Abstract
The antifungal agent flucytosine was found to be active in vitro against Aspergillus fumigatus isolates when the MIC was determined at pH 5.0 instead of pH 7.0. The in vitro MIC at pH 5.0 corresponded to the in vivo efficacy of flucytosine monotherapy in a murine model of invasive aspergillosis.
The efficacy of flucytosine for the treatment of invasive aspergillosis (IA) has been controversial, although the evidence supporting its use for this condition is limited (5, 10, 11). Most guidelines do not recommend the use of the drug for the treatment of IA (1, 13), although use of the combination of amphotericin B and flucytosine for the treatment of Aspergillus osteomyelitis and joint infection was recommended previously (5).
Most isolates of Aspergillus species are not susceptible to flucytosine, with MICs typically being >64 mg/liter (8). However, we previously demonstrated that the activity of flucytosine against Aspergillus species increased when the pH of the medium was lowered from 7.0 to 5.0 (8). Viviani et al. (12) showed that the in vitro activity of flucytosine against Cryptococcus neoformans at pH 5.4 correlated well with the clinical outcome. We investigated the flucytosine MIC distribution of Aspergillus fumigatus at pH 7.0 and pH 5.0 and determined which condition of MIC testing best corresponded with in vivo efficacy in a nonneutropenic murine model of IA.
The in vitro activity of flucytosine against 50 clinical A. fumigatus isolates from our private fungus culture collection was determined by using the microdilution format of the CLSI (formerly the NCCLS) M38-A protocol (6). The isolates were cultured from clinical specimens from patients admitted to the Radboud University Medical Center and other Dutch hospitals between 1999 and 2005. All isolates were tested in duplicate at pH 7.0 and pH 5.0, and the MIC was read as the 50% inhibition of growth compared to the growth for the control. The pH was adjusted to 5.0 by using 100 mM citrate buffer.
Female CD-1 outbred mice (weight, 20 to 29 g; Charles River Laboratories, Sulzfeld, Germany) were used. The animal studies were conducted in accordance with the recommendations of the European Community (Directive 86/609/EEC, 24 November 1986) and were approved by the institutional animal care and use committee of Radboud University. Inocula were prepared as conidial suspensions in saline containing 0.05% Tween 80, counted microscopically, and adjusted to the required concentration. Mice were infected with the 90% lethal dose (LD90) by injection of 0.1 ml of the conidial suspension into the orbital vein.
Flucytosine was dissolved in distilled water, according to the instructions of the manufacturer, and administered intraperitoneally. Treatment was begun 2 h after infection. Groups of 10 mice each were treated for 7 days with 100 mg/kg of body weight every 6 h (q6h), 100 mg/kg every 12 h (q12h), 200 mg/kg q6h, and 200 mg/kg q12h. Control mice were infected but received only distilled water. Animals were checked twice daily for clinical signs and mortality. Mortality was recorded every day for up to 8 days after the end of treatment. Statistical analyses were performed with the GraphPad Prism (version 4.00) program (GraphPad Software, San Diego, CA). Statistical significance was defined as a P value of <0.05.
The median MIC at pH 7.0 was 128 mg/liter (range, 16 to 1,024 mg/liter), whereas at pH 5.0 it was 0.125 mg/liter (range, 0.063 to 512 mg/liter). The geometric mean decrease in the MIC was 9.69 twofold dilution steps (Fig. 1). Only two isolates showed a less significant decrease. One of these (isolate AZN 58, which was flucytosine resistant) was selected to determine the in vivo efficacy of flucytosine monotherapy. The MIC of this isolate at pH 5.0 was 512 mg/liter (Fig. 1). The efficacy of flucytosine against this isolate was compared with that against an isolate (isolate AZN 8196, which was flucytosine susceptible) that was previously shown to respond to flucytosine monotherapy (9). This isolate showed a decrease in the MIC from 512 mg/liter at pH 7.0 to 0.125 mg/liter at pH 5.0.
FIG. 1.
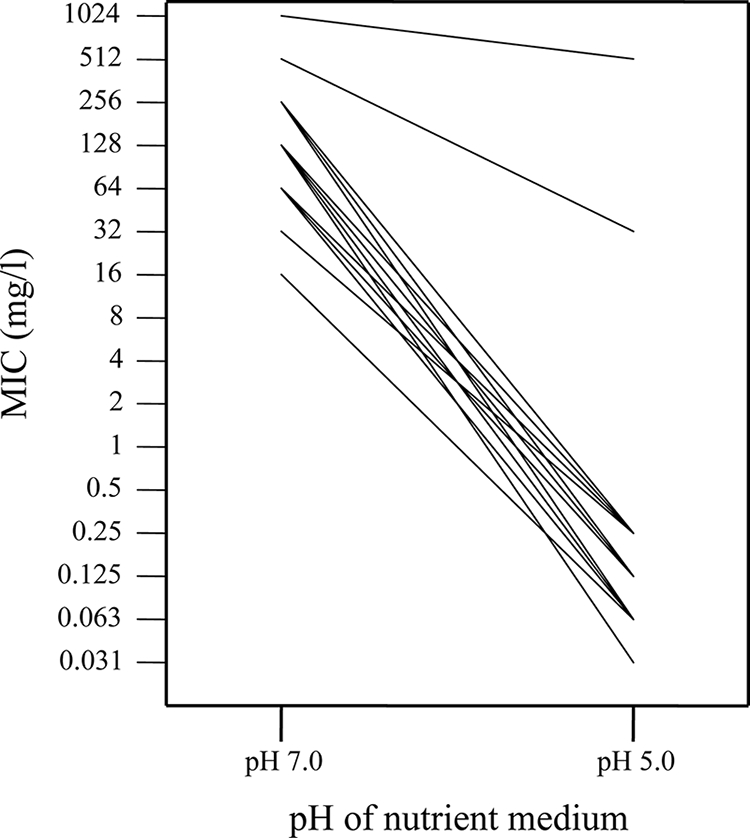
Impact of the pH of the nutrient medium on the in vitro activity of flucytosine against 50 clinical A. fumigatus isolates.
The LD90s were 1 × 107 CFU/mouse for both A. fumigatus isolates, and the survival curves demonstrated that both A. fumigatus isolates caused lethal infections in control mice (Fig. 2). The median survival time of the control mice infected with the flucytosine-resistant A. fumigatus isolate (3.5 days) did not differ significantly from that of control mice infected with the flucytosine-susceptible isolate (4.5 days) (P = 0.33). Figure 2A shows the survival results for mice infected with the flucytosine-resistant A. fumigatus isolate. Treatment with different regimens of flucytosine did not significantly prolong the survival of the treated mice compared to that of the control mice (P > 0.33). Figure 2B shows the survival results for mice infected with the flucytosine-susceptible isolate. Treatment with flucytosine at 100 mg/kg q6h, 200 mg/kg q6h, and 200 mg/kg q12h significantly prolonged the survival of the treated mice compared to that of the control mice (P < 0.01), while treatment with flucytosine at 100 mg/kg q12h did not (P = 0.19).
FIG. 2.
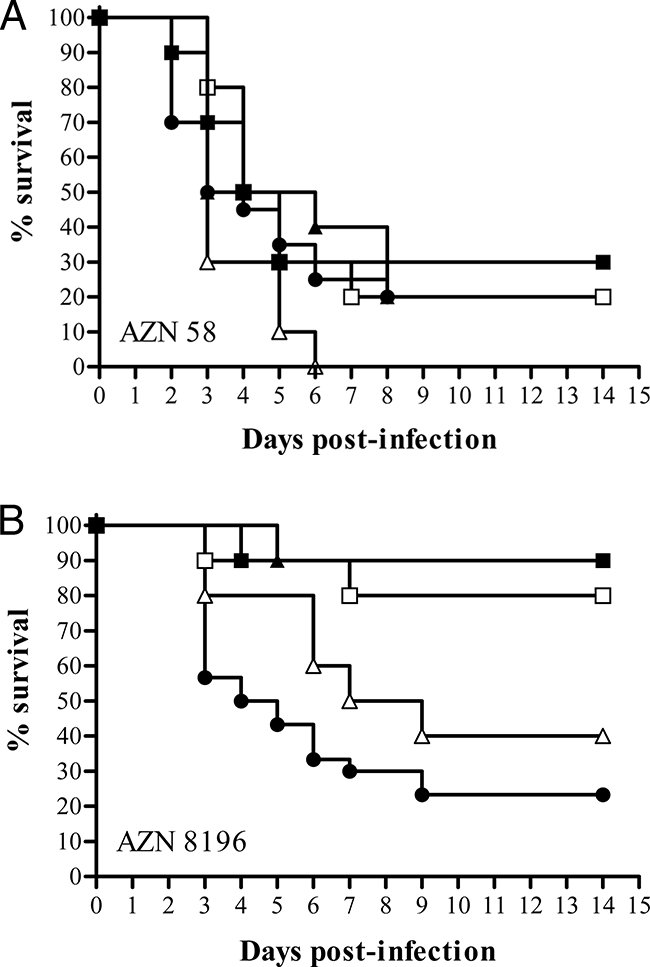
Cumulative mortality of mice infected with a flucytosine-resistant A. fumigatus isolate (isolate AZN 58) (A) compared with that of mice infected with a flucytosine-susceptible isolate (AZN 8196) (B) (9) in groups treated with flucytosine and control groups. Groups of 10 mice each were treated with flucytosine for 7 days starting 2 h after inoculation. ▪, 200 mg/kg q6h; □, 200 mg/kg q12h; ▴, 100 mg/kg q6h; Δ, 100 mg/kg q12h; •, controls.
The in vivo outcome of flucytosine monotherapy against the two A. fumigatus isolates correlated well with the in vitro susceptibilities obtained with RPMI 1640 medium at pH 5.0. Although the CLSI method has improved the intra- and interlaboratory agreements, the correlation between the results obtained by this method and the clinical outcome has not been clearly established for some antifungal agents. In this study, we showed that the in vitro results for flucytosine against A. fumigatus obtained by the CLSI method correlated with the in vivo outcome only when the pH of the medium was 5.0, similar to the observations reported from studies with C. neoformans (12). The results show that efficacy to nearly maximum survival can be obtained with the highest daily dose of flucytosine used (800 mg/kg) if the strain is susceptible. Because the half-life of about 0.5 h in mice is shorter than the one of 2.5 to 5 h in humans, the exposure during this dosing regimen is comparable to that in humans during a regimen of 150 mg/kg/day (1, 9, 13).
Flucytosine is taken up by fungal cells by the enzyme cytosine permease (7). This carrier system is an active process which occurs via H+ symport, which is required for proton expulsion (4). Once it is inside the fungal cell, flucytosine is rapidly deaminated to 5-fluorouracil by means of the enzyme cytosine deaminase (10). In Saccharomyces cerevisiae, the base-transport activity parameters (Km and Vmax) are dependent on the concentration of the cosubstrate H+ (3). Furthermore, an increase in the extracellular proton concentration (i.e., a decrease in the pH) resulted in a decrease in the Km values for adenine, hypoxanthine, and cytosine uptake and a maximum Vmax value at about pH 5.0 (2). This could explain the increased activity of flucytosine against A. fumigatus at pH 5.0, due to the increased uptake of the drug. In IA, the pH at the site of infection might be less than 7.4 due to the metabolism of glucose by Aspergillus and the subsequent formation of organic acids, as suggested before (9).
This study provides evidence that the majority of clinical A. fumigatus isolates are susceptible to flucytosine at pH 5.0 and that for two isolates the MIC at this pH corresponded to in vivo efficacy, but this was not the case for the MICs determined at pH 7.0. Flucytosine could potentially be of clinical benefit for the treatment of abscesses in the brain or bones. Given the increased interest in combination therapy for IA, it might be appropriate to investigate flucytosine further in preclinical studies.
Acknowledgments
Part of this study was supported by an unrestricted grant from Valeant Pharmaceuticals.
Footnotes
Published ahead of print on 15 September 2008.
REFERENCES
- 1.Böhme, A., M. Ruhnke, D. Buchheidt, M. Karthaus, H. Einsele, S. Guth, G. Heussel, C. P. Heussel, C. Junghanss, W. K. Kern, T. Kubin, G. Maschmeyer, O. Sezer, G. Silling, T. Südhoff, H. Szelényi Dagger, and A. J. Ullmann. 2003. Treatment of fungal infections in hematology and oncology—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann. Hematol. 82(Suppl. 2):S133-S140. [DOI] [PubMed] [Google Scholar]
- 2.Brèthes, D., C. Napias, E. Torchut, and J. Chevallier. 1992. Purine-cytosine permease of Saccharomyces cerevisiae. Effect of external pH on nucleobase uptake and binding. Eur. J. Biochem. 210:785-791. [DOI] [PubMed] [Google Scholar]
- 3.Forêt, M., R. Schmidt, and U. Reichert. 1978. On the mechanism of substrate binding in the purine-transport system of Saccharomyces cerevisiae. Eur. J. Biochem. 82:33-43. [DOI] [PubMed] [Google Scholar]
- 4.Griffin, D. H. 1994. Nutrient acquisition: digestion and transport, p. 159-188. In D. H. Griffin (ed.), Fungal physiology. Wiley-Liss, New York, NY.
- 5.Kirby, A., I. Hassan, and J. Burnie. 2006. Recommendations for managing Aspergillus osteomyelitis and joint infections based on a review of the literature. J. Infect. 52:405-414. [DOI] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 7.Polak, A., and M. Grenson. 1973. Evidence for a common transport system for cytosine, adenine and hypoxanthine in Saccharomyces cerevisiae and Candida albicans. Eur. J. Biochem. 32:276-282. [DOI] [PubMed] [Google Scholar]
- 8.te Dorsthorst, D. T. A., J. W. Mouton, C. J. P. van den Beukel, H. A. L. van der Lee, J. F. G. M. Meis, and P. E. Verweij. 2004. Effect of pH on the in vitro activities of amphotericin B, itraconazole, and flucytosine against Aspergillus isolates. Antimicrob. Agents Chemother. 48:3147-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.te Dorsthorst, D. T. A., P. E. Verweij, J. F. G. M. Meis, and J. W. Mouton. 2005. Efficacy and pharmacodynamics of flucytosine monotherapy in a nonneutropenic murine model of invasive aspergillosis. Antimicrob. Agents Chemother. 49:4220-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermes, A., H.-J. Guchelaar, and J. Dankert. 2000. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 46:171-179. [DOI] [PubMed] [Google Scholar]
- 11.Verweij, P. E., J. P. Donnelly, B. J. Kullberg, J. F. Meis, and B. E. De Pauw. 1994. Amphotericin B versus amphotericin B plus 5-flucytosine: poor results in the treatment of proven systemic mycoses in neutropenic patients. Infection 22:81-85. [DOI] [PubMed] [Google Scholar]
- 12.Viviani, M. A., M. C. Esposto, M. Cogliati, and A. M. Tortorano. 2003. Flucytosine and cryptococcosis: which in vitro test is the best predictor of outcome? J. Chemother. 15:124-128. [DOI] [PubMed] [Google Scholar]
- 13.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, T. F. Patterson, et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]


