Abstract
The effect of introduced large herbivores on the abundance of Ixodes ricinus ticks and their Borrelia infections was studied in a natural woodland in The Netherlands. Oak and pine plots, either ungrazed or grazed by cattle, were selected. Ticks were collected weekly by blanket dragging. Borrelia infections were determined by PCR and restriction fragment length polymorphism. Rodent densities were estimated using mark-release-recapture methods. On occasion, the cattle were inspected for tick infestations. Meteorological data were recorded for each habitat. Significantly more ticks were collected in the ungrazed woodland than in the grazed woodland. The ungrazed oak habitat had higher tick densities than the pine habitat, while in the grazed habitats, tick densities were similar. Borrelia infection rates ranged from zero in larvae to 26% in nymphs to 33% in adult ticks, and B. afzelii, B. burgdorferi sensu stricto, B. garinii, and B. valaisiana were the species involved. Coinfections were found in five ticks. There was no effect of the presence of cattle on Borrelia infections in the ticks. In the ungrazed area, Borrelia infections in nymphs were significantly higher in the oak habitat than in the pine habitat. More mice were captured in the ungrazed area, and these had a significantly higher tick burden than mice from the grazed area. Tick burden on cattle was low. The results suggest that grazing has a negative effect on small rodents as well as on ticks but not on Borrelia infections. Implications of these results for management of woodland reserves and risk of Lyme disease are discussed.
The ecology of Lyme disease is complex and is determined by numerous factors, including the vertebrate parasite reservoir, the tick vectors, the blood hosts, the vegetation structure, and the micro- and macroclimates (reviewed in reference 3). In recent years, a rapid rise in the number of Lyme disease cases has been reported in Germany and The Netherlands (8, 13), and it has been postulated that this might be due to an expansion of nature reserves, a steady rise in roe deer populations, and increased outdoor recreation. For the management of woodlands, wardens of nature reserves increasingly turn to grazing by free-ranging bovids, as this can keep the woodlots “open” and helps to keep the vegetation diverse (22). Because large herbivores are often the preferred hosts of adult Ixodes ricinus ticks, the principal vector of Lyme disease in Western Europe (24), we speculate that tick populations benefit from these measures. However, for tick populations to prosper under increased densities of large herbivores, there must also be a population of smaller vertebrate species as hosts for the larval and nymphal stages (5). Several small-rodent species and birds constitute the principal hosts for these life stages of I. ricinus, in particular for the larval stages (10, 11, 15). This implies that the introduction of large herbivores for woodland management should not affect the rodent or bird populations if a rise in tick density is expected. On the other hand, large herbivores might negatively impact local rodent populations (28) and thereby alter the host composition for immature tick stages, as well as the Borrelia reservoir composition. Another factor to consider is the fate of the Borrelia parasites that I. ricinus transmits. Here, high densities of rodents and birds are considered to favor Borrelia maintenance, as most larger herbivores are incompetent for Borrelia parasites (12). If the large herbivores affect the composition of hosts for immature tick stages, Borrelia organisms might be maintained in the area by cofeeding on larger herbivores, where the spirochetes are horizontally transmitted through the local bloodstream without a systemic infection (21). In this paper, we report an investigation of the impact of grazing from introduced cattle on the tick populations and their Borrelia infections in a woodland reserve in The Netherlands. Data from a grazed woodland were compared with those of an adjacent woodland from which the cattle were excluded, as well as from a nearby pasture area.
MATERIALS AND METHODS
Description of the study site.
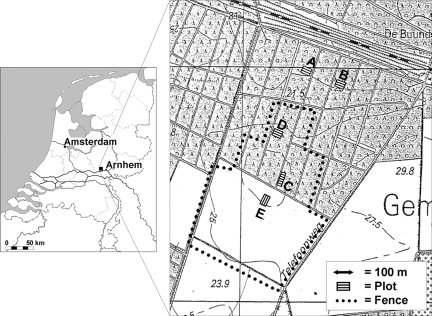
The study was conducted on the estate of Oostereng in The Netherlands, 2 km north of the town of Renkum (52°00′27N, 5°45′20E), 40 m above sea level. The area consists of mixed woodlands (deciduous and evergreen) and is bordered by pasture and agricultural fields to the south. The soils are sandy as a result of the last glacial ice age that covered this part of Europe. The study site covered approximately 1 km2 of pasture, 2 km2 of woodlot accessible to cattle, and 2 km2 of woodlot from which cattle were excluded by fencing (Fig. 1).
FIG. 1.
Map of The Netherlands showing the study site. Inset, detail of the study area. The dotted line marks the fence around the area in which cattle could range freely. Blank areas represent pastures, shaded areas represent forest. Plots: A, oak habitat, cattle excluded; B, pine habitat, cattle excluded; C, oak habitat, cattle resident; D, pine habitat, cattle resident; E, pasture, cattle resident. Plots D and B were also used for rodent captures. Detailed map courtesy of ANWB B.V.
Within the study site, five plots of 400 m2 each were selected: one on an open pasture (plot E), two within a grazed woodlot (plots C and D), and two in an adjacent forest from which cattle were excluded (plots A and B) (Fig. 1). Plots A and C are situated in a habitat that is dominated by oak and plots B and D in a habitat that is dominated by Scots pine. The pasture and adjacent woodlot are grazed by a mixture of Groningen white-headed oxen and Dutch belted oxen, which were introduced in 2001. The cattle are left on the site throughout the year. During the study, an average of 10 animals were present on the study site. In addition to cattle, the study area harbors numerous wildlife; such as roe deer, badgers, foxes, rabbits, hares, and rodents. These animals can pass through the wire fence that surrounds the grazed woodlot and pasture. Also, many birds frequent the site. These animals have all been reported previously to act as natural hosts for I. ricinus (5).
A qualitative vegetation record of the forest plots was made in accordance with the Elton and Miller method, which describes vegetation in terms of the percent coverage (30). Plot E, which is situated within the pasture at 50 m from the edge of the forest, is covered mainly by perennial ryegrass (Lolium perenne), common velvet grass (Holcus lanatus), and red fescue (Festuca rubra). Dominating herbs are spear thistle (Cirsium vulgare), field thistle (Cirsium arvense), persicaria (Persicaria maculosa), ragwort (Senecio jacobae), and patches of stinging nettle (Urtica dioica) and common St. Johnswort (Hypericum perforatum). Plot A is dominated by red oak (Quercus rubra) and European mountain ash (Sorbus aucuparia). The groundcover consists of small patches of wavy hair grass (Deschampsia flexuosa) in between large areas of oak litter. Plot B contains mostly Scots pine (Pinus sylvestris), with a ground cover of wavy hair grass and some patches of blackberry (Rubus fruticosa) and blueberry (Vaccinium myrtillus). Plot C is dominated by common oak (Quercus robur) at a higher density than that of plot A. The ground layer consists mainly of oak litter and small patches of wavy hair grass. Plot D is dominated by Scots pine. Like plot B, the ground layer is dominated by wavy hair grass but has more litter in between. The plot also includes a patch of blueberry and some blackberry.
A few objects (some fallen branches and blackberry branches) that would have obstructed the blanket dragging for tick collection were removed from the study transects prior to the experimental period.
Collection of meteorological data.
Temperature (maximum, minimum, and average) and relative humidity were recorded throughout the study period, using automated data loggers (Gemini Tinytag Plus TPG 1500; Intab Benelux, Cuijk, The Netherlands). Data loggers were suspended, using a metal wire, from a livestock-proof metal cage (Lastec B. V., Wageningen, The Netherlands) 5 cm above the litter layer. The data loggers were protected from the direct impact of rain water by a polyvinylchloride cover, which was attached to the metal wire. The data loggers were programmed to take one measurement per minute. One data logger was placed in the pasture, one in the grazed woodlot, and one in the ungrazed woodlot. Weekly data sets were uploaded to a computer, using Easyview software (version 5.5.1.1, 2002; Intab Benelux).
Collection of ticks.
Tick collections were done weekly over a 15-week period, from March to July 2005 (weeks 11 to 25). Sampling started at 09:00 h and ended at around 13:00 h. In each plot, an area of 200 m2 was sampled according to the method described by Wielinga et al. (32). Ticks were collected by blanket dragging using a white cotton blanket of 1 by 1 m. A metal chain sewn into the lower hem ensured the blanket's contact with the vegetation. The blanket was inspected for ticks at 25-m intervals (32). Ticks of all three stages were counted and placed in 1.5-ml tubes, using forceps. Captured ticks were pooled per 25 m2 and stored at 4°C in 70% ethanol until DNA extraction. Sampling was postponed until the next day when the weather was too wet.
In addition to the tick sampling by blanket dragging, ticks were collected on two occasions from nine of the cattle grazing in the study site to obtain information on the average number of ticks per animal. For this purpose, the cattle were gathered in a crush where they could be restrained by two coworkers using a rope harness around the cows' heads. Once restrained, the cattle were examined as described by L'Hostis et al. (18).
Identification of Borrelia infections.
All life stages of collected I. ricinus ticks were examined for infection with Borrelia spp. DNA extraction was performed as described by Schouls et al. (27), where DNA extracts were produced by boiling individual nymphs and adults in a 4 M ammonium hydroxide solution for 20 min, followed by 20 s of centrifuging at 14,000 rpm, and a final step consisting of 20 min at 90°C in PCR vials with opened caps to evaporate the ammonia. The resulting DNA extracts of approximately 60 μl were stored at −80°C until further analyses. The occurrence of transovarial transmission was examined by extracting DNA from larval I. ricinus as described above. Prior to DNA extraction, larvae from two different sampling dates were pooled for each plot into 75 samples of up to 10 larvae.
DNA extracts were analyzed for the presence of Borrelia spp., using the protocol described by Michel et al. (20), which uses the restriction fragment length polymorphism (RFLP) method with PCR products that were amplified from the B. burgdorferi sensu lato outer surface protein A (OspA) gene. Following a nested-PCR procedure, each B. burgdorferi sensu lato-positive PCR product was digested separately using five different restriction enzymes (SspI, SfuI, BglI, Kpn21, and HindIII) to generate genospecies-specific digestion products. This RFLP technique can distinguish B. afzelii, two different strains of B. valaisiana, five different OspA types (types 3 to 7) of B. garinii, two strains of B. burgdorferi sensu stricto, and one strain each of B. lusitaniae and Borrelia strain A14.
Mark-recapture of rodent populations.
Rodents were trapped in two plots with pine trees, one with and one without cattle, over a 5-day period in June (week 24) of 2005. In each plot, 24 Longworth small-mammal traps (Alana Ecology Ltd., Shropshire, United Kingdom) were placed on the ground in a grid of 4 by 6 m and 5 m apart. The rodent grids were 375 m2 and included the original tick sampling plots.
A prebaiting period of 4 days was performed prior to the trapping period. The traps were placed on the ground and covered with some litter to minimize excessive heat from the sun. The bait consisted of one mealworm and an oatmeal-peanut butter mixture. The bait was refreshed daily. Rodent traps were inspected at 6-h intervals (00:00, 06:00, 12:00, and 18:00 h) to reduce stress endured by insectivorous shrews. All animals captured were identified as to species, and attached ticks were counted. A sample of ticks (as many as practically possible, with a maximum of five ticks) was taken from the rodents, except from shrews, which were set free immediately after inspection. The rodent trapping was approved by the Ethical Animal Experimentation committee of Wageningen University and Research Centre (number 2005056).
Population size estimation.
Captured bank voles and wood mice were gently marked with an individual pattern by clipping off some of the top fur with a pair of scissors. The number and frequency of recaptures were used to estimate the rodent population size as described by Lange et al. (16).
Observation of cattle behavior and counts of cow pats.
The distribution and behavior (resting or foraging) of cattle grazing in the study area were observed over two different periods by walking the study area and using binoculars, without disturbing the cattle. The first observation was done over a 17-h period, from 06:00 to 23:00 h, distributed over 3 days. The second observation was conducted during the rodent capture week.
To estimate the residence time of cattle in different habitats of the study area, cow pats were counted twice in the pasture and in the grazed zones of the pine habitat and in the oak habitat, with a 1-month interval. Pats were marked with a stick to prevent double counts. The recording of cow pats was done in the same 400-m2 plots in which ticks were sampled (1).
Statistical analysis.
Statistical analyses were performed using Genstat software (release version 8.11). The data from the pasture were excluded from the analyses. Data of Borrelia infections in ticks were analyzed using the number of infected ticks as the fraction of the total number of infected ticks analyzed with the RFLP technique. A generalized linear model (GLM; binomial distribution, linked in logit) was used to investigate the effect of the woodland type and the presence of cattle on these fractions. Two-sided t probabilities were calculated to test pairwise differences in means. Adult ticks and larvae were excluded from the statistical analysis of infections. Data for tick abundance, cow pats, and tick burden on rodents were analyzed with GLM (Poisson distribution, linked in logarithm), followed by post-hoc t tests. Effects were considered to be significant at a P value of <0.05.
RESULTS
Meteorological conditions.
The study was conducted from March to July 2005. Minimum and maximum temperatures, relative humidity, and saturation deficit at ground level during the study period are presented as supplemental material. Mean daily minimum temperatures ranged from −4°C to 16°C and mean daily maximum temperatures from 0°C to 32°C. The average values of meteorological parameters collected at the three plots were used to calculate mean relative humidity and temperature at 5 cm above the litter layer in the study area. Minimum temperatures varied from −4° to 1°C and maximum temperatures from 21°C to 32°C. Corresponding values for relative humidity were 39 to 100% and 30 to 100%. Corresponding values of the saturation deficit varied from 0 to 6.4 mm Hg, with highest values in June corresponding with the highest temperatures. On several days, saturation deficit values exceeded 4 mm Hg, which causes I. ricinus to retreat into the litter layer to avoid dehydration. This behavior may put the ticks, in particular larvae, out of reach for the blanket dragging method (23).
Population dynamics of ticks.
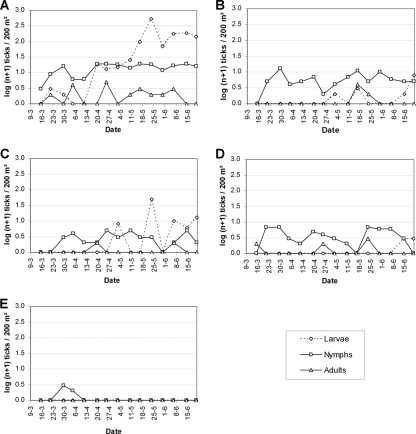
A total of 1,747 ticks were collected with the blanket dragging method during the study period. I. ricinus was the only tick species found. The weekly distribution of the tick collections over the study plots is shown in Fig. 2 for all three life stages. A few larval ticks were already collected in late March, but the majority of larvae appeared only after mid-April, with a peak at the end of May. Nymphal ticks were found for the first time on 10 March 2005 and remained present during the entire study period without a clear peak in population density. Adult ticks were active on nearly each sampling day, albeit in very low numbers.
FIG. 2.
Population dynamics of I. ricinus over the five study plots. Plots: A, oak habitat, cattle excluded; B, pine habitat, cattle excluded; C, oak habitat, cattle resident; D, pine habitat, cattle resident; E, pasture, cattle resident.
Only three ticks were found in the pasture at the beginning of the study. GLM analysis revealed that there was no interaction between habitat and grazing status for larvae (P = 0.85) and adults (P = 0.09). For nymphs, a significant interaction between the factors cattle and habitat was found (P < 0.001). Considerable numbers of larvae were captured in the ungrazed oak plot compared to the other areas (Table 1). However, there were no significant differences in larval densities between the plots due to high spatial within-plot variation. In some plots, larval distribution was significantly biased for one small section, but for the purpose of this paper, we did not take this into account. Both ungrazed plots harbored significantly more nymphs than both grazed plots (P < 0.001 for oak without cattle compared to both plots with cattle; P = 0.002 for pine compared to the grazed oak plot; and P = 0.02 for the ungrazed pine plot compared to the grazed plot). Within the ungrazed area, significantly more nymphs (P < 0.001) were captured in the oak habitat. This difference in nymphal densities between habitats was not found in the grazed area (P = 0.16), which explains the significant interaction between habitat and grazing status for nymphs. Adult tick populations were significantly larger in the ungrazed oak plot than in the other plots (P = 0.04, P = 0.02, and P = 0.04 for ungrazed pine plot, grazed oak plot, and grazed pine plot, respectively). The latter differences can be explained by grazing status (P = 0.02) rather than by habitat (P = 0.09). There were no significant differences between the other plots (all, P > 0.44).
TABLE 1.
Distribution of Ixodes ricinus ticks per life stage and study plota
| Plot | Larvae
|
Nymphs
|
Adults
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SEM | Significance | n | Mean ± SEM | Significance | n | Mean ± SEM | Significance | |
| Oak, cattle absent | 1,283 | 321.8 ± 231.7 | a | 196 | 49.5 ± 4.9 | a | 15 | 3.8 ± 0.9 | a |
| Pine, cattle absent | 11 | 2.8 ± 1.1 | a | 75 | 18.8 ± 1.2 | b | 4 | 1.0 ± 0.4 | b |
| Oak, cattle present | 81 | 20.3 ± 18.9 | a | 27 | 6.8 ± 1.0 | c | 2 | 0.5 ± 0.3 | b |
| Pine, cattle present | 4 | 1.0 ± 0.7 | a | 43 | 10.3 ± 2.3 | c | 4 | 1.0 ± 0.4 | b |
| Pasture, cattle present | 0 | 0 | * | 3 | 1.0 ± 0.5 | * | 0 | 0 | * |
| Total | 1,379 | 344 | 25 | ||||||
Data show the distribution of I. ricinus per life stage and per study plot over the 15-week sampling period. Means ± standard error of the means (SEM) are given for subplots of 50 m2; n, total number of ticks captured in the 200-m2 sampling plot. Different letters within one column indicate significant differences between mean values (P ≤ 0.04). *, not applicable.
Borrelia infection rates.
Seventy-five pools of up to 10 larvae, 340 nymphs, and 24 adult ticks were examined for Borrelia infections. No Borrelia parasites were found in the larvae. On average, 25.6% of nymphs and 33.3% of adult ticks were found infected with Borrelia spp., B. afzelii, B. garinii, B. valaisiana, and B. burgdorferi sensu stricto, with B. burgdorferi sensu stricto being the least abundant (Table 2). Five nymphs were coinfected with two Borrelia genospecies. The B. garinii organisms detected were of four different OspA types: OspA3, OspA5, OspA6, and OspA7 (Table 3). There was no effect of the presence of cattle on Borrelia infections in nymphs (P = 0.70). However, a significant effect of habitat (P = 0.01) was found for nymphal Borrelia infections: more ticks were infected in the oak plots than in the pine plots. There was no interaction between grazing status and habitat (P = 0.71). Nymphs collected in the ungrazed oak plot had a significantly (P = 0.04) higher infection rate than those collected in the ungrazed pine plot (Fig. 3). Numbers of infected adults were too small for statistical analysis.
TABLE 2.
Prevalence of Borrelia infections in adult and nymphal I. ricinus ticksa
| Cattle | Ticks examined
|
% of ticks infected by Borrelia genotype (total no.)b
|
||||||
|---|---|---|---|---|---|---|---|---|
| Stage | No. analyzed | % Infected (total no.) | B. afzelii | B. garinii | Borrelia sp. sensu stricto | B. valaisiana | Borrelia sp. sensu latoc | |
| Absent | Nymph | 272 | 26.1 (71) | 8.8 (24) | 6.3 (17) | 1.1 (3) | 9.9 (27) | 1.8 (5) |
| Adult | 18 | 38.0 (7) | 11.1 (2) | 5.6 (1) | 5.6 (1) | 16.7 (3) | 0 | |
| Present | Nymph | 68 | 23.5 (16) | 5.9 (4) | 2.9 (2) | 0 | 5.9 (4) | 8.8 (6) |
| Adult | 6 | 16.7 (1) | 0 | 0 | 0 | 0 | 16.7(1) | |
Prevalence of Borrelia infections in adult and nymphal I. ricinus ticks in a woodland grazed by cattle and a comparable ungrazed woodland. Where applicable, absolute numbers are given in parentheses.
Five coinfections were found in nymphs collected in the ungrazed oak forest (two coinfections of B. garinii with B. valaisiana, one of B. garinii with B. burgdorferi sensu stricto, one of B. garinii with B. afzelii, and one of B. afzelii with B. valaisiana).
Borrelia sp. sensu lato, genotype could not be determined.
TABLE 3.
B. garinii OspA types in I. ricinus nymphsa
| Cattle | Total no. of nymphs infected with B. garinii | No. of nymphs infected with OspA type:
|
No. of nymphs infected with B. garinii only | ||||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | |||
| Absent | 17 | 1 | 0 | 4 | 11b | 2 | 1 |
| Present | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
Only one adult (not in table) was found infected with B. garinii, which could not be determined to be an OspA type.
Value indicates two coinfections of OspA5 and OspA6.
FIG. 3.
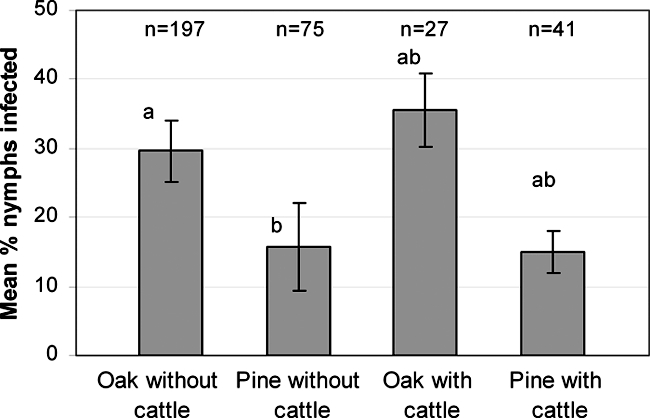
Percentage of I. ricinus nymphs infected with B. burgdorferi sensu lato per plot. n, number of nymphs analyzed. Different letters above bars indicate significant differences (P < 0.05). Error bars represent ±SEM.
Rodents.
A total of 163 wood mice (Apodemus sylvaticus) and 6 bank voles (Clethrionomys glareolus) were captured in the animal traps, as well as 10 common shrews (Sorex araneus) and 5 pygmy shrews (Sorex minutus) (Table 4). On one occasion, a weasel (Mustela nivalis) was caught. Forty percent of the wood mice and 33% of the bank voles were recaptured at least once. Using the mark-recapture method, densities of wood mice were estimated to be 0.2 per m2 in the grazed woodlot and 0.3 per m2 in the ungrazed woodlot. Densities of bank voles were zero and 0.02 per m2, respectively. One male wood mouse, captured in the grazed woodlot, was recaptured 3 days later in the ungrazed woodlot, 500 m from the point where it had been released following the first capture.
TABLE 4.
Rodent populations and rodent larval tick burdens in areas with and without cattlea
| Rodent species | Grazed pine forest
|
Ungrazed pine forest
|
||
|---|---|---|---|---|
| Estimated population (no. per site) | Mean tick burden (n) | Estimated population (no. per site) | Mean tick burden (n) | |
| Wood mouse (Apodemus sylvaticus) | 76 | 2.8 (51) | 96 | 6.1 (51) |
| Bank vole (Clethrionomys glareolus) | 0 | 0 | 7 | 1.25 (4) |
| Common shrew (Sorex araneus) | 5b | 0.2 (5) | 5b | 0.25 (4) |
| Pygmy shrew (Sorex minutus) | 0 | 0 | 5b | 0 (3) |
The n values represent the number of animals inspected for the presence of ticks. Rodent populations were estimated in plots of 375 m2.
No recapture data; shrews were not marked.
Of 102 rodents that were examined during most of the night and early morning captures, a total of 470 I. ricinus ticks were counted, of which 460 were larvae and 10 were nymphs (Table 4). Most wood mice and bank voles carried larvae, despite the fact that during the same period, only a few larvae were collected in both of the pine habitats, using the blanket dragging method. The mean tick burden on wood mice in the grazed plot (2.8 ticks per mouse) was significantly less than that of mice in the ungrazed plot (6.1 ticks per mouse; P = 0.003). No bank voles were caught in the grazed plot, and the mean number of ticks on bank voles in the ungrazed plot was 1.25.
Cattle.
During the cattle observation days, the animals were frequently sighted within the forested area, in particular during the night. Occasionally, roe deer were also seen. Significantly more cow pats (P < 0.001) were counted in the grazed oak area (mean, 15.63 ± 3.12 standard error of the mean [SEM]) than in the pasture (mean, 1.50 ± 0.33 SEM) and pine forest (mean, 1.25 ± 0.31 SEM), whereas no differences were found between the pasture and pine areas. Nine cattle were inspected on two different occasions (5 weeks apart). Four animals were free of ticks; on the other five, the number of ticks collected varied between 1 and 6. With the exception of one nymph, these consisted of adult ticks. All ticks were attached to the cows' upper legs.
DISCUSSION
The presence of cattle had a negative impact on tick populations in our study area, in contrast to our initial hypothesis that livestock would favor the growth of tick populations. We consider it unlikely that differences in densities of roe deer might have caused these effects, as deer lairs were equally common in both the grazed and the ungrazed areas, and deer were also frequently sighted in both areas (F. Gassner, unpublished data). This finding, in combination with the observed abundance of rodents, the wooded vegetation, and the suitable microclimate in the study area, indicates that the area meets all the requirements of an optimal habitat for I. ricinus. The number of ticks collected from the cattle seemed low on both sampling occasions, suggesting that the animals did not encounter questing ticks frequently, even though the animals spent a considerable amount of time during the day in the woodland. In a recent study of 319 cattle in Germany, the tick density per animal varied from 1 to 6 in 75% of the animals (17), suggesting that the tick density on the animals in our study may not have been unusual.
Rodents were abundant at the time of our study, and the tick burden on rodents corresponded to burdens found in studies elsewhere in Europe (4, 6, 10, 19), with a strong variation between individual rodents and rodent species, as reported by Kurtenbach et al. (15). Most ticks on rodents were in the larval stage, similar to data from other studies. As only few nymphs were present on the rodents, it is suggested that in our study area, nymphal stage ticks feed mostly on host species other than wood mice and bank voles.
Our data show that the population density of I. ricinus was significantly higher in the ungrazed woodland than in the grazed area, notably in the oak-dominated forest. The dominant factor differentiating both sites was the presence of cattle. The observed interaction between cattle and habitat for nymphal abundance can be explained by our observations from animal behavior and cow pat counts, indicating that cattle were more often present in the oak area than elsewhere in the forest. During the study, cattle were frequently observed resting in the oak area, thereby inflicting damage to patches of litter layer, which may negatively affect tick survival by increasing tick exposure and making them more vulnerable to desiccation during periods of high saturation deficit. Deer lairs were present in both sites, so large hosts other than cattle were available to the ticks not only in the ungrazed forest but also in the grazed area. Small rodents were numerous in both sites, albeit more numerous and carrying more ticks in the ungrazed woodlot than in the grazed one. Cattle may have had a negative effect on the small rodents (28).
Whereas tick densities were significantly affected by the presence of cattle, the overall Borrelia infection rates in ticks were similar in the grazed and ungrazed woodlots and suggest that the presence of cattle had no effect on infections. Moreover, differences in nymphal infection rate were better explained by habitat type than by grazing status, where nymphs in the ungrazed oak habitat showed a significantly higher infection rate than nymphs in the ungrazed pine habitat. The observed difference in nymphal infection rates between oak and pine forest may have several explanations. The majority of Borrelia infections identified in I. ricinus in The Netherlands are caused by B. afzelii (26, 32), suggesting that most larval ticks feed on small rodents, which are more abundant in oak than in pine forests (28, 29). Hence, infection rates are likely to be higher in ticks present in oak than in pine forests. As B. garinii and B. valaisiana species were also relatively common in the Borrelia infections of the nymphal ticks in our study (Table 2), birds also may have been common hosts for I. ricinus larvae in our study area (7, 9). From the mark-recapture study, it was noted that at least one rodent moved freely between both study areas, so the Borrelia reservoir may have dispersed between both areas, possibly causing a similar proportion of infected rodents in both areas. Assuming a random, nonselective, host-frequenting behavior, questing larval ticks, therefore, would have a similar chance of becoming infected with Borrelia.
Richter and Matuschka (25) recently reported that grazing of cattle had a negative impact on Lyme disease risk. In their study in northern France, however, the effect was caused by significantly different Borrelia infection rates in ticks rather than by tick densities. Unlike our study, where tick densities could be compared because of our rigid sampling strategy, Richter and Matuschka do not mention sampling intensity or record tick densities, although they mention that half as many ticks were found in areas where cattle were present than areas where cattle were absent. The effect of grazing on Lyme disease risk in their study was therefore likely to be even stronger than in our study. Based on Borrelia infection rates in ticks, however, the results of both studies are different. It is possible that differences in vegetation structure and topography between the study areas may have contributed to this result, as Richter and Matuschka collected ticks in a pasture, whereas in our study, ticks were collected from a woodlot where Borrelia-infected mice are likely to be more abundant than in a pasture. As mentioned above, in our study area, the rodents could readily move between both sites, as shown by the dispersal behavior of at least one recaptured mouse, and the probabilities of ticks becoming infected with Borrelia might therefore have been similar in the grazed and the ungrazed sites. We are not aware of any other study reporting the grazing of livestock in woodland areas and the risk of Lyme disease, but the results of both studies, suggesting that grazing cattle can reduce Lyme disease risk, seem to merit additional studies to understand the reported effects.
It is an interesting observation that the oak forest supported a significantly higher tick population than the pine forest. Previously, we found an opposite result in two other areas in The Netherlands, with significantly higher tick densities in pine forest than oak forest (R. Smit, unpublished data). It is likely that this effect is area specific. In the present study, we did not estimate the rodent population in the oak forest. However, given the number of larval ticks found on the rodents, ranging from 2.8 to 6.1 ticks per rodent in the grazed and ungrazed pine forests, respectively (Table 3), it may be assumed that rodents were equally abundant in the oak forests to support such a high density of questing ticks as observed for this study. The ground cover in the oak forest was very different from that in the pine forest and consisted of dead leaves and blueberries in the former and mostly thick grass in the latter. This difference may have resulted in different questing behaviors of ticks between the two sites, so that notably larval populations remained “hidden” from our sampling method in the pine forest.
The pasture area just outside the forest obviously did not support a population of I. ricinus ticks. This was possibly caused by the exposure of the grass to sunlight and subsequent higher saturation deficits in the pasture than in the forest. Larval ticks especially are negatively affected when exposed to a saturation deficit value exceeding 4 (23). We assume that small rodents might have used the pasture for foraging, as there were very many wild plants providing good refuge for numerous sources of rodent food. Yet, this did not cause an increase in the abundance of ticks. Moreover, the pasture could serve as a lethal sink for ticks that have attached to the cattle in the woodlands and dropped from the cattle in the pasture (2). Although we have no data for the feeding frequency of I. ricinus on cattle in our study area, we know from the counts of cow pats that cattle were very often present in the woodlots and therefore were readily available to ticks as blood hosts. We have no comparative data for tick infestation rates of cattle and other large herbivores sharing the same habitat, but data from Sweden mention a tick burden of 428 to 2,072 on roe deer (31), which is much higher than the tick burden on our cattle or on cattle studied in Germany (17) and France (2, 18). Possibly, cattle are less suitable for ticks than roe deer and red deer, which may explain the reduced density of ticks in natural tick habitats grazed by cattle. Our study does not provide an answer to the mechanism that caused the negative impact of cattle on the tick population. Possibly, natural immune responses from cattle following tick bites may be detrimental to tick survival and/or fecundity (14, 33).
The results of this study are of significance for the management of nature reserves in The Netherlands and elsewhere, as these areas are increasingly being stocked with free-ranging livestock such as Scottish highland cattle, Galloway cattle, or Limousin cattle to prevent the areas from turning into closed forests (22). Further studies are needed to elucidate whether livestock have a direct negative impact on the fitness of ticks. If so, a novel tool for the control of Lyme disease is available, which might go hand-in-hand with efficient strategies for the management of nature reserves.
Supplementary Material
Acknowledgments
We thank Jan Wieringa for giving access to his grazing area and for assistance during the collection of ticks from the livestock. Staatsbosbeheer Oost-Gelderland is thanked for permission to use the natural woodlot as a study site. The advice on statistics provided by Saskia Burgers is most appreciated.
Footnotes
Published ahead of print on 3 October 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bookhout, T. A. 1994. Research and management techniques for wildlife and habitats, 5th ed. The Wildlife Society, Bethesda, MD.
- 2.Boyard, C., J. Barnouin, P. Gasqui, and G. Vourc'h. 2007. Local environmental factors characterizing Ixodes ricinus nymph abundance in grazed permanent pastures for cattle. Parasitology 134:987-994. [DOI] [PubMed] [Google Scholar]
- 3.Gassner, F., and L. S. Van Overbeek. 2007. Lyme disease in Europe: facts and no fiction, p. 207-223. In W. Takken and B. G. J. Knols (ed.), Emerging pests and vector-borne diseases in Europe, vol. 1. Wageningen Academic Publishers, Wageningen, The Netherlands. [Google Scholar]
- 4.Gray, J. S. 1999. Risk assessment in Lyme borreliosis. Wien. Klin. Wochenschr. 11:990-993. [PubMed] [Google Scholar]
- 5.Gray, J. S. 1998. Review: the ecology of ticks transmitting Lyme borreliosis. Exp. Appl. Acarol. 22:249-258. [Google Scholar]
- 6.Hanincova, K., S. M. Schäfer, S. Etti, H.-S. Sewell, V. Taragelova, D. Ziak, M. Labuda, and K. Kurtenbach. 2003. Association of Borrelia afzelii with rodents in Europe. Parasitology 126:11-20. [DOI] [PubMed] [Google Scholar]
- 7.Hanincová, K., V. Taragelová, J. Koci, S. M. Schäfer, R. Hails, A. J. Ullmann, J. Piesman, M. Labuda, and K. Kurtenbach. 2003. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 69:2825-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofhuis, A., J. W. van der Giessen, F. H. Borgsteede, P. R. Wielinga, D. W. Notermans, and W. van Pelt. 2006. Lyme borreliosis in the Netherlands: strong increase in GP consultations and hospital admissions in past 10 years. Euro. Surveill. 11:E060622.2. [DOI] [PubMed] [Google Scholar]
- 9.Humair, P. F., D. Postic, R. Wallich, and L. Gern. 1998. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentralbl. Bakteriol. 287:521-538. [PubMed] [Google Scholar]
- 10.Humair, P. F., O. Rais, and L. Gern. 1999. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology 118:33-42. [DOI] [PubMed] [Google Scholar]
- 11.Humair, P. F., N. Turrian, A. Aeschlimann, and L. Gern. 1993. Ixodes ricinus immatures on birds in a focus of Lyme borreliosis. Folia Parasitol. 40:237-242. [PubMed] [Google Scholar]
- 12.Jaenson, T. G., and L. Tälleklint. 1992. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J. Med. Entomol. 29:813-817. [DOI] [PubMed] [Google Scholar]
- 13.Kampen, H., D. C. Rötzel, K. Kurtenbach, W. A. Maier, and H. M. Seitz. 2004. Substantial rise in the prevalence of Lyme borreliosis spirochetes in a region of western Germany over a 10-year period. Appl. Environ. Microbiol. 70:1576-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovar, L. 2004. Tick saliva in anti-tick immunity and pathogen transmission. Folia Microbiol. 49:327-336. [DOI] [PubMed] [Google Scholar]
- 15.Kurtenbach, K., H. Kampen, A. Dizij, S. Arndt, H. M. Seitz, U. E. Schaible, and M. M. Simon. 1995. Infestation of rodents with larval Ixodes ricinus (Acari: Ixodidae) is an important factor in the transmission cycle of Borrelia burgdorferi s.l. in German woodlands. J. Med. Entomol. 32:807-817. [DOI] [PubMed] [Google Scholar]
- 16.Lange, R., A. Van Winden, P. Twisk, J. De Laender, and C. Speer. 1986. Zoogdieren van de Benelux. Herkenning en onderzoek. Jeugdbondsuitgeverij, Amsterdam, The Netherlands.
- 17.Lengauer, H., F. T. Just, R. Edelhofer, and K. Pfister. 2006. Tick infestation and the prevalence of Borrelia burgdorferi and Babesia divergens in cattle in Bavaria. Berl. Munch. Tierarztl. Wochenschr. 119:335-341. [PubMed] [Google Scholar]
- 18.L'Hostis, M., O. Diarra, and H. Seegers. 1994. Sites of attachment and density assessment of female Ixodes ricinus (Acari: Ixodidae) on dairy cows. Exp. Appl. Acarol. 18:681-689. [DOI] [PubMed] [Google Scholar]
- 19.L'Hostis, M., H. Dumon, A. Fusade, S. Lazareff, and A. Gorenflot. 1996. Seasonal incidence of Ixodes ricinus ticks (Acari: Ixodidae) on rodents in western France. Exp. Appl. Acarol. 20:359-368. [DOI] [PubMed] [Google Scholar]
- 20.Michel, H., B. Wilske, G. Hettche, G. Gottner, C. Heimerl, U. Reischl, U. Schulte-Spechtel, and V. Fingerle. 2003. An ospA-polymerase chain reaction/restriction fragment length polymorphism-based method for sensitive detection and reliable differentiation of all European Borrelia burgdorferi sensu lato species and OspA types. Med. Microbiol. Immunol. 193:219-226. [DOI] [PubMed] [Google Scholar]
- 21.Ogden, N. H., P. A. Nuttall, and S. E. Randolph. 1997. Natural Lyme disease cycles maintained via sheep by co-feeding ticks. Parasitology 115:591-599. [DOI] [PubMed] [Google Scholar]
- 22.Olff, H., F. W. Vera, J. Bokdam, E. S. Bakker, J. M. Gleichman, K. de Maeyer, and R. Smit. 1999. Shifting mosaics in grazed woodlands driven by the alternation of plant facilitation and competition. Plant Biol. 1:127-137. [Google Scholar]
- 23.Randolph, S. E., and K. Storey. 1999. Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): implications for parasite transmission. J. Med. Entomol. 36:741-748. [DOI] [PubMed] [Google Scholar]
- 24.Rauter, C., and T. Hartung. 2005. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl. Environ. Microbiol. 71:7203-7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richter, D., and F.-R. Matuschka. 2006. Modulatory effect of cattle on risk for Lyme disease. Emerg. Infect. Dis. 12:1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rijpkema, S. G., J. C. Molkenboer, L. M. Schouls, F. Jongejan, and J. F. Schellekens. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schouls, L., I. van de Pol, S. Rijpkema, and C. S. Schot. 1999. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 37:2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smit, R., J. Bokdam, J. den Ouden, H. Olff, H. Schot-Opschoor, and M. Schrijvers. 2001. Effects of introduction and exclusion of large herbivores on small rodent communities. Plant Ecol. 155:119-127. [Google Scholar]
- 29.Smit, R., F. Dijk, F. Kruidbos, L. M. Schouls, I. van de Pol, B. Docters van Leeuwen, M. Helinski, R. Holtkamp, and W. Takken. 2003. Populatiedynamiek en fenologie van teken in Nederland. Infectieziekten Bull. 14:167-170. [Google Scholar]
- 30.Southwood, T. R. E., and P. A. Henderson. 2000. Ecological methods, 3rd ed. Blackwell Science, Oxford, United Kingdom.
- 31.Tälleklint, L., and T. G. Jaenson. 1997. Infestation of mammals by Ixodes ricinus ticks (Acari: Ixodidae) in south-central Sweden. Exp. Appl. Acarol. 21:755-771. [DOI] [PubMed] [Google Scholar]
- 32.Wielinga, P. R., C. Gaasenbeek, M. Fonville, A. de Boer, A. de Vries, W. Dimmers, G. Jagers Op Akkerhuis, L. M. Schouls, F. Borgsteede, and J. W. van der Giessen. 2006. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in The Netherlands. Appl. Environ. Microbiol. 72:7594-7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willadsen, P. 1999. Immunological control of ectoparasites: past achievements and future research priorities. Genet. Anal. Biomol. Eng. 15:131-137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.