Abstract
No data were available concerning Plasmodium vivax resistance to chloroquine (CQ) in Madagascar. We investigated the therapeutic efficacy of CQ in P. vivax malaria, the prevalence of mutations in the pvcrt-o and pvmdr1 genes before treatment, and the association between mutant parasites and the clinical response of the patients to CQ treatment. Clinical isolates were collected at six sentinel sites located in the three epidemiological strata for malaria throughout Madagascar in 2006. Patients were enrolled, treated, and followed up according to the WHO 2001 guidelines for P. vivax infections. Sequencing was used to analyze polymorphisms of the pvcrt-o (exons 1 to 6) and pvmdr1 genes. The treatment failure rate, after adjustment for genotyping, was estimated at 5.1% for the 105 patients included, ranging from zero in the South to 14.8% in the foothills of the Central Highlands. All samples were wild type for pvcrt-o but mutant for the pvmdr1 gene. Ten nonsynonymous mutations were found in the pvmdr1 gene, including five new mutations, four of which were present at low frequencies (1.3% to 7.5%) while the S513R mutation was present at a much higher frequency (96.3%). The other five mutations, including Y976F, had been described before and had frequencies of 97.8% to 100%. Our findings suggest that CQ-resistant P. vivax isolates are present in Madagascar, particularly in the foothills of the Central Highlands. The 976Y pvmdr1 mutation was found not to be useful for monitoring CQ resistance. Further efforts are required to develop suitable tools for monitoring drug resistance in P. vivax malaria.
Plasmodium vivax remains the most geographically widespread human malaria parasite, affecting almost 40% of the population worldwide (14, 27). Its global burden is estimated at 132 to 391 million clinical malaria infections (17, 33). Most cases of P. vivax infection (80 to 90%) originate in Southeast Asia, the Western Pacific, and the Middle East, but a significant number of cases also occur in Central and South America and in Africa (East and South Africa, including Madagascar). P. vivax malaria is generally considered benign and causes far fewer deaths than Plasmodium falciparum. However, morbidity rates are high, particularly among children and pregnant women (7), and the disease may have a large impact on socioeconomic development (27, 33).
P. falciparum developed resistance to chloroquine (CQ) in various disease foci in the 1950s (47), but the first case of P. vivax resistance to CQ was not reported until 1989, in Papua New Guinea (37). Further sporadic cases were subsequently observed in the Western Pacific (Indonesia, Philippines) (1, 2, 12, 28, 35, 42, 44), Southeast Asia (Myanmar, India, Vietnam) (11, 15, 22, 24, 29, 31, 40), South America (Colombia, Guyana) (32, 41), and the Middle East (Turkey) (21). Despite these reports, it remains difficult to estimate the worldwide prevalence of P. vivax resistance to CQ. Genotyping by using molecular markers is required for in vivo studies of the clinical efficacy of antimalarial drugs, so that the recurrence of parasitemia due to reinfection can be distinguished from recrudescence. However, P. vivax genotyping in the context of antimalarial drug treatment is confounded by the occurrence of relapses due to hypnozoite stages (which can be the same isolate as that at baseline or a different one) or recrudescence (8, 9, 19). In vitro assays, which should provide drug susceptibility data free from the effects of confounding factors, such as host immunity, are still difficult to conduct (45). The molecular mechanisms underlying CQ resistance in P. vivax malaria remain unclear and may involve multigenic loci, but two genes orthologous to the pfmdr1 and pfcrt genes, which encode putative transporters (pvmdr1 [5, 43] and pvcrt-o [39]), have been identified as possible genetic markers of CQ resistance.
Malaria is endemic over three-quarters of Madagascar, with almost 1 million clinical cases reported per year, making this disease a major public health problem (20, 46). Four of the five malaria parasites known to infect human beings are present. Plasmodium vivax, the second most prevalent species after P. falciparum (causing 6.3% of all malaria cases), has a heterogeneous geographical distribution, ranging from 0% in the east coast area to 17.5% to the west of the foothills of the Central Highlands (4). CQ therapeutic efficacy has been assessed in Madagascar for P. falciparum (26) and Plasmodium malariae (3) but not for P. vivax malaria. CQ was replaced by artemisinin-based combination therapy (artesunate plus amodiaquine) as the recommended first-line treatment for uncomplicated malaria in 2005, but CQ is nonetheless still widely used. It is still recommended by the National Malaria Control Programme for the home management of presumed malaria in children (36).
The aims of this study were (i) to assess the therapeutic efficacy of CQ in P. vivax malaria and (ii) to explore the prevalences of mutations in the pvcrt-o and pvmdr1 genes in samples taken before treatment and the association between the presence of a mutant parasite and the clinical response of the patient to CQ treatment.
MATERIALS AND METHODS
Collection of P. vivax clinical isolates.
P. vivax clinical isolates were collected in 2006 from individuals seeking malaria treatment at six sentinel sites located in the three epidemiological strata for malaria throughout Madagascar: Ejeda and Ihosy in the South (an epidemic-prone area), Tsiroanomandidy and Moramanga/Saharevo in the foothills of the Central Highlands (an area of low-level endemicity), and Maevatanana and Miandrivazo in the West (an area of seasonal occurrence and endemicity).
All patients with fever or a history of fever in the 48 h before their arrival at the health center were screened with a rapid diagnostic test, based on the detection of Plasmodium-specific lactate dehydrogenase (OptiMAL-IT; DiaMed AG, Cressier sur Morat, Switzerland). Giemsa-stained thin and thick blood films were prepared for each patient who was positive by the rapid diagnostic test. Plasmodium was identified to species level, and parasite density was assessed by a skilled microscopist. Blood from a finger prick was collected on filter paper once informed consent had been obtained from each adult subject and from at least one of the parents of each minor.
Clinical efficacy of CQ in P. vivax malaria. (i) Patient enrollment, treatment, and follow-up procedures.
The clinical trial was carried out in 2006, at previously identified sentinel sites for assessing the therapeutic efficacy of CQ in P. vivax malaria. Patients with microscopically confirmed P. vivax infections were enrolled according to the inclusion criteria of the 2001 WHO guidelines (48). All patients included were weighed and their medical histories (including previous antimalarial medication) recorded. Clinical examination, including axillary temperature recording and Giemsa staining of serial thick and thin blood films, was carried out on days 0, 1, 2, 3, 7, 14, 21, and 28. Thick and thin films were read by a skilled microscopist. Patients were treated with the standard CQ regimen (25 mg/kg of body weight per day for 3 days) and monitored for 28 days. Patients were directly observed for 30 min after treatment, and the dose was readministered if vomiting occurred. Patients who repeatedly vomited their first dose of study medication were excluded from the study. Blood was blotted onto filter paper during follow-up and was stored at 4°C for DNA analysis. The hemoglobin concentration was determined on days 0 and 28 (HemoCue, Ängelholm, Sweden).
(ii) Outcome measures.
Treatment outcomes were assessed according to WHO 2001 guidelines (48) as “treatment failure” (TF; clinical deterioration due to P. vivax illness requiring hospitalization, with parasitemia and an axillary temperature of ≥37.5°C any time between days 3 and 28, or parasitemia on any day between days 7 and 28, regardless of clinical condition) or “adequate clinical and parasitological response” (ACPR; absence of parasitemia on day 28 without the criteria for TF having been met previously).
Patients with TF were treated with artesunate (4 mg/kg on days 0, 1, and 2) plus amodiaquine (10 mg/kg on days 0, 1, and 2), but their response to rescue therapy was not assessed.
Patients were excluded after enrollment if any of the following occurred: (i) use of antimalarial drugs outside of the study protocol, (ii) detection during follow-up of mixed malarial infections, except at day 28, (iii) parasitemia in the presence of a concomitant febrile illness potentially interfering with the classification of treatment outcome, (iv) withdrawal of consent, (v) loss to follow-up, (vi) protocol violation, or (vii) death due to a nonmalaria illness.
(iii) Laboratory procedures.
Blood smears were stained by incubation with 10% Giemsa stain for 10 min. Asexual- and sexual-stage parasite counts were determined per 200 white blood cells. Parasitemia was calculated based on a white blood cell count of 8,000/μl. Thick-film examinations were considered negative if no parasite was found in 100 high-power fields.
DNA was extracted from blood spots on filter paper with Instagene Matrix resin (Bio-Rad, Marnes la Coquette, France), according to the manufacturer's instructions. The identification of the parasite species was confirmed by real-time PCR using species-specific primers, as described by de Monbrison et al. (10), with a protocol adapted for the RotorGene 3000 thermocycler (Corbett Life Science, Sydney, Australia).
Molecular genotyping techniques were used to distinguish recrudescence from new infection or relapse of liver stages for all patients with TF occurring after day 7. Briefly, blood samples collected on the day of enrollment, on day 1, and on the day of TF were analyzed for polymorphisms in the genes encoding circumsporozoite protein (pvcsp) (18) and merozoite surface protein-3 (pvmsp3) (6) and in six different microsatellite markers, as previously described (4). The PCR products of the genes encoding surface proteins were sequenced, whereas microsatellite PCR products were genotyped on the basis of size, using a GeneScan 500 LIZ size standard on an ABI Prism 3730 XL DNA analyzer. Genotype patterns on the day of TF were compared with those at treatment initiation and on day 1. An outcome was defined as recrudescence if all alleles present at the time of failure were present at the time of treatment initiation or on day 1; otherwise, it was defined as a reinfection or relapse.
Sequencing of pvcrt-o and pvmdr1.
The pvcrt-o (exons 1 to 6) and pvmdr1 genes were amplified by nested PCR using gene-specific primers (Table 1). For the outer pvcrt-o PCR, amplification took place in the following reaction mixture: 2 μl of 10× buffer, 2.5 mM MgCl2, 0.4 mM each deoxynucleoside triphosphate, 0.25 μM each primer, 1 U of FirePol Taq polymerase (Solis Biodyne), and 2 μl of genomic DNA. PCR was performed under the following conditions: heating at 94°C for 5 min, followed by 40 cycles of heating at 94°C for 30 s, 56°C for 30 s, and 72°C for 2 min, and a final extension period at 72°C for 10 min. For the inner pvcrt-o PCR, the following reaction mixture was used: 5.5 μl of 10× buffer, 2.5 mM MgCl2, 0.4 mM each deoxynucleoside triphosphate, 0.27 μM each primer, 2.5 U of FirePol Taq polymerase (Solis Biodyne), and 3 μl of the amplicon. PCR was performed under the following conditions: 94°C for 5 min, followed by 45 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 2 min, and a final extension period at 72°C for 10 min.
TABLE 1.
Sequences of primers used to amplify the pvcrt-o (exons 1 to 6) and pvmdr1 genes
| Primer name | Sequence (5′-3′) | Target gene |
|---|---|---|
| PVCG10 1-6 PF | 5′-GCTACCCCTAACGCACAATG-3′ | pvcrt-o |
| PVCG10 1-6 PR | 5′-GATTTGGGAAAGCACAACGT-3′ | |
| PVCG10 1-6 NF | 5′-GATGAACGTTACCGGGAGTTGG-3′ | |
| PVCG10 1-6 NR | 5′-ATCGGAAGCATCAGGCAGGA-3′ | |
| PVMDR1 PF | 5′-ATGAAAAAGGATCAAAGGCAAC-3′ | pvmdr1 |
| PVMDR1 PR | 5′-CTACTTAGCCAGCTTGACGTAC-3′ | |
| PVMDR1 NF | 5′-TTGAACAAGAAGGGGACGTT-3′ | |
| PVMDR1 NR | 5′-CTTATATACGCCGTCCTGCAC-3′ |
For the first and second rounds of PCR for pvmdr1, PCR conditions were similar to those for the outer and inner pvcrt-o PCRs, except that FirePol Taq polymerase (Solis Biodyne) was replaced by TaKaRa LA Taq polymerase (TaKaRa Bio Inc.) at the same concentration. The first round of PCR was performed under the following conditions: 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 4.5 min, and a final extension period at 72°C for 10 min. The conditions for the second round were as follows: 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 57°C for 30 s, and 72°C for 4 min, and a final extension period at 72°C for 10 min.
PCR products were purified by 96-well plate filtration (Millipore, St. Quentin en Yvelines, France) using a polyacrylamide gel (Bio-Gel P-100; Bio-Rad, Marnes-la-Coquette, France). Sequencing reactions were carried out with the ABI Prism BigDye Terminator cycle sequencing ready reaction kit run on a 3730 XL genetic analyzer (Applied Biosystems, Courtaboeuf, France). Electropherograms were visualized and analyzed with Seqscape software (version 2.0; Applied Biosystems, Courtaboeuf, France). Amino acid sequences were compared with wild-type sequences (GenBank accession no. AF314649 for pvcrt-o and GenBank accession no. AY571984.1 for pvmdr1) using BioEdit Sequence Alignment Editor software (16). Parasites with mixed alleles (both wild-type and mutant alleles present) were considered mutant.
Statistical analysis.
Data were entered and verified using EpiInfo software (version 6.04; Centers for Disease Control and Prevention, Atlanta, GA) and were analyzed with MedCalc software (version 9.1.0.1; MedCalc Software, Mariakerke, Belgium). The frequency of TF at the sentinel sites was unknown. We therefore included all patients with microscopically confirmed P. vivax malaria during the duration of the in vivo study, between February and June 2006 (26). Efficacy data were assessed by a per-protocol analysis including all patients who completed the study. An age-stratified analysis for patients <5 years old, 5 to 14 years old, and ≥15 years old was planned. Categorical variables were compared using chi-square or Fisher's exact tests, and continuous variables were compared using an independent-samples ANOVA (analysis of variance) or Mann-Whitney test.
The primary end point was the therapeutic response to CQ, based on parasitological and clinical cure by day 28 and adjusted by genotyping. The secondary end points included fever clearance, parasite clearance, and a change in hemoglobin levels between day zero and the last day of follow-up. Hypotheses were tested by determining the difference in risk, exact 95% confidence intervals (95% CI), and P values. A P value (two-tailed) of <0.05 was considered statistically significant.
Ethical approval.
The study protocol was reviewed and approved by the Ethics Committee of the Ministry of Health of Madagascar (007/SANPF/2007). Informed written consent was provided by all patients or their parents/guardians before inclusion in the study.
Nucleotide sequence accession numbers.
The exact sequence of each mutant allele has been submitted to GenBank (accession no. EU683813 to EU683819).
RESULTS
Clinical and parasitological response to CQ.
Among the 5,484 patients screened for malaria in health facilities, 139 (2.5%) tested positive for P. vivax. In total, 105 (75.5%) of these patients met all the inclusion criteria and were enrolled. The baseline characteristics of the included patients are presented by site in Table 2 and by age group in Table 3. No significant differences were observed between sites, except for the proportions of children under the age of 5 years, which were similar in the South (16.6%) and the West (18.6%) but higher in the Central Highlands (62.1%) (P = 0.0006). Similarly, no significant differences were observed between age groups except for mean hemoglobin levels at day zero, which were similar for individuals 5 to 14 years old and those >15 years old (10.7 g/dl) but significantly lower for those <5 years old (9.1 g/dl) (P = 0.03).
TABLE 2.
Baseline characteristics of the enrolled patients by site, Madagascar, 2006
| Parameter | Value for all patients | Value for the following areaa:
|
P | ||
|---|---|---|---|---|---|
| South | West | Central Highlands | |||
| Total no. of patients | 105 | 6 | 70 | 29 | |
| No. (%) of female patients | 53 (51.0) | 3 (50.0) | 33 (47.1) | 17 (60.7) | 0.39b |
| No. (%) of children <5 yr old | 32 (31.0) | 1 (16.6) | 13 (18.6) | 18 (62.1) | 0.0006b |
| Mean (range) age (yr) | 11.2 (0.8-54) | 10.3 (3-19) | 12.9 (0.8-54) | 7.36 (1-47) | 0.08c |
| Median (range) axillary temp (°C) | 37.9 (36.2-41.1) | 39.0 (37.5-40.0) | 37.7 (36.2-41.1) | 38.0 (37.5-40.1) | 0.11c |
| Geometric mean (range) parasite density (no./μl) | 3,122 (200-52,000) | 3,190 (750-52,000) | 3,256 (200-33,000) | 2,810 (250-37,750) | 0.87c |
| No. (%) of patients with previous antimalarial treatment | 9 (8.9) | 0 | 5 (7.1) | 4 (13.8) | 0.37b |
| Mean (range) hemoglobin concn (g/dl) | 10.2 (4.4-16.3) | 11.8 (9.2-14.7) | 10.6 (5.2-15.3) | 9.0 (4.4-16.3) | 0.01c |
South sites, Ejeda and Ihosy; West sites, Maevatanana and Miandrivazo; Central Highlands sites, Tsiroanomandidy and Moramanga/Saharevo.
By the chi-square test.
By ANOVA.
TABLE 3.
Baseline characteristics of the enrolled patients by age group, Madagascar, 2006
| Parameter | Value for the following age group:
|
P | ||
|---|---|---|---|---|
| <5 yr | 5-14 yr | ≥15 yr | ||
| Total no. of patients | 32 | 48 | 25 | |
| No. (%) of female patients | 17 (53.1) | 23 (47.9) | 13 (53.0) | 0.85a |
| Median (range) axillary temp (°C) | 38.0 (37.5-40.0) | 37.7 (36.3-40.1) | 38.0 (36.2-41.1) | 0.37b |
| Geometric mean (range) parasite density (no./μl) | 3,821 (360-52,000) | 3,205 (250-31,000) | 2,294 (200-33,000) | 0.32c |
| No. (%) of patients with previous antimalarial treatment | 2 (6.7) | 4 (8.7) | 3 (12.0) | 0.78a |
| Mean (range) hemoglobin concn (g/dl) | 9.1 (4.4-16.3) | 10.7 (5.2-15.3) | 10.7 (5.5-16.0) | 0.03c |
By the chi-square test.
By the Mann-Whitney test.
By ANOVA.
Clinical and parasitological monitoring was completed up to day 28 for 98 (93.3%) of the 105 enrolled patients. Five patients were lost to follow-up, and two were excluded because of the emergence of P. falciparum infection at day 21.
Results for response to CQ treatment are presented by study site in Table 4 and by age group in Table 5. Eighty-eight patients (89.8%) successfully cleared P. vivax parasitemia after CQ treatment, whereas 10 patients (10.2%; 95% CI, 5.0% to 18.0%) experienced recurrences: 1 (10%) on day 7, 3 (30%) on day 21 (2 with mixed P. falciparum-P. vivax infections), and 6 (60%) on day 28 (1 with fever). After PCR correction, five cases of recurrence were confirmed as TF, giving a failure rate of 5.1% (95% CI, 1.7% to 11.5%). The frequency of TF was significantly higher in the Central Highlands (14.8%; 95% CI, 4.2% to 33.7%) than in the South (0%; 95% CI, 0% to 45.9%) and West (1.5%; 95% CI, 0% to 8.3%) (P = 0.02), regardless of the age group considered.
TABLE 4.
Response to CQ treatment by site, Madagascar, 2006
| Treatment response on day 28 | Valuea for all patients | Valuea for the following area:
|
Pc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Southb | Westb | Central Highlandsb | ||||||||
| Unadjusted by genotyping | ||||||||||
| TF | 10 (10.2 [5.0-18.0]) | 0 (0 [0-45.9]) | 4 (6.2 [1.7-15.0]) | 6 (22.2 [8.6-42.3]) | 0.04 | |||||
| ACPR | 88 (89.8 [82.0-95.0]) | 6 (100) | 61 (93.8 [85.0-98.3]) | 21 (77.8 [57.7-91.4]) | ||||||
| Adjusted by genotyping | ||||||||||
| TF | 5 (5.1 [1.7-11.5]) | 0 (0 [0-45.9]) | 1 (1.5 [0-8.3]) | 4 (14.8 [4.2-33.7]) | 0.02 | |||||
| ACPR | 93 (94.9 [88.5-98.3]) | 6 (100) | 64 (98.5 [91.7-100]) | 23 (85.2 [66.3-95.8]) | ||||||
All values are given as number of patients (percentage of patients [95% CI]).
South sites, Ejeda and Ihosy; West sites, Maevatanana and Miandrivazo; Central Highlands sites, Tsiroanomandidy and Moramanga/Saharevo.
By the chi-square test.
TABLE 5.
Response to CQ treatment by age group, Madagascar, 2006
| Treatment response on day 28 | Valuea for the following age group:
|
Pb | ||||||
|---|---|---|---|---|---|---|---|---|
| <5 yr | 5-14 yr | ≥15 yr | ||||||
| Unadjusted by genotyping | ||||||||
| TF | 6 (20.0 [7.7-38.6]) | 3 (6.5 [1.4-17.9]) | 1 (4.5 [0.1-22.8]) | 0.10 | ||||
| ACPR | 24 (80.0 [61.4-92.3]) | 43 (93.5 [82.1-98.6]) | 21 (95.5 [77.2-99.9]) | |||||
| Adjusted by genotyping | ||||||||
| TF | 3 (10.0 [2.1-26.5]) | 1 (2.2 [0.1-11.5]) | 1 (4.5 [0.1-22.8]) | 0.31 | ||||
| ACPR | 27 (90.0 [73.5-97.9]) | 45 (97.8 [88.5-99.9]) | 21 (95.5 [77.2-99.9]) | |||||
Given as number of patients (percentage of patients [95% CI]).
By the chi-square test.
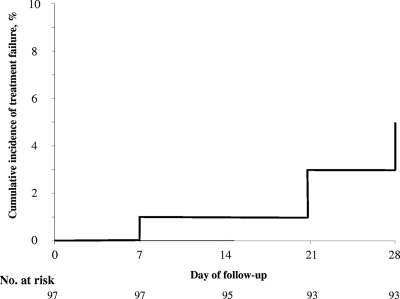
Figure 1 shows the cumulative incidence of TF over the 28-day follow-up period, adjusted by genotyping.
FIG. 1.
Cumulative incidence of treatment failure over the 28-day follow-up period, Madagascar, 2006.
For patients who were treated successfully, fever abated after 1.1 ± 0.5 days and parasite clearance took 2.1 ± 1.3 days. On day 28, hematological recovery was estimated by calculating the median of individual increases in hemoglobin concentration, 1.6 g/dl (95% CI, 1.3 to 1.9 g/dl). No severe adverse effects attributable to the study medication were observed during the follow-up period.
Analysis of the pvcrt-o gene.
The pvcrt-o gene was successfully sequenced in 95 of the 105 P. vivax isolates collected (90.5%). All isolates were wild type, including those from patients displaying recrudescence. Only synonymous mutations or insertions (in introns) were found.
Analysis of the pvmdr1 gene.
The pvmdr1 gene was successfully sequenced in 80 of the 105 isolates collected (76.2%), and at least four nonsynonymous mutations were detected in each case.
Ten nonsynonymous mutations were identified: five mutations described in previous studies (G698S, M908L, T958M, Y976F, and F1076L) and five new mutations (F194Y, S510T, S513R, I636T, and A829V) (Table 6). Six mutations were highly frequent and were found in almost all isolates tested (from 96.3% to 100%): the five previously identified mutations and the S513R mutation. The new mutations (other than S513R) were found only in isolates from the West and had a low prevalence (from 1.3% to 7.5%). Seven different haplotypes were identified, with four to seven amino acid substitutions (Table 7). The most common haplotype was the 513R 698S 908L 958M 976F 1076L sextuple-mutant type (85.0%). This haplotype was the only haplotype found at the South and Central Highlands sites. All seven haplotypes were found at sites in western Madagascar.
TABLE 6.
Frequency distribution of mutations in the pvmdr1 gene by sampling site, Madagascar, 2006a
| Mutation in the pvmdr1 gene | No. (%) of mutant-type alleles by sampling siteb
|
Total no. (%) | ||
|---|---|---|---|---|
| South (n = 5) | West (n = 53) | Central Highlands (n = 22) | ||
| F194Y | 0 (0) | 6 (11) | 0 (0) | 6 (7.5) |
| S510T | 0 (0) | 3 (6) | 0 (0) | 3 (3.8) |
| S513R | 5 (100) | 50 (94) | 22 (100) | 77 (96.3) |
| I636T | 0 (0) | 1 (2) | 0 (0) | 1 (1.3) |
| G698S | 5 (100) | 51 (96) | 22 (100) | 78 (97.8) |
| A829V | 0 (0) | 1 (2) | 0 (0) | 1 (1.3) |
| M908L | 5 (100) | 53 (100) | 22 (100) | 80 (100) |
| T958M | 5 (100) | 53 (100) | 22 (100) | 80 (100) |
| Y976F | 5 (100) | 52 (98) | 22 (100) | 79 (98.8) |
| F1076L | 5 (100) | 53 (100) | 22 (100) | 80 (100) |
New mutations are shown in boldface.
South sites, Ejeda and Ihosy; West sites, Maevatanana and Miandrivazo; Central Highlands sites, Tsiroanomandidy and Moramanga/Saharevo.
TABLE 7.
Frequency distribution of pvmdr1 haplotypes by sampling site and treatment outcome
| pvmdr1 haplotype | No. (%) by sampling sitea
|
Total no. (%) | No. (%) by treatment outcome
|
|||
|---|---|---|---|---|---|---|
| South (n = 5) | West (n = 53) | Central Highlands (n = 22) | ACPR (n = 80) | TF (n = 4) | ||
| No mutation (wild type) | 0 | 0 | 0 | 0 | 0 | 0 |
| Quadruple mutant (510T 908L 958M 1076L) | 0 | 1 | 0 | 1 (1.3) | 1 | 0 |
| Quintuple mutant (513R 908L 958M 976F 1076L) | 0 | 1 | 0 | 1 (1.3) | 1 | 0 |
| Sextuple mutants | ||||||
| 513R 698S 908L 958M 976F 1076L | 5 | 41 | 22 | 68 (85.0) | 64 | 4 |
| 510T 698S 908L 958M 976F 1076L | 0 | 2 | 0 | 2 (2.5) | 2 | 0 |
| Septuple mutants | ||||||
| 513R 698S 829V 908L 958M 976F 1076L | 0 | 1 | 0 | 1 (1.3) | 1 | 0 |
| 513R 636T 698S 908L 958M 976F 1076L | 0 | 1 | 0 | 1 (1.3) | 1 | 0 |
| 194Y 513R 698S 908L 958M 976F 1076L | 0 | 6 | 0 | 6 (7.5) | 6 | 0 |
South sites, Ejeda and Ihosy; West sites, Maevatanana and Miandrivazo; Central Highlands sites, Tsiroanomandidy and Moramanga/Saharevo.
Characteristics of patients and parasites and clinical response to CQ.
No significant differences were found between patients with and without recrudescence in terms of the characteristics of the patients or of their isolates before treatment (Table 8). Isolates obtained before treatment from patients who subsequently displayed recrudescence were wild type for the pvcrt-o gene and carried the most common haplotype (513R 698S 908L 958M 976F 1076L) for the pvmdr1 gene (Table 5). DNA obtained on the day of treatment failure was successfully amplified and sequenced for the pvcrt-o gene in four of five samples and for pvmdr1 in three of five samples. The genotype identified (wild type for the pvcrt-o gene and sextuple-mutant type for the pvmdr1 gene) was similar to the genotype identified at the day of enrollment for each patient.
TABLE 8.
Demographic, clinical, and parasitological characteristics of baseline isolates from patients with and without recrudescence
| Characteristic | Value for patients:
|
P | ||||
|---|---|---|---|---|---|---|
| With recrudescence (n = 5) | Without recrudescence (n = 98) | |||||
| Patients | ||||||
| % Female (95% CI) | 60.0 (14.7-94.7) | 48.4 (37.9-59.0) | 0.86a | |||
| Mean (range) age (yr) | 8.4 (1.3-27.0) | 10.7 (0.8-54.0) | 0.33b | |||
| Mean (range) temp (°C) | 37.9 (37.5-38.4) | 38.1 (36.2-41.1) | 0.68b | |||
| % with previous fever (95% CI) | 100.0 | 92.4 (84.9-96.9) | 0.73a | |||
| % with previous malaria treatment (95% CI) | 20.0 | 7.8 (3.2-15.4) | 0.36a | |||
| Mean (range) hemoglobin concn (g/dl) | 10.8 (5.5-16.3) | 10.3 (4.4-16.0) | 0.84b | |||
| Baseline isolates | ||||||
| Geometric mean (range) parasite density (no./μl) | 4,285 (2,000-8,000) | 3,098 (200-52,000) | 0.55b | |||
| % (95% CI) with the following mutant allele in the pvmdr1 gene: | ||||||
| 194Y | 0 (0-60.2) | 7.0 (2.3-15.7) | 0.75c | |||
| 510T | 0 (0-60.2) | 4.2 (0.9-11.9) | 0.84c | |||
| 513R | 100.0 | 95.8 (88.1-99.1) | 0.84c | |||
| 636T | 0 (0-60.2) | 1.4 (0-7.6) | 0.94c | |||
| 698S | 100.0 | 97.2 (90.2-99.7) | 0.89c | |||
| 908L | 100.0 | 100.0 | 1c | |||
| 958M | 100.0 | 100.0 | 1c | |||
| 976F | 100.0 | 98.6 (92.4-100.0) | 0.94c | |||
| 1076L | 100.0 | 100.0 | 1c | |||
By the chi-square test.
By the Mann-Whitney test.
By Fisher's exact test.
DISCUSSION
CQ continues to be widely used in Madagascar, despite having been officially replaced by artemisinin combination therapy as the first-line treatment for uncomplicated P. falciparum malaria in 2005. CQ was recommended for more than 50 years for both treatment and prophylaxis and has been used both in health facilities and in the community. Prepackaged CQ is still recommended by the Ministry of Health and Family Planning for the home management of presumed malaria in children under the age of 5 years. Recent studies have investigated the susceptibilities of P. falciparum (26) and P. malariae (3) to CQ in Madagascar. P. malariae remains sensitive to CQ, but high levels of CQ resistance (from 19% to 64%) have been reported for P. falciparum isolates.
This study was the first to investigate the susceptibility of P. vivax to CQ in Madagascar. We assessed the efficacy of CQ at the national level by carrying out our clinical trial at six sentinel sites, selected as representative of the various epidemiological strata for malaria. Treatment outcome was assessed after adjustment based on molecular genotyping techniques to distinguish between recurrences of parasitemia due to new infection and those due to recrudescence or relapse. Since primaquine is not available in Madagascar, this drug was not used to treat hypnozoite stages in this study. The occurrence of relapse can be due to the same isolate as that observed at baseline or a different one. It can lead to misclassification of the recurrent parasitemia, as recrudescence (if the antimalarial drug level is under the MIC) in the first case or as a new infection in the second case. The interpretation of genotyping in this context is uncertain, and it is recommended to measure the concentration of the antimalarial drug in the blood (25). The absence of measurements of blood CQ concentrations in patients displaying recrudescence is the main limitation of this study. Overall, our data suggest that CQ-resistant P. vivax isolates are present in Madagascar, particularly in the foothills of the Central Highlands. We have previously found a high frequency of sulfadoxine-pyrimethamine TF in patients with P. vivax malaria in the same area (4).
In vivo studies remain the “gold standard” method for assessing antimalarial drug efficacy. However, due to the difficulties involved in determining TF rates, particularly for P. vivax malaria, molecular markers seem to be useful for monitoring the drug resistance of malaria parasites. The K76T mutation in the P. falciparum crt gene is known to be involved in CQ resistance, but very few data are available concerning the possible relationship between the P. vivax crt-o and mdr1 genes and CQ resistance. Only a few studies have been carried out, and associations between TF and nonsynonymous mutations in isolates obtained before treatment have not yet been clearly established (5, 30, 38). However, the Y976F substitution in the pvmdr1 gene is thought to be involved in CQ resistance in P. vivax (23, 43). Suwanarusk et al. found that the geometric mean 50% inhibitory concentration of CQ was significantly higher in P. vivax isolates carrying the Y976F mutation than in isolates with the wild-type allele (43).
We used a sequencing approach to screen for the presence of mutations in the target genes in order to confirm the involvement of these two genetic markers, pvcrt-o and pvmdr1, in CQ resistance in P. vivax. The sequences analyzed in pvcrt-o were those of exons 1 to 6, in which mutations have already been described (30). All the Malagasy P. vivax isolates sequenced were wild type, with only single-nucleotide polymorphisms or insertions in intron sequences. However, isolates were more polymorphic for the pvmdr1 gene, with 11 synonymous mutations and 10 nonsynonymous mutations, 5 of which had not been identified previously. Six of these mutations, including Y976F, were highly frequent (from 96.3% to 100%). These findings may reflect the CQ resistance situation of P. falciparum, in which the pfmdr1 mutation N86Y is highly prevalent even in the absence of the K76T pfcrt mutation (34). Comparison with the SalI reference strain showed all samples to be of the mutant type, with haplotype patterns comprising four to seven mutations. We also found that the 510R and 513T amino acid substitutions were mutually exclusive, suggesting that two different stepwise mutant selection processes had occurred. For the 510R amino acid substitution, the 510T 908L 958M 1076L haplotype must have been selected first and the 510T 698S 908L 958M 976F 1076L haplotype second, whereas for the 513T amino acid substitution, the 513R 908L 958M 976F 1076L haplotype must have been selected first, followed by the 513R 698S 908L 958M 976F 1076L haplotype and, finally, the 513R 698S 829V 908L 958M 976F 1076L or 513R 636T 698S 908L 958M 976F 1076L haplotype.
Unlike the investigators in another recent study (43), we found no significant correlation between the pvmdr1 Y976F mutation and CQ treatment outcome, essentially because this mutation was already fixed in the Malagasy P. vivax population. There are two possible reasons for this. First, the Y976F mutation may have been selected by CQ drug pressure over the last 50 years in Madagascar. This possibility implies a role of this mutation in CQ resistance. Second, the Malagasy P. vivax population may have been introduced into Madagascar at the beginning of the Christian era from Indonesia (13), where the prevalence of the Y976F mutation is high (43). If that is the case, this mutation is unlikely to play any role in CQ resistance.
The monitoring of the resistance of malaria parasites to CQ through molecular markers remains complex. The usefulness of marker sets may differ for different areas and needs to be evaluated for a given area before use. These findings call into question the validity of in vitro tests. Major efforts have been made to develop a continuous system for the maintenance of P. vivax isolates in culture (45), but this remains difficult. Furthermore, the determination of antimalarial drug activity based on in vitro tests and the schizont maturation method is more complicated for P. vivax than for P. falciparum, due to the asynchrony of P. vivax blood parasites. Furthermore, the parasite response to antimalarial dugs may depend on the parasite development stage (43).
Our study provides new data concerning the susceptibility of P. vivax to CQ in Madagascar and suggests that CQ-resistant isolates have been selected in Madagascar, particularly in the foothills of the Central Highlands. However, levels of resistance to CQ remain much lower in P. vivax than in P. falciparum (5.1% versus 44%) (26). The 976Y pvmdr1 mutation was found not to be a useful tool for monitoring CQ resistance in Madagascar, and further efforts are required to develop surveillance tools for the temporal and geographical mapping of emerging drug resistance in P. vivax malaria.
Acknowledgments
We thank the patients and health care workers involved in the national network for the surveillance of malaria resistance in Madagascar (Réseau d'Etude de la Résistance [RER]), from which these samples were obtained, and the staff of the Ministry of Health of Madagascar for their collaboration. We thank Laurence Randrianasolo, Rogelin Raherinjafy, Arthur Randriamanantena, Hanitra Ranaivosoa, Didier Ralaizandry, Diamondra Raveloariseheno, and Vony Rabekotorina for helping with field work.
This study was supported by grants from Natixis/Impact Malaria through the Observatoire de la Résistance aux Antipaludiques Project and the Genomics Platform, Pasteur Génopôle, Pasteur Institute, France. Sample collection was funded by the Global Fund project for Madagascar, round 3 (Community Action to Roll Back Malaria grant MDG-304-G05-M). C. Barnadas is a Ph.D. student supported by the Fondation Jeunesse Internationale (Fondation de France), BioMérieux (Prix BioMérieux Infectiologie 2006), Association des Internes et Anciens Internes en Pharmacie des Hôpitaux de Lyon (Prix R. Rizard), and Hospices Civils de Lyon.
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Baird, J. K., H. Basri, Purnomo, M. J. Bangs, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. K., M. F. Sustriayu Nalim, H. Basri, S. Masbar, B. Leksana, E. Tjitra, R. M. Dewi, M. Khairani, and F. S. Wignall. 1996. Survey of resistance to chloroquine by Plasmodium vivax in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 90:409-411. [DOI] [PubMed] [Google Scholar]
- 3.Barnadas, C., A. Ratsimbasoa, H. Ranaivosoa, D. Ralaizandry, D. Raveloariseheno, V. Rabekotonorina, S. Picot, and D. Ménard. 2007. Prevalence and chloroquine sensitivity of Plasmodium malariae in Madagascar. Am. J. Trop. Med. Hyg. 77:1039-1042. [PubMed] [Google Scholar]
- 4.Barnadas, C., M. Tichit, C. Bouchier, A. Ratsimbasoa, L. Randrianasolo, R. Raherinjafy, M. Jahevitra, S. Picot, and D. Ménard. 2008. Plasmodium vivax dhfr and dhps mutations in isolates from Madagascar and therapeutic response to sulphadoxine-pyrimethamine. Malar. J. 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brega, S., B. Meslin, F. de Monbrison, C. Severini, L. Gradoni, R. Udomsangpetch, I. Sutanto, F. Peyron, and S. Picot. 2005. Identification of the Plasmodium vivax mdr-like gene (pvmdr1) and analysis of single-nucleotide polymorphisms among isolates from different areas of endemicity. J. Infect. Dis. 191:272-277. [DOI] [PubMed] [Google Scholar]
- 6.Bruce, M. C., M. R. Galinski, J. W. Barnwell, G. Snounou, and K. P. Day. 1999. Polymorphism at the merozoite surface protein-3α locus of Plasmodium vivax: global and local diversity. Am. J. Trop. Med. Hyg. 61:518-525. [DOI] [PubMed] [Google Scholar]
- 7.Cattani, J. A., J. L. Tulloch, H. Vrbova, D. Jolley, F. D. Gibson, J. S. Moir, P. F. Heywood, M. P. Alpers, A. Stevenson, and R. Clancy. 1986. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am. J. Trop. Med. Hyg. 35:3-15. [DOI] [PubMed] [Google Scholar]
- 8.Chen, N., A. Auliff, K. Rieckmann, M. Gatton, and Q. Cheng. 2007. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J. Infect. Dis. 195:934-941. [DOI] [PubMed] [Google Scholar]
- 9.Cui, L., A. A. Escalante, M. Imwong, and G. Snounou. 2003. The genetic diversity of Plasmodium vivax populations. Trends Parasitol. 19:220-226. [DOI] [PubMed] [Google Scholar]
- 10.de Monbrison, F., C. Angei, A. Staal, K. Kaiser, and S. Picot. 2003. Simultaneous identification of the four human Plasmodium species and quantification of Plasmodium DNA load in human blood by real-time polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 97:387-390. [DOI] [PubMed] [Google Scholar]
- 11.Dua, V. K., P. K. Kar, and V. P. Sharma. 1996. Chloroquine resistant Plasmodium vivax malaria in India. Trop. Med. Int. Health 1:816-819. [DOI] [PubMed] [Google Scholar]
- 12.Fryauff, D. J., S. Tuti, A. Mardi, S. Masbar, R. Patipelohi, B. Leksana, K. C. Kain, M. J. Bangs, T. L. Richie, and J. K. Baird. 1998. Chloroquine-resistant Plasmodium vivax in transmigration settlements of West Kalimantan, Indonesia. Am. J. Trop. Med. Hyg. 59:513-518. [DOI] [PubMed] [Google Scholar]
- 13.Goodman, S. M., and P. B. Benstead. 2003. The natural history of Madagascar. University of Chicago Press, Chicago, IL.
- 14.Guerra, C. A., R. W. Snow, and S. I. Hay. 2006. Mapping the global extent of malaria in 2005. Trends Parasitol. 22:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthmann, J. P., A. Pittet, A. Lesage, M. Imwong, N. Lindegardh, M. Min Lwin, T. Zaw, A. Annerberg, X. de Radigues, and F. Nosten. 2008. Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop. Med. Int. Health 13:91-98. [DOI] [PubMed] [Google Scholar]
- 16.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 17.Hay, S. I., C. A. Guerra, A. J. Tatem, A. M. Noor, and R. W. Snow. 2004. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect. Dis. 4:327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imwong, M., S. Pukrittayakamee, A. C. Gruner, L. Renia, F. Letourneur, S. Looareesuwan, N. J. White, and G. Snounou. 2005. Practical PCR genotyping protocols for Plasmodium vivax using Pvcs and Pvmsp1. Malar. J. 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imwong, M., G. Snounou, S. Pukrittayakamee, N. Tanomsing, J. R. Kim, A. Nandy, J. P. Guthmann, F. Nosten, J. Carlton, S. Looareesuwan, S. Nair, D. Sudimack, N. P. Day, T. J. Anderson, and N. J. White. 2007. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J. Infect. Dis. 195:927-933. [DOI] [PubMed] [Google Scholar]
- 20.Institut National de la Statistique et ORC Macro. 2005. Enquête Démographique et de Santé de Madagascar 2003-2004. INSTAT et ORC Macro, Calverton, MD. http://pdf.dec.org/pdf_docs/Pnadd779.pdf.
- 21.Kurcer, M. A., Z. Simsek, and Z. Kurcer. 2006. The decreasing efficacy of chloroquine in the treatment of Plasmodium vivax malaria, in Sanliurfa, south-eastern Turkey. Ann. Trop. Med. Parasitol. 100:109-113. [DOI] [PubMed] [Google Scholar]
- 22.Luxemburger, C., M. van Vugt, S. Jonathan, R. McGready, S. Looareesuwan, N. J. White, and F. Nosten. 1999. Treatment of vivax malaria on the western border of Thailand. Trans. R. Soc. Trop. Med. Hyg. 93:433-438. [DOI] [PubMed] [Google Scholar]
- 23.Marfurt, J., I. Mueller, A. Sie, P. Maku, M. Goroti, J. C. Reeder, H. P. Beck, and B. Genton. 2007. Low efficacy of amodiaquine or chloroquine plus sulfadoxine-pyrimethamine against Plasmodium falciparum and P. vivax malaria in Papua New Guinea. Am. J. Trop. Med. Hyg. 77:947-954. [PubMed] [Google Scholar]
- 24.Marlar-Than, Myat-Phone-Kyaw, Aye-Yu-Soe, Khaing-Khaing-Gyi, Ma-Sabai, and Myint-Oo. 1995. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans. R. Soc. Trop. Med. Hyg. 89:307-308. [DOI] [PubMed] [Google Scholar]
- 25.Medicines for Malaria Venture and World Health Organization. 2008. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Informal consultation organized by the Medicines for Malaria Venture and cosponsored by the World Health Organization, 29-31 May 2007, Amsterdam, The Netherlands. World Health Organization, Geneva, Switzerland. http://www.mmv.org/IMG/pdf/MalariaGenotyping2.pdf.
- 26.Ménard, D., A. Ratsimbasoa, M. Randrianarivelojosia, L. Rabarijaona, L. Raharimalala, O. Domarle, L. Randrianasolo, A. Randriamanantena, M. Jahevitra, V. Andriantsoanirina, M. A. Rason, R. Raherinjafy, E. Rakotomalala, L. Tuseo, and A. Raveloson. 2008. Assessment of the efficacy of antimalarial drugs recommended by the National Malaria Control Programme in Madagascar: up-dated baseline data from randomized and multi-site clinical trials. Malar. J. 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendis, K., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97-106. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, G. S., H. Basri, Purnomo, E. M. Andersen, M. J. Bangs, D. L. Mount, J. Gorden, A. A. Lal, A. R. Purwokusumo, S. Harjosuwarno, et al. 1993. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet 341:96-100. [DOI] [PubMed] [Google Scholar]
- 29.Myat-Phone-Kyaw, Myint-Oo, Myint-Lwin, Thaw-Zin, Kyin-Hla-Aye, and Nwe-Nwe-Yin. 1993. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans. R. Soc. Trop. Med. Hyg. 87:687. [DOI] [PubMed] [Google Scholar]
- 30.Nomura, T., J. M. Carlton, J. K. Baird, H. A. del Portillo, D. J. Fryauff, D. Rathore, D. A. Fidock, X. Su, W. E. Collins, T. F. McCutchan, J. C. Wootton, and T. E. Wellems. 2001. Evidence for different mechanisms of chloroquine resistance in 2 Plasmodium species that cause human malaria. J. Infect. Dis. 183:1653-1661. [DOI] [PubMed] [Google Scholar]
- 31.Phan, G. T., P. J. de Vries, B. Q. Tran, H. Q. Le, N. V. Nguyen, T. V. Nguyen, S. H. Heisterkamp, and P. A. Kager. 2002. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam. Trop. Med. Int. Health 7:858-864. [DOI] [PubMed] [Google Scholar]
- 32.Phillips, E. J., J. S. Keystone, and K. C. Kain. 1996. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin. Infect. Dis. 23:1171-1173. [DOI] [PubMed] [Google Scholar]
- 33.Price, R. N., E. Tjitra, C. A. Guerra, S. Yeung, N. J. White, and N. M. Anstey. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79-87. [PMC free article] [PubMed] [Google Scholar]
- 34.Rason, M. A., H. B. Andrianantenaina, F. Ariey, A. Raveloson, O. Domarle, and M. Randrianarivelojosia. 2007. Prevalent pfmdr1 n86y mutant Plasmodium falciparum in Madagascar despite absence of pfcrt mutant strains. Am. J. Trop. Med. Hyg. 76:1079-1083. [PubMed] [Google Scholar]
- 35.Ratcliff, A., H. Siswantoro, E. Kenangalem, M. Wuwung, A. Brockman, M. D. Edstein, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 101:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratsimbasoa, A., M. Randrianarivelojosia, P. Millet, J. L. Soares, L. Rabarijaona, B. Rakotoson, D. Malvy, and D. Ménard. 2006. Use of pre-packaged chloroquine for the home management of presumed malaria in Malagasy children. Malar. J. 5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 38.Sá, J. M., T. Nomura, J. Neves, J. K. Baird, T. E. Wellems, and H. A. del Portillo. 2005. Plasmodium vivax: allele variants of the mdr1 gene do not associate with chloroquine resistance among isolates from Brazil, Papua, and monkey-adapted strains. Exp. Parasitol. 109:256-259. [DOI] [PubMed] [Google Scholar]
- 39.Sá, J. M., M. M. Yamamoto, C. Fernandez-Becerra, M. F. de Azevedo, J. Papakrivos, B. Naude, T. E. Wellems, and H. A. Del Portillo. 2006. Expression and function of pvcrt-o, a Plasmodium vivax ortholog of pfcrt, in Plasmodium falciparum and Dictyostelium discoideum. Mol. Biochem. Parasitol. 150:219-228. [DOI] [PubMed] [Google Scholar]
- 40.Singh, N., S. S. Mishra, M. P. Singh, and V. P. Sharma. 2000. Seasonality of Plasmodium vivax and P. falciparum in tribal villages in central India (1987-1995). Ann. Trop. Med. Parasitol. 94:101-112. [DOI] [PubMed] [Google Scholar]
- 41.Soto, J., J. Toledo, P. Gutierrez, M. Luzz, N. Llinas, N. Cedeno, M. Dunne, and J. Berman. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 65:90-93. [DOI] [PubMed] [Google Scholar]
- 42.Sumawinata, I. W., Bernadeta, B. Leksana, A. Sutamihardja, Purnomo, B. Subianto, Sekartuti, D. J. Fryauff, and J. K. Baird. 2003. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am. J. Trop. Med. Hyg. 68:416-420. [PubMed] [Google Scholar]
- 43.Suwanarusk, R., B. Russell, M. Chavchich, F. Chalfein, E. Kenangalem, V. Kosaisavee, B. Prasetyorini, K. A. Piera, M. Barends, A. Brockman, U. Lek-Uthai, N. M. Anstey, E. Tjitra, F. Nosten, Q. Cheng, and R. N. Price. 2007. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS ONE 2:e1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tjitra, E., J. Baker, S. Suprianto, Q. Cheng, and N. M. Anstey. 2002. Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob. Agents Chemother. 46:3947-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Udomsangpetch, R., S. Somsri, T. Panichakul, K. Chotivanich, J. Sirichaisinthop, Z. Yang, L. Cui, and J. Sattabongkot. 2007. Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol. Int. 56:65-69. [DOI] [PubMed] [Google Scholar]
- 46.UNICEF. 2000. Multiple Indicator Cluster Survey (MICS) 2000, Madagascar, rapport complet. http://www.childinfo.org/files/madagascar.pdf.
- 47.Wellems, T. E., and C. V. Plowe. 2001. Chloroquine-resistant malaria. J. Infect. Dis. 184:770-776. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. 2001. Monitoring antimalarial drug resistance. Report of a WHO consultation, Geneva, December 2001. Document WHO/CDS/RBM 2002.39. WHO, Geneva, Switzerland.