Abstract
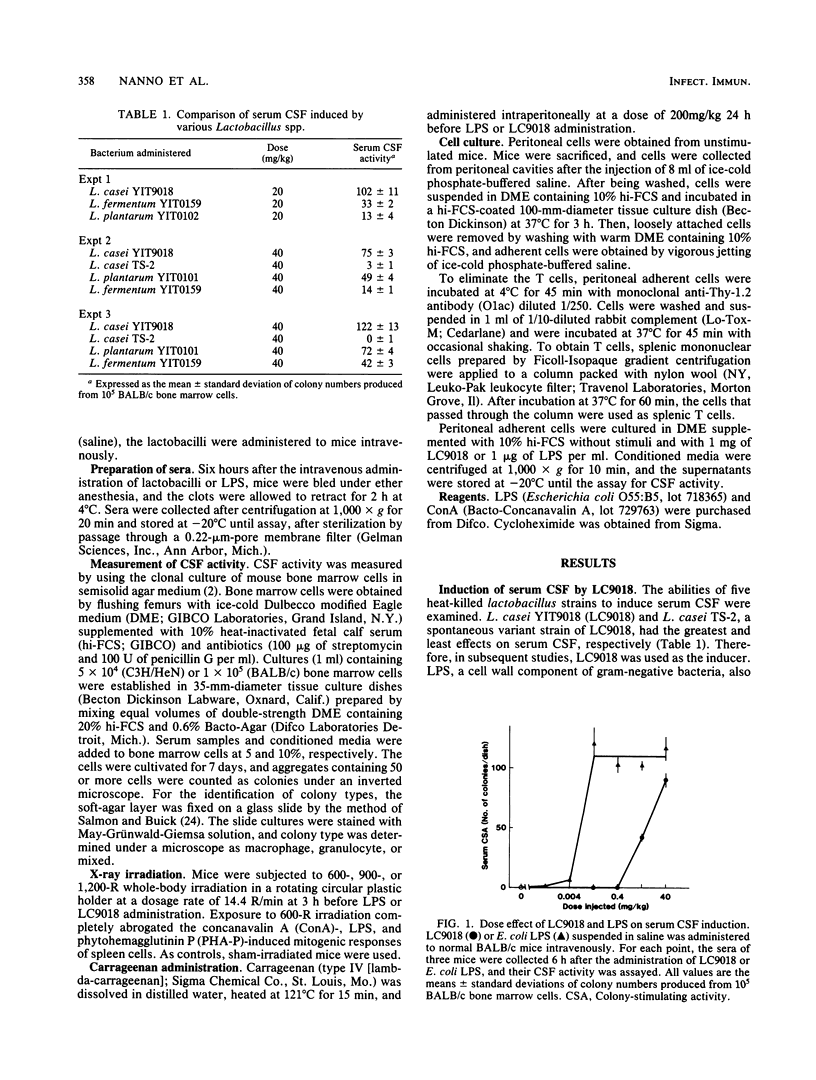
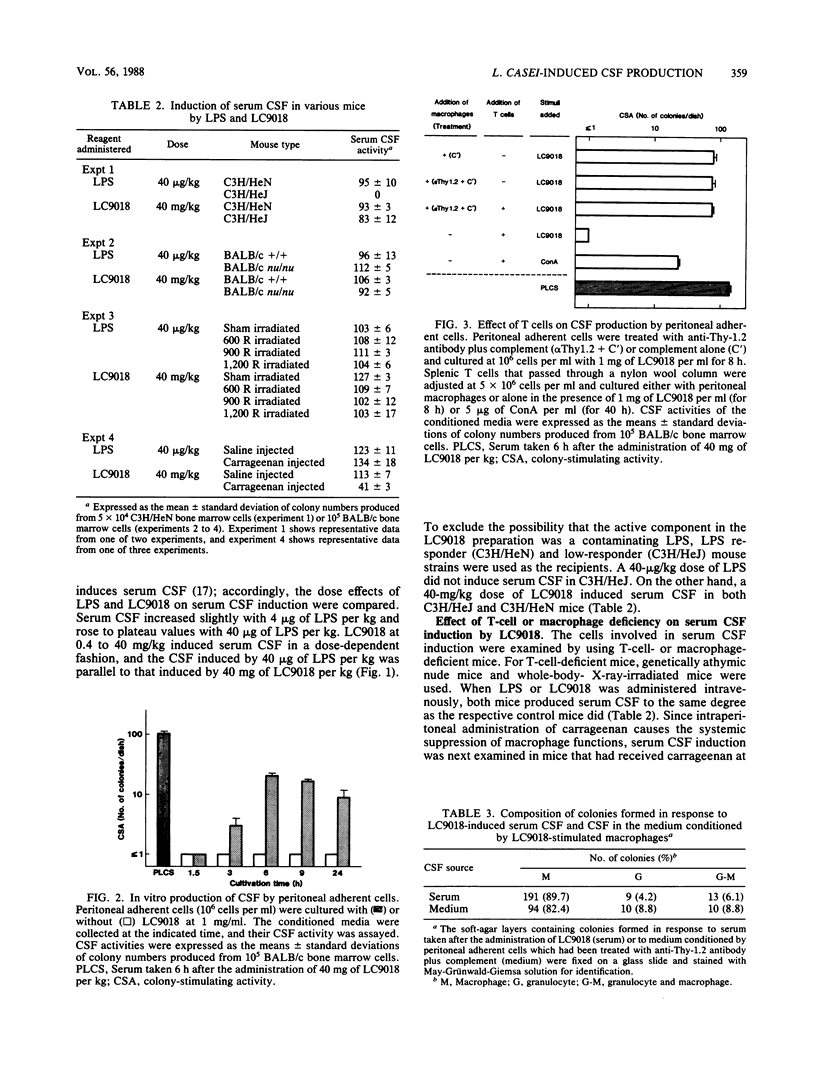
Heat-killed Lactobacillus casei YIT9018 (LC9018), when injected intravenously into mice at a dose of 4 to 40 mg/kg, induced the production of serum colony-stimulating factor (CSF). Since this induction was observed in both C3H/HeJ and C3H/HeN mice, LC9018 was considered to act differently from lipopolysaccharide. The amount of serum CSF induced by LC9018 in nude mice and whole-body-X-ray-irradiated mice was similar to that in control mice, but the induction of serum CSF was suppressed by the previous administration of carrageenan, indicating that macrophages, but not T cells, were responsible for serum CSF induction by LC9018. To determine whether macrophages themselves produce CSF or help other cells produce CSF in response to LC9018, we prepared adherent cells from the peritoneal cavity of normal mice and examined CSF activity in their conditioned media. Peritoneal adherent cells did not produce CSF without LC9018, but when cultivated with 1 mg of LC9018 per ml, they produced CSF at the same time that serum CSF was induced after the intravenous administration of LC9018. Additionally, in vitro-induced CSF formed macrophage, granulocyte, and mixed colonies, as serum CSF did. CSF production by peritoneal adherent cells was completely inhibited by cycloheximide (50 micrograms/ml), and neither the elimination of T cells from the peritoneal adherent cells by treating them with anti-Thy-1.2 antibody plus complement nor the addition of T cells affected CSF production. These results suggest that heat-killed LC9018 induces serum CSF in mice via direct stimulation of macrophages to produce CSF de novo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby G. C., Jr, Rigas V. D., Bennett R. M., Vandenbark A. A., Garewal H. S. Interaction of lactoferrin, monocytes, and T lymphocyte subsets in the regulation of steady-state granulopoiesis in vitro. J Clin Invest. 1981 Jul;68(1):56–63. doi: 10.1172/JCI110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Byrne P. V., Guilbert L. J., Stanley E. R. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Stone D. K., Kamen R., Wong G. G., Sehgal P. K., Nathan D. G., Clark S. C. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. 1986 Jun 26-Jul 2Nature. 321(6073):872–875. doi: 10.1038/321872a0. [DOI] [PubMed] [Google Scholar]
- Eaves A. C., Bruce W. R. In vitro production of colony-stimulating activity. I. Exposure of mouse peritoneal cells to endotoxin. Cell Tissue Kinet. 1974 Jan;7(1):19–30. doi: 10.1111/j.1365-2184.1974.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Foster R. S., Jr, MacPherson B. R., Browdie D. A. Effect of Corynebacterium parvum on colony-stimulating factor and granulocyte-macrophage colony formation. Cancer Res. 1977 May;37(5):1349–1355. [PubMed] [Google Scholar]
- Freedman V. H., Calvelli T. A., Silagi S., Silverstein S. C. Macrophages elicited with heat-killed bacillus Calomette-Guérin protect C57BL/6J mice against a syngeneic melanoma. J Exp Med. 1980 Sep 1;152(3):657–673. doi: 10.1084/jem.152.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galelli A., Chedid L. Modulation of myelopoiesis in vivo by synthetic adjuvant-active muramyl peptides: induction of colony-stimulating activity and stimulation of stem cell proliferation. Infect Immun. 1983 Dec;42(3):1081–1085. doi: 10.1128/iai.42.3.1081-1085.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galelli A., Lefrancier P., Chedid L. Colony-stimulating activity induced by synthetic muramyl peptides: variation with chemical structure and association with anti-infectious activity. Infect Immun. 1984 Nov;46(2):495–500. doi: 10.1128/iai.46.2.495-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Ikehara S., Pahwa R. N., Fernandes G., Hansen C. T., Good R. A. Functional T cells in athymic nude mice. Proc Natl Acad Sci U S A. 1984 Feb;81(3):886–888. doi: 10.1073/pnas.81.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Kobayashi S., Yokokura T., Mutai M. Antitumor activity of Lactobacillus casei in mice. Gan. 1981 Aug;72(4):517–523. [PubMed] [Google Scholar]
- Kato I., Yokokura T., Mutai M. Macrophage activation by Lactobacillus casei in mice. Microbiol Immunol. 1983;27(7):611–618. doi: 10.1111/j.1348-0421.1983.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Kelso A., Metcalf D. Clonal heterogeneity in colony stimulating factor production by murine T lymphocytes. J Cell Physiol. 1985 Apr;123(1):101–110. doi: 10.1002/jcp.1041230115. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of cell surface receptors for different hematopoietic growth factors on myeloid leukemic cells. EMBO J. 1986 Sep;5(9):2163–2170. doi: 10.1002/j.1460-2075.1986.tb04480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Nanno M., Ohwaki M., Mutai M. Induction by Lactobacillus casei of increase in macrophage colony-forming cells and serum colony-stimulating activity in mice. Jpn J Cancer Res. 1986 Jul;77(7):703–710. [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Metcalf D. Similar molecular properties of granulocyte-macrophage colony-stimulating factors produced by different mouse organs in vitro and in vivo. J Biol Chem. 1979 Jun 25;254(12):5290–5299. [PubMed] [Google Scholar]
- Olivotto M., Bomford R. In vitro inhibition of tumour cell growth and DNA synthesis by peritoneal and lung macrophages from mice injected with Corynebacterium parvum. Int J Cancer. 1974 Apr 15;13(4):478–488. doi: 10.1002/ijc.2910130406. [DOI] [PubMed] [Google Scholar]
- Platzer E., Rubin B. Y., Lu L., Welte K., Broxmeyer H. E., Moore M. A. OKT3 monoclonal antibody induces production of colony-stimulating factor(s) for granulocytes and macrophages in cultures of human T lymphocytes and adherent cells. J Immunol. 1985 Jan;134(1):265–271. [PubMed] [Google Scholar]
- Prestidge R. L., Watson J. D., Urdal D. L., Mochizuki D., Conlon P., Gillis S. Biochemical comparison of murine colony-stimulating factors secreted by a T cell lymphoma and a myelomonocytic leukemia. J Immunol. 1984 Jul;133(1):293–298. [PubMed] [Google Scholar]
- Salmon S. E., Buick R. N. Preparation of permanent slides of intact soft-agar colony cultures of hematopoietic and tumor stem cells. Cancer Res. 1979 Mar;39(3):1133–1136. [PubMed] [Google Scholar]
- Staber F. G., Hültner L., Marcucci F., Krammer P. H. Production of colony-stimulating factors by murine T cells in limiting dilution and long-term cultures. Nature. 1982 Jul 1;298(5869):79–82. doi: 10.1038/298079a0. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Sullivan R., Gans P. J., McCarroll L. A. The synthesis and secretion of granulocyte-monocyte colony-stimulating activity (CSA) by isolated human monocytes: kinetics of the response to bacterial endotoxin. J Immunol. 1983 Feb;130(2):800–807. [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Williams Z., Hertogs C. F., Pluznik D. H. Use of mice tolerant to lipopolysaccharide to demonstrate requirement of cooperation between macrophages and lymphocytes to generate lipopolysaccharide-induced colony-stimulating factor in vivo. Infect Immun. 1983 Jul;41(1):1–5. doi: 10.1128/iai.41.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Ampel N. M., Waheed A., Shadduck R. K. Macrophage colony-stimulating factor (M-CSF) enhances the capacity of murine macrophages to secrete oxygen reduction products. J Immunol. 1985 Sep;135(3):2052–2056. [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasutake N., Kato I., Ohwaki M., Yokokura T., Mutai M. Host-mediated antitumor activity of Lactobacillus casei in mice. Gan. 1984 Jan;75(1):72–80. [PubMed] [Google Scholar]
- Yasutake N., Ohwaki M., Mutai M., Koide Y., Yoshida T. Anti-tumour effect of humoral and cellular immunities mediated by a bacterial immunopotentiator, Lactobacillus casei, in mice. Cancer Immunol Immunother. 1985;20(2):109–116. doi: 10.1007/BF00205676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokokura T., Nomoto K., Shimizu T., Nomoto K. Enhancement of hematopoietic response of mice by subcutaneous administration of Lactobacillus casei. Infect Immun. 1986 Apr;52(1):156–160. doi: 10.1128/iai.52.1.156-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


