Abstract
Bacterial biofilms cause numerous problems in health care and industry; notably, biofilms are associated with a large number of infections. Biofilm-dwelling bacteria are particularly resistant to antibiotics, making it hard to eradicate biofilm-associated infections. Bacteria rely on efflux pumps to get rid of toxic substances. We discovered that efflux pumps are highly active in bacterial biofilms, thus making efflux pumps attractive targets for antibiofilm measures. A number of efflux pump inhibitors (EPIs) are known. EPIs were shown to reduce biofilm formation, and in combination they could abolish biofilm formation completely. Also, EPIs were able to block the antibiotic tolerance of biofilms. The results of this feasibility study might pave the way for new treatments for biofilm-related infections and may be exploited for prevention of biofilms in general.
Bacteria often live as complex surface-associated communities rather than as planktonic cells. These compact microbial consortia, referred to as biofilms, are commonly associated with many health problems (for reviews, see references 9, 10, 13, and 47). Biofilms are ubiquitous, and biofilms that occur in dental plaque, in lung infections, and in infections related to the use of medical devices, such as urinary catheters, are a few common examples. Many persistent and chronic bacterial infections, including biliary tract infections, endocarditis, otitis media periodontitis, prostatitis, and many urinary tract infections, are now thought to be linked to biofilm formation. Virtually all medical implants are prone to colonization and formation of biofilms by pathogenic bacteria, and these biofilms often serve as a source of recurrent infections (9). Biofilm-linked infections are particularly problematic, because biofilm-associated bacteria can withstand host immune defenses, antibiotics, biocides, and hydrodynamic shear forces far better than the corresponding planktonic bacteria. For example, the MIC of ampicillin for a strain of Klebsiella pneumoniae growing in a biofilm is 2,500-fold higher than the MIC of this antibiotic for planktonic cells of the same strain (2). Also, outside the medical field biofilms cause a host of problems, such as surface fouling and blocking equipment (6).
In order to get rid of toxic substances and waste products, bacteria have efficient efflux systems. Efflux refers to the pumping of a solute out of a cell. Efflux pump proteins occur as both single-component and multicomponent systems, and the latter are found exclusively in gram-negative bacteria (24). Whereas the single-component systems extrude their substrates into the periplasmic space, multicomponent systems, like the multiple drug resistance (MDR) pumps AcrAB-TolC (Escherichia coli) and MexAB-OprM (Pseudomonas aeruginosa), traverse both the inner and outer membranes and are therefore able to export their substrates directly from the cytoplasm to the surrounding medium (24). Since they are able to transport a wide range of antibiotics, MDR pumps play a crucial role in the increasing prevalence of multidrug-resistant bacteria (30, 36). The exact role of efflux pumps in biofilm growth and their importance in biofilm-mediated antibiotic resistance are, however, elusive, and studies of gram-negative bacteria have produced somewhat incongruous results. In E. coli, the putative multidrug resistance pump YhcQ was reported to be involved in antibiotic resistance of biofilms (26), and the AcrAB-TolC pump has been found to be upregulated under stress conditions, such as stationary-phase growth or, as in the natural habitat of this species, exposure to bile acids and other harmful agents (43). Interestingly, exposure to these agents renders E. coli resistant to lipophilic antibiotics, and it has been suggested that this finding is connected to the upregulation of AcrAB-TolC (43).
In P. aeruginosa, the MexAB-OprM and MexCD-OprJ efflux pumps have been shown to be involved in biofilm-specific mechanisms for resistance (17), particularly mechanisms for resistance to the macrolide azithromycin (17). In contrast, De Kievit et al. found that neither of these two pumps was upregulated in a developing biofilm (12). However, it was pointed out that the expression of these pumps may be influenced by factors like the growth rate and accumulation of metabolites. Zhang et al. recently reported on a novel efflux pump in P. aeruginosa, PA1874-1877, which shows a higher level of expression in a biofilm than during planktonic growth and which appears to be involved in biofilm-mediated antibiotic resistance (48). Most probably, biofilm-mediated resistance is achieved through multiple factors, such as slow growth due to deficient nutrition, reduced penetration due to production of protective extracellular polysaccharides, and efficient efflux, to mention a few (for reviews, see references 28 and 35). Additionally, Mah and coworkers found that the ndvB gene, coding for a glucosyltransferase, is a genetic determinant of antibiotic resistance in P. aeruginosa (29).
Efflux pump inhibitors (EPIs) are substances that block the activity of efflux pumps. EPIs have attracted a great deal of attention over the years due to their ability to block some types of antibiotic resistance in bacteria (for reviews, see references 30 and 36); the EPIs known to block MDR pumps are of particular interest, and three well-known EPIs of this type were assessed here (Table 1 and Fig. 1C). We studied the potency of EPIs as antibiofilm drugs, and we report below that EPIs can block biofilm formation in many bacteria. We also found that in some cases the inherent antibiotic resistance of biofilms can be blocked by EPIs.
TABLE 1.
MDR pumps inhibited by the EPIs used in this study
| MDR pump(s) | Organism | Substrates | EPI(s) | Reference(s) |
|---|---|---|---|---|
| AcrAB | E. coli | Bile salts, fatty acids, antibiotics (tetracycline, chloramphenicol, quinolones, fluoroquinolones, rifampin), dyes, detergentsa | NMP, PAβN | 3, 7, 8, 21, 27, 31, 33 |
| AcrEF | E. coli | Hydrophobic uncouplers, multiple structurally dissimilar antibiotics, basic dyes | NMP | 3, 33, 38 |
| AcrAB | K. pneumoniae | Multiple structurally dissimilar antibiotics | PAβN | 4 |
| MexAB, MexCD, MexEF | P. aeruginosa | Multiple structurally dissimilar antibiotics | NMP, PAβN | 1, 23, 39, 40 |
| NorA | S. aureus | Tetracycline, chloramphenicol, quinolones, fluoroquinolones, reserpine, plant alkaloidsa | Thioridazineb | 30, 36 |
The quinolones include nalidixic acid, levofloxacin, and ciprofloxacin.
It should be noted that thioridazine has been shown to reduce vancomycin resistance in the gram-negative bacterium Enterococcus faecalis.
FIG. 1.
EPIs significantly reduce biofilm formation by E. coli UTI strain 83972, E. coli wild-type strain F18, and K. pneumoniae i222-86. The EPIs thioridazine (T) (50 μg/ml), PAβN (50 μg/ml), and NMP (100 μg/ml) or combinations of thioridazine and PAβN (50 μg/ml each) or of thioridazine and NMP (50 and 100 mg/ml, respectively) were added to static cultures at the time of inoculation, and the amount of biofilm after 24 h was determined by crystal violet staining. (A) Addition of either thioridazine or PAβN significantly reduced biofilm formation up to 80%, and addition of the combinations significantly reduced biofilm formation up to 99% compared with controls without EPIs (P < 0.001, as determined by a paired two-tailed t test for at least three independent experiments). However, NMP alone did not have a significant effect. (B) Biofilm formation by E. coli UTI strain 83972-yfp in the presence of the EPIs thioridazine and NMP (50 and 100 μg/ml, respectively) was monitored using scanning confocal laser microscopy at 16 and 24 h postinoculation. In the presence of the combination of EPIs biofilm formation was reduced 54% at 16 h and 83% at 24 h. Scale bars = 30 μm. (C) Molecular structures of the three EPIs that were used to study bacterial biofilm formation.
MATERIALS AND METHODS
Strains and growth media.
The strains used for biofilm formation in microtiter plates were E. coli urinary tract infection (UTI) strains 83972, VR50 and VR89; E. coli wild-type strain F18; K. pneumoniae strains i222-86, i225-86, i113-96, and i133-96; Staphylococcus aureus strain 8324, a kind gift from Hanne Ingmer and Dorte Frees, Department of Veterinary Pathology, University of Copenhagen (16); and P. putida KT2442 (18). Due to strain differences the strains were grown in LB, FBA minimal medium (AB minimal medium with FeCl3 replaced by 10 μM Fe-EDTA and supplemented with 0.02% glucose, 0.02% Casamino Acids, and 1.0 μg/ml thiamine), tryptic soy broth (with 2.5% glucose), or pooled sterile human urine (42), with EPIs and/or antibiotics when appropriate. Mutants 83972ΔtolC, 83972ΔyqgA, and 83972ΔaaeX were constructed using the λRed recombinase gene replacement system (11). Briefly, the npt gene from plasmid pKD4 was amplified using three sets of primers containing 40-nucleotide homology extensions of the target genes (i.e., tolC, yqgA, and aaeX). The PCR products were purified from agarose gels and transformed into strain 83972(pKD46), and kanamycin-resistant colonies were selected. The λRed helper plasmid pKD4 was cured by growth at 37°C, and the correct double-crossover and recombination events were confirmed by PCR. MICs of EPIs were determined by using LB (E. coli UTI strain 83972, E. coli wild-type strain F18, K. pneumoniae strain i222-86, and P. putida KT2442), FBA minimal medium (E. coli UTI strain 83972), or tryptic soy broth (S. aureus strain 8324) (32), and the concentrations used in the biofilm experiments were between one-half and one-quarter the MICs in liquid cultures for each strain to ensure that the concentrations were not toxic.
Biofilm formation in microtiter plates.
Cells were allowed to form biofilms overnight at 37°C in 24-well (Iwaki), 48-well (Iwaki), or 96-well (TPP) flat-bottom microplates, and biofilm formation was monitored by crystal violet staining, as described previously (14). In the experiment in which tetracycline and ethidium bromide (EtBr) were added, as well as for P. putida biofilm growth, the biofilm was dispersed, diluted, and plated on LB agar plates to determine the number of CFU/well.
Biofilm formation in flow cell chambers.
Flow cell experiments were performed at 37°C using FBA minimal medium, essentially as described previously (5, 14) except that there was an additional overnight wash of the flow cell system with sterile MilliQ water. Biofilm formation in the presence and in the absence of a combination of the EPIs thioridazine (50 μg/ml) and 1-(1-naphthylmethyl)-piperazine (NMP) (100 μg/ml) was monitored at 16 and 24 h postinoculation. The images acquired were processed for display using the IMARIS software (Bitplane), and the relative coverage of the substratum by the biofilm was estimated using the COMSTAT software (20). Each experiment in flow chambers was performed in triplicate channels and repeated twice.
RESULTS
Efflux pump and drug transporter genes are highly upregulated during biofilm growth.
We previously studied global gene expression of biofilms of two E. coli UTI strains grown in human urine and compared these biofilms with planktonic growth in the same medium (19). When we reanalyzed the global transcription profiles of E. coli strains 83972 and VR50 during biofilm growth and compared the results with the results for planktonic growth with a focus on efflux pump genes, it was apparent that 128 of 600 upregulated genes encoded efflux pumps and transporters; many of these genes were among the most highly upregulated genes overall (Table 2). Notably, the genes in the aaeXAB operon, which normally is not expressed at a significant level (46), were some of the most upregulated genes. Recently, it has been shown that the aaeAB genes encode an aromatic carboxylic acid efflux pump whose physiological role may be as a metabolic relief valve to alleviate toxic effects of imbalanced metabolism (46). Many of the efflux systems involved in removal of toxic substances, including many antibiotics, from the cells were highly upregulated during biofilm growth (Table 2). Furthermore, the multidrug transporter genes yieO, acrD, and acrF were induced 5.4-, 4.5-, and 2.9-fold, respectively, in a strain 83972 biofilm. The upregulation of these genes encoding transport systems for drugs and metabolites, together with the induction of many stress-related genes, indicates that the biofilm growth mode indeed exerts great pressure on the cells, leading to metabolic upset due to intracellular stress conditions.
TABLE 2.
Twenty efflux and transport genes upregulated during biofilm growth
| Gene | b no. | Change (fold) during biofilm formationa
|
Product | |
|---|---|---|---|---|
| 83972 | VR50 | |||
| yqgA | b2966 | 39.45 | 10.42 | Putative transport protein |
| mdtL | b3710 | 11.46 | 15.27 | Multidrug efflux system protein (increases resistance to fosfomycin and chloramphenicol) |
| aaeX | b3242 | 24.78 | 9.21 | Membrane protein of efflux system |
| mdtG | b1053 | 8.82 | 11.64 | Predicted drug efflux system (increases resistance to fosfomycin and chloramphenicol) |
| aaeA | b3241 | 30.89 | 4.07 | p-Hydroxybenzoic acid efflux system component |
| setB | b2170 | 7.42 | 5.96 | Lactose/glucose:proton efflux pump (MFS family) |
| yjeH | b4141 | 8.96 | 2.82 | Predicted transporter |
| ynfM | b1596 | 3.62 | 7.39 | Predicted transporter (MFS family) |
| cmr | b0842 | 6.86 | 4.89 | Multidrug efflux system protein (increased resistance to tetracycline, chloramphenicol, erythromycin, and EtBr) |
| yfhD | b2558 | 4.1 | 5.31 | Putative periplasmic binding protein of transport system |
| aaeB | b3240 | 12.39 | 1.96 | p-Hydroxybenzoic acid efflux system component |
| ybjL | b0847 | 7.63 | 2.17 | Predicted transporter |
| ycdZ | b1036 | 6.97 | 2.83 | Putative transport protein |
| yojI | b2211 | 4.66 | 1.94 | Predicted multidrug/microcin J25 efflux pump |
| ygaY | b2680 | 2.78 | 3.79 | Uncharacterized transport protein (MFS family) |
| yhaO | b3110 | 2.21 | 7.66 | Inner membrane transport protein |
| sugE | b4148 | 3.51 | 2.48 | Multidrug efflux system protein |
| mdtI | b1599 | 3.59 | 1.77 | Multidrug efflux system transporter |
| yejA | b2177 | 2.31 | 3.49 | Microcin C transport protein (ABC superfamily) |
| mdtJ | b1600 | 2.02 | 2.99 | Multidrug efflux system transporter |
Changes in a urine biofilm compared with planktonic growth in urine. Bold type indicates that there was significant upregulation.
EPIs inhibit bacterial biofilm formation.
Arguably, the upregulation of efflux pump-encoding genes could be due to the cramped conditions in biofilms, in which cells are close together. We therefore speculated that biofilm-associated cells might have a waste management problem. This would make efflux pumps attractive targets in the fight against biofilm-associated populations and prime targets for antibiofilm drugs. With this in mind, we investigated the effect of inhibitors of efflux pumps on biofilm formation.
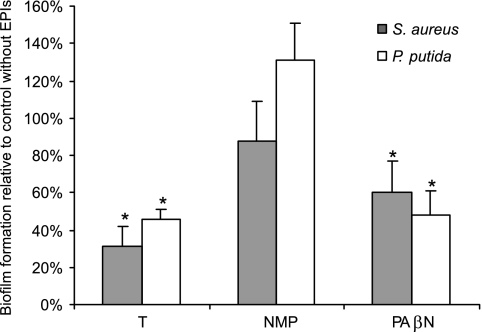
When three known inhibitors of bacterial efflux pumps were tested, in almost all cases they were able to reduce bacterial biofilm formation significantly (Fig. 1). Thus, addition of the inhibitor thioridazine, Phe-Arg β-naphthylamide (PAβN), or NMP significantly reduced formation of biofilms by several strains representative of the bacteria mentioned above (Fig. 1A and B). Furthermore, addition of thioridazine resulted in 84 to 89% and 63 to 91% reductions in the formation of biofilms by E. coli UTI strains VR50 and VR89 and by the uropathogenic Klebsiella sp. strains i225-86, i113-96, and i133-96, respectively (P < 0.001, as determined by a paired two-tailed t test for five independent experiments) (data not shown). To briefly assess whether the effect of EPIs on biofilm formation also applies to bacteria not belonging to the family Enterobacteriaceae, we included S. aureus strain 8324 and P. putida strain KT2442 in our study. Addition of 20 μg/ml thioridazine and PAβN resulted in 50 to 70% and 40 to 50% reductions, respectively, in the formation of biofilms by S. aureus 8324 and P. putida KT2442, while addition of 20 μg/ml NMP (Fig. 2) did not result in significant reductions in the formation of biofilms by these two strains.
FIG. 2.
Effects of the EPIs thioridazine, NMP, and PAβN on biofilm formation by S. aureus and P. putida. Thioridazine (T) (20 μg/ml), NMP (20 μg/ml), or PAβN (20 μg/ml) was added to static microtiter cultures of S. aureus 8324 and P. putida KTT2442 at the time of inoculation, and the amount of biofilm after 24 h was determined by crystal violet staining. Addition of thioridazine and addition of PAβN resulted in 50 to 70% and 40 to 50% reductions, respectively, in biofilm formation by S. aureus and P. putida compared with the biofilm formation in the absence of EPIs (*, P < 0.01, as determined by a paired two-tailed t test for five independent experiments).
Thioridazine has been shown to act as an EPI for major facilitator superfamily proteins in S. aureus (36). The dipeptide PAβN and NMP are known to inhibit resistance-nodulation-cell division pumps in gram-negative bacteria, particularly the AcrAB-TolC system of E. coli (4) and the MexAB-OprM system of P. aeruginosa (41). Both PAβN and NMP have been shown to be efficient EPIs in clinical isolates (21), improving the potency of antibiotics for resistant bacteria, although their effector profiles differ (25). The MICs of all three EPIs were determined (Table 3). The concentrations of EPIs used in the biofilm experiments were well below the MICs of interest (see Materials and Methods), and under these conditions the EPIs did not have a substantial effect on planktonic growth when the final optical cell densities and growth rates of the strains grown in the presence and in the absence of the EPIs were compared.
TABLE 3.
Susceptibility of planktonic growth to the EPIs tested
| Strain | MIC (μg/ml) of:
|
||||
|---|---|---|---|---|---|
| Thioridazine | PAβN | NMP | Thioridazine + PAβNa | Thioridazine + NMPa | |
| E. coli UTI strain 83972 | 150b | >200 | >200b | 100 | 100b |
| E. coli wild-type strain F18 | 150 | >200 | >150 | 100 | 100 |
| K. pneumoniae UTI strain i222-86 | 150 | >200 | >150 | 100 | 100 |
| P. putida KT2442c | 80 | ||||
| S. aureus 8324c | 80 | ||||
100 μg/ml of each EPI.
The MICs determined in LB and FBA minimal medium were the same.
Strains KT2442 and 8324 were tested only with thioridazine.
Synergistic action of inhibitors.
Bacteria generally have several different types of efflux pumps (36). Therefore, the most efficient way of abolishing efflux pump activity might be simultaneous use of two or more types of EPIs with extended ranges. Combinations of EPIs, such as thioridazine with PAβN or thioridazine with NMP, acted synergistically, and the effects of these combinations on biofilm formation were even greater than the additive effects of the individual EPIs (Fig. 1A). In the case of thioridazine plus PAβN, both E. coli UTI strain 83972 and Klebsiella sp. strain i222-86 showed up to >95% decreases in biofilm formation compared with controls without EPIs, and a similar reduction in formation of biofilms by E. coli 83972 was observed with a combination of thioridazine and NMP (Fig. 1A). This pattern should be interpreted in light of the fact that individual bacterial strains have several efflux pump systems and inhibition of more than one type seems to be required for efficient suppression of biofilm formation. This observation is in line with the finding that simultaneous expression of efflux pumps that are different structural types results in a level of antibiotic resistance higher than that conferred by expression of a single type of pump (24). However, with S. aureus and P. putida no synergistic effect was observed when combinations of the EPIs were assessed (data not shown). This was probably a reflection of the different types of efflux pumps present in these bacteria compared to members of the family Enterobacteriaceae.
The urinary tract is a highly hydrodynamic environment. To better mimic the conditions in the urinary tract, we investigated the effect of EPIs on the biofilm-forming ability of E. coli UTI strain 83972 in the flow cell chamber system. To do this, a yellow fluorescent protein-producing version of E. coli 83972 (E. coli 83972-yfp) was used (15). There was an 83% reduction in the formation of biofilms by E. coli 83972-yfp at 24 h postinoculation in the presence of a combination of the EPIs thioridazine and NMP (Fig. 1B).
EPIs enhance the killing effect of antimicrobial agents on bacterial biofilms.
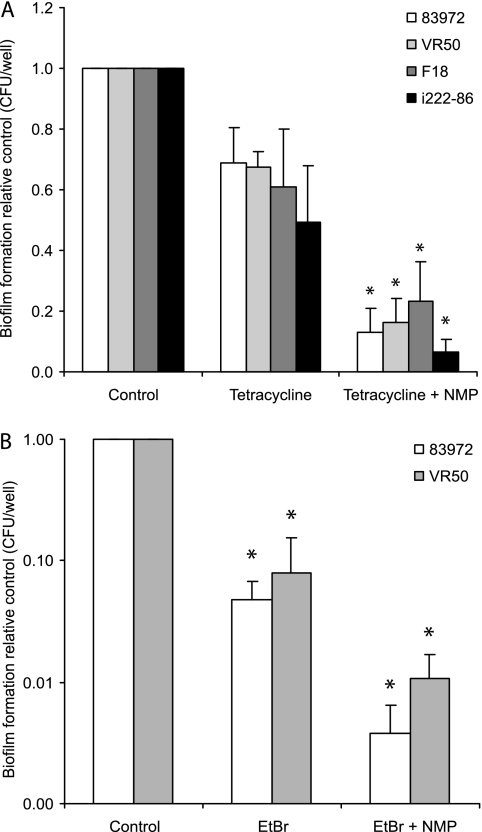
Biofilm-dwelling bacteria are known to be highly tolerant or resistant to antibiotics. Meanwhile, the detailed mechanisms underlying this important phenomenon are largely unknown. The expression of genes encoding efflux pumps was highly upregulated. Arguably, the cramped conditions of the biofilm growth mode confer severe waste management problems on the bacteria. Arguably, the enhanced pump activity not only rids the bacteria of “normal” waste but also pumps out many antibiotics. Therefore, the tolerance of bacterial biofilms to antibiotics, at least in some cases, may be a consequence of the enhanced efflux pump activity in biofilms. The least efficient of the three EPIs, NMP (Fig. 1A), was chosen in order to better visualize any significant effect that occurred in combination with an antimicrobial agent. The antibiotic tetracycline was used in combination with the selected EPI; NMP has been shown to have a significant effect on the susceptibility of clinical isolates of E. coli to tetracycline (21), and furthermore, it has also been shown that tetracycline rapidly reaches all the constituent cells of E. coli biofilms (45). Addition of tetracycline to overnight biofilms of E. coli UTI strains 83972 and VR50, E. coli F18, and K. pneumoniae i222-86 revealed that there was significantly higher sensitivity to tetracycline in the presence of the EPI NMP (Fig. 3A). While 33 to 51% of the biofilm-forming bacteria were killed by tetracycline after a 2 h of exposure, between 77 and 93% of the biofilm bacteria were killed by a combination of tetracycline and the EPI NMP, resulting in 5.2-, 4.2-, 2.6-, and 7.5-fold-increased killing effects on strains 83972, VR50, F18, and i222-86, respectively, while use of NMP alone did not result in any significant reduction in biofilm formation (Fig. 1A). To investigate whether the increased killing effect of tetracycline induced by NMP could be seen with another antimicrobial agent, EtBr was selected and tested with the two UTI strains. Significant effects of NMP on the MICs of EtBr have been observed for a large fraction of clinical isolates of E. coli; the MICs were reduced more than fourfold in 51 of 60 isolates (85%) by the presence of NMP, and this effect on EtBr MICs was observed when EtBr was used in combination with NMP but not when it was used with another EPI, PAβN (21). For our two clinical E. coli isolates, 83972 and VR50, there were 92 to 95% reductions in biofilm formation after 2 h of exposure to 200 μg/ml EtBr, and even though the effect of NMP on UTI strains 83972 and VR50 was limited (Fig. 1A) (no significant decrease in biofilm formation was observed), NMP increased the killing effect of EtBr 12.8- and 7.5-fold compared with the effect observed with EtBr alone (Fig. 3B). The presence of the EPI NMP in combination with 2 h of exposure to EtBr resulted in 264- and 93-fold reductions in the formation of biofilms by UTI strains 83972 and VR50, respectively.
FIG. 3.
Biofilm formation in the presence of antimicrobial agents in combination with the EPI NMP. (A) Biofilms of E. coli UTI strains 83972 and VR50, E. coli F18, and K. pneumoniae were significantly more sensitive to tetracycline in the presence of the EPI NMP. Tetracycline (100 μg/ml) was added to overnight biofilms that had been grown in media with and without NMP. After 2 h of exposure to tetracycline the biofilm density was determined by determining the number of CFU per microtiter well. In the presence of NMP (100 μg/ml) the killing effect of tetracycline was increased 2.6- to 7.5-fold (*, P < 0.01, as determined by a paired two-tailed t test for three independent experiments). (B) Biofilms of UTI strains 83972 and VR50 showed large increases in EtBr susceptibility in the presence of NMP. Overnight biofilms were exposed to EtBr (200 μg/ml) for 2 h after growth in media with and without NMP. The size of the viable biofilm was determined by determining the number of CFU per microtiter well. In the presence of NMP (100 μg/ml) the killing effect of EtBr was increased 13- and 7.5-fold for strains 83972 and VR50, respectively (*, P < 0.01, as determined by a paired two-tailed t test for five independent experiments).
Sensitivity of efflux pump knockout to EPIs.
Two of the most highly upregulated genes during biofilm growth of E. coli UTI strain 83972 were aaeX and yqgA (Table 2), which encode a membrane protein component of the AaeAB efflux pump and a putative transport protein, respectively. Compared with the wild-type strain, ΔaaeX and ΔyqgA knockout mutants of E. coli UTI strain 83972 showed 33 and 26% reductions in biofilm formation. Furthermore, 83972ΔyqgA was more sensitive to the EPI thioridazine than the wild type; the reduction in biofilm formation in the presence of thioridazine was 36% greater for this strain than for strain 83972.
Multicomponent efflux pumps, like the AcrAB-TolC (E. coli) and MexAB-OprM (P. aeruginosa) systems, are dependent on outer membrane channel proteins (like TolC and OprM) to extrude their substrates directly into the external medium (34). A wide range of EPIs are known; some are highly system specific, whereas others can inhibit the action of several types of efflux pumps. The compositions of structurally diverse pumps also are different in different bacteria, and since the EPIs tested here might also act on pumps other than the AcrAB-TolC type, a strain not capable of producing TolC would be suspected to be more sensitive to EPIs than its nonmutated counterpart. Indeed, the MIC of thioridazine was four times lower for the knockout mutant E. coli UTI strain 83972ΔtolC than that for the nonmutated strain. When the organism was exposed to 10 μg/ml thioridazine, the biofilm formation by the ΔtolC mutant was reduced 88% compared with the biofilm formation in the absence of thioridazine (P < 0.01, as determined by a paired two-tailed t test for three independent experiments).
DISCUSSION
Bacterial biofilms can become established on almost any surface and cause numerous problems in industry and health care. We are particularly interested in bacterial biofilms in medical settings. Most chronic and persistent human infectious diseases are associated with biofilm growth, a fact that has compounded the emergence and rapid spread of multidrug-resistant bacteria. It has been known for decades that biofilm-associated bacteria can tolerate antibacterial agents like detergents, biocides, and antibiotics far better than planktonic cells (2). There seem to be multiple mechanisms underlying this phenomenon (for a review, see reference 28). However, in only a few cases are the underlying mechanisms known in detail (29). Our findings concerning the role of efflux pumps in E. coli during biofilm growth could help elucidate this phenomenon further. Arguably, the relatively cramped conditions in a biofilm with tightly compacted cells require efficient waste management in the form of increased efflux pump activity. Since many types of antibiotic resistance in bacteria are known to be conferred by efflux pumps (37), it is not surprising that enhanced efflux pump activity occurs concomitant with enhanced antibiotic resistance. In line with this reasoning, interference with efflux pump activity during biofilm growth should be predicted to impact the biofilm-forming capacity. Indeed, when we added EPIs in our experiments, we observed significant reductions in biofilm formation. Several different EPIs had this capacity.
Five main families of bacterial efflux pumps are known, and individual bacteria possess multiple efflux pumps belonging to these families. In light of this it is not surprising that one type of EPI was not able to fully suppress biofilm formation. However, when two complementary EPIs, potentially acting on different types of pumps, were used together, we observed close to 100% inhibition (Fig. 1A). The data resulting from biofilm inhibition with EPIs were also corroborated by using knockout mutants having genetic lesions in efflux pump-encoding genes. E. coli mutants with ΔaaeX and ΔyqgA knockout mutations exhibited significantly reduced biofilm formation compared to their parents. Also, such mutants were notably more sensitive to EPIs.
Our work has focused mainly on uropathogenic E. coli and Klebsiella strains. These organisms are significant pathogens, and UTI is one of the top bacterial diseases in humans, with more than 150 million cases per year globally (44). However, in order to get a rough idea of the extent of the phenomenon, two well-characterized biofilm-forming bacterial strains not belonging to the family Enterobacteriaceae were tested to determine the effect of EPI activity on biofilm formation. These strains were S. aureus strain 8324, a gram-positive organism, and P. putida strain KT2442, a gram-negative organism. In both cases biofilm formation could be significantly reduced by EPIs (Fig. 2). Although our results do not indicate that EPIs can abolish or reduce biofilm formation in all types of bacteria, they do suggest that EPI inhibition is not restricted to the family Enterobacteriaceae.
The intrinsic tolerance of biofilm-associated bacteria to external agents like detergents, biocides, and antibiotics makes biofilm-associated infections particularly recalcitrant to treatment. New types of antibiotics are, therefore, urgently needed; development of candidate drugs that target bacterial adhesion and biofilm formation should be of great importance for combating diseases (22). By virtue of their anti-efflux pump activities, it seems that EPIs have great potential as antibiofilm drugs. EPIs are generally simple, robust, and cheap chemicals. Also, in cases where they have been tested, EPIs are well tolerated by humans (30, 36). Indeed, EPIs could well constitute a long-needed new class of antibiotics. Furthermore, it is possible that EPIs could be used as enhancers of “classical” antibiotics when such antibiotics are used to treat biofilm-related diseases.
Acknowledgments
We thank Birthe Jul Jørgensen for expert technical assistance.
This work was supported by grants from the Danish Medical Research Council (grant 271-07-0291), Lundbeckfonden (R19-A2191), and FØSU (grant 2101-06-0009).
Footnotes
Published ahead of print on 3 October 2008.
REFERENCES
- 1.Aires, J., T. Köhler, H. Nikaido, and P. Plésiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderl, J. N., M. J. Franklin, and P. S. Stewart. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohnert, J., and W. Kern. 2005. Selected arylpiperazines are capable of reversing multidrug resistance in Escherichia coli overexpressing RND efflux pumps. Antimicrob. Agents Chemother. 49:849-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chevalier, J., J. Bredin, A. Mahamoud, M. Malléa, M. Barbe, and J. Pagés. 2004. Inhibitors of antibiotic efflux in resistant Enterobacter aerogenes and Klebsiella pneumoniae strains. Antimicrob. Agents Chemother. 48:1043-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, B., C. Sternberg, J. Andersen, R. Palmer, A. Toftgaard Nilsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 6.Coetser, S. E., and T. E. Cloete. 2005. Biofouling and biocorrosion in industrial water systems. Crit. Rev. Microbiol. 31:213-232. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, S. P., D. C. Hooper, J. S. Wolfson, K. S. Souza, L. M. McMurry, and S. B. Levy. 1988. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob. Agents Chemother. 32:1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, S. P., L. M. McMurry, D. C. Hooper, J. S. Wolfson, and S. B. Levy. 1989. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob. Agents Chemother. 33:1318-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costerton, J., S. Stewart, and E. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 10.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K., and B. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Kievit, T., M. Parkins, R. Gillis, R. Srikumar, H. Ceri, K. Poole, B. Iglewski, and D. Storey. 2001. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 45:1761-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrières, L., V. Hancock, and P. Klemm. 2007. Biofilm exclusion of uropathogenic bacteria by selected asymptomatic bacteriuria Escherichia coli strains. Microbiology 153:1711-1719. [DOI] [PubMed] [Google Scholar]
- 15.Fexby, S., T. Bjarnsholt, P. O. Jensen, V. Roos, N. Hoiby, M. Givskov, and P. Klemm. 2007. A biological Trojan horse: antigen 43 provides specific bacterial uptake and survival in human neutrophils. Infect. Immun. 75:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frees, D., A. Chastanet, S. Qazi, K. Sørensen, P. Hill, T. Msadek, and H. Ingmer. 2004. Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol. Microbiol. 54:1445-1462. [DOI] [PubMed] [Google Scholar]
- 17.Gillis, R., K. White, K. Choi, V. Wagner, H. Schweizer, and B. Iglewski. 2005. Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 49:3858-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gjermansen, M., P. Ragas, C. Sternberg, S. Molin, and T. Tolker-Nielsen. 2005. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms. Environ. Microbiol. 7:894-904. [DOI] [PubMed] [Google Scholar]
- 19.Hancock, V., and P. Klemm. 2007. Global gene expression profiling of asymptomatic bacteriuria Escherichia coli during biofilm growth in human urine. Infect. Immun. 75:966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heydorn, A., A. Nielsen, M. Henzer, C. Sternberg, M. Givskov, B. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 21.Kern, W., P. Steinke, A. Schumacher, S. Schuster, H. von Baum, and J. Bohnert. 2006. Effect of 1-(1-naphtylmethyl)-piperazine, a novel putative efflux pump inhibitor, on a antimicrobial drug susceptibility in clinical isolates of Escherichia coli. J. Antimicrob. Chemother. 57:339-343. [DOI] [PubMed] [Google Scholar]
- 22.Klemm, P., V. Hancock, M. Kvist, and M. Schembri. 2007. Candidate targets for new antivirulence drugs: selected cases of bacterial adhesion and biofilm formation. Future Microbiol. 2:643-653. [DOI] [PubMed] [Google Scholar]
- 23.Kohler, T., M. Michea-Hamzehpour, S. F. Epp, and J. C. Pechere. 1999. Carbapenem activities against Pseudomonas aeruginosa: respective contributions of OprD and efflux systems. Antimicrob. Agents Chemother. 43:424-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, A., W. Mao, M. S. Warren, A. Mistry, K. Hoshino, R. Okumura, H. Ishida, and O. Lomovskaya. 2000. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J. Bacteriol. 182:3142-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomovskaya, O., H. Zgurskaya, M. Totrov, and W. Watkins. 2007. Waltzing transporters and ‘the dance macabre’ between humans and bacteria. Nat. Rev. 6:56-65. [DOI] [PubMed] [Google Scholar]
- 26.Lynch, S., L. Dixon, M. Benoit, E. Brodie, M. Keyhan, P. Hu, D. Ackerley, G. Andersen, and A. Matin. 2007. Role of the rapA gene in controlling antibiotic resistance of Escherichia coli biofilms. Antimicrob. Agents Chemother. 51:3650-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma, D., D. Cook, M. Alberti, N. Pon, H. Nikaido, and J. Hearst. 1993. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J. Bacteriol. 175:6299-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mah, T., and G. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 29.Mah, T., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 30.Marquez, B. 2005. Bacterial efflux systems and efflux pump inhibitors. Biochimie 87:1137-1147. [DOI] [PubMed] [Google Scholar]
- 31.Moniot-Ville, N., J. Guibert, N. Moreau, J. Acar, E. Collatz, and L. Gutmann. 1991. Mechanisms of quinolone resistance in a clinical isolate of Escherichia coli highly resistant to fluoroquinolones but susceptible to nalidixic acid. Antimicrob. Agents Chemother. 35:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standards. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 33.Nelson, M. 2002. Modulation of antibiotic efflux in bacteria. Curr. Med. Chem. 1:35-54. [Google Scholar]
- 34.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Toole, G., and P. Stewart. 2005. Biofilms strike back. Nat. Biotechnol. 23:1378-1379. [DOI] [PubMed] [Google Scholar]
- 36.Piddock, L. J. V. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole, K. 2005. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 56:20-51. [DOI] [PubMed] [Google Scholar]
- 38.Poole, K. 2000. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob. Agents Chemother. 44:2233-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole, K., K. Tetro, Q. Zhao, S. Neshat, D. Heinrichs, and N. Bianco. 1996. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 40:2021-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renau, T. E., R. Leger, E. M. Flamme, J. Sangalang, M. W. She, R. Yen, C. L. Gannon, D. Griffith, S. Chamberland, O. Lomovskaya, S. J. Hecker, V. J. Lee, T. Ohta, and K. Nakayama. 1999. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 42:4928-4931. [DOI] [PubMed] [Google Scholar]
- 42.Roos, V., and P. Klemm. 2006. Global gene expression profiling of the asymptomatic bacteriuria Escherichia coli strain 83972 in the human urinary tract. Infect. Immun. 74:3565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg, E. Y., D. Bertenthal, M. L. Nilles, K. P. Bertrand, and H. Nikaido. 2003. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 48:1609-1619. [DOI] [PubMed] [Google Scholar]
- 44.Stamm, W., and S. Norrby. 2001. Urinary tract infections: disease panorama and challenges. J. Infect. Dis. 183:S1-S4. [DOI] [PubMed] [Google Scholar]
- 45.Stone, G., P. Wood, L. Dixon, M. Keyhan, and A. Matin. 2002. Tetracycline rapidly reaches all the constituent cells of uropathogenic Escherichia coli biofilms. Antimicrob. Agents Chemother. 46:2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Dyk, T. K., L. J. Templeton, K. A. Cantera, P. L. Sharpe, and F. S. Sariaslani. 2004. Characterization of the Escherichia coli AeaAB efflux pump: a metabolic relief valve? J. Bacteriol. 186:7196-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren, J. W. 2001. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 17:299-303. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, L., and T.-F. Mah. 2008. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 190:4447-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]