Abstract
Raltegravir is a novel human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor with potent in vitro activity against HIV-1 (95% inhibitory concentration = 31 nM in 50% human serum). The possible effects of ritonavir and efavirenz on raltegravir pharmacokinetics were separately examined. Two clinical studies of healthy subjects were conducted: for ritonavir plus raltegravir, period 1, 400 mg raltegravir; period 2, 100 mg ritonavir every 12 h for 16 days with 400 mg raltegravir on day 14; for efavirenz plus raltegravir, period 1, 400 mg raltegravir; period 2, 600 mg efavirenz once daily for 14 days with 400 mg raltegravir on day 12. In the presence of ritonavir, raltegravir pharmacokinetics were weakly affected: the plasma concentration at 12 h (C12 h) geometric mean ratio (GMR) (90% confidence interval [CI]) was 0.99 (0.70, 1.40), area under the concentration-time curve from zero to infinity (AUC0-∞) was 0.84 (0.70, 1.01), and maximum concentration of drug in serum (Cmax) was 0.76 (0.55, 1.04). In the presence of efavirenz, raltegravir pharmacokinetics were moderately to weakly reduced: C12 h GMR (90% CI) was 0.79 (0.49, 1.28); AUC0-∞ was 0.64 (0.52, 0.80); and Cmax was 0.64 (0.41, 0.98). There were no substantial differences in the time to maximum concentration of drug in plasma or the half-life. Plasma concentrations of raltegravir were not substantially affected by ritonavir. Though plasma concentrations of raltegravir were moderately to weakly reduced by efavirenz, the degree of this reduction was not clinically meaningful. No dose adjustment is required for raltegravir with coadministration with ritonavir or efavirenz.
There are several issues associated with many of the currently available therapies for human immunodeficiency virus (HIV) that can lead to discontinuation of therapy and treatment failure. Drug-drug interactions are particularly problematic among many of the marketed antiretroviral agents and often result in complications in the administration of appropriate antiretroviral cocktails and other supportive medications. Thus, the investigation of potential drug-drug interactions is of paramount importance. Virtually all of the nonnucleoside reverse transcriptase inhibitors and protease inhibitors are known to affect the metabolism of coadministered agents (2). Two commonly prescribed agents, ritonavir and efavirenz, are among these.
Ritonavir is a potent inhibitor of cytochrome 3A (CYP3A) and CYP2D6 and also appears to induce CYP3A and other drug metabolizing enzymes, including glucuronosyl transferases (7). Similar to ritonavir, efavirenz is an inducer of a number of drug metabolizing enzymes and has been shown to have inhibitive characteristics in vitro (20). The list of drugs that may have significant interactions with these frequently used antiretroviral agents is extensive.
Development of compounds with little propensity for such interactions will provide a significant benefit to patients. Raltegravir is a member of a promising new class of drug for the treatment of HIV infection. Raltegravir is a novel HIV type 1 (HIV-1) integrase strand transfer inhibitor with potent in vitro activity against HIV-1 (95% inhibitory concentration = 31 nM in 50% human serum) (13, 18, 19; Isentress package insert [Merck & Co., Inc.]). Raltegravir has been shown to be well tolerated and effective in reducing viral loads in treatment-naive HIV-1-infected patients when dosed as monotherapy for 10 days (10). Clinical studies of treatment-experienced patients when drug was administered in concert with optimal background therapy have further supported the safety and efficacy profile (1, 4, 5, 16), with a dose of 400 mg administered twice daily established as the therapeutic dose and schedule.
Raltegravir is primarily cleared by metabolism, predominantly by glucuronidation via the isozyme UGT1A1 (9). Raltegravir is neither an inducer nor an inhibitor of enzymes involved in drug metabolism (8). Since the current treatment paradigm for HIV involves the administration of anti-HIV agents in combination, to boost efficacy and to control the emergence of resistance, it is currently planned that raltegravir will be administered in concert with other anti-HIV drugs, including ritonavir and efavirenz. The two studies presented here examined the safety, tolerability, and pharmacokinetics of raltegravir when coadministered with either ritonavir or efavirenz in healthy male subjects.
(The information in this article was presented in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2006 [8a]).
MATERIALS AND METHODS
Study subjects.
A mutually exclusive set of subjects participated in each of the two studies. Healthy male subjects between the ages of 18 to 45 years and within 30% of ideal body weight were eligible for the studies. Subjects were excluded from the studies if they were smokers; infected with HIV; had a significant history of psychiatric, neurologic, endocrine, cardiovascular, rheumatologic, gastrointestinal, hepatic, renal, genitourinary, hematologic, or neoplastic disease; had donated blood or experienced a significant loss of blood within 4 weeks prior to the start of the studies; or had anticipated needing any prescription or nonprescription drugs during the studies. Females were excluded from the studies presented in the manuscript due to the fact that these studies were conducted prior to availability of data from preclinical developmental and reproductive toxicology studies and female pharmacokinetic studies; these data were provided later in the development program, supporting inclusion of females in other clinical studies.
All subjects provided written informed consent to participate in their respective study. The protocols were approved by the institutional or ethical review boards of the respective study centers. The protocols were conducted in accordance with the guidelines on good clinical practice and with the ethical standards for human experimentation established by the Declaration of Helsinki.
Ritonavir study design (study I).
The ritonavir interaction study was a double-blind, randomized, placebo-controlled, two-period study of young, healthy male subjects. In period 1, subjects received a 400-mg single oral dose of raltegravir (lactose formulation) or placebo. This was followed by at least a 4-day washout interval for each subject prior to the start of period 2. In period 2, all subjects received 100-mg oral doses of ritonavir (Norvir; Abbott Laboratories, Abbott Park, IL) twice a day for 16 days. On day 14, subjects received their morning dose of ritonavir in combination with a 400-mg single oral dose of raltegravir or a placebo. Ritonavir was administered in an open-label fashion, while raltegravir was administered in a double-blind fashion. The same two subjects in each period received placebo versus raltegravir based on a computer-generated randomized allocation schedule. All doses of study drug were administered following a moderate-fat meal, including those doses administered on pharmacokinetic sampling days. Ritonavir is recommended to be administered with food, and prior results from a food effect study with the lactose formulation of raltegravir indicated that food had a minimal effect on raltegravir pharmacokinetics (14).
Efavirenz study design (study II).
The efavirenz interaction study was a double-blind, randomized, placebo-controlled, two-period study of young, healthy male subjects. In period 1, subjects received a 400-mg single oral dose of raltegravir (lactose formulation) or a placebo. This was followed by at least a 4-day washout interval for each subject prior to the start of period 2. In period 2, all subjects received 600-mg oral doses of efavirenz (Stocrin; Merck & Co., Inc., Whitehouse Station, NJ) once daily for 14 days. On day 12, subjects received their daily dose of efavirenz in combination with a 400-mg single oral dose of raltegravir or a placebo. Efavirenz was administered in an open-label fashion, while raltegravir was administered in a double-blind fashion. The same two subjects in each period received placebo versus raltegravir based on a computer-generated randomized allocation schedule. All doses of study drug were administered to subjects in the fasted state. Efavirenz was administered at bedtime, as recommended in the package insert (Stocrin package insert; Merck & Co., Inc.).
Analytical and pharmacokinetic measurements.
Whole blood samples were collected for each study for the quantification of raltegravir in plasma. In study I, samples were collected at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 32, 48, and 72 h postdose after raltegravir dosing in each treatment period. Time points were similar in study II with the exception of a collection at 36 h rather than 32 h.
Plasma samples were analyzed for raltegravir concentrations using a validated reverse-phase high-pressure liquid chromatography tandem mass spectrometry method, as previously described (12). The lower limit of quantitation for the plasma assay was 2 ng/ml (4.5 nM), and the linear calibration range was 2 to 1,000 ng/ml. For study I, the interday accuracy of the plasma quality control samples was 96.8 to 108.3% and the interday precision was 1.4 to 2.6%. For study II, the interday accuracy of the plasma quality control samples was 90.9 to 98.8% and the interday precision was 2.6 to 5.4%.
Plasma raltegravir concentrations were converted into molar units (nM) using the molecular weight of 444.4 and were subsequently employed to determine the pharmacokinetic parameters, including the plasma concentration at 12 h (C12 h), area under the concentration-time curve from zero to infinity (AUC0-∞), maximal plasma concentration (Cmax), time to maximal plasma concentration (Tmax), and apparent half-life (t1/2) for each subject. The software program WinNonlin (Pharsight Corporation, Mountain View, CA) was used in the calculation of pharmacokinetic parameter values. The distribution and elimination phases of each plasma concentration profile were fit to a biexponential equation (concentration = A × e−αt + B × e−βt) using the Gauss-Newton (Levenberg and Hartley) minimization method and a weighting of 1/(predicted concentration)2. Onset of the α phase was determined by inspection. t1/2 values for each phase were calculated as the quotient of ln(2) and α or β. The AUC to the last time point with a detectable plasma concentration was calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations. The AUC0-∞ was estimated as the sum of the AUC to the last time point with a detectable plasma concentration and the extrapolated area given by the quotient of the last measured concentration and β. Cmax and Tmax were obtained by inspection of the plasma concentration data. Provided that the actual observed time of Tmax did not differ in a meaningful way from the nominal plasma sampling time, nominal plasma sampling times were used to determine the Tmax value. C12 h values were assessed from the plasma concentrations determined for the nominal sampling times at 12 h postdose.
Safety.
Safety and tolerability were assessed in each study by clinical evaluation (including physical examinations, vital signs, and 12-lead electrocardiograms) and laboratory measurements (including hematology, serum chemistry, and urinalysis). Adverse experiences were monitored throughout the study. Investigators evaluated all clinical adverse experiences in terms of intensity (mild, moderate, or severe), duration, seriousness, outcome, and relationship to study drug.
Statistical methods.
The parameters C12 h, Cmax, and AUC0-∞ were natural-log transformed before analysis. Ninety-percent confidence intervals (CIs) were calculated for the mean treatment difference (raltegravir with ritonavir or efavirenz-raltegravir alone) of C12 h, Cmax, and AUC0-∞ and were based on an analysis-of-variance model with treatment as a fixed effect and with subject as a random effect. Confidence intervals constructed on the log scale were back transformed to obtain confidence intervals for the geometric mean ratios (GMR) of C12 h, Cmax, and AUC0-∞.
For Tmax, t1/2 α, and t1/2 β, the Hodges-Lehman estimate of the median difference (raltegravir with ritonavir or efavirenz-raltegravir alone) was computed, as was a 90% CI for the true median difference.
All statistical analyses were conducted using the SAS software program, version 8 (SAS Institute Inc., Cary, NC).
RESULTS
Demographics and baseline characteristics.
For study I, a total of 14 male subjects was enrolled in the study. All subjects were nonsmokers and weighed within ±30% of ideal body weight, with a mean weight of 79.6 kg (range, 63.3 to 114.8 kg) and a mean age of 28 years (range, 18 to 41 years). Subjects were HIV negative and in good general health according to routine medical history, physical examination, vital signs, and laboratory data. Eight of the fourteen enrolled subjects were African-American and six were Caucasian.
For study II, a total of 14 male subjects was enrolled in the study. All subjects were nonsmokers and weighed within ±30% of ideal body weight, with a mean weight of 79.6 kg (range, 62.4 to 95.5 kg) and a mean age of 34 years (range, 19 to 45 years). Subjects were HIV negative and in good general health according to routine medical history, physical examination, vital signs, and laboratory data. All of the enrolled subjects were Caucasian.
Pharmacokinetics.
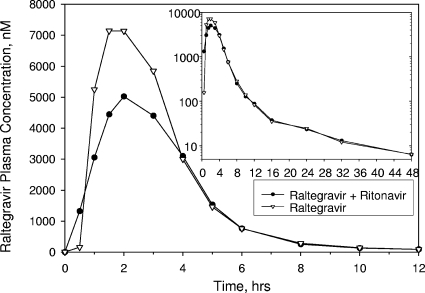
The arithmetic mean raltegravir plasma concentration-time profiles for both the ritonavir and efavirenz studies are shown in Fig. 1 and 2, respectively. The pharmacokinetic parameter values for each of these studies are summarized in Tables 1 and 2. The administration of multiple doses of 100 mg of ritonavir twice daily for 14 days weakly affected the pharmacokinetic profile of a single dose of raltegravir. The C12 h GMR (raltegravir plus ritonavir-raltegravir) was 0.99 with a corresponding 90% CI of 0.70, 1.40. The AUC0-∞ and Cmax GMRs (90% CI) were 0.84 (0.70, 1.01) and 0.76 (0.55, 1.04), respectively. As shown in Table 2, there were no substantial differences observed in Tmax or t1/2 values of raltegravir in the presence versus the absence of ritonavir.
FIG. 1.
Arithmetic mean raltegravir plasma concentrations following a single oral dose of 400-mg raltegravir with or without multiple oral doses of 100-mg ritonavir twice daily to young, healthy male subjects (inset, semilog scale; lines connecting data points are interpolations between the points).
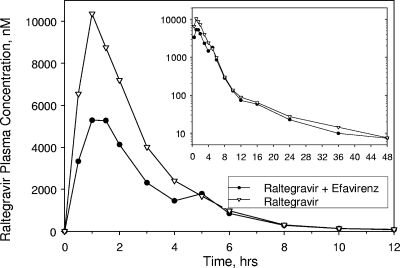
FIG. 2.
Arithmetic mean raltegravir plasma concentrations following a single oral dose of 400-mg raltegravir with or without multiple oral doses of 600-mg efavirenz once daily to young, healthy male subjects (inset, semilog scale; lines connecting data points are interpolations between the points).
TABLE 1.
Plasma pharmacokinetic parameters for raltegravir following single oral doses of 400-mg raltegravir with or without administration of 100-mg ritonavir twice daily
| Pharmacokinetic parameter | No. of subjects | Geometric mean
|
Value for raltegravir + ritonavir/value for raltegravir
|
||
|---|---|---|---|---|---|
| Raltegravir + ritonavir | Raltegravir | GMR (90% Cl) | P value | ||
| C12 h (nM)a | 10 | 76.5 | 77.1 | 0.99 (0.70, 1.40) | 0.965 |
| AUC0-∞ (μM · h)a | 10 | 19.32 | 22.88 | 0.84 (0.70, 1.01) | 0.122 |
| Cmax (μM)a | 10 | 7.23 | 9.57 | 0.76 (0.55, 1.04) | 0.143 |
| Tmax (h) | 10 | 2.0b | 1.8b | 0.5 (−1.0, 2.0)c | 0.555 |
| t1/2 α (h) | 10 | 0.97d | 1.06d | −0.11 (−0.16, −0.05)c | 0.078 |
| t1/2 β (h) | 10 | 10.0d | 10.8d | 0.3 (−2.5, 3.8)c | 0.826 |
Geometric mean computed from least-squares estimate from an analysis of variance performed on the natural-log transformed values.
Median reported for Tmax.
Hodges-Lehman estimate of median treatment difference with corresponding 90% CI for true median treatment difference.
Harmonic mean reported for t1/2.
TABLE 2.
Plasma pharmacokinetic parameters for raltegravir following single oral dose of 400-mg raltegravir with or without multiple oral doses of 600-mg efavirenz
| Pharmacokinetic parameter | No. of subjects | Geometric mean
|
Value for raltegravir + efavirenz/value for raltegravir
|
||
|---|---|---|---|---|---|
| Raltegravir + efavirenz | Raltegravir | GMR (90% CI) | P value | ||
| C12 h (nM)a | 9 | 62.5 | 79.1 | 0.79 (0.49, 1.28) | 0.388 |
| AUC0-∞ (μM · h)a | 9 | 18.14 | 28.15 | 0.64 (0.52, 0.80) | 0.006 |
| Cmax (μM)a | 9 | 6.45 | 10.13 | 0.64 (0.41, 0.98) | 0.089 |
| Tmax (h) | 9 | 1.5b | 1.5b | −0.1 (−1.0, 2.2)c | 0.984 |
| t1/2 α (h) | 9 | 0.95d | 0.98d | −0.06 (−0.14, 0.16)c | 0.414 |
| t1/2 β (h) | 9 | 9.1d | 10.8d | −0.6 (−4.4, 2.7)c | 0.820 |
Geometric mean computed from least-squares estimate from an analysis of variance performed on the natural-log transformed values.
Median reported for Tmax.
Hodges-Lehman estimate of median treatment difference with corresponding 90% CI for true median treatment difference.
Harmonic mean reported for t1/2.
Multiple-dose administration of efavirenz prior to and coadministered with a single oral dose of raltegravir resulted in a moderately lower C12 h GMR (raltegravir plus efavirenz-raltegravir) of 0.79 with a corresponding 90% CI of 0.49, 1.28. Moderately to weakly reduced raltegravir AUC0-∞ and Cmax values were also observed: the GMR (90% CI) of the AUC0-∞ was 0.64 (0.52, 0.80), and that of the Cmax was 0.64 (0.41, 0.98). As shown in Table 2, there were no substantial differences observed in the Tmax or t1/2 values of raltegravir in the presence versus absence of efavirenz.
Safety and tolerability.
Single-dose administration of raltegravir alone and in combination with either ritonavir or efavirenz was generally well tolerated in young, healthy male subjects. In study I, there were no serious clinical or laboratory adverse experiences observed during the study. No subject discontinued treatment due to an adverse experience. Twelve of the 14 enrolled subjects completed the study. Two subjects discontinued the study for personal reasons unrelated to study safety. All 14 subjects enrolled in the study were included in the evaluation of safety, since both subjects who discontinued had received at least one dose of raltegravir. Five subjects reported a total of seven nonserious clinical adverse experiences, three of which were determined by the study investigator to possibly be drug related (dyspepsia, concentration impairment, concentration loss), with all three of these determined to be mild in intensity and limited in duration. None of the adverse experiences reported occurred in subjects treated with raltegravir alone, with raltegravir in combination with ritonavir, or with a placebo. The three drug-related adverse experiences all occurred during administration of ritonavir alone. There were no laboratory adverse experiences reported.
In study II, there were no serious clinical or laboratory adverse experiences. Twelve of the fourteen enrolled subjects completed the efavirenz study. Two subjects discontinued the study due to nonserious clinical adverse experiences judged by the investigator to be unrelated to raltegravir. A total of 114 nonserious clinical adverse experiences were reported by 14 subjects enrolled in the study, 97 of which were judged to be drug related. Of these 97 adverse experiences, 92 were related to efavirenz, all of which were consistent with adverse experiences reported in the label for this drug. There were no laboratory adverse experiences reported.
DISCUSSION
A primary aim of the two studies reported was to assess the potential pharmacokinetic interactions of raltegravir when coadministered with either ritonavir or efavirenz, two drugs which are known to have substantive effects on the pharmacokinetic profiles of multiple drugs. Data from these two current studies indicate that the propensity for ritonavir and efavirenz to meaningfully affect the pharmacokinetic profile of raltegravir is not substantial.
Interpretation of deviations of raltegravir pharmacokinetics secondary to extrinsic factors, such as concomitant medicinal product use, is based on the overall efficacy and safety database. For other classes of antiretroviral agents, there is a reasonable but imperfect association of efficacy with doses that achieve trough concentrations (Ctrough) in excess of the protein-binding-adjusted 95% inhibitory concentration in the HIV spread assay (3). Analyses of the raltegravir viral response measures associated with pharmacokinetic measures did not identify any clinically meaningful correlations with Ctrough or other pharmacokinetic parameter values (21). However, pharmacokinetic/pharmacodynamic (PK/PD) analyses of short-term viral response identified a potential association of day-10 HIV RNA and slope of decline in HIV RNA with the Ctrough value (or C12 h on the therapeutic schedule). Although these results did not translate into differences in longer-term efficacy, these short-term PK/PD results suggested that Ctrough is likely a sensitive pharmacokinetic parameter for predicting the viral response (11). In patients receiving combination therapy with raltegravir, little concentration dependency was seen, with long-term efficacy. As such, the lower bound of pharmacokinetic exposures defined the reduction in the C12 h value that has been demonstrated not to be associated with an increased risk of reduced long-term efficacy. Pharmacokinetic and efficacy data in the phase II and III studies established that a 60% decrease in Ctrough concentration (C12 h) is not considered to be clinically relevant (11).
As is the case with many drugs, AUC and/or Cmax are the pharmacokinetic parameters that are most likely to be associated with toxicity. Because there have been no acute safety findings in the raltegravir clinical program that were temporally associated with peak concentrations, the AUC was determined to be the most appropriate raltegravir pharmacokinetic parameter from which to judge the clinical significance of elevations in raltegravir concentrations (11). Overall, raltegravir has been generally well tolerated, with no dose-related toxicities identified thus far; therefore, the upper bound of broad clinical experience was used to define the elevation in AUC demonstrated not to be associated with an increased risk of clinically meaningful alterations in safety and tolerability. Pharmacokinetic and safety data in the phase II and III clinical studies established an upper comparability bound of up to a twofold increase in exposure not having clinical relevance (11).
In the ritonavir interaction study, the results did not demonstrate bioequivalence. However, with respect to clinical no-effect bounds, the raltegravir C12 h value was essentially unaffected, with a C12 h GMR (90% CI) of 0.99 (0.70, 1.40) and the lower bound of the confidence interval greater than the lower bound of clinical relevance (0.40). The AUC and Cmax of raltegravir were weakly reduced in the presence of ritonavir versus its absence. Ritonavir is an inducer of glucuronidation and an inhibitor of CYP3A and CYP2D6 metabolism. Ritonavir is also an inhibitor of the transporter P-glycoprotein (7). The main route of elimination of raltegravir is glucuronidation mediated by UGT1A1 (9), and results of this study support that a commonly used boosting dose of ritonavir (100 mg twice daily) has no clinically meaningful effect on the pharmacokinetics of raltegravir, despite the potential for induction of UGT1A1 by ritonavir. Due to the multiple effects of ritonavir on enzymes and transporters noted above, a balance of competing effects of induction and inhibition cannot be ruled out, although based on preclinical and clinical data, it is not anticipated that the pharmacokinetics of raltegravir would be altered by inhibition of CYP3A, CYP2D6, or P-glycoprotein.
In the efavirenz study, the coadministration of efavirenz and raltegravir moderately to weakly reduced plasma concentrations of raltegravir (AUC0-∞, Cmax, and C12 h) without otherwise altering the plasma pharmacokinetic profile (Tmax and apparent t1/2). Similar to the case with ritonavir, bioequivalence was not demonstrated; however, the lower bound of the C12 h 90% CI (0.49) was greater than the lower bound of clinical relevance. In vitro studies have shown that efavirenz inhibits CYP2C9, CYP2C19, and CYP3A4. Efavirenz has also been shown to induce CYP450 enzymes and has the potential to induce its own metabolism (CYP3A4 and 2B6 are the major isozymes responsible for efavirenz metabolism). Efavirenz is known to activate the pregnane X receptor (6), which is involved in regulating activity of CYP3A4 and that of UGT1A1 (17). Due to its effects on the pregnane X receptor, efavirenz is a potential inducer of UGT1A1. Evidence for such induction has been reported in a study of antiretroviral therapy-associated hyperbilirubinemia, in which the use of efavirenz therapy resulted in significantly decreased bilirubin levels compared to results for patients using other antiretroviral therapies, presumably due to increased clearance of bilirubin catalyzed by increased UGT1A1 activity (15). The observed interaction with raltegravir is most consistent with a minor inductive effect of efavirenz on UGT1A1, and the lack of a substantial effect of efavirenz on the t1/2 of raltegravir suggests that efavirenz may primarily affect first-pass metabolism. Due to the multiple effects of efavirenz on enzymes as noted above, a balance of competing effects of induction and inhibition cannot be ruled out, although based on preclinical and clinical data, it is not anticipated that the pharmacokinetics of raltegravir would be altered by induction of CYP450 enzymes or by inhibition of CYP3A4, CYP2C9, or CYP2C19.
Based on the collective clinical data obtained in phase II and phase III efficacy studies in population pharmacokinetic analyses and PK/PD correlation analyses, the degree of interaction for ritonavir or efavirenz with raltegravir pharmacokinetics is not clinically meaningful since perturbations fall within the bounds of clinical relevance.
Drug-drug interactions are particularly important considerations in the treatment of the HIV-infected population and can be problematic in many instances. Both ritonavir and efavirenz have been shown to exert significant effects on the pharmacokinetics of many antiretroviral agents, as well as several other drugs commonly used with this population (2). Since ritonavir and efavirenz are commonly used agents for this population, it would be advantageous to have other antiretroviral drugs that are not negatively impacted by ritonavir or efavirenz. Overall, an antiretroviral compound with little propensity for drug interactions, such as raltegravir, would be highly advantageous.
It should be noted that the studies discussed in this article were conducted with healthy volunteers rather than with HIV-1-infected individuals. Furthermore, the sample size of the studies was small, representing a limited demographic range. However, demographic effects on raltegravir pharmacokinetics have been assessed with no evidence that factors such as age, race, sex, or HIV infection status influence pharmacokinetics (11). Despite the limitations in these healthy volunteer studies, the data imply a limited propensity for clinically meaningful drug-drug interactions in the target population.
In summary, raltegravir in combination with ritonavir or efavirenz was generally well tolerated in healthy subjects. In the population studied, plasma concentrations of raltegravir are not substantially affected when subjects are codosed with ritonavir. Though plasma concentrations of raltegravir are moderately to weakly reduced when subjects are codosed with efavirenz, the degree of this reduction is likely not clinically meaningful. Based on these data, no dose adjustment is required for raltegravir with coadministration with ritonavir or efavirenz.
Acknowledgments
We thank Ruth Lachaert for her assistance in the conduct of the clinical studies and the volunteers involved in the clinical studies.
Both of these studies were performed on behalf of Merck & Co., Inc, Whitehouse Station, NJ.
Authors who are employees of Merck may own stock and/or stock options in the company. Authors who are not employees of Merck have received grant support, consultant fees, and/or lecture honoraria.
Footnotes
Published ahead of print on 6 October 2008.
REFERENCES
- 1.Cooper, D. A., R. T. Steigbigel, J. M. Gatell, J. K. Rockstroh, C. Katlama, P. Yeni, A. Lazzarin, B. Clotet, P. N. Kumar, J. E. Eron, M. Schechter, M. Markowitz, M. R. Loutfy, J. L. Lennox, J. Zhao, J. Chen, D. M. Ryan, R. R. Rhodes, J. A. Killar, L. R. Gilde, K. M. Strohmaier, A. R. Meibohm, M. D. Miller, D. J. Hazuda, M. L. Nessly, M. J. DiNubile, R. D. Isaacs, H. Teppler, B.-Y. Nguyen, and the BENCHMRK-1 Study Teams. 2008. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N. Engl. J. Med. 359:355-365. [DOI] [PubMed] [Google Scholar]
- 2.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. 10 October 2006, posting date. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 3.Gerber, J. G., and E. P. Acosta. 2003. Therapeutic drug monitoring in the treatment of HIV-infection. J. Clin. Virol. 27:117-128. [DOI] [PubMed] [Google Scholar]
- 4.Grinsztejn, B., B.-Y. Nguyen, C. Katlama, J. M. Gatell, A. Lazzarin, D. Vittecoq, C. J. Gonzalez, J. Chen, C. M. Harvey, R. D. Isaacs, and the Protocol 005 Team. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261-1269. [DOI] [PubMed] [Google Scholar]
- 5.Grinsztejn, B., B.-Y. Nguyen, C. Katlama, J. Gatell, A. Lazzarin, D. Vittecoq, J. Chen, and the Protocol 005 Team. 2007. 48 week efficacy and safety of MK-0518, a novel HIV-1 integrase inhibitor, in patients with triple-class resistant virus, abstr. H-713. Abstr. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL.
- 6.Hariparsad, N., S. C. Nallani, R. S. Sane, D. J. Buckley, A. R. Buckley, and P. B. Desai. 2004. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J. Clin. Pharmacol. 44:1273-1281. [DOI] [PubMed] [Google Scholar]
- 7.Hsu, A., G. R. Granneman, and R. J. Bertz. 1998. Ritonavir—clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharmacokinet. 35:275-291. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto, M., K. Kassahun, M. D. Troyer, W. D. Hanley, P. Lu, A. Rhoton, A. S. Petry, K. Ghosh, E. Mangin, E. P. DeNoia, L. A. Wenning, J. A. Stone, K. M. Gottesdiener, and J. A. Wagner. 2008. Lack of a pharmacokinetic interaction of raltegravir on midazolam—in vitro/in vivo correlation. J. Clin. Pharmacol. 48:209-214. [DOI] [PubMed] [Google Scholar]
- 8a.Iwamoto, M., L. A. Wenning, A. S. Petry, T. Laethem, M. De Smet, J. T. Kost, S. Merschman, E. Mangin, N. Azrolan, H. E. Greenberg, W. Haazen, J. Stone, K. M. Gottesdiener, and J. A. Wagner. 2006. Minimal effect of ritonavir (RTV) and efavirenz (EFV) on the pharmacokinetics (PK) of MK-0518. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-373.
- 9.Kassahun, K., I. McIntosh, D. Cui, D. Hreniuk, S. Merschman, K. Lasseter, N. Azrolan, M. Iwamoto, J. A. Wagner, and L. A. Wenning. 2007. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab. Dispos. 35:1657-1663. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz, M., J. O. Morales-Ramirez, B. Y. Nguyen, C. M. Kovacs, R. T. Steigbigel, D. A. Cooper, R. Liporace, R. Schwartz, R. Isaacs, L. R. Gilde, L. Wenning, J. Zhao, and H. Teppler. 2006. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 43:509-515. [DOI] [PubMed] [Google Scholar]
- 11.Merck & Co., Inc. 2007. Raltegravir advisory committee meeting background package. Food and Drug Administration, Rockville, MD. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4314b1-01-Merck.pdf.
- 12.Merschman, S. A., P. T. Vallano, L. A. Wenning, B. K. Matuszewski, and E. J. Woolf. 2007. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 857:15-24. [DOI] [PubMed] [Google Scholar]
- 13.Miller, M., M. Witmer, K. Stillmock, P. Felock, L. Ecto, J. Flynn, W. Schleif, G. Dornadula, R. Danovich, and D. Hazuda. 2006. Biochemical and antiviral activity of MK-0518, a potent HIV integrase inhibitor, abstr. THAA0302. Abstr. AIDS 2006: 16th International AIDS Conference, Toronto, Canada.
- 14.Petry, A. S., L. A. Wenning, T. Laethem, M. De Smet, J. T. Kost, S. Merschman, K. M. Strohmaier, S. Ramael, K. Lasseter, J. Stone, K. M. Gottesdiener, J. A. Wagner, and M. Iwamoto. 2006. Safety, tolerability, and pharmacokinetics after single- and multiple-doses of MK-0518 in healthy subjects, abstr. A-1424. Abstr. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA.
- 15.Rotger, M., P. Taffe, G. Bleiber, H. F. Gunthard, H. Furrer, P. Vernazza, H. Drechsler, E. Bernasconi, M. Rickenbach, and A. Telenti. 2005. Gilbert syndrome and the development of antiretroviral therapy-associated hyperbilirubinemia. J. Infect. Dis. 192:1381-1386. [DOI] [PubMed] [Google Scholar]
- 16.Steigbigel, R. T., D. A. Cooper, P. N. Kumar, J. E. Eron, M. Schechter, M. Markowitz, M. R. Loufty, J. L. Lennox, J. M. Gatell, J. K. Rockstroh, C. Katlama, P. Yeni, A. Lazzarin, B. Clotet, J. Zhao, J. Chen, D. M. Ryan, R. R. Rhodes, J. A. Killar, L. R. Gilde, K. M. Strohmaier, A. R. Meibohm, M. D. Miller, D. J. Hazuda, M. L. Nessly, M. J. DiNubile, R. D. Isaacs, B.-Y. Nguyen, H. Teppler, and the BENCHMRK-2 Study Team. 2008. Raltegravir with optimized background therapy for resistant HIV-1 infection. N. Engl. J. Med. 359:339-354. [DOI] [PubMed] [Google Scholar]
- 17.Sugatani, J., S. Nishitani, K. Yamakawa, K. Yoshinari, T. Sueyoshi, M. Negishi, and M. Miwa. 2005. Transcriptional regulation of human UGT1A1 gene expression: activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol. Pharmacol. 67:845-855. [DOI] [PubMed] [Google Scholar]
- 18.Summa, V., P. Pace, A. Petrocchi, R. Laufer, R. Cortese, D. Hazuda, M. Miller, W. Schleif, J. Vacca, S. Young, and M. Rowley. 2006. Discovery of MK-0518 a novel, potent and selective HIV integrase inhibitor in phase III clinical trials. AIDS 2006: International AIDS Conference, Toronto, Canada.
- 19.Summa, V., P. Pace, A. Petrocchi, P. Jones, J. P. Vacca, M. Rowley, E. Monteagudo, F. Fiore, P. O. Gonzalez, R. Laufer, K. A. Stillmock, and D. J. Hazuda. 2007. Discovery of raltegravir—a novel, potent and selective HIV integrase inhibitor. 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, Sydney, Australia.
- 20.Vrouenraets, S. M. E., F. W. N. M. Wit, J. Van Tongeren, and J. M. A. Lange. 2007. Efavirenz: a review. Expert Opin. Pharmacother. 8:851-871. [DOI] [PubMed] [Google Scholar]
- 21.Wenning, L., B.-Y. Nguyen, X. Sun, E. Hwang, Y. Chen, H. Teppler, C. Harvey, R. Rhodes, D. Ryan, N. Azrolan, and J. Stone. 2008. Pharmacokinetic/pharmacodynamic (PK/PD) analyses for raltegravir (RAL) in phase II and III studies in treatment experienced HIV-infected patients, abstr. 021. Abstr. 9th International Workshop on Clinical Pharmacology of HIV Therapy, New Orleans, LA.