The zoonotic protozoan parasite Cryptosporidium parvum poses a significant risk to public health and has become a global concern for water resource management (10). In order to identify the risk of potential contamination, knowledge about the survival of Cryptosporidium oocysts in the environment is required. Cryptosporidium oocysts can retain infectivity for months and resist environmental stresses more readily than many other pathogens because of a hard protective wall (10, 15, 41). As a result, the characterization of the die-off dynamics of C. parvum oocysts in the environment has received much attention (26). In this paper, we review the published data of the last two decades and the derived understanding of the relationships between temperature, one of the most important environmental stresses, and the die-off of C. parvum in water, soils, and feces.
In general, the inactivation of Cryptosporidium oocysts in the environment slows down exponentially with time, presenting shoulder and tailing effects (31, 38). To cater for these two functions, a first-order exponential formula has usually been used to simulate the die-off curves for oocysts in water (5, 9, 18, 21), in soils (8, 20, 28), and in feces (30, 35), with equation 1 as follows:
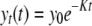 |
(1) |
where K is the die-off rate coefficient (dimensionless) and y0 and yt are the oocyst numbers at time zero, under the initial condition, and at time t (any suitable unit of time), respectively. If normalized by the initial oocyst number, equation 1 can be rewritten as follows:
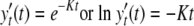 |
(2) |
where
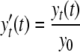 |
(3) |
In equation 2, K is independent of the initial oocyst number and represents a constant die-off rate over the entire incubation period. Alternatively, for a given percentage of inactivated oocysts, K is inversely proportional to the incubation time. By using equation 2, it is possible to estimate the infectivity of oocysts at a given time. For example, if K is 0.01 day−1, the inactivation of 99.9% of oocysts requires 690 days, compared to 138 days when K is 0.05 day−1. In addition, it is possible to find relationships between K and other quantifiable environmental factors.
King and Monis (26) reviewed many critical environmental factors affecting Cryptosporidium oocyst survival, ranging from the abiotic stresses of temperature, pH, ammonia, salinity, desiccation, and solar radiation to biotic antagonism. Oocyst survival in soils and feces has received less attention than that in water, perhaps because more complicated stresses occur in terrestrial than in aquatic environments. Aside from the temperature, moisture level (or soil water potential), mineralogy, pH, and presence and composition of organic matter, other physical, chemical, and biological properties may play a potential role in the infectivity of oocysts in soils (9, 20, 24, 28, 37). Additional stresses such as the composition of manure, the concentration of ammonia, and pile style may also influence oocyst fate in feces and slurry spread on land (19, 22, 35). Therefore, in any environment, multiple concomitant factors such as those aforementioned complicate any attempt to predict the survival of Cryptosporidium oocysts.
There have been a number of reviews on the fate and transport of Cryptosporidium in the aquatic environment (2, 11, 16, 31, 38). However, defining a quantified relationship between the die-off rate and environmental stress has received little attention in the literature. In this study, we focus on the die-off rate for Cryptosporidium oocysts in water, soils, and feces as affected primarily by temperature. Temperature has been regarded as one of the most critical factors governing the fate of oocysts in the environment. This is because oocyst ability to initiate infection is linked to finite carbohydrate energy reserves in the form of amylopectin, which is metabolized in direct response to ambient environmental temperatures (15). Inactivation at higher temperatures is a function of increased oocyst metabolic activity, with a close relationship between infectivity and the level of ATP in the oocyst (25). There have been many studies on the effect of temperature on the survival of C. parvum oocysts in different environments. However, no attempt to generalize the function of this specific factor has been described in a published report.
Data on the survival of C. parvum oocysts in the environment as affected by temperature, which was constant or nearly so (±2°C) during the entire incubation period, were compiled from studies published in the last two decades. The die-off rate coefficient values were taken directly from published data. Some authors presented K values after fitting equation 2 to observed data, while in other studies, K values were not calculated but plots or tables detailing viable oocyst concentrations with time allowed the extraction of data with the Plot Digitizer program (http://plotdigitizer.sourceforge.net) and the fitting of equation 2 in order to establish K. If K values were calculated with the first-order die-off rates based on the logarithm of base 10 in the original studies, they were recalculated based on the natural logarithm. These data provide a source for determining the relationship between K and temperature in the environment, and they can also be used to evaluate the risk of oocyst contamination for public health.
OOCYST SURVIVAL IN WATER
Sterilized water.
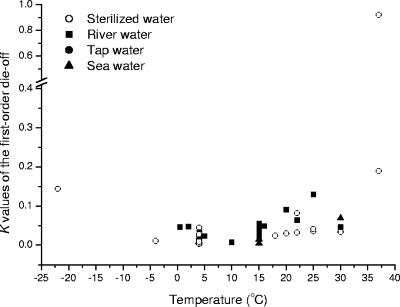
Sterilized water was defined to include distilled water, deionized water, and reverse osmosis water as referred to in the original papers. In sterilized water, temperature is the most important factor influencing oocyst survival. In many studies, the most common methods for determining oocyst viability were DAPI (4′,6-diamidino-2-phenylindole)/propidium iodide (PI) vital dye and in vitro excystation analyses. There was a high level of correlation between the results of the two methods (4, 19). However, the results obtained by other methods, such as cell culture, fluorescence in situ hybridization (FISH), and reverse transcription-PCR, did not show such strong correlations (18, 23, 25). To overcome discrepancies due to the methods used, the die-off rate coefficient K was initially determined only for DAPI/PI vital dye permeability or in vitro excystation data (Fig. 1). The lowest K value occurred at 4°C, which agrees with the findings of Walker and Stedinger (38), who stated that the mortality rate of oocysts increases as the temperature increases above 4°C. For 4°C, the mean of collated K values was 0.0086 day−1, with the values ranging from 0.0029 day−1 up to 0.0272 day−1 (19, 20, 30, 32-36, 40). With increases in temperature up to 37°C, K increased exponentially. There have been a limited number of studies with temperatures above 37°C. Fayer (12) found that oocysts are inactivated when exposed to a temperature of 72.4°C for 1 min or 64.2°C for more than 2 min. Using DAPI/PI dye permeability assays, Jenkins et al. (19) reported K values of 0.140, 1.83, and 6.04 min−1 at 70, 80, and 100°C, respectively. These K values predicted the exposure times before inactivation to be somewhat longer than those reported by Fayer (12).
FIG. 1.
K values of the first-order die-off in distilled and raw water as a function of temperature, as determined by a dye permeability assay or in vitro excystation. Data sources for temperatures above 4°C are listed in Table 1. The K value for −22°C is from reference 35, that for −4°C is from reference 30, and those for 0.5 to 2°C are from reference 5.
Based on K values obtained from the studies with temperatures in the range of 4 to 37°C, which covers most situations in the aquatic environment, it is proposed that the relationship between K and temperature can be represented by an exponential function, as follows:
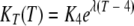 |
(4) |
where K4 and KT are the die-off rate coefficients at 4°C and temperature T, respectively, and λ is a dimensionless modifier of temperature. For sterilized water with temperatures ranging from 4 to 37°C, K4 is estimated to be 0.0029 day−1 and λ is 0.118. The coefficient of correlation (R) between the die-off rate for oocysts and the temperature is 0.767 (P < 0.001). The two parameters of K4 and λ in equation 4 can be used to estimate the inactivation rate at other temperatures in this range in a risk assessment for cryptosporidiosis.
Temperatures below freezing also detrimentally affect oocyst survival due to physical damage. Unfortunately, few published K values for sterilized water below 4°C were available, but a negative exponential trend can be identified (Fig. 1) (30, 35). Freezing enhanced the inactivation of oocysts, as demonstrated using neonatal BALB/c mice and oocysts kept at temperatures from 5 down to −70°C (14). Oocysts held at 5°C for 168 h did not lose their ability to infect mice, while those held at −20°C for 24 h or −70°C for 1 h became completely inactivated. As shown by DAPI/PI staining, only 33% of oocysts remained viable after incubation for 21 h at −22°C and 98% of oocysts lost infectivity after 600 h (35), which is much longer than the times reported by Fayer et al. (14). According to Robertson et al. (35), the estimated die-off rate at −22°C is 0.144 day−1, with a correlation coefficient of 0.843 (P < 0.01). The K value at −4°C was estimated to be 0.011 day−1 (R = 0.920; P < 0.001) by using data reported by Olsen et al. (30). Based on the aforementioned results, it is predicted that to inactivate 99.99% of oocysts, 853 days at −4°C or 64 days at −22°C is required.
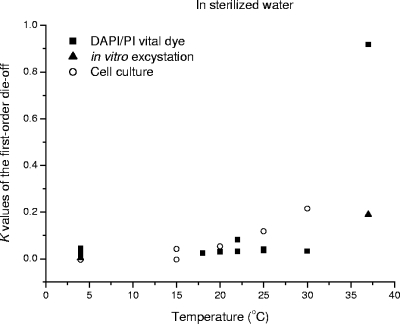
For oocysts held at about −20°C (14, 35) and 70°C (12, 19), the viability determined by DAPI/PI dye permeability assays appears to be overestimated compared with that determined by the infection of neonatal BALB/c mice. The oral infection of BALB/c mice is considered to be the “gold standard” test for determining the infectivity of Cryptosporidium oocysts. The results of cell culture inoculation compared more favorably with the results of this test than those of other oocyst viability tests such as FISH, reverse transcription-PCR, and amylopectin assays (23). There was no significant difference in the K values for oocysts kept in sterilized water over a temperature range of 4 to 20°C whose viability was determined by either DAPI/PI vital dye, in vitro excystation, or cell culture analysis (Fig. 2). When the temperature is over 25°C, however, K values from cell culture are notably higher than those from DAPI/PI and excystation methods, as shown in Fig. 2. After the fitting of equation 4 to these data, λ for cell culture is 0.141. This value is greater than the λ value calculated from the DAPI/PI vital dye data (λ = 0.118) (Table 1). Similar trends for raw water can be found (Fig. 3). Thus, given the data in Fig. 2 and 3, DAPI/PI vital dye and in vitro excystation analyses should be as reliable as the more costly and time-consuming mouse infection assay over the range of 4 to 20°C. This finding explains why some researchers (19) found good correlation between DAPI/PI results for oocysts and mouse infectivity after storage at 4°C but others pointed out that the dye-based assay and in vitro excystation significantly overestimated oocyst viability at 22°C (1) and at 37°C (3, 29) compared to that determined by the neonatal mouse infectivity assay.
FIG. 2.
Comparison of the oocyst die-off rate K values for sterilized water as determined by a DAPI/PI vital dye assay (19, 20, 30, 32-36), in vitro excystation (40), and cell culture (25, 28).
TABLE 1.
Parameters of equation 4 for the different aquatic environments at temperatures above 4°C as determined by DAPI/PI vital dye (or in vitro excystation) and cell culture methods
| Method | Water sample type | K4 (day−1) | λ | Ra | n | Reference(s) |
|---|---|---|---|---|---|---|
| DAPI/PI or excystation | Sterilized water | 0.0029 | 0.118 | 0.767*** | 19 | 19, 20, 30, 32-36, 40 |
| analysis | River water | 0.0093 | 0.095 | 0.616** | 18 | 5, 25, 27, 28, 32, 35, 36 |
| Tap water | 2 | 35 | ||||
| Seawater | 0.009 | 0.064 | 0.891** | 6 | 28, 35 | |
| Cell culture | Sterilized water | 0.0055 | 0.141 | 0.982*** | 6 | 25, 28 |
| Raw water | 0.0051 | 0.158 | 0.933*** | 16 | 18, 25 |
** and *** indicate significance at P values of <0.01 and <0.001, respectively.
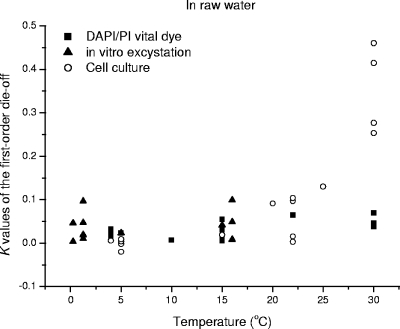
FIG. 3.
Comparison of the oocyst die-off rate K values for raw water as determined by a DAPI/PI vital dye assay (27, 28, 32, 35, 36), in vitro excystation (5, 27), and cell culture (18, 25).
Raw water.
Raw water herein includes surface water (river and lake water), groundwater, seawater, and tap water. In these types of water, the natural die-off of C. parvum oocysts is likely to be affected by a combination of abiotic and biotic stresses. Most of the chemical and physical water properties generally tested do not have a significant effect on oocyst survival, with the exception of the temperature, pH, and ammonia level (17, 35). Calculated K values for river water, tap water, and seawater combine the effects of chemical, physical, and biological water properties on oocyst survival as determined by DAPI/PI vital dye or in vitro excystation analysis only (Fig. 1). Consequently, the data are very variable with respect to temperature, but an exponential relationship between K and temperature in raw water can be identified (Table 1). Although these three types of raw water have different physical, chemical, and biological properties, their K values over the range of 4 to 30°C are not significantly different (Fig. 1). For example, the means ± standard deviations of K values for river water, tap water, and seawater at 4°C are 0.193 ± 0.011, 0.0194 ± 0.006, and 0.009 ± 0.017 (P > 0.05), respectively.
The biological antagonism and potential predation of Cryptosporidium may enhance the die-off of oocysts. Table 2 summarizes K values over a range of temperatures from 0.5 up to 25°C. The die-off rate for oocysts in river water at 5°C is similar to that in autoclaved river water, but at 15°C, oocyst die-off occurs more rapidly in natural than in autoclaved river water (27). The difference is perhaps due to increased biological or biochemical activity at 15°C, as suggested by the authors of the study (27). Chauret et al. (5) reported that oocyst survival in samples from the St. Lawrence River and Ottawa River was better in water filtered through a 0.2-μm-pore-size membrane than in unfiltered water, suggesting that biological antagonism may play a vital role in the fate of the infective stage of the parasite in the aquatic environment. King et al. (25) did not find any difference in oocyst survival between untreated water and autoclaved water containing antibiotics, suggesting that microbial degradation is a major factor affecting oocyst viability. An effect of microbial activity on oocyst survival was not observed by Pokorny et al. (32) using tissue culture assays for the comparison of raw river water and river water passed through a sterile 11-μm-pore-size nitrocellulose filter.
TABLE 2.
Comparisons of die-off rate K values for raw water and sterilized raw water as a function of biological antagonism
| Water source (reference) | Method | Temp (°C) | K value (day−1) for raw water | Sterilization step | K value (day−1) for sterilized water |
|---|---|---|---|---|---|
| St. Lawrence River, Canada (5) | Analysis of in vitro excystation (in dialysis cassette)a | 0.5-2.0 | 0.097 | Filtering through 0.2-mm-pore-size membrane in dialysis cassette | 0.047 |
| Analysis of in vitro excystation (in microtube) | 0.5-2.0 | 0.018 | Filtering through 0.2-mm-pore-size membrane in microtube | 0.020 | |
| River Meuse, The | DAPI/PI vital dye assay | 5 | 0.023 | Autoclaving | 0.023 |
| Netherlands (27) | DAPI/PI vital dye assay | 15 | 0.055 | Autoclaving | 0.014 |
| In vitro excystation analysis | 5 | 0.023 | Autoclaving | 0.023 | |
| In vitro excystation analysis | 15 | 0.041 | Autoclaving | 0.025 | |
| Hope Valley, | Cell culture | 4 | 0.005 | Autoclaving | 0.017 |
| Australia (25) | Cell culture | 15 | 0.019 | Autoclaving | 0.020 |
| Cell culture | 20 | 0.091 | Autoclaving | 0.088 | |
| Cell culture | 25 | 0.130 | Autoclaving | 0.132 | |
| Speed River, Canada (32) | Tissue culture assay | 21-23 | 0.064 | Filtering through sterile 11-mm-pore-size nitrocellulose filter | 0.082 |
Fed with raw water continuously.
OOYCST SURVIVAL IN SOILS
Soil is more complex than water due to the presence of gaseous, water, and solid phases, numerous chemicals, microorganisms, macrofauna, and plants and a range of other physical properties, which can vary over very small distances. For these reasons, K values determined for different soils (9, 20, 28, 30, 39) at near saturation present a wide variation with respect to temperature (Fig. 4). It is also difficult to make comparisons among previous studies as the method of soil sampling and the method of determining oocyst survival may account for some variation, in addition to that caused by soil properties. Using FISH, Davies et al. (9) found that the inactivation rates for Cryptosporidium oocysts in loam and clay loam soils at 4°C were approximately 0.005 day−1. Jenkins et al. (20) indicated that K values for oocysts incubated at 4°C in a sentinel chamber in which one end was exposed to free water varied from 0.0014 day−1 to 0.0032 day−1 for three soils as determined using DAPI/PI vital dye. An oocyst degradation rate of 0.027 day−1 at 4°C in a silty clay loam soil maintained at a 17% water content in a sealed system was reported by Olson et al. (30). Nasser et al. (28) demonstrated that the viability of oocysts in a saturated loamy soil at 15°C remained unchanged during the incubation, as determined by an immunofluorescence assay and PCR. However, from these published works, regardless of soil types (Fig. 4), we can estimate average K values of 0.0055, 0.011, and 0.076 day−1 at 4, 20, and 30°C, respectively.
FIG. 4.
K values of the first-order die-off as a function of temperature (T) in soils as determined by a DAPI/PI vital dye assay (20, 30), cell culture (28), and FISH (9).
Besides temperature, oocysts in soils are subjected to stress driven by the interaction of the water content and the texture. The soil water potential indicates whether oocysts are exposed to wet or dry conditions. Desiccation is probably lethal to oocysts (26, 35). Walker et al. (39) reported that decreasing the soil water potential by adjusting NaCl solution linearly increases the rate of population degradation. Nasser et al. (28) also found that incubation for 10 days in dry loamy soil at 32°C resulted in a 3-log10 reduction in oocyst infectivity but that a saturated soil at a similar temperature caused only a 1-log10 reduction. Jenkins et al. (22) reported that the estimated K values for soil at 25°C were increased from 0.014 to 0.416 day−1 when the soil water potential was decreased from −0.10 down to −3.2 MPa, but they also pointed out that when the soil water potential indicated a water content in excess of the field capacity (>−0.10 MPa), then the effect on oocyst infectivity was negligible. Jenkins et al. (20) did not find any effect of soil water potentials in the range of −0.033 to −1.5 MPa on oocyst inactivation by using a sentinel chamber. This discrepancy from the data in other reports perhaps resulted from the one open end of the sentinel chamber's being exposed to free water, through which the water potential of the soil in the chamber could be mediated by the moisture of the surrounding soil. Kato et al. (24) found that soil moisture did not affect oocyst survival within a range of −2 to 1°C by using a sealed sentinel chamber in the field. Although they did not measure the water content of the soil inside the chamber, the soil moisture could be kept constant over the experimental period due to the sealed conditions. Davies et al. (9) reported little difference in K values between soils at the field capacity (relatively wet, corresponding to the water content of soils after free drainage) and the wilting point (very dry, the point at which plants fail to recover with later wetting). Published results suggest that the soil water potential or moisture content can influence the oocyst die-off rate; however, it is still not clear how this abiotic stress affects oocyst metabolic activity and whether oocysts recover and loose infectivity during natural wetting and drying cycles. More effort is required to determine the generalized relationship among the soil water potential and oocyst survival and metabolic activity, although it is generally accepted that desiccation is lethal to oocysts (26, 35).
Soil texture, a static property of the soil, also appears to affect oocyst survival in the soil environment. Jenkins et al. (20) reported that the rate of oocyst survival in a silt loam soil (50% silt and 16% clay) appeared to be significantly greater than those in silty clay loam (69% silt and 29% clay) and loamy sand (14% silt and 5% clay). Davies et al. (9) found that oocysts were inactivated more quickly in loam soil (27% silt and 24% clay) than in clay loam (55% silt and 38% clay). In their review, King and Monis (26) stated that soil texture has been identified as an important factor for oocyst survival, but how it influences oocyst viability is unclear. Soil particles probably do not directly affect oocyst survival, but they may modify oocyst metabolic activity through changing other physiochemical and biological soil properties and their attachment properties, although further research is required to confirm these hypotheses.
Many soil properties are thought to influence the fate and transport of oocysts (16, 31). Laboratory results showed that oocyst degradation in soil was faster than that in sterilized water (30). However, a field experiment found little difference in oocyst survival rates in soil under corn, woodland soil, and pasture soil (24). Using a sentinel chamber buried under in situ conditions, Udeh et al. (37) could not clearly define the effects of chemical and biological soil properties on oocyst survival. With the present knowledge, it is not possible to define the roles of organic matter, the nutrient concentration, and the microorganism communities in soil for oocyst survival. As shown in Table 1, λ for soil is smaller (0.093) than λ for sterilized water (0.118), indicating that oocyst inactivation in almost-saturated soils is slower than that in soils with lower water contents. Furthermore, oocysts in soil are less sensitive to air temperature and solar radiation as a consequence of their ability to attach to soil particles. It would appear that high temperature, desiccation, and UV radiation may be lethal only to oocysts lying free on or very near the soil surface. Oocysts deep in the soil profile are to a large extent protected from inactivation by these stresses. Soil can thus be regarded as a habitat that may promote survival and provide a source of oocysts, especially after the spreading of animal manure and slurry. Secondary soil structure (pedality and transpedal macropores) may result in a preferential flow of oocysts, thus creating a transport pathway that bypasses much of the soil volume (7, 8). Such preferential flow may offer pathways to the contamination of surface water and groundwater that may not be readily predicted by studying the effects of bulk soil properties on oocyst survival. It is apparent from our review of the literature that there are many gaps in our knowledge of how physiochemical and biological soil properties influence oocyst fate and transport.
OOCYST SUIVIVAL IN FECES
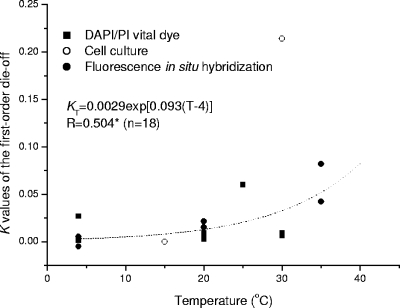
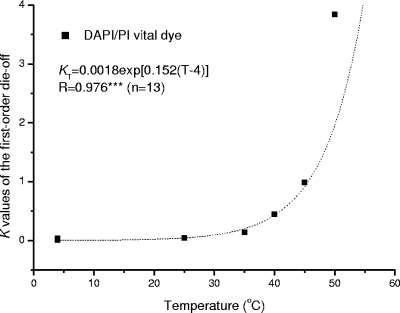
Temperature is a key factor influencing oocyst survival in feces as reported in published data (19, 22, 30, 35). An analysis of these data indicates that there is a strong exponential relationship between the die-off rate, K, and the temperature and that K4 is 0.0018 day−1 and λ is 0.152 (R = 0.976; P < 0.001) (Fig. 5). The temperature modifier λ is much greater for feces than for sterilized water (λ = 0.118), indicating that other stresses in addition to temperature are involved. Olson et al. (30) reported that oocyst degradation in feces is more rapid than that in water, because other fecal microorganisms such as bacteria may be associated with the degradation process. Similarly, Jenkins et al. (22) found that the proportions of potentially infective oocysts in dung heap material decreased more rapidly than those of controls exposed only to buffer solution or water, indicating that factors other than temperature affect oocyst inactivation in dung heaps. Ammonia, which may be present in feces in high concentrations, was identified as a significant inactivation agent for oocysts (13, 21), but the levels of ammonium in surface water and drinking water are not high enough to have an impact on oocyst viability (2). Fecal material may also play a vital role in oocyst survival. Robertson et al. (35) reported that the degradation rates for oocysts varied with different stool samples. Jenkins et al. (22) also reported K values for two fecal samples to be quite different, 0.0246 day−1 (R = 0.803; P < 0.01) and 0.0162 day−1 (R = 0.930; P < 0.001). However, Collick et al. (6) did not find a significant difference in the viability of oocysts excreted by calves in solar housing and those in conventional housing, although there was a significant difference in the temperature of the bedding materials in the two rearing systems (P < 0.05). Besides temperature, other factors may account for these differences (22, 35). However, it could not be ascertained from the reports reviewed what these additional factors are for feces.
FIG. 5.
K values of the first-order die-off in feces, determined as a function of temperature (T) by a DAPI/PI vital dye assay (19, 22, 30, 35).
SUMMARY
Overall, based on the published data obtained for water, soils, and feces, C. parvum oocysts exhibit a first-order die-off like that of any other microorganism in the aquatic and terrestrial environments, regardless of the detection methods. Temperature appears to be the most lethal factor affecting oocysts in the environment. The exponential relationship between the die-off rate (K) and temperature and parameterization in different environments as defined in this work can provide us with a better understanding of C. parvum survival and permit improved assessment of the risk to public health. However, oocyst survival in aquatic environments is less complicated than that in terrestrial environments. The water contents and compositions of soils and feces may pose other factors important for oocyst survival that are generally excluded from water. These interactive environmental effects complicate the relationship between oocyst survival and stresses. Unlike temperature, the other factors affecting oocyst survival in the environment have not been defined clearly and need further study.
Fortunately, outbreaks involving the waterborne transmission of zoonotic C. parvum in urban populations are rare, but they remain a possibility. In the absence of more detailed information on the survival and passage of oocysts through the environmental matrices, the die-off rates (K) reported here for water, soils, and feces may be useful in conjunction with ambient temperature for assessing the potential risk for urban outbreaks of cryptosporidiosis for water resource management.
Acknowledgments
This work was funded by the European Commission Framework Programme 6 Marie Curie Transfer of Knowledge project “Cryptonet.ie” (MTKD-CT-2005-029454).
Footnotes
Published ahead of print on 10 October 2008.
REFERENCES
- 1.Black, E. K., G. R. Finch, R. Taghi-Kilani, and M. Belosevic. 1996. Comparison of assays for Cryptosporidium parvum oocysts viability after chemical disinfection. FEMS Microbiol. Lett. 135:187-189. [DOI] [PubMed] [Google Scholar]
- 2.Brookes, J. D., J. Antenucci, M. Hipsey, M. D. Burch, N. Ashbolt, and C. Ferguson. 2004. Fate and transport of pathogens in lakes and reservoirs. Environ. Int. 30:741-759. [DOI] [PubMed] [Google Scholar]
- 3.Bukhari, Z., M. M. Marshall, D. G. Korich, C. R. Fricker, H. V. Smith, J. Rosen, and J. L. Clancy. 2000. Comparison of Cryptosporidium parvum viability and infectivity assays following ozone treatment of oocysts. Appl. Environ. Microbiol. 66:2972-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, A. T., L. J. Robertson, and H. V. Smith. 1992. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl. Environ. Microbiol. 58:3488-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauret, C., K. Nolan, P. Chen, S. Springthorpe, and S. Sattar. 1998. Aging of Cryptosporidium parvum oocysts in river water and their susceptibility to disinfection by chlorine and monochloramine. Can. J. Microbiol. 44:1154-1160. [DOI] [PubMed] [Google Scholar]
- 6.Collick, A. S., E. A. Fogarty, P. E. Ziegler, M. T. Walter, D. D. Bowman, and T. S. Steenhuis. 2006. Survival of Cryptosporidium parvum oocysts in calf housing facilities in the New York watersheds. J. Environ. Qual. 35:680-687. [DOI] [PubMed] [Google Scholar]
- 7.Darnault, C. J. G., P. Garnier, Y. J. Kim, K. J. Oveson, T. S. Steenhuis, J.-Y. Parlange, M. B. Jenkins, W. C. Ghiorse, and P. C. Baveye. 2003. Preferential transport of Cryptosporidium parvum oocysts in variably saturated subsurface environments. Water Environ. Res. 75:113-120. [DOI] [PubMed] [Google Scholar]
- 8.Darnault, C. J. G., T. S. Steenhuis, P. Garnier, Y.-J. Kim, M. B. Jenkins, W. C. Ghiorse, P. C. Baveye, and J. Y. Parlange. 2004. Preferential flow and transport of Cryptosporidium parvum oocysts through the vadose zone: experiments and modeling. Vadose Zone J. 3:262-270. [Google Scholar]
- 9.Davies, C. M., N. Altavilla, M. Krogh, C. M. Ferguson, D. A. Deere, and N. J. Ashbolt. 2005. Environmental inactivation of Cryptosporidium oocysts in catchment soils. J. Appl. Microbiol. 98:308-317. [DOI] [PubMed] [Google Scholar]
- 10.Fayer, R. 2008. General biology, p. 1-42. In R. Fayer and L. Xiao (ed.), Cryptosporidium and cryptosporidiosis, 2nd ed. CRC Press and IWA Publishing, Boca Raton, FL.
- 11.Fayer, R. 2004. Cryptosporidium: a water-borne zoonotic parasite. Vet. Parasitol. 126:37-56. [DOI] [PubMed] [Google Scholar]
- 12.Fayer, R. 1994. Effect of high temperature on infectivity of Cryptosporidium parvum oocysts in water. Appl. Environ. Microbiol. 60:2732-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fayer, R., and T. Nerad. 1996. Effects of low temperatures on viability of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 62:1431-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayer, R., M. Trout, and M. C. Jenkins. 1998. Infectivity of Cryptosporidium parvum oocysts stored in water at environmental temperatures. J. Parasitol. 84:1165-1169. [PubMed] [Google Scholar]
- 15.Fayer, R., T. K. Graczyk, M. R. Cranfield, and J. M. Trout. 1996. Gaseous disinfection of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 62:3908-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson, C., A. M. R. Husman, N. Altavilla, D. Deere, and N. Ashbolt. 2003. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 33:299-361. [Google Scholar]
- 17.Hsu, B., C. Huang, and J. R. Pan. 2001. Filtration behaviors of Giardia and Cryptosporidium—ionic strength and pH effects. Water Res. 35:3777-3782. [DOI] [PubMed] [Google Scholar]
- 18.Ives, R. L., A. M. Kamarainen, D. E. John, and J. B. Rose. 2007. Use of cell culture to assess Cryptosporidium parvum survival rates in natural groundwaters and surface waters. Appl. Environ. Microbiol. 73:5968-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins, M. B., L. J. Anguish, D. D. Bowman, M. J. Walker, and W. C. Ghiorse. 1997. Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 63:3844-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins, M. B., D. D. Bowman, E. A. Fogarty, and W. Ghiorse. 2002. Cryptosporidium parvum oocyst inactivation in three soil types at various temperatures and water potentials. Soil Biol. Biochem. 34:1101-1109. [Google Scholar]
- 21.Jenkins, M. B., D. D. Bowman, and W. Ghiorse. 1998. Inactivation of Cryptosporidium parvum oocysts by ammonia. Appl. Environ. Microbiol. 64:784-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins, M. B., M. J. Walker, D. D. Bowman, L. C. Anthony, and W. C. Ghiorse. 1999. Use of a sentinel system for field measurements of Cryptosporidium parvum oocyst inactivation in soil and animal waste. Appl. Environ. Microbiol. 65:1998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins, M. B., J. M. Trout, J. Higgins, M. Dorsch, D. Veal, and R. Fayer. 2003. Comparison of tests for viable and infectious Cryptosporidium parvum oocysts. Parasitol. Res. 89:1-5. [DOI] [PubMed] [Google Scholar]
- 24.Kato, S., M. B. Jenkins, E. A. Fogarty, and D. D. Bowman. 2004. Cryptosporidium parvum oocyst inactivation in field soil and its relation to soil characteristics: analyses using the geographic information systems. Sci. Total Environ. 321:47-58. [DOI] [PubMed] [Google Scholar]
- 25.King, B. J., A. R. Keegan, P. T. Monis, and C. P. Saint. 2005. Environmental temperature controls Cryptosporidium oocyst metabolic rate and associated retention of infectivity. Appl. Environ. Microbiol. 71:3848-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King, B. J., and P. T. Monis. 2007. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology 134:309-323. [DOI] [PubMed] [Google Scholar]
- 27.Medema, G. J., M. Bahar, and F. M. Schets. 1997. Survival of Cryptosporidium parvum, Escherichia coli, faecal enterococci and Clostridium perfringens in river water: influence of temperature and autochthonous microorganisms. Water Sci. Technol. 35:249-252. [Google Scholar]
- 28.Nasser, A. M., E. Tweto, and Y. Nitzan. 2007. Die-off of Cryptosporidium parvum in soil and wastewater effluents. J. Appl. Microbiol. 102:169-176. [DOI] [PubMed] [Google Scholar]
- 29.Neumann, N. F., L. L. Gyürek, G. R. Finch, and M. Belosevic. 2000. Intact Cryptosporidium parvum oocysts isolated after in vitro excystation are infectious to neonatal mice. FEMS Microbiol. Lett. 183:331-336. [DOI] [PubMed] [Google Scholar]
- 30.Olson, M. E., J. Goh, M. Philips, N. Guselle, and T. A. McAllister. 1999. Giardia cyst and Cryptosporidium oocyst survival in water, soil, and cattle feces. J. Environ. Qual. 28:1991-1996. [Google Scholar]
- 31.Pachepsky, Y. A., A. M. Sadeghi, S. A. Bradford, D. R. Shelton, A. K. Guber, and T. Dao. 2006. Transport and fate of manure-borne pathogens: modeling perspective. Agric. Water Manage. 86:81-92. [Google Scholar]
- 32.Pokorny, N. J., S. C. Weir, R. A. Carreno, J. T. Trevors, and H. Lee. 2002. Influence of temperature on Cryptosporidium parvum oocyst infectivity in river water samples as detected by tissue culture assay. J. Parasitol. 88:641-643. [DOI] [PubMed] [Google Scholar]
- 33.Reinoso, R., and E. Bécares. 2008. Environmental inactivation of Cryptosporidium parvum oocysts in waste stabilization ponds. Microb. Ecol. 56:585-592. [DOI] [PubMed] [Google Scholar]
- 34.Reinoso, R., E. Bécares, and H. V. Smith. 2008. Effect of various environmental factors on the viability of Cryptosporidium parvum oocysts. J. Appl. Microbiol. 104:980-986. [DOI] [PubMed] [Google Scholar]
- 35.Robertson, L. J., A. T. Campbell, and H. V. Smith. 1992. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl. Environ. Microbiol. 58:3494-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson, L. J., and B. K. Gjerde. 2006. Fate of Cryptosporidium oocysts and Giardia cysts in the Norwegian aquatic environment over winter. Microb. Ecol. 52:597-602. [DOI] [PubMed] [Google Scholar]
- 37.Udeh, P. J., G. John, and J. N. Veenstra. 2003. Field inactivation of oocysts exposed to agricultural land. Water Air Soil Pollut. 142:211-228. [Google Scholar]
- 38.Walker, F. R., and J. R. Stedinger. 1999. Fate and transport model of Cryptosporidium. J. Environ. Eng. 125:325-333. [Google Scholar]
- 39.Walker, M. J., K. Leddy, and E. Hagar. 2001. Effects of combined water potential and temperature stresses on Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 67:5526-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware, M. W., and F. W. Schaefer III. 2005. The effects of time and temperature on flow cytometry enumerated live Cryptosporidium parvum oocysts. Lett. Appl. Microbiol. 41:385-389. [DOI] [PubMed] [Google Scholar]
- 41.Xiao, L., and R. Fayer. 2008. Molecular characterisation of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int. J. Parasitol. 38:1239-1255. [DOI] [PubMed] [Google Scholar]