Abstract
The recent emergence of group B streptococcal isolates exhibiting increased penicillin MICs at the Funabashi Municipal Medical Center and other hospitals in Japan prompted a comparative analysis of the penicillin-binding proteins (PBPs) from those strains with the PBPs from penicillin-susceptible strains comprising four neonatal invasive strains isolated from 1976 to 1988 and two recent isolates. The PBP sequences of the penicillin-susceptible strains were highly conserved, irrespective of their isolation date. Of six strains with reduced susceptibility to penicillin (penicillin MICs, 0.25 to 0.5 μg/ml), strains R1, R2, R5, and R6 shared a unique set of five amino acid substitutions, including V405A adjacent to the 402SSN404 motif in PBP 2X and one in PBP 2B. The remaining two strains, R3 and R4, shared several substitutions, including Q557E adjacent to the 552KSG554 motif in PBP 2X, in addition to the substitutions in PBP 2B, which are commonly found among penicillin-insusceptible strains. Strains R7 and R8, which had a penicillin MIC of 1 μg/ml, shared a unique set of eight amino acid substitutions (two in PBP 2X; two in PBP 2B, including G613R adjacent to the 614KTG616 motif; three in PBP 1A; and one in PBP 2A), and the Q557E substitution in PBP 2X was common to R3 and R4. The binding of Bocillin FL was reduced or not detected in some PBPs, including PBP 2X of penicillin-insusceptible strains, but no significant reduction in the level of pbp2x transcription was found in such strains. The results of phylogenetic comparative analyses imply the absence of epidemic penicillin-insusceptible strains, and several genetic lineages of penicillin-insusceptible strains have been independently emerging through the accumulation of mutations in their pbp genes, especially in pbp2x.
Group B streptococcus (GBS), which composes a part of the normal vaginal flora in 10 to 35% of healthy women, is one of the most important causes of neonatal sepsis and meningitis (29, 31). GBS also causes cutaneous and invasive infections in pregnant women and nonpregnant adults, including elderly individuals and immunocompromised patients (8, 32). Penicillin is the first-line antibiotic for GBS disease therapy as well as for intrapartum chemoprophylaxis. Resistance to this agent has so far not been reported among GBS isolates, while survey studies have shown a high prevalence of resistance to macrolides, followed by fluoroquinolones, among invasive and noninvasive isolates (3, 9, 14, 18, 24, 40). However, a trend toward an increase in the MICs of β-lactam antibiotics has occasionally been noted in recent reports (7, 16, 22), although no detailed analysis of the molecular mechanism associated with the loss of susceptibility had been conducted before the recently described studies (6, 19). This phenomenon elicits concern for the future prevalence of GBS strains with increased resistance to β-lactams, as has been noted for Streptococcus pneumoniae, in which penicillin-intermediate resistant pneumococcal strains were reported in the late 1960s (1, 13), followed by the reporting of more highly resistant strains (MICs, ≥2 μg/ml) whose drug resistance expanded not only to β-lactams but also to other antimicrobial agents in the late 1970s (2, 20).
Penicillin resistance in gram-positive organisms, including S. pneumoniae, is essentially due to the production of altered, low-affinity target enzymes, penicillin-binding proteins (PBPs) that catalyze the terminal stage of bacterial cell wall peptidoglycan synthesis. In PBPs, three conserved motifs, SXXK, SXN, and KT(S)G, commonly found in transpeptidase domains form the catalytic center; and alterations within or adjacent to these motifs are associated with their reduced affinity for β-lactams (10, 34). As for S. pneumoniae, in particular, substitutions in the amino acid residues of PBP 2B, PBP 2X, and PBP 1A, which are among the five high-molecular-weight PBPs, confer the development of β-lactam resistance in this organism; altered PBP 2B and PBP 2X enzymes confer low-level resistance to piperacillin and cefotaxime, respectively (12), and an additional alteration in PBP 1A confers high-level resistance to β-lactams (23, 33).
We recently encountered several GBS strains insusceptible to penicillin and other β-lactams at the Funabashi Municipal Medical Center and other hospitals in Japan. The emergence of resistance to those drugs in GBS would have significant clinical implications, which prompted us to seek to gain an in-depth understanding of the isolates with reduced susceptibility to penicillins. In a very recent work, Kimura et al. reported that altered PBP 2X makes a major contribution to the reduction of GBS susceptibility to β-lactams (19). The present study investigated the association between the amino acid substitutions found in high-molecular-weight PBPs, the affinities of PBPs to penicillins, and the levels of increased penicillin MICs for isolates with reduced susceptibilities to penicillins. Our analysis is unique in that it was supported by the inclusion of a group of penicillin-susceptible strains comprising four neonatal invasive strains isolated from 1976 to 1988 and two recently isolated strains for phylogenetic comparative analysis.
MATERIALS AND METHODS
Bacterial strains.
For PBP analysis, eight GBS clinical strains (strains R1 to R8) with penicillin MICs of 0.25 to 1 μg/ml recovered during 2003 and 2004 were selected from our bacterial culture collection. Included were penicillin-susceptible strains, four of which (strains N1 to N4) were from neonatal invasive infections recovered from 1976 to 1988, and two of which, strains C1 and C2, were recovered from vaginal and stool specimens obtained in 2004. The origins of these strains are presented in Table 1.
TABLE 1.
Origins and MICs of GBS isolatesa
| Strain | Strain or geographic origin in Japan | Ward(s) | Patient
|
Specimen | Date of isolation (mo/yr) | Serotype | MIC (μg/ml)
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex, age | Underlying disease(s) | Prior therapy | PEN | AMC | CTX | CTM | CFM | FEP | CZOP | CDN | MEM | CLR | ERY | CLI | LVX | TET | ||||||
| 2603 V/R | ATCC BAA-611 | V | 0.06 | 0.12 | ≤0.06 | ≤0.5 | 0.5 | ≤0.5 | 0.12 | ≤0.06 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | 0.5 | >4 | ||||||
| NEM 316 | ATCC 12403 | III | 0.06 | 0.12 | ≤0.06 | ≤0.5 | 0.5 | ≤0.5 | 0.12 | ≤0.06 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | 0.5 | ≤0.5 | ||||||
| N1 | Hokkaido (FMC collection) | Pediatrics | —, 50 days | Meningitis | None | CSF | 1976 | Ia | 0.06 | 0.12 | 0.12 | ≤0.5 | 0.5 | ≤0.5 | 0.12 | ≤0.06 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | 1 | >4 |
| N2 | Hokkaido (FMC collection) | Pediatrics | —, 90 days | Meningitis | None | CSF | 1988 | Ib | 0.06 | 0.25 | 0.12 | ≤0.5 | 1 | ≤0.5 | 0.12 | ≤0.06 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | 0.5 | ≤0.5 |
| N3 | FMC Funabashi, Chiba | Pediatrics | M, 90 days | Meningitis | None | CSF | 1983 | III | 0.06 | 0.12 | 0.12 | ≤0.5 | 0.5 | ≤0.5 | 0.12 | ≤0.06 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | 0.5 | >4 |
| N4 | Hokkaido (FMC collection) | Pediatrics | —, 1 day | Sepsis, pneumonia | None | BL | 1985 | VI | 0.06 | 0.12 | ≤0.06 | ≤0.5 | 0.5 | ≤0.5 | 0.12 | ≤0.06 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | 0.5 | >4 |
| C1 | FMC Funabashi, Chiba | Gynecology | F, 27 yr | None | None | VA | 03/2004 | V | 0.06 | 0.12 | ≤0.06 | ≤0.5 | 0.5 | ≤0.5 | 0.12 | ≤0.06 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | 0.5 | >4 |
| C2 | FMC Funabashi, Chiba | Neurosurgery | F, 75 yr | Diabetes mellitus | LVFX, CEZ | ST | 11/2004 | Ib | 0.06 | 0.25 | 0.12 | ≤0.5 | 0.5 | ≤0.5 | 0.12 | ≤0.06 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | >8 | ≤0.5 |
| R1 | FMC Funabashi, Chiba | Neurosurgery | M, 74 yr | Acute subdural hematoma, brain contusion | TOB, CAZ | TTA | 10/2003 | VI | 0.5 | 0.5 | 0.5 | 4 | >1 | 1 | 0.5 | 0.25 | 0.25 | ≤0.12 | ≤0.12 | ≤0.12 | 8 | >4 |
| R2 | FMC Funabashi, Chiba | Neurosurgery | M, 55 yr | Cerebral infarction | CEZ, GM CAZ | TTA | 11/2003 | VI | 0.5 | 0.5 | 0.5 | 4 | >1 | 1 | 1 | 0.25 | 0.5 | ≤0.12 | ≤0.12 | ≤0.12 | 8 | >4 |
| R3 | FMC Funabashi, Chiba | Urology, ophthalmology | M, 84 yr | Multiple cerebral infarction | None | CS | 03/2004 | Ib | 0.5 | 0.25 | 0.5 | 4 | >1 | ≤0.5 | 1 | 0.25 | 0.25 | ≤0.12 | ≤0.12 | ≤0.12 | >8 | ≤0.5 |
| R4 | Hospital A Choshi, Chiba | Internal medicine | F, 89 yr | — | — | SP | 12/2004 | VI | 0.5 | 0.25 | 0.5 | 4 | >1 | 1 | 0.5 | 0.25 | 0.25 | >2 | >2 | >1 | 2 | >4 |
| R5 | Hospital B Kodaira, Tokyo | Internal medicine | F, 96 yr | — | — | PHA | 12/2003 | VI | 0.25 | 0.5 | 0.5 | 2 | >1 | 1 | 0.5 | 0.25 | 0.25 | ≤0.12 | ≤0.12 | ≤0.12 | 4 | >4 |
| R6 | Hospital C Tachikawa, Tokyo | Internal medicine | F, adult | — | — | SP | 09/2004 | VI | 0.5 | 0.5 | 0.5 | 4 | >1 | 1 | 0.5 | 0.25 | 0.5 | ≤0.12 | ≤0.12 | ≤0.12 | 4 | >4 |
| R7 | Hospital D Ichinoseki, Iwate | Internal medicine | M, adult | — | — | SP | 09/2004 | Ia | 1 | 0.5 | 1 | >4 | >1 | 2 | 2 | 0.5 | 0.25 | 2 | >2 | ≤0.12 | >8 | >4 |
| R8 | Hospital D Ichinoseki, Iwate | Internal medicine | F, 80 yr | — | — | SP | 01/2004 | NT | 1 | 0.5 | 1 | >4 | >1 | 1 | 1 | 0.5 | 0.25 | 1 | >2 | ≤0.12 | >8 | >4 |
PEN, penicillin; AMC, ampicillin; CTX, cefotaxime; CTM, cefotiam; CFM, cefixime; FEP, cefepime; CZOP, cefozopran; CDN, cefditoren; LVFX, levofloxacin; MEM, meropenem; CLR, clarithromycin; ERY, erythromycin; CLI, clindamycin; TET, tetracycline; LVX, levofloxacin; CEZ, cefazolin; CAZ, ceftazidime; GM, gentamicin; FMC, Funabashi Medical Center; M, male; F, female; TTA, transtracheal aspirate; VA, vaginal discharge; CS, conjunctival sac discharge; ST, stool; PHA, pharyngeal swab; SP, sputum; CSF, cerebrospinal fluid; BL, blood; NT, nontypeable; —, unknown.
The GBS strains were grown in Todd-Hewitt broth (BBL Microbiology Systems, Cockeysville, MD) at 37°C.
The GBS clinical strains were serotyped with antisera (Denka Seiken, Tokyo, Japan) to the type-specific capsular polysaccharides Ia, Ib, II, III, IV, V, VI, VIII, and 7271. Strains 2603 V/R (ATCC BAA-611; GenBank accession number NC 004116) and NEM316 (ATCC 12403; GenBank accession number NC 004368) were used as reference strains for comparative characterization.
No β-lactamase activity was detected by the acidimetric method in any of the GBS strains subjected to this study.
Antimicrobial susceptibility testing.
MICs were determined by a broth microdilution method with a MicroScan MICroFAST panel type 3J system (Dade Behring Inc., Tokyo, Japan) by following the guidelines recommended by the Clinical and Laboratory Standards Institute (CLSI) (4, 5). MIC determinations were performed five times for each strain to ensure the reproducibility of the MICs by using quality control strain S. pneumoniae ATCC 49619.
Analysis of pbp gene sequences.
For all 14 clinical strains, five pbp genes encoding high-molecular-weight PBPs 1A, 1B, 2A, 2B, and 2X identified in the strain 2603 V/R genome available from the GenBank database were sequenced. Genomic DNAs were extracted from each strain with a Wizard genomic DNA purification kit (Promega, Madison, WI) and 20 U of mutanolysin (27). The entire coding regions of the pbp1a, pbp1b, pbp2a, pbp2b, and pbp2x genes were amplified with primer pairs f1 and r1, designed on the basis of the sequence of the corresponding gene of strain 2603 V/R, as listed in Table 2. PCRs were carried out with Pyrobest DNA polymerase (Takara Bio Inc., Shiga, Japan) and the following parameters: initial denaturation at 98°C for 1 min, denaturation at 98°C for 10 s, primer annealing at 55°C for 1 min, and extension at 72°C for 2.5 min, repeated for 30 cycles, and a final extension at 72°C for 7 min. The amplified DNA fragments were purified with a Wizard SV Gel and PCR cleanup system (Promega). For each pbp gene, several internal forward and reverse sequencing primers were designed on the basis of the published sequence of strain 2603 V/R (Table 2) and were used for the sequencing reactions to provide complete coverage of the sequences as well as to ensure the accuracy of the sequence data. Sequencing of both strands was performed with a BigDye Terminator (version 3.1) cycle sequencing kit (Applied Biosystems, Foster City, CA) and an ABI Prism model 3100 genetic analyzer (Applied Biosystems).
TABLE 2.
Oligonucleotide primers
| Procedure(s) and target gene | Primer name | Sequencea | Amplicon size (bp) |
|---|---|---|---|
| PCR and sequencing | |||
| pbp1a PCR | f1 | 5′-CGGAATTCATGGGATTTATTATCTTAGCTA-3′ | 2,209 |
| r1 | 5′-ACGTCGACTTAATTACCGTTAGGTACTGTA-3′ | ||
| pbp1a sequencing primers | f1b | 5′-ACACCAAAGAAGAAATTCTTAC-3′ | |
| f2 | 5′-TAAAGCAAAAATCTACTTATCC-3′ | ||
| f2b | 5′-GTAGTGAGAAAATGGCAGCGGC-3′ | ||
| f3 | 5′-GCCTACATGATGACGGATATGC-3′ | ||
| f3b | 5′-CAAAATTCTGGACAGTCAAGTC-3′ | ||
| r2 | 5′-TCCAATCTGCACTGTATCCGCC-3′ | ||
| r3 | 5′-TAGCTGCTTTAGTACCAGTACC-3′ | ||
| r4 | 5′-CAGCGGCTTCAAGTGCTCTGAC-3′ | ||
| r5 | 5′-TGACTTTACCATTAGTCGCATC-3′ | ||
| r6 | 5′-TTTTATCTTGATACATCTGCTG-3′ | ||
| pbp1b PCR | f1 | 5′-CGGAATTCATGTTTAAAGGTAATAAGAAGT-3′ | 2,314 |
| r1 | 5′-ACGTCGACTTATCGTTTTCCACCCAAAGTA-3′ | ||
| pbp1b sequencing primers | f1b | 5′-GGTTTGGAGAGAGTAGCGG-3′ | |
| f2 | 5′-CTATTGTATATTCTCCTTATAC-3′ | ||
| f2b | 5′-GTATACTATTAAAACTACTATC-3′ | ||
| f3 | 5′-TGATGTAAAAAACTATATGGAG-3′ | ||
| f3b | 5′-CCTGTCCGTGTCTTTTCGAAAG-3′ | ||
| r2 | 5′-GTGTAGAAAGCATCAACCAAAC-3′ | ||
| r3 | 5′-GAGCAACTGACGTATCAATACC-3′ | ||
| r4 | 5′-GATCAATAGCAATTCCGTAAGG-3′ | ||
| r5 | 5′-TTTTTAAATCATGCTCTGAAAC-3′ | ||
| r6 | 5′-GTAAACCTGCAAGGAAAGCTGC-3′ | ||
| pbp2a PCR | f1 | 5′-CGGGATCCATGAAATTATTTGATAAGTTTA-3′ | 2,338 |
| r1 | 5′-ACGTCGACCTATCTAAAGTAGTCCTTTAGA-3′ | ||
| pbp2a sequencing primers | f1b | 5′-TGCTCTAAAAACAACCACCACC-3′ | |
| f2 | 5′-ATCTTAATAACTCTTATTTTGG-3′ | ||
| f2b | 5′-GGTATGAAAAATAGATTAGCAG-3′ | ||
| f3 | 5′-TCCTGCTGTTTATACTTTAGAC-3′ | ||
| f3b | 5′-ACTCGAATTGAGACAGCTAATG-3′ | ||
| r2 | 5′-CTGTCAAATAATGGTGTTTATC-3′ | ||
| r3 | 5′-ATGAGCGCGATGCATTATACCG-3′ | ||
| r4 | 5′-GTTCTTTATCTATTGACCATCC-3′ | ||
| r5 | 5′-TATAGCCATTATTGACAATATC-3′ | ||
| r6 | 5′-TCAAATTAGCAGCACTGGTTCC-3′ | ||
| pbp2b PCR | f1 | 5′-CGGAATTCATGTTGAATCGTAAAAAAAGGT-3′ | 2,062 |
| r1 | 5′-ACGTCGACTTATTGTCCTGTGAACTGTGAA-3′ | ||
| pbp2b sequencing primers | f1b | 5′-TTCATCTCAGTCTATCAAAGAG-3′ | |
| f2 | 5′-CAACTCTAATGGAATCGTTCGG-3′ | ||
| f2b | 5′-CTATTTCTACAGAAAAGGCAGG-3′ | ||
| f3 | 5′-AGAAAGTATCTTGAAACAATAC-3′ | ||
| f3b | 5′-TGGACAAACAGTTTCTACCTAC-3′ | ||
| r2 | 5′-CTATCTTATTTAGTGTTTTAGG-3′ | ||
| r3 | 5′-GATAGCCTCGATCAGTTAAAGC-3′ | ||
| r4 | 5′-CATGATCATTTTTCAGACCAGC-3′ | ||
| r5 | 5′-CTCGGTCATTCAGTGAATAGCC-3′ | ||
| r6 | 5′-TAGCGCTCACTGGAACTGCAGC-3′ | ||
| pbp2x PCR | f1 | 5′-CGGAATTCGTGACTTTTTTTAAAAAGCTAA-3′ | 2,275 |
| r1 | 5′-ACGTCGACTTAATCTCCTATTGTAATTTTG-3′ | ||
| pbp2x sequencing primers | f1b | 5′-AACTATACGACAGCTACAGGTC-3′ | |
| f2 | 5′-GTAGTGGGAATGTTCTTTTAGG-3′ | ||
| f2b | 5′-TCTAAGCATTTTAACTCTACTG-3′ | ||
| f3 | 5′-AAGAAGCAGCTAGTAAAACACG-3′ | ||
| f3b | 5′-GAAAATCCAGGTCATGTAGCGG-3′ | ||
| r2 | 5′-GAACCAGATTACGACGTAATTC-3′ | ||
| r3 | 5′-CAGATTTTACTGCAACTGATTG-3′ | ||
| r4 | 5′-ATGAGCTCATAGCGATAGTTAC-3′ | ||
| r5 | 5′-TTGCAGAGGCTAGAGTCATTAC-3′ | ||
| r6 | 5′-CCGCCCTACGTTCTGTTGTTGC-3′ | ||
| r7 | 5′-AAGACAATCCTGAACCTGAACTTCC-3′ | ||
| r8 | 5′-TATCTGTACCAACGATGATGAC-3′ | ||
| Real-time RT-PCR | |||
| pbp2x | f1 | 5′-ACCGGGATTGAATGACTCAG-3′ | 109 |
| r1 | 5′-TGGCTGAACCAGATTACGAC-3′ | ||
| pros | f1 | 5′-AATGCCAAGTGATGCTCAGG-3′ | 84 |
| r1 | 5′-GCATAGATTCCAGCCGAAAC-3′ |
Restriction sites are underlined.
The sequences obtained were assembled into contigs with BioEdit (version 5.0.9) software (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Phylogenetic analyses for the sequences determined were conducted with the MEGA program (version 3.1) (21). The sequences were aligned with Clustal W software (37), and distance-based analyses were conducted by using Kimura's two-parameter model distance matrices at the nucleotide level. Phylogenetic trees were constructed by the neighbor-joining method in the MEGA program. A bootstrap analysis (500 repeats) was performed to evaluate the topology of the phylogenetic tree.
Membrane preparation and labeling of PBPs with fluorescent penicillin.
Membrane proteins were prepared as described by van Asselt et al. (39), except that the cells were disrupted by passage through a French press twice at a pressure of 120 MPa, and aliquots of the membrane proteins were stored at −80°C at a concentration of 10 mg/ml until use. The quantity of protein was determined with a BCA protein assay kit (bicinchoninic acid method; Pierce, Rockville, IL).
For detection of PBPs, 400 μg of the membrane proteins was mixed with 12.5 μM Bocillin FL (Molecular Probes, Inc., Eugene, OR), incubated at 37°C for 30 min, and denatured by adding 50 μl of 3× Laemmli sample buffer and heating to 100°C for 3 min. The labeled PBPs (40 μg protein/lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 5 or 10% gels and were visualized by fluorography with an LAS-3000 multicolor image analyzer (excitation at 460 nm; Fujifilm, Tokyo, Japan).
Immunodetection of PBP 2X.
To identify PBP 2X, immunoblotting was performed with a polyclonal antibody. Anti-PBP 2X was developed in rabbits by using as an immunogen a synthetic peptide (GKVTYEKDRSGNVL) corresponding to amino acids 237 to 250 of PBP 2X from S. agalactiae 2603 V/R conjugated to keyhole limpet hemocyanin via an N-terminal added cysteine residue (19).
The membrane proteins were separated by SDS-PAGE as described above and electroblotted onto a Hybond-P polyvinylidene difluoride membrane (GE Healthcare, Piscataway, NJ). The immobilized proteins were probed with the anti-PBP 2X antibody (1:1,000) and a goat anti-rabbit immunoglobulin G horseradish peroxidase antibody (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA). The immunoblots were detected by chemilumigraphy with an ECL Western blotting detection system (GE Healthcare) and an LAS-3000 multicolor image analyzer.
Real-time RT-PCR analysis of pbp2x expression.
The GBS strains were grown to an optical density at 600 nm of 0.3, and total RNA was isolated by using the RNAprotect bacterial reagent and an RNeasy mini kit (Qiagen GmbH, Hilden, Germany), as described previously (26), and the integrity was checked by agarose gel electrophoresis. The RNA was quantified spectrophotometrically and stored in aliquots at −70°C. DNase-treated RNA was reverse transcribed with a PrimeScript reverse transcription (RT) reagent kit (Takara), according to the instructions of the manufacturer, with 5 μM random 6-mers. Real-time PCR analysis was performed in triplicate on the SmartCycler system (Cepheid, Sunnyvale, CA) with 2 μl of cDNA (200 pg to 80 ng), SYBR Premix Ex Taq (Takara), and 0.2 μM each of the specific primers (Table 2), according to the manufacturer's protocol. The levels of the target transcripts were normalized to those of the internal reference gene, the gene for prolyl-tRNA synthetase (pros) (11), in each sample. The values for the pbp2x transcripts of strain 2603 V/R were normalized to 1, and other data were calculated relative to this value. For comparison between penicillin-insusceptible and -susceptible strains, statistical analysis of variance, followed by the unpaired Student t test, was performed. A P value of <0.05 was considered statistically significant.
Chromosomal DNA restriction profiles.
Molecular analysis of the chromosomal DNAs was performed as previously described by Nagano et al. (25), with some modifications. Briefly, chromosomal DNAs were extracted with mutanolysin in agarose gel plugs and were then incubated overnight at 30°C with 20 U of either SmaI (Takara) or ApaI (Takara). Bacteriophage lambda DNA ladders (48.5 kb to 1 Mb; Takara) were used as molecular size markers.
Epidemiological study.
Epidemiological analysis of the MIC distributions was performed with 442 clinical isolates collected from various regions in Japan between March 2005 and February 2006 by the Miroku Medical Laboratory.
Nucleotide sequence accession numbers.
The nucleotide sequences of the pbp genes from all the GBS strains tested in this study were deposited in the EMBL/GenBank through DDBJ under accession numbers AB368300 to AB368369.
RESULTS
MICs.
The MICs of several antimicrobial agents for S. pneumoniae ATCC 49619 were all within the quality control ranges defined by the CLSI (5), and reproducible MIC results were obtained for the GBS strains tested against all antimicrobials (Table 1). Strains N1 to N4, C1, C2, 2603 V/R, and NEM316 were all susceptible to β-lactams: the penicillin MICs were 0.06 μg/ml, the ampicillin MICs ranged from 0.12 to 0.25 μg/ml, the cefotaxime MICs ranged from ≤0.06 to 0.12 μg/ml, and the cefepime MICs were ≤0.5 μg/ml. Strains R1 to R6 were all insusceptible to penicillin (MICs, 0.25 to 0.5 μg/ml), whereas the ampicillin MICs were 0.25 μg/ml for R3 and R4 and 0.5 μg/ml (insusceptible) for R1, R2, R5, and R6. The cefepime MICs were ≤0.5 μg/ml for R3 and 1 μg/ml (insusceptible) for R1, R2, and R4 to R6. These six strains were susceptible to cefotaxime (MIC, 0.5 μg/ml), but this MIC was higher than the MICs for strains N1 to N4, C1, C2, 2603 V/R, and NEM316. Strains R7 and R8 were insusceptible to these β-lactams; the MICs of penicillin, ampicillin, cefotaxime, and cefepime were 1 μg/ml, 0.5 μg/ml, 1 μg/ml, and 1 to 2 μg/ml, respectively. Increased MICs of the other β-lactams, especially those of cefotiam and cefozopran, were also noted in strains R1 to R8. A trend toward higher MICs of levofloxacin was found for the recently isolated strains.
Nucleotide and deduced amino acid sequences of pbp genes.
DNA sequencing of the pbp genes revealed that the pbp2x and pbp2b genes of penicillin-insusceptible strains possessed many nucleotide mutations compared with the nucleotide sequences of the corresponding genes of strains 2603 V/R and NEM316. Additionally, many nucleotide mutations were detected in the pbp1a genes, especially those from strains R7 and R8. Among the penicillin-susceptible strains, only strain N3 carried many nucleotide mutations, and these were especially detected in the pbp1a gene. From the viewpoint of amino acid substitutions, many of the nucleotide mutations detected in pbp2a were silent (Table 3).
TABLE 3.
Nucleotide mutations in pbp genesa
| Strain |
pbp2x
|
pbp2b
|
pbp1a
|
pbp2a
|
pbp1b
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Ns | Total | Ns | Total | Ns | Total | Ns | Total | Ns | |
| N1 | 0 | 0 | 1 | 1 | 0 | 0 | 10 | 1 | 3 | 1 |
| N2 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1 | 3 | 1 |
| N3 | 2 | 1 | 4 | 1 | 7 | 2 | 13 | 3 | 0 | 0 |
| N4 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1 | 3 | 1 |
| C1 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1 | 3 | 1 |
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1 | 3 | 1 |
| R1 | 11 | 5 | 1 | 1 | 0 | 0 | 10 | 1 | 3 | 1 |
| R2 | 11 | 5 | 1 | 1 | 0 | 0 | 10 | 1 | 3 | 1 |
| R3 | 3 | 2 | 2 | 2 | 1 | 0 | 2 | 0 | 3 | 1 |
| R4 | 2 | 1 | 2 | 2 | 0 | 0 | 3 | 1 | 3 | 1 |
| R5 | 10 | 5 | 1 | 1 | 0 | 0 | 10 | 1 | 3 | 1 |
| R6 | 11 | 6 | 1 | 1 | 0 | 0 | 11 | 2 | 3 | 1 |
| R7 | 9 | 4 | 6 | 2 | 12 | 4 | 1 | 1 | 0 | 0 |
| R8 | 9 | 4 | 6 | 2 | 12 | 4 | 1 | 1 | 0 | 0 |
Total, total number of nucleotide substitutions compared to the sequences od S. agalactiae 2603 V/R and NEM316; Ns, number of nonsynonymous substitutions.
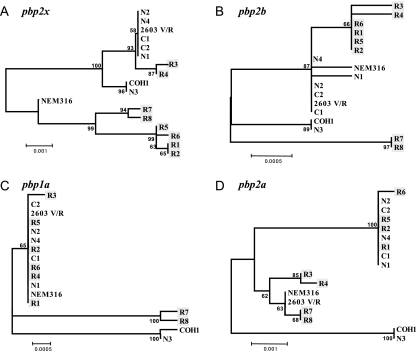
A phylogenic tree constructed with the entire pbp sequences showed that the pbp2x and pbp2b genes of three groups of strains (strains R1, R2, R5, and R6; strains R3 and R4; and strains R7 and R8) and penicillin-susceptible strains N1, N2, N4, C1, C2, and 2603 V/R formed distinct genetic lineages. In particular, the pbp2b as well as the pbp1a genes of strains R7 and R8 (MIC, 1 μg/ml) formed a lineage distinct from the major lineage that included the other strains. The pbp2x, pbp2b, pbp1a, and pbp2a genes of penicillin-susceptible strain N3 belonged to a distinct lineage (Fig. 1). The phylogeny of the pbp1b gene showed no notable relationship with penicillin susceptibility (data not shown).
FIG. 1.
Nucleotide phylogram of pbp genes from GBS strains and S. agalactiae strains 2603 V/R, NEM316, and COH1. Penicillin-insusceptible strains R1 to R8 are shaded. The nucleotide sequences were aligned by using the Clustal W program and were subjected to phylogenetic analysis by the neighbor-joining method based on Kimura's two-parameter model distance matrices with the MEGA program (version 3.1). The resulting trees were bootstrapped 500 times, and the bootstrap values are shown as percentages. The scale bars indicate the expected number of changes per sequence position.
Amino acid substitutions in PBP 2X, PBP 2B, and PBP 1A.
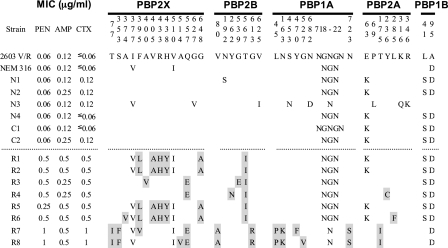
Figure 2 shows the amino acid substitutions in PBP 2X, PBP 2B, PBP 1A, PBP 2A, and PBP 1B identified in comparison with the published sequences, as described in the Materials and Methods. Except for strains N1 and N3, no amino acid substitutions were found in PBP 2X, PBP 2B, or PBP 1A of the penicillin-susceptible strains, irrespective of the dates of their isolation except. Strains N1 and N3 possessed one and four substitutions, respectively. The amino acid substitutions found in strains N1 and N3 were not found among the strains with reduced penicillin susceptibility. Of the six penicillin-insusceptible strains for which the penicillin MICs were 0.25 to 0.5 μg/ml, strains R1, R2, R5, and R6 shared a unique set of five substitutions, F395L, V405A, R433H, H438Y, and G648A in PBP 2X and a T567I substitution in PBP 2B. The first four substitutions were located in the transpeptidase domain of PBP 2X, and among these, F395L and V405A were at positions close to the 402SSN404 motif. Only strain R6 had an additional substitution, A374V, in PBP 2X. The remaining strains, strains R3 and R4, shared Q557E, which is in proximity to the 552KSG554 motif of PBP 2X, in addition to the T567I substitution in PBP 2B, which was commonly found among the six penicillin-insusceptible strains. Other substitutions detected were A400V, close to the 402SSN404 motif of PBP 2X, and G539E in PBP 2B for R3 and Y262N in PBP 2B for R4. Among those six strains, no amino acid substitutions were detected in PBP 1A. A total of eight substitutions were shared by two penicillin-insusceptible strains, strains R7 and R8, for which the penicillin MICs were 1 μg/ml. Among those substitutions, seven substitutions (T77I and S353F in PBP 2X; V80A and G613R in PBP 2B; and L45P, N163K, and N723S in PBP 1A) were unique to those strains. The S353F and G613R substitutions were in proximity to the 344STMK347 motif of PBP 2X and the 614KTG616 motif of PBP 2B, respectively. Another substitution in PBP 2X, Q557E, was common to strains R3 and R4. There were two additional substitutions in each of two strains: F395V in PBP 2X and Y470F in PBP 1A for strain R7 and A514V in PBP 2X and G527V in PBP 1A for strain R8.
FIG. 2.
Deduced amino acid substitutions in five high-molecular-mass PBPs from GBS isolates. Residues mutated in PBPs from penicillin-insusceptible strains are shaded. Strain 2603 V/R is ATCC BAA-611, and strain NEM316 is ATCC 12403. PEN, penicillin; AMP, ampicillin; CTX, cefotaxime.
Furthermore, the sequence data for PBP 1A revealed that five amino acid residues (residues 718 to 722) consisting of NGNGN in strain 2603 V/R differed among NEM316 and the other strains tested, regardless of their penicillin susceptibility status.
Amino acid substitutions in PBP 2A and PBP 1B.
In PBP 2A, an E63K substitution was observed in four of eight clinical strains with reduced penicillin susceptibilities as well as in all penicillin-susceptible strains except strain N3, which had three other substitutions. One substitution each was found in strain R4 (Y236C) and strain R6 (L285F). Strains R7 and R8 shared the T175I substitution. Sequencing data for PBP 1B showed that an L41S substitution was shared by six of eight clinical strains with reduced penicillin susceptibilities and by all penicillin-susceptible strains except strain N3 (Fig. 2).
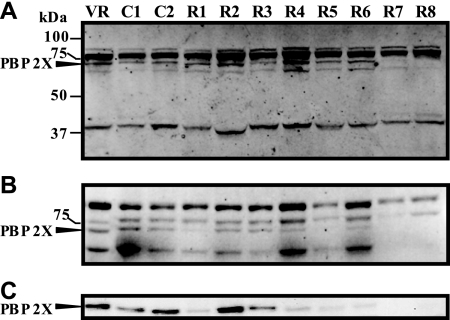
Detection of PBPs and immunodetection of PBP 2X.
Figure 3A shows the PBP profiles detected by the Bocillin FL-binding assays. Five bands with molecular masses of 84, 77, 72, and 66 kDa (high-molecular-mass PBPs) and 42 kDa (low-molecular-mass PBP) were detected in strain 2603 V/R, in which strong signals were produced from the 77-kDa band, which comprised overlapping bands of two PBPs on 10% SDS-PAGE and which thus indicates that six bands were detected by the Bocillin FL-binding assays. The PBP profiles of the penicillin-insusceptible strains were similar to those of susceptible strains of 2603 V/R, C1, and C2, except for the lowering of the intensities of some bands among the high-molecular-mass PBPs for strains R1 to R8 in an analysis on a 5% SDS-PAGE gel, on which the highest-molecular-mass band consisted of two overlapping PBPs (Fig. 3B). Thus, the intensities of the second-highest-molecular-mass band were decreased 60% and 30% in strains R7 and R8, respectively. The intensity of the third band (molecular mass, 72 kDa) was decreased 30 to 60% in strains R1 to R6 or the band was not recognized in strains R7 and R8. The intensity of the fourth band (molecular mass, 66 kDa) was decreased 40 to 60% in strains R1 to R3 but was not recognized in strains R7 and R8. As shown in Fig. 3C, a positive reaction was obtained with the 72-kDa band by immunoblotting of the membrane proteins of strains probed with anti-PBP 2X antibodies recognizing a conserved 14-residue epitope. However, the amount of the antibody bound to the 72-kDa band was not equivalent among strains. Separately from the six PBPs, unstable Bocillin FL binding to a 45-kDa band was observed among all strains tested (data not shown).
FIG. 3.
Binding of penicillin to GBS PBPs and immunodetection of PBP 2X. Membrane proteins were incubated with Bocillin FL, separated on a 10% (A) or a 5% (B) SDS-polyacrylamide gel, and detected by fluorography, as described in Materials and Methods. The molecular sizes of the protein standards (Precision Plus; Bio-Rad Laboratories, Hercules, CA) are provided on the left. (C) Membrane proteins separated on a 5% SDS-polyacrylamide gel were subjected to Western blotting with anti-PBP 2X antibody as the primary antibody, as described in Materials and Methods. Arrows indicate the position of PBP 2X. VR, S. agalactiae 2603 V/R.
pbp2x gene expression.
Expression of the pbp2x gene was quantitatively assessed by real-time RT-PCR. The relative amounts of pbp2x transcripts in penicillin-susceptible strains 2603 V/R, C1, and C2 ranged from 0.79 to 1.00, with a mean ± standard deviation of 0.88 ± 0.11, while those in penicillin-insusceptible strains R1 to R8 ranged from 0.74 to 1.32, with a mean ± standard deviation of 1.03 ± 0.23. No statistically significant differences in relative transcript levels were detected between these two groups (data not shown).
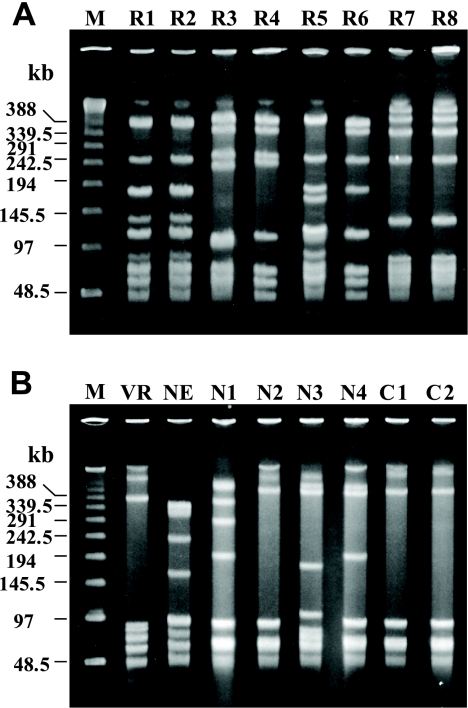
PFGE analysis.
Analysis of SmaI-digested genomic DNA revealed six distinguishable patterns among all eight penicillin-insusceptible strains (Fig. 4A). Strains R1 and R2, which were serotype VI and which were detected from cultures of transtracheal aspirates from different inpatients in the neurosurgery ward of the Funabashi Municipal Medical Center over a 1-month interval, shared a pulsed-field gel electrophoresis (PFGE) profile. Strain R7, which was of serotype Ia, and nontypeable strain R8 shared another PFGE profile. Of note, these two strains originated from different inpatients in the same hospital (hospital D) 8 months apart. These findings were in agreement with the ApaI profiles (data not shown). Penicillin-susceptible strains did not share any PFGE profiles with penicillin-insusceptible strains (Fig. 4B). The PFGE profiles of strains C1 and C2, which had different serotypes, were largely similar by PFGE with SmaI but differed by more than 10 distinguishable bands by PFGE with ApaI (data not shown).
FIG. 4.
PFGE profiles of genomic DNA of penicillin-insusceptible (A) and -susceptible (B) GBS isolates digested with SmaI. Lanes M, bacteriophage lambda DNA ladder as molecular size markers; VR, S. agalactiae 2603 V/R; NE, S. agalactiae NEM316.
Epidemiology.
The distributions of the MICs of representative β-lactams for 442 recent clinical isolates are shown in Table 4. There was a unimodal distribution for each β-lactam, with an elevated MIC outside the specified susceptible range (4, 5). Reduced susceptibilities to penicillin (MICs, >0.12 μg/ml), ampicillin (MICs, >0.25 μg/ml), and cefepime (MICs, >0.5 μg/ml) were demonstrated for 2.3%, 2.3%, and 0.9% of the isolates, respectively.
TABLE 4.
Distribution of MICs of β-lactams against GBS clinical isolatesa
| Antimicrobial agent | No. (%) of strains with MIC (μg/ml) of:
|
||||||
|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | |
| Penicillin | 34 (7.7) | 280 (63.3) | 118 (26.7) | 7 (1.6) | 3 (0.7) | ||
| Ampicillin | 52b (11.8) | 313 (70.8) | 67 (15.1) | 10 (2.3) | |||
| Cefotaxime | 301b (68.1) | 114 (25.8) | 16 (3.6) | 11 (2.5) | |||
| Cefepime | 438b (99.1) | 3 (0.7) | 1 (0.2) | ||||
| Cefozopran | 35b (7.9) | 316 (71.5) | 72 (16.3) | 17 (3.8) | 2 (0.5) | ||
| Cefditoren | 399b (90.3) | 25 (5.7) | 11 (2.5) | 6 (1.3) | 1 (0.2) | ||
| Meropenemc | 365b (94.6) | 19 (4.9) | 2 (0.5) | ||||
A total of 442 isolates were tested.
Includes isolates with MICs less than the given value.
The number of isolates tested was 386.
DISCUSSION
High rates of tetracycline resistance are noted in GBS, and increasing incidences of macrolide resistance as well as the emergence of fluoroquinolone resistance have been reported in recent years (3, 9, 14, 18, 24, 40). Although this study did not focus on quinolone-resistant strains, a trend toward increasing fluoroquinolone MICs has clearly been noted among recently emerged strains. However, confirmed resistance to β-lactams, including penicillin, has not been recognized among GBS isolates, although strains with reduced susceptibilities have been described in several reports (7, 16, 22). This is exemplified by the fact that the CLSI lists interpretive criteria for susceptibility for strains of beta-hemolytic streptococci, that is, MICs of ≤0.12 μg/ml, ≤0.25 μg/ml, and ≤0.5 μg/ml, only for penicillin, ampicillin, and cephems (cefotaxime, cefepime, and ceftriaxone) and meropenem, respectively; and it comments that strains with MICs greater than those breakpoints have not been observed (5). Very recently, Kimura et al., on the basis of molecular-based analyses, first described the alterations in high-molecular-mass PBPs in GBS clinical isolates exhibiting increased MICs of β-lactams and the major role of altered PBP 2X in reducing their susceptibilities to these agents (19). In the present study, we investigated the amino acid sequences of five high-molecular-mass PBPs among clinical isolates with reduced susceptibilities to penicillin recovered during 2003 and 2004 in five geographically separate hospitals. The analysis included penicillin-susceptible strains comprising four strains from neonates with systemic infections isolated from 1976 to 1988 and two recently isolated strains. Thus, this study allowed us to verify whether or not the amino acid substitutions in PBPs reported previously (19) were common to our penicillin-insusceptible isolates and to investigate whether or not alterations in PBPs have accumulated over time, as well as whether such alterations could mediate the insusceptibilities to penicillins.
The deduced amino acid sequences of PBP 2X, PBP 2B, PBP 1A, and PBP 2A were highly conserved among six penicillin-susceptible strains, although strains N1 and N3 possessed one and seven substitutions, respectively. Because these amino acid substitutions were not found among the penicillin-insusceptible strains tested, they are probably unrelated to penicillin resistance. Of the six penicillin-insusceptible strains for which the penicillin MICs were 0.25 to 0.5 μg/ml, strains R1, R2, R5, and R6 shared a unique set of five amino acid substitutions, including V405A in PBP 2X and one in PBP 2B, that have been identified in some GBS isolates with reduced penicillin susceptibilities (19). The remaining two strains, strains R3 and R4, shared the Q557E substitution in PBP 2X, in addition to the T567I substitution in PBP 2B, which was commonly found among the six penicillin-insusceptible strains. Strains R7 and R8, for which the penicillin MICs were 1 μg/ml, shared a unique set of eight amino acid substitutions (two each in PBP 2X and PBP 2B, three in PBP 1A, and one in PBP 2A), in addition to the Q557E substitution in PBP 2X that was common to strains R3 and R4. Thus, our study confirmed previous findings about PBP alterations (19), and some other amino acid substitutions were also suggested to be involved in the elevation in the rate of penicillin insusceptibility. Our findings did not include amino acid substitutions within conserved motifs. However, the Q557E substitution, which was in proximity to the 552KSG554 motif of PBP 2X, corresponds to the Q552E substitution in S. pneumoniae that has been reported to be responsible for the most of the reduction in susceptibility to β-lactams (28). The significance of each amino acid substitution located adjacent to conserved motifs, such as S353F, F395L, and A400V in PBP 2X and G613R in PBP 2B, needs to be assessed in future studies. Moreover, it will be necessary to look into additional possibilities that other substitutions located in the transpeptidase domain might also contribute to penicillin insusceptibility by providing compensatory structural and functional alterations in PBPs.
The amino acid substitutions found in PBPs imply that during evolution three groups of strains (strains R1, R2, R5, and R6; strains R3 and R4; and strains R7 and R8) each accumulated different genetic mutations for the acquisition of penicillin insusceptibility, and this finding is also suggested by the phylogram. Strains R1 and R2 had indistinguishable PFGE profiles as well as completely identical nucleotide sequences in their pbp genes; hence, their nosocomial transmission was strongly suspected. Strains R7 and R8 were isolated from different patients in the same hospital over an 8-month interval and shared the same PFGE profile. However, these two strains each had one different substitution in PBP 2X and PBP 1A and also shared many substitutions, which may lead us to speculate that they are derivatives of a strain that independently accumulated mutations, which allowed them to have common substitutions. These penicillin-insusceptible strains may possibly survive stably and could be further transmitted among patients in a clinical environment. The nucleotide sequences of the PBP 2X, PBP 2B, and PBP 1A genes of five of the six penicillin-susceptible strains, the exception being strain N3, were highly homologous with those of two penicillin-susceptible reference strains, irrespective of the dates of their isolation, and mostly fell into the same phylogenetic groups. In contrast, strain N3, which carried altered pbp2x, pbp2b, pbp1a, and pbp2a genes, formed an independent evolutionary lineage in the phylogram. In previous studies, it was demonstrated that this strain fell into a certain genotype among serotype III GBS strains which had been found at a high frequency in severe neonatal infections and was suggested to be a more virulent genotype than the other strains included in this serotype (35, 36). Interestingly, the nucleotide sequences of the pbp genes of strains N3 were almost 100% identical to the homologous genes of Sreptococcus agalactiae COH1 (GenBank accession number AAJR01000000), a highly virulent serotype III wild-type strain recovered from a neonatal patient with sepsis. Thus, they formed a distinct genetic lineage (Fig. 1). Few studies have addressed the role of PBPs in the pathogenicity of GBS. Jones et al. found that the ponA gene, which encodes extracytoplasmic PBP 1A, promoted resistance to phagocytic killing independently of the capsular polysaccharide (17). Our results are of interest from the standpoint of the analysis of a possible association between PBPs and pathogenicity in this organism.
Six Bocillin FL-labeled PBPs with estimated molecular masses of 84, 77 (which comprised overlapping bands of two PBPs), 72, 66, and 42 kDa on a 10% SDS-polyacrylamide gel were detected in strain 2603 V/R as well as in penicillin-susceptible strains, which is somewhat similar to PBP patterns with molecular masses of 86 (which comprised overlapping bands of two PBPs), 80, 76, 70, and 42 kDa identified in a GBS strain with 3H-labeled penicillin (15), while four PBPs with molecular masses of 101, 90, 82, and 66 kDa have been visualized in a GBS strains by Bocillin FL labeling (17). Among the penicillin-insusceptible strains, the decrease or the absence of intensities for some bands was noted among high-molecular-mass PBPs. A correlation was found between the decrease in the levels of the band intensities and the increase in the penicillin MICs, so the most likely explanation for the phenomenon is that altered PBPs may reduce their affinities for Bocillin FL. However, unexpectedly, the amount of the anti-PBP 2X antibody bound to the 72-kDa PBP was not equivalent among strains but, rather, paralleled the band intensity of Bocillin FL-labeled PBP 2X. If the amount of antibody bound to PBP 2X reflects the amount of PBP 2X, then the possibility that an altered PBP 2X or that the intrinsic instability of an altered PBP 2X results in a reduction in the levels of expression cannot be excluded. However, the relative levels of the pbp2x transcript between penicillin-insusceptible and -susceptible strains were statistically indistinguishable by Student's t test analysis, indicating that such a decrease in the signal intensity of Bocillin FL for altered PBP 2X cannot be accounted for by a reduction in the level of expression. Complete understanding would require examination of whether or not the stability is affected in the altered PBP 2X, then this study should be expanded to PBP 2B, PBP 1A, and PBP 2A, although no antibodies for these molecules are presently available.
Neonatal GBS meningitis is a life-threatening infection, and even if the treatment is started in the early stage, it has a poor prognosis, in that many cases may develop severe neurological sequelae. Rapid and accurate clinical diagnosis is therefore crucial, and most clinical laboratory efforts have focused on the prompt identification of the causative agent by performing a rapid antigen detection test with cerebrospinal fluid specimens as well as Gram staining. Once the test result is positive for the GBS antigen, specific therapy is initiated with penicillin or ampicillin plus gentamicin (30, 38), because GBS is considered to be uniformly susceptible to these drugs. In the present study, no penicillin insusceptibility was detected among invasive GBS isolates. In addition, the clinical significance of the in vitro penicillin insusceptibility of invasive isolates in antimicrobial chemotherapy and also in intrapartum prophylaxis remains unclear. However, our epidemiological findings revealed a unimodal MIC distribution for β-lactams, including penicillin, with the MIC elevated above the specified susceptible range. Hence, the emergence of such strains with reduced penicillin susceptibility should trigger awareness of the appropriate therapeutic strategy for dealing with severe GBS infections and the strategy for intrapartum prophylaxis for GBS carriers.
Acknowledgments
This work was supported by a grant (H18-Shinkou-011) from the Ministry of Health, Labor and Welfare of Japan.
We are grateful to Kunikazu Yamane, Jun-ichi Wachino, and Naohiro Shibata for technical advice.
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis. 15:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C., A. Bhamjee, J. N. Scragg, A. F. Hallett, A. J. Bowen, and R. C. Cooper. 1977. Streptococcus pneumoniae resistant to penicillin and chloramphenicol. Lancet ii:995-997. [DOI] [PubMed] [Google Scholar]
- 3.Biedenbach, D. J., J. M. Stephen, and R. N. Jones. 2003. Antimicrobial susceptibility profile among beta-haemolytic Streptococcus spp. collected in the SENTRY Antimicrobial Surveillance Program—North America, 2001. Diagn. Microbiol. Infect. Dis. 46:291-294. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6, 6th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing. Supplement M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Dahesh, S., M. E. Hensler, N. M. Van Sorge, R. E. Gertz, Jr., S. Schrag, V. Nizet, and B. W. Beall. 2008. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to β-lactam antibiotics. Antimicrob. Agents Chemother. 52:2915-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Azavedo, J. C., M. McGavin, C. Duncan, D. E. Low, and A. McGeer. 2001. Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B streptococcus isolates from Ontario, Canada. Antimicrob. Agents Chemother. 45:3504-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farley, M. M., R. C. Harvey, T. Stull, J. D. Smith, A. Schuchat, J. D. Wenger, and D. S. Stephens. 1993. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N. Engl. J. Med. 328:1807-1811. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez, M., M. E. Hickman, and C. J. Baker. 1998. Antimicrobial susceptibilities of group B streptococci isolated between 1992 and 1996 from patients with bacteremia or meningitis. Antimicrob. Agents Chemother. 42:1517-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goffin, C., and J. M. Ghuysen. 1998. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 62:1079-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham, M. R., L. M. Smoot, C. A. Migliaccio, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, M. J. Federle, G. J. Adams, J. R. Scott, and J. M. Musser. 2002. Virulence control in group A streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 99:13855-13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grebe, T., and R. Hakenbeck. 1996. Penicillin-binding proteins 2B and 2X of Streptococcus pneumoniae are primary resistance determinants for different classes of beta-lactam antibiotics. Antimicrob. Agents Chemother. 40:829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansman, D., and M. M. Bullen. 1967. A resistant pneumococcus. Lancet ii:264-265. [DOI] [PubMed] [Google Scholar]
- 14.Heelan, J. S., M. E. Hasenbein, and A. J. McAdam. 2004. Resistance of group B streptococcus to selected antibiotics, including erythromycin and clindamycin. J. Clin. Microbiol. 42:1263-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horne, D., and A. Tomasz. 1981. pH-dependent penicillin tolerance of group B streptococci. Antimicrob. Agents Chemother. 20:128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsueh, P.-R., L.-J. Teng, L.-N. Lee, S.-W. Ho, P.-C. Yang, and K.-T. Luh. 2001. High incidence of erythromycin resistance among clinical isolates of Streptococcus agalactiae in Taiwan. Antimicrob. Agents Chemother. 45:3205-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, A. L., R. H. Needham, A. Clancy, K. M. Knol, and C. E. Rubens. 2003. Penicillin-binding proteins in Streptococcus agalactiae: a novel mechanism for evasion of immune clearance. Mol. Microbiol. 47:247-256. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura, Y., H. Fujiwara, N. Mishima, Y. Tanaka, A. Tanimoto, S. Ikawa, Y. Itoh, and T. Ezaki. 2003. First Streptococcus agalactiae isolates highly resistant to quinolones with point mutations in gyrA and parC. Antimicrob. Agents Chemother. 47:3605-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura, K., S. Suzuki, J. Wachino, H. Kurokawa, K. Yamane, N. Shibata, N. Nagano, H. Kato, K. Shibayama, and Y. Arakawa. 2008. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob. Agents Chemother. 52:2890-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klugman, K. P. 1990. Pneumococcal resistance to antibiotics. Clin. Microbiol. Rev. 3:171-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa, Y., M. Kitazato, C. Katsukawa, and A. Tamaru. 2003. Prevalence of cefotaxime resistance in group B streptococcus isolates from Osaka, Japan. J. Infect. Chemother. 9:131-133. [DOI] [PubMed] [Google Scholar]
- 23.Munoz, R., C. G. Dowson, M. Daniels, T. J. Coffey, C. Martin, R. Hakenbeck, and B. G. Spratt. 1992. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 6:2461-2465. [DOI] [PubMed] [Google Scholar]
- 24.Murdoch, D. R., and L. B. Reller. 2001. Antimicrobial susceptibilities of group B streptococci isolated from patients with invasive disease: 10 year perspective. Antimicrob. Agents Chemother. 45:3623-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagano, N., N. Shibata, Y. Saitou, Y. Nagano, and Y. Arakawa. 2003. Nosocomial outbreak of infections by Proteus mirabilis that produces extended-spectrum CTX-M-2 type β-lactamase. J. Clin. Microbiol. 41:5530-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagano, N., Y. Nagano, and F. Taguchi. 2002. High expression of a C protein β antigen gene among invasive strains from certain clonally related groups of type Ia and Ib group B streptococci. Infect. Immun. 70:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagano, N., Y. Nagano, R. Nakano, R. Okamoto, and M. Inoue. 2006. Genetic diversity of the C protein β-antigen gene and its upstream regions within clonally related groups of type Ia and Ib group B streptococci. Microbiology 152:771-778. [DOI] [PubMed] [Google Scholar]
- 28.Pernot, L., L. Chesnel, A. Le Gouellec, J. Croize, T. Vernet, O. Dideberg, and A. Dessen. 2004. A PBP2x from a clinical isolate of Streptococcus pneumoniae exhibits an alternative mechanism for reduction of susceptibility to beta-lactam antibiotics. J. Biol. Chem. 279:16463-16470. [DOI] [PubMed] [Google Scholar]
- 29.Regan, J. A., M. A. Klebanoff, R. P. Nugent, et al. 1991. The epidemiology of group B streptococcal colonization in pregnancy. Obstet. Gynecol. 77:604-610. [PubMed] [Google Scholar]
- 30.Saez-Llorens, X., and G. H. McCracken, Jr. 2003. Bacterial meningitis in children. Lancet 361:2139-2148. [DOI] [PubMed] [Google Scholar]
- 31.Schuchat, A. 1998. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin. Microbiol. Rev. 11:497-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuchat, A. 1999. Group B streptococcus. Lancet 353:51-56. [DOI] [PubMed] [Google Scholar]
- 33.Smith, A. M., and K. P. Klugman. 1998. Alterations in PBP 1A essential for high-level penicillin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spratt, B. G., and K. D. Cromie. 1988. Penicillin-binding proteins of gram-negative bacteria. Rev. Infect. Dis. 10:699-711. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi, S., E. E. Adderson, Y. Nagano, N. Nagano, M. R. Briesacher, and J. F. Bohnsack. 1998. Identification of a highly encapsulated, genetically related group of invasive type III group B streptococci. J. Infect. Dis. 177:1116-1119. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi, S., Y. Nagano, N. Nagano, O. Hayashi, F. Taguchi, and Y. Okuwaki. 1995. Role of C5a-ase in group B streptococcal resistance to opsonophagocytic killing. Infect. Immun. 63:4764-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunkel, A. R., B. J. Hartman, S. L. Kaplan, B. A. Kaufman, K. L. Roos, W. M. Scheld, and R. J. Whitley. 2004. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39:1267-1284. [DOI] [PubMed] [Google Scholar]
- 39.van Asselt, G. J., G. de Kort, and J. A. M. van de Klundert. 1995. Differences in penicillin-binding protein patterns of penicillin tolerant and nontolerant group A streptococci. J. Antimicrob. Chemother. 35:67-74. [DOI] [PubMed] [Google Scholar]
- 40.Wehbeh, W., R. Rojas-Diaz, X. Li, N. Mariano, L. Grenner, S. Segal-Maurer, B. Tommasulo, K. Drlica, C. Urban, and J. J. Rahal. 2005. Fluoroquinolone-resistant Streptococcus agalactiae: epidemiology and mechanism of resistance. Antimicrob. Agents Chemother. 49:2495-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]