Abstract
With disk diffusion, the following zone diameters are suggested to be resistant and susceptible breakpoints, respectively: for susceptibility testing of Campylobacter coli, no inhibition zone and 15 mm or more for erythromycin, and 20 mm or less and 25 mm or more for ciprofloxacin, in the absence or presence of an inhibition zone around the nalidixic acid disk; and for susceptibility testing of C. coli and Campylobacter jejuni, 20 mm or less and 26 mm or more for tetracycline.
Campylobacter coli is a significant human pathogen responsible for 5% to 15% of enterocolitis cases caused by Campylobacter spp. (2, 6). This bacterium may also cause bacteremia (1). Such enteric infections need to be treated with an antimicrobial agent in less than 50% of cases (2, 6, 15). Macrolides and fluoroquinolones are first- and second-choice antimicrobials for that purpose (2, 4, 5, 6). C. coli is reported to be more frequently resistant to erythromycin than is Campylobacter jejuni subsp. jejuni (C. jejuni), with actual resistance rates between 0 and 50% (7). Resistance of C. coli to fluoroquinolones is increasing over time, with the actual resistance rate at 30.8% in the United States (7). Routine susceptibility testing of C. coli has become a very important tool for appropriate antimicrobial treatment when needed (2, 6-11, 14-16). The main erythromycin resistance mechanism of C. coli strains (mutation of 23S ribosomal rRNA and proteins) confers high-level resistance (13). The main fluoroquinolone resistance mechanism of C. coli strains [mutation(s) of DNA gyrase A] can confer a low, intermediate, or high resistance level to ciprofloxacin and, for most isolates, a high resistance level to nalidixic acid (5, 16). Even with resistance rates between 46 and 70%, tetracycline is the third-choice antimicrobial agent for the treatment of C. coli and C. jejuni enteric infections (2, 6-11, 14-16), and resistance is mainly mediated by a plasmid (16). In a previous study, using disk diffusion, zone diameters were suggested to be resistant and susceptible breakpoints for erythromycin and ciprofloxacin susceptibility testing of C. jejuni (12).
Antimicrobial susceptibility testing of Campylobacter spp. with an agar dilution method has been standardized by the CLSI (3), and the erythromycin, ciprofloxacin, tetracycline, and doxycycline MIC interpretative criteria for C. jejuni and C. coli have been reported recently (4). The agar dilution method, however, is not convenient for testing a few isolates at a time, and disk diffusion has not yet been standardized.
The aims of this study were to compare the results obtained by the disk diffusion method with those obtained by the reference agar dilution method to test the susceptibility of C. coli isolates to erythromycin, tetracycline, and ciprofloxacin and of C. jejuni isolates to tetracycline.
Within a total of 100 C. coli isolates tested, one isolate per patient, 54 were isolated from 1999 to 2007 from a collection at Hôpital Saint-Luc (HSL), and 46 were isolated from 1983 to 1993 and obtained from the HSL and from the Laboratoire de santé publique du Québec (LSPQ). Identification at the genus and species levels of the 100 C. coli strains was confirmed by the LSPQ. All 100 C. coli isolates were microaerobic spiral gram-negative rods that were negative on the hippurate tube test. All C. coli strains isolated from 1999 to 2007 and the three C. coli strains that were isolated before 1999 and resistant to erythromycin were subjected to hippuricase gene PCR, performed at the National Laboratory of Microbiology, Winnipeg, Canada, and they were negative for this test. A total of 289 C. jejuni organisms isolated at HSL from 2002 to 2006 were studied and identified as reported previously (12).
Erythromycin and tetracycline (Sigma Chemical Co., St. Louis, MO) and ciprofloxacin (Bayer, Leverkusen, Germany) MICs of the C. coli isolates and tetracycline MICs of the C. jejuni isolates were determined at HSL by the agar dilution method at 37°C, as recommended by the CLSI (3). C. jejuni ATCC 33560 served as the control strain (3). The MIC interpretive criteria for these three antimicrobial agents were those prescribed by the CLSI for C. jejuni and C. coli (4).
Erythromycin, tetracycline, and ciprofloxacin disk diffusion testing of the 100 C. coli isolates and tetracycline disk diffusion testing of the 289 C. jejuni isolates were performed at HSL. The presence or absence of an inhibition zone with nalidixic acid disks was also investigated at HSL for the 100 C. coli isolates. Inocula, prepared in Mueller-Hinton broth at a density adjusted to a 0.5 McFarland turbidity standard, were delivered onto 5% sheep blood Mueller-Hinton agar plates (Difco, Becton Dickinson, Sparks, MD). Zone diameters were measured with slipping calipers. Incubation occurred in a microaerobic incubator at 37°C for 48 h. Erythromycin (15 μg), tetracycline (30 μg), ciprofloxacin (5 μg), and nalidixic acid (30 μg) disks (BBL; Becton Dickinson) were tested.
Erythromycin susceptibility testing.
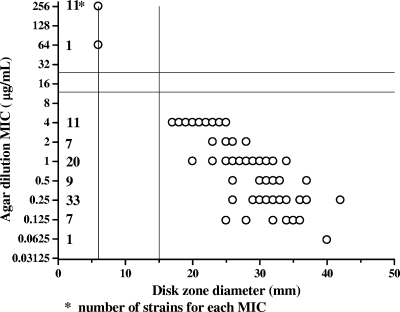
In Fig. 1, the results of disk diffusion testing with 100 C. coli isolates were compared to those of agar dilution for erythromycin by scattergram. No inhibition zone and diameters of ≥15 mm around the erythromycin disk are suggested to be resistant and susceptible breakpoints, respectively, of C. coli isolates. MIC determination by the agar dilution method for isolates with an inhibition zone of less than 15 mm is recommended. No isolates of the 260 C. jejuni strains reported previously (12) and no isolates of the 100 C. coli strains tested in this study would have needed erythromycin MIC testing. The 215 susceptible C. jejuni strains tested previously (12) and the 88 susceptible C. coli strains tested in this study had inhibition zones around the erythromycin disk. The 45 resistant C. jejuni strains tested previously (12) and the 12 resistant C. coli strains tested in this study had no inhibition zone around the erythromycin disk.
FIG. 1.
Correlation between disk diffusion results and MICs of erythromycin for 100 C. coli isolates.
Ciprofloxacin susceptibility testing.
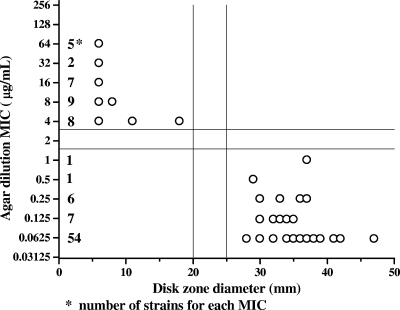
In Fig. 2, the results of disk diffusion testing with 100 C. coli isolates were compared to those of agar dilution for ciprofloxacin by scattergram. Zone diameters of ≤20 mm and ≥25 mm around the ciprofloxacin disk and the absence or presence of an inhibition zone around the nalidixic acid disk are suggested to be resistant and susceptible breakpoints, respectively, of C. coli isolates. If the results obtained with both disks are consistent, the isolate could be reported accordingly. If the two disks give discordant results and reidentification confirms a C. coli isolate, we suggest obtaining a ciprofloxacin MIC by the agar dilution method. One susceptible isolate of the 260 C. jejuni strains reported previously (12) and no isolates of the 100 C. coli strains tested in the present study would have needed ciprofloxacin MIC testing. The 154 ciprofloxacin-susceptible C. jejuni strains tested previously (12) and the 69 susceptible C. coli strains tested in this study had inhibition zones around the nalidixic acid disk. The 124 ciprofloxacin-resistant and the two ciprofloxacin-intermediate C. jejuni strains tested previously (12) and the 31 resistant C. coli strains tested in this study had no inhibition zone around the nalidixic acid disk.
FIG. 2.
Correlation between disk diffusion results and MICs of ciprofloxacin for 100 C. coli isolates.
Tetracycline susceptibility testing.
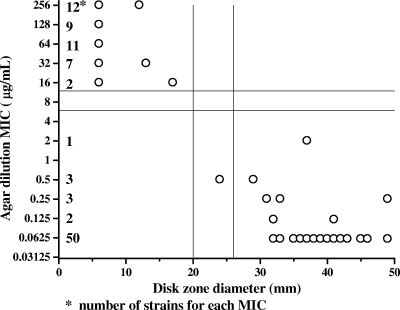
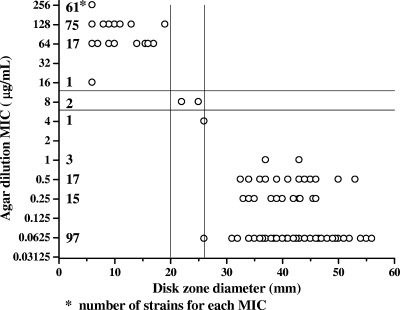
In Fig. 3 and 4, the results of disk diffusion testing with 100 C. coli and 289 C. jejuni isolates, respectively, were compared to the results of agar dilution testing for tetracycline by scattergram. Zone diameters of ≤20 mm and ≥26 mm around the tetracycline disk are suggested to be resistant and susceptible breakpoints, respectively, of C. jejuni and C. coli isolates. MIC determination by the agar dilution method for isolates with a zone diameter of more than 20 mm or of less than 26 mm is recommended. Of the 289 C. jejuni and 100 C. coli strains tested, two intermediate C. jejuni isolates and one susceptible C. coli isolate would have needed tetracycline MIC testing.
FIG. 3.
Correlation between disk diffusion results and MICs of tetracycline for 100 C. coli isolates.
FIG. 4.
Correlation between disk diffusion results and MICs of tetracycline for 289 C. jejuni isolates.
Concluding remarks.
Disk diffusion was an easy and inexpensive method for testing the susceptibilities of C. coli and C. jejuni. The resistant and susceptible zone diameter breakpoints defined in the present study were reliable for determining the susceptibilities of C. coli and C. jejuni isolates for the three antimicrobial agents. None of the 100 C. coli isolates tested was intermediate to erythromycin, tetracycline, and ciprofloxacin. The resistance mechanisms were not determined for the isolates tested. At Hôpital Sacré-Cœur, the disk diffusion method, done at 42°C, gave results similar to ours with erythromycin and nalidixic acid disks for C. jejuni (12) and with erythromycin, nalidixic acid, and tetracycline disks for C. coli (data not shown). In our hospital (HSL), C. jejuni and C. coli species represented 95.3% of 516 Campylobacter spp. isolated from 1999 to 2007 (our unpublished data). Tetracycline susceptibility was also an epidemiological marker of C. jejuni isolates (11). The CLSI recommends C. coli and C. jejuni testing for susceptibility to erythromycin and to ciprofloxacin; no inhibition zone by disk diffusion indicates erythromycin and ciprofloxacin resistance, and any zone of inhibition would require obtaining an MIC to determine the susceptibility category (4). Until it becomes standardized, the disk diffusion method for determining erythromycin (with the erythromycin disk), tetracycline (with the tetracycline disk), and ciprofloxacin (with ciprofloxacin and nalidixic acid disks) susceptibilities of C. coli and for determining tetracycline (with the tetracycline disk) susceptibility of C. jejuni should be considered for screening purposes. To implement this method in a laboratory, we suggest using C. jejuni ATCC 33560 and C. coli and C. jejuni isolates for which the MICs are known as quality controls.
Acknowledgments
We thank France Boucher for her technical assistance and Manon Lorange and Louise Ringuette from Laboratoire de santé publique du Québec (LSPQ) for providing some of the strains isolated from 1983 to 1993. The editorial work on the manuscript by Ovid Da Silva, Research Support Office, Research Centre, CHUM, is appreciated.
Footnotes
Published ahead of print on 6 October 2008.
REFERENCES
- 1.Arai, A., A. Kitano, E. Sawabe, H. Kanegane, T. Miyawaki, and O. Miura. 2007. Relapsing Campylobacter coli bacteremia with reactive arthritis in a patient with X-linked agammaglobulinemia. Intern. Med. 46:605-609. [DOI] [PubMed] [Google Scholar]
- 2.Blaser, M. J. 2005. Campylobacter jejuni and related species, p. 2548-2557. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 6th ed. Elsevier Churchill Livingston, Philadelphia, PA.
- 3.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement; no. M100-S15 (M7-A6), vol. 25, no. 1. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for antimicrobial dilution and disk susceptibility testing for infrequently-isolated or fastidious bacteria: approved guidelines. Publication no. M45-A. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed]
- 5.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald, C., and I. Nachamkin. 2007. Campylobacter and Arcobacter, p. 933-946. In P. R. Murray, E. J. Baron, H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. American Society for Microbiology, Washington, DC.
- 7.Fitzgerald, C., J. Whichard, and I. Nachamkin. 2008. Diagnosis and antimicrobial susceptibility of Campylobacter species, p. 227-243. In I. Nachamkin, C. M. Szymanski, and M. J. Blaser (ed.), Campylobacter, 3rd ed. American Society for Microbiology, Washington, DC.
- 8.Gaudreau, C., and H. Gilbert. 1997. Comparison of disc diffusion and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni subsp. jejuni and Campylobacter coli. J. Antimicrob. Chemother. 39:707-712. [DOI] [PubMed] [Google Scholar]
- 9.Gaudreau, C., and H. Gilbert. 1998. Antimicrobial resistance of clinical strains of Campylobacter jejuni subsp. jejuni isolated from 1985 to 1997 in Quebec, Canada. Antimicrob. Agents Chemother. 42:2106-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudreau, C., and H. Gilbert. 2003. Antimicrobial resistance of Campylobacter jejuni subsp. jejuni strains isolated from humans in 1998 to 2001 in Montréal, Canada. Antimicrob. Agents Chemother. 47:2027-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudreau, C., and S. Michaud. 2003. Cluster of erythromycin- and ciprofloxacin-resistant Campylobacter jejuni subsp. jejuni from 1999 to 2001 in men who have sex with men, Québec, Canada. Clin. Infect. Dis. 37:131-136. [DOI] [PubMed] [Google Scholar]
- 12.Gaudreau, C., Y. Girouard, L. Ringuette, and C. Tsimiklis. 2007. Comparison of disk diffusion and agar dilution methods for erythromycin and ciprofloxacin susceptibility testing of Campylobacter jejuni subsp. jejuni. Antimicrob. Agents Chemother. 51:1524-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibreel, A., V. N. Kos, M. Keelan, C. A. Trieber, S. Levesque, S. Michaud, and D. E. Taylor. 2005. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob. Agents Chemother. 49:2753-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachance, N., C. Gaudreau, F. Lamothe, and F. Turgeon. 1993. Susceptibilities of β-lactamase-positive and -negative strains of Campylobacter coli to β-lactam agents. Antimicrob. Agents Chemother. 37:1174-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ternhag, A., T. Asikainen, J. Giesecke, and K. Ekdahl. 2007. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin. Infect. Dis. 44:696-700. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Q., and P. J. Plummer. 2008. Mechanisms of antibiotic resistance in Campylobacter, p. 263-276. In I. Nachamkin, C. M. Szymanski, and M. J. Blaser (ed.), Campylobacter, 3rd ed. American Society for Microbiology, Washington, DC.