Abstract
The ink of sea hares (Aplysia californica) contains escapin, an l-amino acid oxidase that metabolizes l-lysine, thereby producing a mixture that kills microbes and deters attacking predators. This secretion contains H2O2, ammonia, and an equilibrium mixture of “escapin intermediate product” (EIP-K) that includes α-keto-ɛ-aminocaproic acid and several other molecules. Components of the equilibrium mixture react nonenzymatically with H2O2 to form “escapin end product” (EEP-K), which contains δ-aminovaleric acid and δ-valerolactam. The proportions of the molecules in this equilibrium mixture change with pH, and this is biologically important because the secretion is pH 5 when released but becomes pH 8 when fully diluted in seawater. The goal of the current study was to identify which molecules in this equilibrium mixture are bactericidal. We show that a mixture of H2O2 and EIP-K, but not EEP-K, at low mM concentrations is synergistically responsible for most of the bactericidal activity of the secretion against Escherichia coli, Vibrio harveyi, Staphylococcus aureus, and Pseudomonas aeruginosa. Low pH enhances the bactericidal effect, and this does not result from stress associated with low pH itself. Sequential exposure to low mM concentrations of EIP-K and H2O2, in either order, does not kill E. coli. Reaction products formed when l-arginine is substituted for l-lysine have almost no bactericidal activity. Our results favor the idea that the bactericidal activity is due to unstable intermediates of the reaction of α-keto-ɛ-aminocaproic acid with H2O2.
Defensive chemical secretions are produced by many animals, including a marine snail, the sea hare Aplysia californica. When vigorously attacked by predators, sea hares release an inky secretion (see Fig. S1 in the supplemental material). This ink secretion is a mixture of the products of two glands: a purplish ink from the ink gland and a whitish, mucousy ink from the opaline gland (7, 21). This secretion protects sea hares through a diverse number of chemicals and mechanisms, some acting on predators to abate their attack and others acting on conspecific sea hares as alarm cues to evoke escape behaviors (3, 4, 7-9, 12-14, 17, 19).
One of the bioactive ingredients in the ink gland secretion of A. californica is escapin, an l-amino acid oxidase (l-AAO). Escapin has orthologs in the ink of other sea hare species and paralogs in the eggs and other tissues of A. californica (reviewed in references 3 and 10). Substrates of escapin and its homologs include l-lysine and l-arginine (22). However, l-lysine is the major natural substrate, since the concentration of l-lysine in the secretion is much higher than that of l-arginine. In the A. californica secretion, l-lysine and l-arginine are present at concentrations of 145 and 0.5 mM, respectively (4, 8).
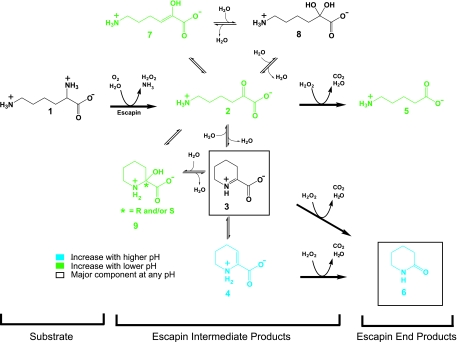
The chemistry of the escapin/l-lysine pathway is summarized in Fig. 1. This series of chemical reactions produces products that interact with each other to form a diverse group of molecules at low millimolar concentrations within a few seconds of the release of the secretion (M. Kamio, K.-C. Ko, S. Zheng, B. Wang, S. L. Collins, G. Gadda, P. C. Tai, and C. D. Derby, submitted for publication). The first step is escapin's oxidative deamination of l-lysine (compound 1), which produces an equilibrium mixture of compounds that we call “escapin intermediate products of lysine” (EIP-K); they include α-keto-ɛ-aminocaproic acid (compound 2), Δ1-piperideine-2-carboxylic acid (compound 3), Δ2-piperideine-2-carboxylic acid (compound 4), 6-amino-2-hydroxy-hex-2-enoic acid (compound 7), possibly 6-amino-2,2-dihydroxy-hexanoic acid (compound 8), 2-hydroxy-piperidine-2-carboxylic acid (compound 9), H2O2, and ammonium. Three of these compounds, compounds 2, 3, and 4, then react nonenzymatically with H2O2 to yield a mixture of δ-aminovaleric acid (compound 5) and δ-valerolactam (compound 6), which we call “escapin end products of l-lysine” (EEP-K). The pH of A. californica ink is ∼5.0 at full strength, in contrast to a pH of ∼8.0 for seawater (18). This is significant because pH affects the equilibrium among the escapin reaction products: though the cyclic forms 3 and 6 dominate at any pH, the naturally low pH of the secretion favors the linear forms 2 and 5 (9a).
FIG. 1.
Summary of the chemistry of the reaction of escapin with l-lysine, including the effects of pH on the relative composition of the molecular species in the equilibrium mixture.
Sea hare l-AAOs have been of interest primarily to biomedical researchers in search of antitumor and antimicrobial compounds (reviewed in references 3 and 10). There is very little known about antipredatory functions of molecules in the pathway of sea hare l-AAOs (1, 9, 19). Most studies of sea hare and other gastropod l-AAOs identified H2O2 as the primary antimicrobial product of this pathway, though other compounds are implicated (reviewed in references 3, 10, 11, and 15). Escapin was tested on 11 types of microbes, and it was found to be bacteriostatic for all (22). Against Escherichia coli, H2O2 at concentrations below 10 mM accounts for most of the bacteriostatic effects of escapin's products, but it contributes relatively little (10× reduction in bacterial number) compared to the powerful bactericidal effects of escapin's products (up to 107× reduction) (22). The reaction of escapin with l-arginine produces a mixture with almost no bactericidal activity, even though the product contains more H2O2 than the product of the reaction of escapin with l-lysine (22). This demonstrates that compounds other than H2O2 are responsible for the bactericidal effects of escapin.
The goal of this study was to identify the bioactive components of the complex equilibrium mixture of the escapin/l-lysine pathway by examining their bactericidal effects. We show that only a few of these compounds, in specific combinations, are bactericidal.
MATERIALS AND METHODS
Animals.
Sea hares (Aplysia californica Cooper 1863) were collected in California by Marinus Scientific (Garden Grove, CA). The sea hares were dissected on the day of arrival in our laboratory. Experiments were performed according to Georgia State University regulations and national guidelines.
Collection of ink and isolation of escapin.
Ink glands were dissected from anesthetized animals and frozen at −80°C until they were used. Purple ink was collected by gently squeezing dissected ink glands in a petri dish with the blunt end of a scalpel handle. Escapin (ATCC accession no. AY615888) was isolated and purified by using an ÄKTA 100 automated fast protein liquid chromatography system. A two-step purification process involving an initial size separation followed by a purification using a cation-exchange Mono S column was performed according to the method of Yang et al. (22).
Preparation of the products of oxidation of l-amino acids by escapin.
To produce EIP-K or EIP-R, we typically incubated 55 mM l-lysine or l-arginine monohydrochloride, 1 × 10−3 mg/ml escapin, and 0.13 mg/ml catalase in deionized water at 30°C on a shaker for up to 24 h. This solution was filtered using an Amicon Ultra-4 centrifugal filter device (Millipore Corp., Billerica, MA) to remove escapin and catalase and then stored it at −80°C until it was used further. To produce EEP-K or EEP-R, we performed the same procedure except catalase was not added to it. The concentration of the EIP-K mixture was expressed as the starting concentration of l-lysine.
Chemicals and the synthesis of components in EIP-K.
l-Lysine (compound 1), δ-aminovaleric acid (compound 5), δ-valerolactam (compound 6), l-arginine, catalase, and H2O2 were purchased from Sigma-Aldrich (St. Louis, MO). We synthesized a solution containing α-keto-ɛ-aminocaproic acid (compound 2), the dehydropipecolinic acid enamine/imine tautomer mixture (compounds 3 and 4), 6-amino-2-hydroxy-hex-2-enoic acid (compound 7), 2-hydroxy-piperidine-2-carboxylic acid (compound 9), and possibly 6-amino-2,2-dihydroxy-hexanoic acid (compound 8), as performed by Kamio et al. (9a); we call this synthetic EIP-K. Since both synthetic EIP-K and natural EIP-K are dominated by compound 3, we quantified their concentration using the NMR signal of compound 3 with 50 nmol of 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TSP-d4; Sigma-Aldrich) as an internal standard and expressed the concentration relative to the starting concentration of l-lysine.
Bactericidal assay.
Escherichia coli strains MC4100 and C921-b2, Vibrio harveyi strain BB170, Staphylococcus aureus strain ATCC 6835, and Pseudomonas aeruginosa strain PAO1 were used in our assays. Bacteria were incubated until they reached a density of ∼3 × 108 cells/ml in Luria-Bertani medium (LB) at pH 7, and they were then transferred to LB at pH 7 or LB with adjusted pH buffer. Bacteria were treated with chemicals and controls in a Thermomixer (37°C; Eppendorf) for 10 min. Samples were serially diluted and plated onto petri dishes with solid LB at pH 7 and incubated overnight at 37°C. Viable cell counts were determined by enumeration of CFU with appropriate dilutions. Given that l-lysine is present at ∼145 mM in full-strength secretion (4) and given the kinetics of escapin under natural conditions, escapin is expected to generate 1 to 10 mM of H2O2 and other intermediate products within 10 s (9a). Thus, most of our bioassays used low mM concentrations of products. The statistical significance of bactericidal effects was analyzed with paired t tests or analysis of variance with an α value of 0.05.
RESULTS AND DISCUSSION
Bactericidal activity of the products of escapin's intermediate and end products of l-lysine and l-arginine.
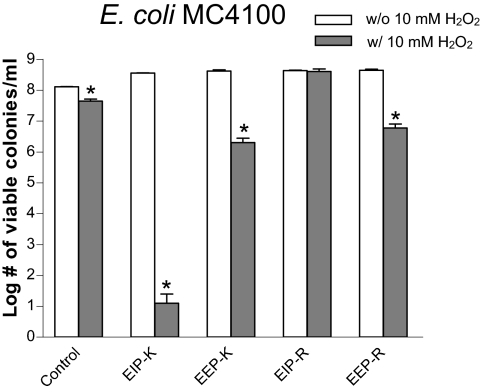
We used Escherichia coli MC4100 to test the bactericidal activity of products generated by the oxidative deamination of l-lysine or l-arginine by escapin at pH 7 (Fig. 2). The mixture of 45 mM EIP-K and 10 mM H2O2 produced by far the greatest bactericidal activity, a >7-log unit reduction in cell number. Its effect was significantly greater than that of either EIP-K alone (no reduction from the control) or H2O2 alone (∼1-log unit reduction, which is significantly greater than that of its control). The mixture of 45 mM EEP-K and 10 mM H2O2 produced a ∼2-log unit reduction in cell number, which was a greater bactericidal effect than that seen with H2O2 but much less than that seen with EIP-K and H2O2. The mixture of EIP-R and H2O2 was not bactericidal (the same as EIP-R alone), even though l-arginine was as effective as l-lysine as a substrate for escapin. EEP-R was not bactericidal. EEP-R with H2O2 was significantly though mildly bactericidal, producing a ∼2-log unit reduction in cell number.
FIG. 2.
Bactericidal effects of escapin intermediate products and escapin end products in the absence (w/o) or presence (w/) of 10 mM H2O2, at pH 7, on E. coli MC4100. Concentrations of the intermediates and end products are based on a starting substrate concentration of 45 mM l-lysine (for EIP-K and EEP-K) or 45 mM l-arginine (for EIP-R and EEP-R). Values are means ± standard errors of the means for three replicates from a representative experiment. Asterisks indicate that the value for product with H2O2 is significantly lower than that for product without H2O2 (P < 0.05; paired t test).
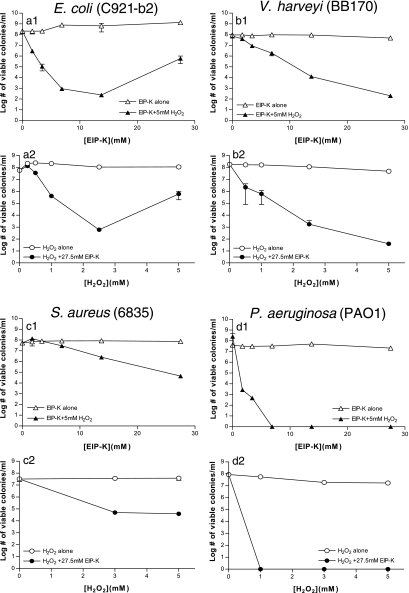
The bactericidal effects of EIP-K, H2O2, and EIP-K + H2O2 were then compared for four bacteria: Escherichia coli C921-b2, a nonvirulent form of a pathogenic strain of this gram-negative species; Vibrio harveyi, a gram-negative marine species; Staphylococcus aureus, a gram-positive and pathogenic species; and Pseudomonas aeruginosa PAO1, a gram-negative pathogenic species (Fig. 3). These four species were used because escapin was previously shown to be inhibitory against each of them (22). Our results show that EIP-K alone up to 27.5 mM (Fig. 3a1 to d1) or H2O2 alone up to 5 mM (Fig. 3a2 to d2) was not bactericidal against any of them. However, when mixed, EIP-K and H2O2 were potently and significantly bactericidal, in a concentration-dependent manner (P < 0.05; analysis of variance). The effect was strongest for P. aeruginosa and weakest for S. aureus. The bactericidal effect on E. coli was lower at the highest concentration, 27.5 mM EIP-K + 5 mM H2O2. Our finding that P. aeruginosa is extremely sensitive to these chemicals is of interest, given that this bacterium is a human pathogen that is often highly resistant to antimicrobial agents (2).
FIG. 3.
Bactericidal effects of EIP-K, H2O2, and EIP-K + H2O2, for four species of bacteria: Escherichia coli strain C921-b2, Vibrio harveyi strain BB170, Staphylococcus aureus strain 6835, and Pseudomonas aeruginosa strain PAO1. Each figure depicts results of one to four experiments, with three replicates per experiment. Panels a1 to d1 depict concentration-response functions for EIP-K alone or EIP-K with 5 mM H2O2. Panels a2 to d2 depict concentration-response functions for H2O2 alone or H2O2 with 27.5 mM EIP-K. The antimicrobial effect was greater for the mixture of EIP-K and H2O2 than for either individual component.
We used E. coli C921-b2 as a representative species in all subsequent experiments to determine mechanisms of the bactericidal effect.
Identification of bactericidal compounds in escapin's pathway.
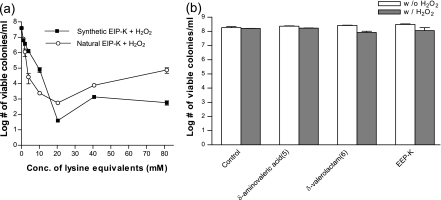
We compared the bactericidal activity of natural EIP-K (generated as in the previous experiment using escapin) and the bactericidal activity of synthetic EIP-K (synthesized as performed by Kamio et al. [9a] and which contains an equilibrium mixture of compounds 2 to 4 and 7 to 9). Both are mixtures dominated by compound 3. The two mixtures, when in the presence of H2O2, showed similar bactericidal activities (Fig. 4a). The bioactivity was concentration dependent up to 20 mM. The similarity of these functions supports the conclusion that the bactericidal activity of natural EIP-K is due to the interaction of one or more of the components of the equilibrium mixture of compounds 2 to 4 and 7 to 9 with H2O2. EIP-K, either natural or synthetic, at high concentrations and in the presence of 5 mM H2O2 was less bactericidal than EIP-K at some lower concentrations (Fig. 4a; see also Fig. 3). This type of concentration-response relationship is known as the Eagle effect and has been reported for a variety of microbes and antimicrobial agents (5, 6, 20). The mechanism responsible for the Eagle effect in our study is unknown, but it is worth noting that the curve was U-shaped for either a variable concentration of EIP-K in a constant concentration of H2O2 or a variable concentration of H2O2 in a constant concentration of EIP-K (Fig. 3a1 and a2). Also, the concentrations at which the inflection in the curve occurred differed, though inflections typically occurred at >20 mM EIP-K, as can be seen by the relatively high variance for this concentration of EIP-K (Fig. 3a1 and a2).
FIG. 4.
Bactericidal effects of escapin products on E. coli C921-b2. (a) Effects of naturally produced intermediates of action of escapin on l-lysine versus synthesized lysine intermediate products, tested in the presence of 5 mM H2O2 at pH 7. Both mixtures are dominated by compound 3, so concentrations are defined in lysine equivalents using the nuclear magnetic resonance signal of compound 3 and TSP-d4 as a standard (see Materials and Methods). Conc., concentration. Values are means ± standard errors of the means (two to four experiments, each run in triplicate). (b) Bactericidal effects of 55 mM EEP-K and identified end products, δ-aminovaleric acid (compound 5) and δ-valerolactam (compound 6), whose concentrations are the same as those in EEP-K in the absence (w/o) or presence (w/) of 5 mM H2O2 at pH 7. Values are means ± standard errors of the means (two to four experiments, each run in triplicate).
The components of EEP-K, i.e., δ-aminovaleric acid (compound 5) and δ-valerolactam (compound 6), presented either alone or in combination with 5 mM H2O2 had very little bactericidal activity or no bactericidal activity (Fig. 4b).
These results, together with those shown in Fig. 2 and 3, demonstrate that the interaction of H2O2 with one or more of the compounds 2 to 4 or 7 to 9, but not compound 5 or 6, is responsible for the bactericidal activity.
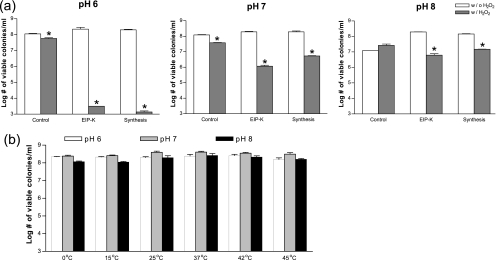
Influence of pH in the bactericidal activity.
The compositions of the reaction products of escapin change with pH of the solution (Fig. 1). The linear forms 2 and 5 become relatively more abundant under acidic conditions, although the cyclic forms 3 and 6 always dominate (9a). We expect that pH changes will affect the bactericidal activity of the mixture if some components are more bactericidal than others. Thus, to determine which components of the escapin pathway are bactericidal, we examined the effect of pH on the bactericidal activities of EIP-K and the synthetic product in the presence or absence of H2O2 (Fig. 5). The intensity of the bactericidal effect of EIP-K + H2O2 and synthetic product-H2O2 changed with pH, being greatest at pH 6, intermediate at pH 7, and virtually absent at pH 8 (Fig. 5a). This suggests that the linear form 2, when mixed with H2O2, has bactericidal activity and that the cyclic forms 3 and 4, when mixed with H2O2, do not. To determine if the stronger bactericidal effect at a lower pH was caused by attenuation of the viability of bacteria under stress, we treated bacteria with another form of stress, temperature shock, at pH values of 6 to 8 (Fig. 5b). Our results showed that pH did not affect bacterial viability at temperatures as high as 45°C, suggesting that the effect of pH is not due to a nonspecific stress but rather is due to a chemical-specific effect: the mixture of compound 2 + H2O2 is responsible for most of the bactericidal activity of escapin.
FIG. 5.
(a) Effect of pH on bactericidal activity of EIP-K and synthetic products (both at 27.5 mM) in the absence (w/o) or presence (w/) of 5 mM H2O2. Asterisks indicate that the value for the product with H2O2 is significantly lower than that for the product without H2O2 (P < 0.05; paired t test). (b) Viability of bacteria at pH values of 6 to 8 at temperatures from 0 to 45°C. Values are means ± standard errors of the means (two to four experiments, each run in triplicate).
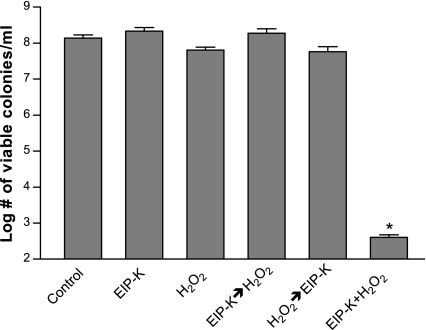
Bactericidal effect of EIP-K and H2O2 requires simultaneous presentation.
The bactericidal effect of the mixture of EIP-K and H2O2 might result either from an unstable intermediate of the reaction of H2O2 with compound 2 or from H2O2 and compound 2 acting in combination to kill bacteria. To explore the bactericidal mechanism, we presented EIP-K and H2O2 sequentially rather than simultaneously and also presented them in different orders. If the sequential presentation is bactericidal, this would be evidence against the bactericidal components being unstable intermediates. The results showed that EIP-K and H2O2 must be simultaneously presented to be bactericidal (Fig. 6), thus supporting the idea that the bioactive molecules are unstable intermediates of the reaction of compound 2 and H2O2.
FIG. 6.
Bactericidal effect of EIP-K and H2O2 presented in different temporal combinations. EIP-K (27.5 mM) and H2O2 (5 mM) were presented to bacteria at pH 7 singly for 10 min, simultaneously for 10 min (EIP-K + H2O2), sequentially with EIP-K first for 10 min followed by rinsing and then by H2O2 for 10 min (EIP-K→H2O2), sequentially with H2O2 first for 10 min followed by rinsing with LB then by EIP-K for 10 min (H2O2→EIP-K), or with neither EIP-K nor H2O2 (Control). Only EIP-K + H2O2 produced significant and substantial bactericidal activity. Values are means ± standard errors of the means (two to four experiments, each run in triplicate). An asterisk indicates that the value for the product with H2O2 is significantly lower than that for the product without H2O2 (P < 0.05; paired t test).
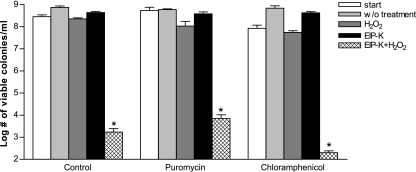
Bactericidal effect of EIP-K and H2O2 does not require protein synthesis.
The bactericidal effect of the mixture of EIP-K and H2O2 was not affected by exposure to a protein synthesis inhibitor, either puromycin or chloramphenicol (Fig. 7). This result is consistent with the results of Yang et al. (22), who used escapin in LB, and supports the conclusion that this bactericidal mechanism does not require new protein synthesis.
FIG. 7.
Effect of a protein synthesis inhibitor, chloramphenicol or puromycin, on the bactericidal activity of EIP-K + H2O2. Chloramphenicol (50 μg/ml), puromycin (100 μg/ml), or H2O (control) was presented to bacteria for 10 min and then rinsed with LB (start). EIP-K (27.5 mM), H2O2 (5 mM), EIP + H2O2, or H2O (w/o treatment) was presented to bacteria separately for another 10 min. Values are means ± standard errors of the means (two to four experiments, each run in triplicate). Asterisks indicate that the value for the product with H2O2 is significantly lower than that for the product without H2O2 (P < 0.05; paired t test).
Conclusions.
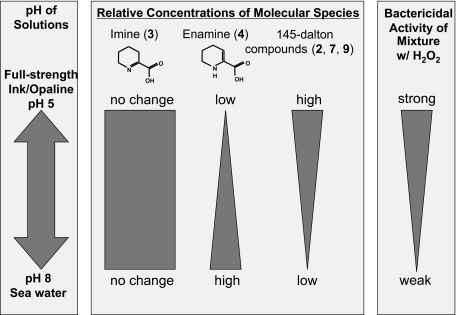
The defensive secretion of sea hares is a complex mixture, of which the molecules generated by escapin, an l-AAO that uses l-lysine as its substrate, form an equilibrium mixture whose balance depends on pH, as summarized in Fig. 1. These escapin products are both bacteriostatic and bactericidal. H2O2 accounts for most of the bacteriostatic activity and a significant amount though relatively small portion of the bactericidal activity: H2O2 at up to 10 mM kills ∼90% of the bacteria, but this is much less than the value corresponding to the mixture, which reduces bacterial levels to 10−7 times the preexposure levels. The bactericidal effect is rapid and does not require protein synthesis. A strong correlation between the effect of pH on the relative concentrations and on the bactericidal activity of molecular species in the equilibrium mixture strongly suggests that the mixture of H2O2 and α-keto-ɛ-aminocaproic acid (compound 2) accounts for most of the bactericidal activity of escapin, as depicted in Fig. 8. Tests using simultaneous or sequential presentation of compounds strongly suggest that the bactericidal effects are unstable intermediates generated by chemical interactions between compound 2 and H2O2, but the effects are not due to δ-aminovaleric acid (compound 5), the end product of the reaction of compound 2 and H2O2. Our work is consistent with other studies on l-AAOs of various types showing that H2O2 is a potent antimicrobial agent (e.g., references 15 and 16), but it provides the novel finding that other molecules generated by reactions between products of l-AAOs are much more potent antimicrobials. Future studies can focus on detailed mechanisms of this unusual synergistic bactericidal effect, identifying antipredatory components of the escapin pathway, and testing if similar effects occur for other l-AAOs.
FIG. 8.
Correlation of pH, relative concentrations of molecular species in the equilibrium mixture of EIP-K, and bactericidal effect of the mixture.
Supplementary Material
Acknowledgments
We thank Shilong Zheng for synthesizing compounds used in this study, Michiya Kamio for discussions and assistance with preparing Fig. 1 and 8, Hsiuchin Yang for technical guidance, Jonathan Beckwith (Harvard Medical School) and June Scott (Emory University) for providing E. coli MC4100 and C921-b2, respectively, and Hanan El-Mayas for critical comments on the manuscript.
This work was supported by NSF grants IBN-0324435 and 0614685, NIH grant GM-34766, a grant from the Georgia State University Brains & Behavior program, a fellowship to Ko-Chun Ko from the Georgia State University Molecular Basis of Disease Program, and the Georgia Research Alliance.
Footnotes
Published ahead of print on 13 October 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Aggio, J. F., and C. D. Derby. 2008. Hydrogen peroxide and other components in the ink of sea hares are chemical defenses against predatory spiny lobsters acting through non-antennular chemoreceptors. J. Exp. Mar. Biol. Ecol. 363:28-34. [Google Scholar]
- 2.Cornelis, P. (ed.). 2008. Pseudomonas: genomics and molecular biology. Caister Academic Press, Norfolk, United Kingdom.
- 3.Derby, C. D. 2007. Escape by inking and secreting: marine molluscs avoid predators through a rich array of chemicals and mechanisms. Biol. Bull. 213:274-289. [DOI] [PubMed] [Google Scholar]
- 4.Derby, C. D., C. E. Kicklighter, P. M. Johnson, and X. Zhang. 2007. Chemical composition of inks of diverse marine molluscs suggests convergent chemical defenses. J. Chem. Ecol. 33:1105-1113. [DOI] [PubMed] [Google Scholar]
- 5.Eagle, H., and A. D. Musselman. 1948. The rate of bactericidal action of penicillin in vitro as a function of its concentration, and its paradoxically reduced activity at high concentrations against certain organisms. J. Exp. Med. 88:99-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischhacker, M., C. Radecke, B. Schultz, and M. Ruhnke. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 27:127-131. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, P. M., and A. O. D. Willows. 1999. Defense in sea hares (Gastropoda, Opisthobranchia, Anaspidea): multiple layers of protection from egg to adult. Mar. Freshw. Behav. Physiol. 32:147-180. [Google Scholar]
- 8.Johnson, P. M., C. E. Kicklighter, M. Schmidt, M. Kamio, H. Yang, D. Elkin, W. C. Michel, P. C. Tai, and C. D. Derby. 2006. Packaging of chemicals in the defensive secretory glands of the sea hare Aplysia californica. J. Exp. Biol. 209:78-88. [DOI] [PubMed] [Google Scholar]
- 9.Kamio, M., C. Kicklighter, K.-C. Ko, M. Nusnbaum, J. Aggio, M. Hutchins, and C. Derby. 2007. Defense through chemoreception: an L-amino acid oxidase in the ink of sea hares deters predators through their chemical senses. Chem. Senses 32:A37. [Google Scholar]
- 9a.Kamio, M., K.-C. Ko, S. Zheng, B. Wang, S. Collins, G. Gadda, P. C. Tai, and C. D. Derby. The chemistry of escapin: identification and quantification of the components in the complex mixture generated by an l-amino acid oxidase in the defensive secretion of the sea snail Aplysia california. Chemistry—A European Journal, in press. [DOI] [PubMed]
- 10.Kamiya, H., R. Sakai, and M. Jimbo. 2006. Bioactive molecules from sea hares, p. 215-239. In G. Cimino and M. Gavagnin (ed.), Molluscs. From chemo-ecological study to biotechnological application. Marine molecular biotechnology. Progress in molecular and subcellular biology. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 11.Kanzawa, N., S. Shintani, K. Ohta, S. Kitajima, T. Ehara, H. Kobayashi, H. Kizaki, and T. Tsuchiya. 2004. Achacin induces cell death in HeLa cells through two different mechanisms. Arch. Biochem. Biophys. 422:103-109. [DOI] [PubMed] [Google Scholar]
- 12.Kicklighter, C. E., S. Shabani, P. M. Johnson, and C. D. Derby. 2005. Sea hares use novel antipredatory chemical defenses. Curr. Biol. 15:549-554. [DOI] [PubMed] [Google Scholar]
- 13.Kicklighter, C. E., and C. D. Derby. 2006. Multiple components in ink of the sea hare Aplysia californica are aversive to the sea anemone Anthopleura sola. J. Exp. Mar. Biol. Ecol. 334:256-268. [Google Scholar]
- 14.Kicklighter, C. E., M. W. Germann, M. Kamio, and C. D. Derby. 2007. Molecular identification of alarm cues in the defensive secretions of the sea hare Aplysia californica. Anim. Behav. 74:1481-1492. [Google Scholar]
- 15.Kitani, Y., N. Kikuchi, G. Zhang, S. Ishizaki, K. Shimakura, K. Shiomi, and Y. Nagashima. 2008. Antibacterial action of L-amino acid oxidase from the skin mucus of rockfish Sebastes schlegelii. Comp. Biochem. Physiol. B 149:394-400. [DOI] [PubMed] [Google Scholar]
- 16.Mai-Prochnow, A., P. Lucas-Elío, S. Egan, T. Thomas, J. S. Webb, A. Sanchez-Amat, and S. Kjelleberg. 2008. Hydrogen peroxide linked to lysine oxidase activity facilitates biofilm differentiation and dispersal in several gram-negative bacteria. J. Bacteriol. 190:5493-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolen, T. G., P. M. Johnson, C. E. Kicklighter, and T. Capo. 1995. Ink secretion by the marine snail Aplysia californica enhances its ability to escape from a natural predator. J. Comp. Physiol. A 176:239-254. [Google Scholar]
- 18.Shabani, S., S. Yaldiz, L. Vu, and C. D. Derby. 2007. Acidity enhances the effectiveness of active chemical defensive secretions of sea hares, Aplysia californica, against spiny lobsters, Panulirus interruptus. J. Comp. Physiol. A 193:1195-1204. [DOI] [PubMed] [Google Scholar]
- 19.Sheybani, A., M. Nusnbaum, J. Caprio, and C. D. Derby. Responses of the sea catfish, Ariopsis felis, to chemical defenses from the sea hare, Aplysia californica. J. Exp. Mar. Biol. Ecol., in press.
- 20.Stevens, D. L., A. E. Gibbons, R. Bergstron, and V. Winn. 1988. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 158:23-28. [DOI] [PubMed] [Google Scholar]
- 21.Wägele, H., and A. Kussmann-Kolb. 2005. Opisthobranchia (Mollusca, Gastropoda)—more than just slimy slugs. Shell reduction and its implications on defence and foraging. Front. Zool. 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, H., P. M. Johnson, K.-C. Ko, M. Kamio, M. W. Germann, P. C. Tai, and C. D. Derby. 2005. Cloning, characterization, and expression of escapin, a broadly antimicrobial FAD-containing L-amino acid oxidase from ink of the sea hare Aplysia californica. J. Exp. Biol. 208:3609-3622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.