highly susceptible to the evaluated carbapenems (≥99%S). Similar to those of meropenem, the MIC50 and MIC90 of doripenem against E. cloacae (0.12 μg/ml and 0.25 μg/ml, respectively) were fourfold higher against derepressed AmpC isolates than against isolates suspected to have nonderepressed AmpC expression (nondepressed AmpC isolates). This trend was not observed with derepressed AmpC isolates of Citrobacter spp. for doripenem, where the MIC50 and MIC90 of derepressed AmpC isolates were identical to or within 1 doubling dilution of those for nonderepressed AmpC isolates.
P. aeruginosa and Acinetobacter spp.
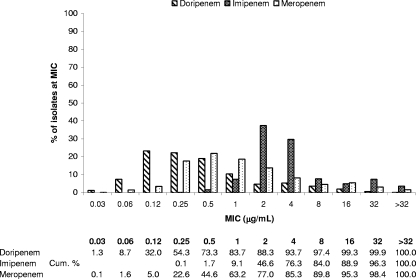
As shown in Table 3, based on MIC50 and MIC90 data, doripenem was the most potent carbapenem tested against P. aeruginosa, regardless of ceftazidime susceptibility status. Doripenem had MIC90s of 2 μg/ml and 16 μg/ml against ceftazidime-susceptible and -resistant isolates, respectively, compared to meropenem (8 μg/ml and 32 μg/ml, respectively) and imipenem (16 μg/ml and ≥32 μg/ml, respectively). More isolates overall were susceptible to doripenem (88.3%) than to meropenem (85.3%) and imipenem (76.3%). Of P. aeruginosa isolates, 73.3% were inhibited by doripenem at concentrations of ≤0.5 μg/ml, relative to 44.6% and 1% of isolates inhibited by meropenem and imipenem at these concentrations, respectively (Fig. 2). Doripenem also was more active in vitro against P. aeruginosa isolates from both inpatients (excluding intensive care unit [ICU] patients) (MIC50 = 0.25 μg/ml, MIC90 = 8 μg/ml, 87.9%S) and ICU patients (MIC50 = 0.5 μg/ml, MIC90 = 16 μg/ml, 78.4%S) than were both meropenem (inpatient: MIC50 = 1 μg/ml, MIC90 = 16 μg/ml, 83.9%S; ICU: MIC50 = 1 μg/ml, MIC90 = 32 μg/ml, 74.1%S) and imipenem (inpatient and ICU: MIC50 = 4 μg/ml, MIC90 = 32 μg/ml, 74.9%S and 67.6%S, respectively) (Table 4).
FIG. 2.
MIC distributions and cumulative susceptibilities of doripenem and comparator MICs against P. aeruginosa (n = 875).
TABLE 4.
In vitro activities of doripenem and comparators against P. aeruginosa according to patient status
| Antimicrobial agent and patient statusa | MIC (μg/ml)
|
%S | %Ic | %Rd | |||
|---|---|---|---|---|---|---|---|
| Range | Mode | MIC50 | MIC90 | ||||
| Doripenemb | |||||||
| Inpatient (n = 355) | 0.03-32 | 0.25 | 0.25 | 4 | 87.9 | ||
| ICU (n = 139) | 0.06-32 | 0.25 | 0.5 | 8 | 78.4 | ||
| Imipenem | |||||||
| Inpatient | 0.25->32 | 2 | 4 | 32 | 74.9 | 7.9 | 17.2 |
| ICU | 1->32 | 2 | 4 | 32 | 67.6 | 5.8 | 26.6 |
| Meropenem | |||||||
| Inpatient | 0.06->32 | 0.5 | 1 | 16 | 83.9 | 3.7 | 12.4 |
| ICU | 0.06->32 | 0.25 | 1 | 32 | 74.1 | 7.9 | 18.0 |
| Cefepime | |||||||
| Inpatient | 0.25->32 | 4 | 4 | 16 | 86.8 | 8.2 | 5.1 |
| ICU | 0.06->32 | 4 | 4 | 16 | 79.9 | 13.7 | 6.5 |
| Ceftazidime | |||||||
| Inpatient | 0.5->32 | 2 | 2 | 32 | 87.3 | 2.3 | 10.4 |
| ICU | 0.06->32 | 2 | 2 | 32 | 79.9 | 5.8 | 14.4 |
| Piperacillin-tazobactam | |||||||
| Inpatient | 0.5->128 | 8 | 16 | >128 | 87.0 | 0.0 | 13.0 |
| ICU | 0.5->128 | 16 | 16 | >128 | 78.4 | 0.0 | 21.6 |
The inpatient category consists of non-ICU patients.
FDA interpretive criteria used for doripenem test results: ≤2 μg/ml for susceptibility against P. aeruginosa; nonsusceptibility interpretation not available.
I, intermediate.
R, resistant.
Doripenem activity was also evaluated against imipenem-nonsusceptible P. aeruginosa isolates (Table 3). Based on cumulative MICs (data not shown) and MIC50/MIC90, doripenem was the most potent carbapenem tested against this resistant subpopulation. Among these imipenem-nonsusceptible isolates, 55.6% were susceptible to doripenem (MICs, ≤2 μg/ml) while 42.5% were meropenem susceptible (MICs, ≤4 μg/ml). Finally, nearly 90% (89.9%) of the imipenem-nonsusceptible strains were inhibited by ≤8 μg/ml of doripenem (data not shown).
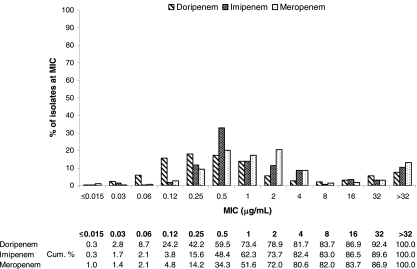
Against imipenem-susceptible Acinetobacter spp., the doripenem MIC90 (2 μg/ml) was twofold lower than the MIC90 of either imipenem or meropenem (MIC90 = 4 μg/ml) (Table 3), though by current FDA criteria, only 89.1% of these isolates were susceptible to doripenem (MICs, ≤1 μg/ml) relative to the 97.9% and 100% observed with meropenem and imipenem, respectively (MICs, ≤4 μg/ml). Nonetheless, the MIC distribution (Fig. 3) of the evaluated carbapenems against these isolates illustrates the finding that doripenem is more potent than imipenem and meropenem over the lower range of concentrations tested as reflected by the lower MIC50 of doripenem (0.25 μg/ml), which is twofold lower than that of imipenem (0.5 μg/ml) and fourfold lower than that of meropenem (1 μg/ml). However, as was the case with meropenem and imipenem, the MIC90 of doripenem was >32 μg/ml against imipenem-nonsusceptible isolates of Acinetobacter (Table 3).
FIG. 3.
MIC distributions and cumulative susceptibilities of doripenem and comparator MICs against Acinetobacter spp. (n = 5,029).
DISCUSSION
Doripenem, although intended for use primarily against significant gram-negative pathogens, was also active against clinically relevant gram-positive cocci at levels consistent with previous reports (1, 6, 8, 14, 35). Against methicillin-susceptible staphylococci (both methicillin-susceptible S. aureus and methicillin-susceptible CoNS), doripenem had a MIC90 of 0.06 μg/ml, comparable to those of imipenem and meropenem. The MIC90 for doripenem rose to 8 μg/ml against methicillin-resistant S. aureus and methicillin-resistant CoNS, similar to the other evaluated carbapenems. Doripenem, along with comparator carbapenems imipenem and meropenem, was potent against susceptible isolates of S. pneumoniae at a MIC90 of ≤0.015 μg/ml, but comparable increases in MICs for all three carbapenems coincided with decreased susceptibility to penicillin. Regardless of resistance phenotype, doripenem, imipenem, and meropenem were consistently more potent against the gram-positive cocci tested than was ertapenem.
Doripenem was potent against all Enterobacteriaceae isolates tested, including those resistant to “advanced-generation” cephalosporins and screen-positive ESBL isolates. Among Enterobacteriaceae, doripenem was most active against E. coli, K. pneumoniae, E. cloacae, and Citrobacter spp. (MIC90s, 0.03 to 0.25 μg/ml) and was less active against P. mirabilis and S. marcescens (MIC90s, 0.25 to 0.5 μg/ml). Regardless, Enterobacteriaceae isolates were highly susceptible (≥99%S) to doripenem and the comparator carbapenems evaluated in this study. This pattern of activity for doripenem against Enterobacteriaceae was similar to that observed in previous studies (1, 6, 8, 13, 14, 35). As observed previously (1, 6, 8, 13), doripenem activity was similar to that of meropenem against enteric bacilli by MIC90, including ceftazidime-nonsusceptible and ESBL isolates. Consistent with previous reports (1, 6, 8, 13, 14), both meropenem and doripenem were more potent than imipenem against all species and resistance phenotypes of Enterobacteriaceae examined. Although doripenem activity was similar to that of meropenem against Enterobacteriaceae, doripenem appeared to be slightly more potent than meropenem at low concentrations of drug against Enterobacteriaceae (Fig. 1). The increased activity of doripenem relative to meropenem at low concentrations of drug was also reflected in the lower modes and MIC50s exhibited by doripenem in this study against E. coli, K. pneumoniae, E. cloacae, and Citrobacter spp. However, this difference in doripenem activity against the abovementioned species was not apparent by MIC50 in previous surveillance reports where activity against small numbers of isolates of each enteric species from diverse geographic locations was assessed (1, 6, 8, 13, 14) and, as mentioned above, did not translate to any marked differences in susceptibility of these isolates to the evaluated carbapenems.
By MIC90, %S, and cumulative MIC analysis, doripenem maintained its activity against all ceftazidime-nonsusceptible isolates of Enterobacteriaceae (MIC90s, 0.25 μg/ml for nonsusceptible isolates [96.7%S], 0.12 μg/ml for susceptible isolates [99.7%S]) and ESBL isolates (MIC90s, 0.12 μg/ml for both ESBL-positive and -negative isolates). MIC90s against resistant enteric isolates by species were either equal or comparable (1 doubling dilution higher) to those observed with susceptible isolates, with the exception of E. cloacae and Citrobacter spp., for which the doripenem MIC90 was fourfold higher among ceftazidime-nonsusceptible isolates, as observed in both this study and a previous report (8). The activity that doripenem and other carbapenems maintain against enteric bacilli resistant to other β-lactams is due in large part to their stability to hydrolysis by β-lactamases commonly encountered among these organisms. A previous study by Jones et al. (15) examined the activity of doripenem against E. coli isolates with characterized ESBLs and showed that doripenem maintained its level of activity against isolates with variable expression of a variety of β-lactamases common among ESBL isolates. Separate studies (15, 25) also demonstrated the stability of doripenem against high-level AmpC producers commonly encountered among Enterobacter spp., Citrobacter spp., and S. marcescens.
While carbapenems are stable to many of the class A and class C β-lactamases that have activity against broad-spectrum cephalosporins, they remain susceptible to hydrolysis by metallo-β-lactamases (IMP-type and VIM-type), some class A enzymes (SPE-type, IMI-type, and K. pneumoniae carbapenemase [KPC-type]), and a subset of class D enzymes (oxacillin [OXA-type]) (12, 34). The in vitro activities of doripenem and other carbapenems have been shown to be greatly diminished against isolates possessing β-lactamases with carbapenemase activity (15, 25, 26). In addition to carbapenemases, resistance to carbapenems among gram-negative bacteria can also occur through the variable expression or mutation of porins through which carbapenems gain access to the periplasmic space (34). Although carbapenem resistance is more commonly associated with isolates of P. aeruginosa and Acinetobacter spp. as discussed below, the penetration of carbapenemases into Enterobacteriaceae, where plasmid-mediated class A carbapenemases (KPCs) have emerged primarily in K. pneumoniae (17, 27), is a growing cause for concern.
Comparatively few Enterobacteriaceae with high MICs (intermediate or resistant) to both the tested carbapenems (imipenem and meropenem) were encountered throughout the course of this surveillance (three K. pneumoniae, two E. cloacae, and two S. marcescens isolates). Doripenem maintained some in vitro activity against these isolates of K. pneumoniae (MICs of 4, 8, and 32 μg/ml) and E. cloacae (MICs of 1 and 4 μg/ml) but was inactive (MICs of >32 μg/ml) against carbapenem-resistant S. marcescens. A few imipenem-resistant, meropenem-susceptible isolates were observed among Enterobacteriaceae isolates tested, primarily among P. mirabilis isolates. There were 16 P. mirabilis isolates which were either intermediate or resistant to imipenem, but all of these isolates were susceptible to meropenem (MIC range, 0.12 to 0.5 μg/ml) and had low MICs for doripenem (MIC range, 0.25 to 1 μg/ml). A single isolate of Citrobacter which was resistant to imipenem but susceptible to meropenem (MIC = 4 μg/ml) had a doripenem MIC of 1 μg/ml. Because of the continuous potential for carbapenem resistance to become more widely distributed among enteric populations, continued surveillance of the activities of doripenem and other carbapenems is needed.
Against P. aeruginosa isolates tested in this study and in other studies (1, 6, 14, 15, 35, 36), doripenem was the most potent carbapenem tested. Activity of doripenem, at a MIC90 of 4 μg/ml, was fourfold higher than that of meropenem and eightfold higher than that of imipenem against the tested P. aeruginosa isolates. Overall, 88.3% of isolates were susceptible to doripenem, 85.3% to meropenem, and 76.3% to imipenem. As doripenem is intended primarily for use among hospitalized patients, the in vitro activity of doripenem relative to other carbapenems against P. aeruginosa from both inpatients and patients in intensive care was also evaluated. By MIC50 and MIC90, doripenem was fourfold more active than meropenem and four to eightfold more potent than imipenem against P. aeruginosa isolates from these inpatient populations.
Though the in vitro activities of doripenem and imipenem vary against both enteric gram-negative bacilli and P. aeruginosa as described above, imipenem has been shown to be an acceptable surrogate marker for determining the susceptibility of gram-negative clinical isolates to doripenem (16). However, it is important that while susceptibility to imipenem is a suitable marker for susceptibility to doripenem, this type of testing does not account for clinical isolates which are nonsusceptible to imipenem but remain susceptible to doripenem. For example, in this study 13% of P. aeruginosa isolates tested as nonsusceptible to imipenem but were susceptible to doripenem. It is also important that due to the assignment of lower breakpoints to doripenem than to the other agents within its class, differences in in vitro potencies of doripenem relative to these agents, in particular against Enterobacteriaceae and Acinetobacter spp., are not well reflected in percent susceptibility as doripenem-susceptible breakpoints are up to fourfold lower than those of imipenem and meropenem. As breakpoints of previously approved agents continue to be reevaluated per FDA guidelines pursuant to section 1111 of the FDA Amendments Act, it will be important to reassess the issue of surrogate testing and comparative activities of agents within classes should interpretive criteria be changed.
As mentioned above, there exist a variety of resistance mechanisms that limit the effectiveness of β-lactams against P. aeruginosa including the production of β-lactamases (AmpC, ESBLs, and metallo-β-lactamases), the mutations/loss of porins (OprD), and the presence of multidrug efflux pumps (MexAB). These mechanisms have decreased the utility of cephalosporins as therapeutics for P. aeruginosa infections due to the increasing frequency of ceftazidime-nonsusceptible isolates, isolates which are also nonsusceptible to cefepime (MIC90s, ≥32 μg/ml in this study). Carbapenem MICs are also increased against ceftazidime-nonsusceptible isolates of P. aeruginosa, although a significant proportion of these isolates (43% in the current study) remain susceptible to treatment with imipenem. This illustrates that some, but not all, of the mechanisms conferring resistance to cephalosporins in P. aeruginosa also confer resistance to carbapenems. Doripenem was the most potent carbapenem tested against the ceftazidime-nonsusceptible population of P. aeruginosa isolates tested in this study. Of these isolates, 61% had doripenem MICs of ≤2 μg/ml, which is the susceptibility breakpoint recently issued by the FDA for doripenem against P. aeruginosa. Furthermore, the MIC50 and MIC90 of doripenem against these isolates (MIC50 = 1 μg/ml, MIC90 = 16 μg/ml) were lower than those observed with imipenem (MIC50 of 4 μg/ml, MIC90 of >32 μg/ml) and meropenem (MIC50 = 4 μg/ml, MIC90 = 32 μg/ml), which inhibited only 34% and 49% of ceftazidime-nonsusceptible isolates at 2 μg/ml, respectively. Similar trends in doripenem activity relative to other carbapenems were observed against ceftazidime-nonsusceptible P. aeruginosa in a previous study by Tsuji et al. (35).
The emergence of carbapenem resistance among P. aeruginosa is also a growing concern, as carbapenems remain one of the few available drugs with demonstrable activity against resistant P. aeruginosa. β-Lactam resistance in P. aeruginosa is mediated primarily by the production of AmpC and metallo-β-lactamases (IMP and VIM) and the loss or mutation of porin OprD (19, 34). Mushtaq et al. (26) showed that the in vitro activities of doripenem, imipenem, and meropenem against P. aeruginosa were adversely affected by all of these resistance mechanisms. In this study, doripenem maintained activity against imipenem-nonsusceptible isolates of P. aeruginosa as evidenced by the cumulative MICs for doripenem and its comparators among P. aeruginosa imipenem-nonsusceptible isolates. Doripenem was also observed to be more active in vitro against these isolates than were both meropenem and imipenem. The activity of doripenem against imipenem-nonsusceptible isolates was similar to that observed in other studies (13, 15, 26, 35). Interestingly, only 2.5% of the imipenem-resistant P. aeruginosa isolates in this study had high doripenem MICs (>16 μg/ml) that would be indicative of isolates that produce either VIM or IMP carbapenemases, suggesting that VIM or IMP carbapenemases may not be prevalent among the isolates in this study and that resistance to carbapenems in this instance may be driven by other means (e.g., OprD loss or mutation).
A number of studies have also been conducted to evaluate the potential for the emergence among P. aeruginosa isolates of resistance to doripenem relative to other carbapenems. A recent report from Japan (33) suggests that mutation frequencies resulting in carbapenem-resistant P. aeruginosa were lower with doripenem than with either meropenem or imipenem. A separate study also showed that mutants could be selected after repeated passage of P. aeruginosa in the presence of doripenem but that fewer mutants with lower MICs were observed relative to other carbapenems (26). The highest MIC observed against mutants in that study was 8 μg/ml, and the majority of the mutants were altered in OprD, a carbapenem-specific porin, which further illustrates that among P. aeruginosa isolates, porin expression impacts sensitivity to carbapenems. The generation of doripenem-resistant mutants by serial passage was notably reduced when doripenem was administered in combination with an aminoglycoside (10). The potential for the further emergence and spread of carbapenem resistance within P. aeruginosa warrants the continued surveillance of the activity of doripenem and other carbapenems against clinical isolates.
Against Acinetobacter spp., doripenem was slightly more potent than imipenem by both MIC50 (0.25 μg/ml versus 0.5 μg/ml) and MIC90 (2 μg/ml versus 4 μg/ml) against imipenem-susceptible isolates. Against imipenem-nonsusceptible isolates, the MIC50s and MIC90s of both imipenem and doripenem were ≥32 μg/ml. Doripenem and other carbapenems have been shown to be inactive against Acinetobacter spp. with characterized metallo-β-lactamases or OXA-type carbapenemases (25). In contrast to P. aeruginosa, doripenem was not active against imipenem-nonsusceptible isolates of Acinetobacter spp., which may be due in part to carbapenemase phenotype (OXA expression), membrane permeability, or variable porin/outer membrane protein expression among these organisms (5, 29).
In conclusion, doripenem is a broad-spectrum carbapenem with in vitro activity similar to those of imipenem and meropenem against gram-positive cocci and activity similar to that of meropenem but superior to that of imipenem against challenging gram-negative pathogens, including resistant enteric bacilli and P. aeruginosa. Based on the activity profile presented in this study and others, doripenem appears to be a promising new agent for the treatment of infections caused by severe gram-negative pathogens commonly encountered in the hospital including cephalosporin-resistant enteric bacilli and multidrug-resistant P. aeruginosa and Acinetobacter spp., excluding those with metallo-β-lactamases (IMP and VIM), other carbapenemases (OXA and KPC), and altered permeability (due to variable porin expression, porin loss, or mutation). Because its primary use will be associated with the hospital, where plasmid-mediated resistance to carbapenems among some gram-negative bacterial isolates has already been documented, it is important to continue to monitor the activity of doripenem throughout its clinical development and after its introduction into clinical use.
Acknowledgments
This work was supported by Johnson & Johnson Pharmaceutical Research and Development, LLC, Raritan, NJ.
Footnotes
Published ahead of print on 8 September 2008.
REFERENCES
- 1.Brown, S. D., and M. M. Traczewski. 2005. Comparative in vitro antimicrobial activity of a new carbapenem, doripenem: tentative disc diffusion criteria and quality control. J. Antimicrob. Chemother. 55:944-949. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard—7th ed. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. CLSI document M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Dalhoff, A., N. Janjic, and R. Echols. 2006. Redefining penems. Biochem. Pharmacol. 71:1085-1095. [DOI] [PubMed] [Google Scholar]
- 5.del Mar Tomas, M., A. Beceiro, A. Perez, D. Velasco, R. Moure, R. Villanueva, J. Martinez-Beltran, and G. Bou. 2005. Cloning and functional analysis of the gene encoding the 33- to 36-kilodalton outer membrane protein associated with carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:5172-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritsche, T. R., M. G. Stilwell, and R. N. Jones. 2005. Antimicrobial activity of doripenem (S-4661): a global surveillance report (2003). Clin. Microbiol. Infect. 11:974-984. [DOI] [PubMed] [Google Scholar]
- 7.Gaynes, R., and J. R. Edwards. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848-854. [DOI] [PubMed] [Google Scholar]
- 8.Ge, Y., M. A. Wikler, D. F. Sahm, R. S. Blosser-Middleton, and J. A. Karlowsky. 2004. In vitro antimicrobial activity of doripenem, a new carbapenem. Antimicrob. Agents Chemother. 48:1384-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gniadkowski, M. 2001. Evolution and epidemiology of extended-spectrum beta-lactamases (ESBLs) and ESBL-producing microorganisms. Clin. Microbiol. Infect. 7:597-608. [DOI] [PubMed] [Google Scholar]
- 10.Huynh, H. K., D. J. Biedenbach, and R. N. Jones. 2006. Delayed resistance selection for doripenem when passaging Pseudomonas aeruginosa isolates with doripenem plus an aminoglycoside. Diagn. Microbiol. Infect. Dis. 55:241-243. [DOI] [PubMed] [Google Scholar]
- 11.Iso, Y., T. Irie, Y. Nishino, K. Motokawa, and Y. Nishitani. 1996. A novel 1 beta-methylcarbapenem antibiotic, S-4661. Synthesis and structure-activity relationships of 2-(5-substituted pyrrolidin-3-ylthio)-1 beta-methylcarbapenems. J. Antibiot. (Tokyo) 49:199-209. [DOI] [PubMed] [Google Scholar]
- 12.Jacoby, G. A., and L. S. Munoz-Price. 2005. The new beta-lactamases. N. Engl. J. Med. 352:380-391. [DOI] [PubMed] [Google Scholar]
- 13.Jones, R. N., H. K. Huynh, and D. J. Biedenbach. 2004. Activities of doripenem (S-4661) against drug-resistant clinical pathogens. Antimicrob. Agents Chemother. 48:3136-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, R. N., H. K. Huynh, D. J. Biedenbach, T. R. Fritsche, and H. S. Sader. 2004. Doripenem (S-4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluations. J. Antimicrob. Chemother. 54:144-154. [DOI] [PubMed] [Google Scholar]
- 15.Jones, R. N., H. S. Sader, and T. R. Fritsche. 2005. Comparative activity of doripenem and three other carbapenems tested against Gram-negative bacilli with various beta-lactamase resistance mechanisms. Diagn. Microbiol. Infect. Dis. 52:71-74. [DOI] [PubMed] [Google Scholar]
- 16.Jones, R. N., H. S. Sader, T. R. Fritsche, and M. J. Janechek. 2007. Selection of a surrogate beta-lactam testing agent for initial susceptibility testing of doripenem, a new carbapenem. Diagn. Microbiol. Infect. Dis. 59:467-472. [DOI] [PubMed] [Google Scholar]
- 17.Landman, D., S. Bratu, S. Kochar, M. Panwar, M. Trehan, M. Doymaz, and J. Quale. 2007. Evolution of antimicrobial resistance among Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae in Brooklyn, N.Y. J. Antimicrob. Chemother. 60:78-82. [DOI] [PubMed] [Google Scholar]
- 18.Livermore, D. M. 2002. The impact of carbapenemases on antimicrobial development and therapy. Curr. Opin. Investig. Drugs 3:218-224. [PubMed] [Google Scholar]
- 19.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 20.Livermore, D. M. 2003. The threat from the pink corner. Ann. Med. 35:226-234. [DOI] [PubMed] [Google Scholar]
- 21.Livermore, D. M., and D. F. Brown. 2001. Detection of beta-lactamase-mediated resistance. J. Antimicrob. Chemother. 48(Suppl. 1):59-64. [DOI] [PubMed] [Google Scholar]
- 22.Mikamo, H., K. Izumi, Y. X. Hua, Y. Hayasaki, Y. Sato, and T. Tamaya. 2000. In vitro and in vivo antibacterial activities of a new injectable carbapenem, S-4661, against gynaecological pathogens. J. Antimicrob. Chemother. 46:471-474. [DOI] [PubMed] [Google Scholar]
- 23.Moland, E. S., N. D. Hanson, J. A. Black, A. Hossain, W. Song, and K. S. Thomson. 2006. Prevalence of newer beta-lactamases in gram-negative clinical isolates collected in the United States from 2001 to 2002. J. Clin. Microbiol. 44:3318-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller. 2003. Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 25.Mushtaq, S., Y. Ge, and D. M. Livermore. 2004. Comparative activities of doripenem versus isolates, mutants, and transconjugants of Enterobacteriaceae and Acinetobacter spp. with characterized beta-lactamases. Antimicrob. Agents Chemother. 48:1313-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mushtaq, S., Y. Ge, and D. M. Livermore. 2004. Doripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants, and transconjugants and resistance selection potential. Antimicrob. Agents Chemother. 48:3086-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in Gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 28.Paterson, D. L. 2006. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 34:S20-S28. [DOI] [PubMed] [Google Scholar]
- 29.Poirel, L., and P. Nordmann. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 12:826-836. [DOI] [PubMed] [Google Scholar]
- 30.Potz, N. A., R. Hope, M. Warner, A. P. Johnson, and D. M. Livermore. 2006. Prevalence and mechanisms of cephalosporin resistance in Enterobacteriaceae in London and South-East England. J. Antimicrob. Chemother. 58:320-326. [DOI] [PubMed] [Google Scholar]
- 31.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21:510-515. [DOI] [PubMed] [Google Scholar]
- 32.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 33.Sakyo, S., H. Tomita, K. Tanimoto, S. Fujimoto, and Y. Ike. 2006. Potency of carbapenems for the prevention of carbapenem-resistant mutants of Pseudomonas aeruginosa: the high potency of a new carbapenem doripenem. J. Antibiot. (Tokyo) 59:220-228. [DOI] [PubMed] [Google Scholar]
- 34.Thomson, J. M., and R. A. Bonomo. 2005. The threat of antibiotic resistance in Gram-negative pathogenic bacteria: beta-lactams in peril! Curr. Opin. Microbiol. 8:518-524. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji, M., Y. Ishii, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1998. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob. Agents Chemother. 42:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe, A., H. Takahashi, T. Kikuchi, T. Kobayashi, K. Gomi, S. Fujimura, Y. Tokue, and T. Nukiwa. 2000. Comparative in vitro activity of S-4661, a new parenteral carbapenem, and other antimicrobial agents against respiratory pathogens. Chemotherapy 46:184-187. [DOI] [PubMed] [Google Scholar]