Abstract
The antifungal activity of proteinaceous compounds from different food matrices was investigated. In initial experiments, water-soluble extracts of wheat sourdoughs, cheeses, and vegetables were screened by agar diffusion assays with Penicillium roqueforti DPPMAF1 as the indicator fungus. Water-soluble extracts of sourdough fermented with Lactobacillus brevis AM7 and Phaseolus vulgaris cv. Pinto were selected for further study. The crude water-soluble extracts of L. brevis AM7 sourdough and P. vulgaris cv. Pinto had a MIC of 40 mg of peptide/ml and 30.9 mg of protein/ml, respectively. MICs were markedly lower when chemically synthesized peptides or partially purified protein fractions were used. The water-soluble extract of P. vulgaris cv. Pinto showed inhibition toward a large number of fungal species isolated from bakeries. Phaseolin alpha-type precursor, phaseolin, and erythroagglutinating phytohemagglutinin precursor were identified in the water-soluble extract of P. vulgaris cv. Pinto by nano liquid chromatography-electrospray ionization-tandem mass spectrometry. When the antifungal activity was assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, all three proteins were inhibitory. A mixture of eight peptides was identified from the water-soluble extract of sourdough L. brevis AM7, and five of these exhibited inhibitory activity. Bread was made at the pilot plant scale by sourdough fermentation with L. brevis AM7 and addition of the water-soluble extract (27%, vol/wt; 5 mg of protein/ml) of P. vulgaris cv. Pinto. Slices of bread packed in polyethylene bags did not show contamination by fungi until at least 21 days of storage at room temperature, a level of protection comparable to that afforded by 0.3% (wt/wt) calcium propionate.
Contamination by fungi is the most common source of microbial spoilage and is a costly problem in bakery processes. In many cases, it is the major factor governing shelf life. Besides the repellent sight of visible growth, fungi may be responsible for off-flavors and synthesize mycotoxins and allergenic compounds. Overall, the most widespread species of fungi that contaminate bakery products belong to the genera Eurotium, Aspergillus, Penicillium (1, 15), Monilia, Mucor, Endomyces, Cladosporium, Fusarium, and Rhizopus (18, 19).
The use of chemical preservatives, novel ingredients with mold-inhibiting properties, and packaging techniques are all technology options that, together with hygiene during processing, may contribute to decreasing the growth of fungi in baked goods (9, 25). Salts of propionic, sorbic, and benzoic acids are routinely used as chemical preservatives. Propionic acid is inhibitory to fungi and Bacillus spores, and it is largely used for bread preservation (24). Its antifungal activity strictly depends on the concentration of the undissociated form that prevails at low pH. The European directive on preservatives (11) has decreased the allowable concentrations of sorbate (0.2%, wt/wt) and propionate (0.3%, wt/wt). Furthermore, the appearance of cancer-like tumors in rats fed propionic acid at concentrations of up to 4% has led to prohibition of the use of calcium propionate in some European countries and/or to the limited use of organic acid preservatives in foods (24). Prolonged preservation of rye bread with a high calcium propionate concentration showed a strong inhibitory effect toward many fungi, but after the lag phase of growth, it also stimulated resistant strains of Penicillium roqueforti (32). Ethanol is also inhibitory to fungi in baked goods. Although it has the status of being generally recognized as safe, the use of ethanol is permitted only up to 2% (wt/wt). The inhibitory concentration of ethanol varies, depending on the fungal species, and in some cases it is not sufficient to prevent contamination (10).
Modern consumers require high quality, preservative-free, safe, but mildly processed foods with extended shelf life and preserved sensory properties. These considerations have led to the search for new preservatives, especially those derived from natural sources. Antifungal compounds from plants comprise several proteins and peptides that are thought to play an important role in disease resistance (38). Antifungal compounds include peptides such as thionins from barley and wheat tissues, as well as proteins and peptides from the seeds of many different species of leguminous plants (22, 35), including Phaseolus spp. Overall, antifungal proteins and peptides from plants are classified based on their structures and/or functions into chitinases, glucanases, thaumatin-like proteins, thionins, and cyclophilin-like proteins. Some well-known proteins, such as lectins, ribosome-inactivating proteins, ribonucleases, deoxyribonucleases, peroxidases, and protease inhibitors, also exhibit antifungal activity (22).
Among natural preservatives, sourdough has been largely considered for extending the shelf life of baked goods. Sourdough lactic acid bacteria synthesize a range of antifungal compounds such as phenyllactic acid, cyclic dipeptides, and short- or medium-chain fatty acids (18, 28, 29, 31). Recently, a small peptide (ca. 3 kDa) that is heat stable and active in a pH range of 3 to 6 was liberated during growth of Lactobacillus coryniformis Si3 (20). Two cyclic dipeptides, Phe-Pro and Phe-OH-Pro, were also isolated in culture media or food matrices during growth of Lactobacillus plantarum MiLAB393 (31), Pediococcus pentosaceus, Lactobacillus sakei, and L. coryniformis (21). This suggests that these peptides could represent common metabolites of lactic acid bacteria. Most of the above studies demonstrated antifungal activity only under laboratory conditions, and very little information was given on the mechanism of mold inhibition and on their potential activity under pilot plant or industrial conditions.
Herein, we describe the systematic screening and characterization of novel antifungal compounds isolated from Phaseolus vulgaris cv. Pinto and sourdough fermented by Lactobacillus brevis AM7. The mechanism of fungal inhibition was investigated, and the inhibitory activity toward a large spectra of bakery-related fungi was tested. The inhibitory activity was confirmed during long-term shelf life testing under pilot plant conditions.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Twenty-seven strains of various species of sourdough lactic bacteria belonging to the Culture Collection of the Department of Plant Protection and Applied Microbiology were used. Lactobacillus plantarum was excluded from the study since it was well characterized for antifungal activity previously (9, 18, 33). Lactic acid bacteria were routinely propagated for 24 h at 30°C in MRS (Oxoid, Hampshire, United Kingdom) with the addition of fresh yeast extract (5%, vol/vol) and 28 mM maltose at a final pH of 5.6.
P. roqueforti DPPMAF1 was isolated from contaminated bread and used as the indicator microorganism for antifungal assays since it is one of the fungi that are more resistant to chemical preservatives (32). Penicillium polonicum CBS 112490, Penicillium chrysogenum CBS 111214, Penicillium paneum CBS 101032, Penicillium bialowiezense CBS 110102, Penicillium brevicompactum CBS 289.97, Penicillium albocoremium CBS 109582, Penicillium chermesinum CBS 117279, Penicillium carneum CBS 112297, Eurotium herbarioum CBS 117336, Eurotium rubrum CBS 150.92, Aspergillus parasiticus CBS 971.97, and Aspergillus versicolor CBS 117286 from the Culture Collection of the Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands) were also used. All of the above species corresponded to some of the most related spoilage fungi in baked goods (18, 32). Fungi were grown in potato dextrose agar (PDA; Oxoid) for 24 to 72 h at 25°C. PDA was also used for agar diffusion assays.
Wheat flour hydrolysate (WFH) was produced as described previously (12). A suspension of wheat flour (20%, wt/vol, in tap water) was incubated for 18 h at 30°C while being stirred (ca. 200 rpm). After incubation, the suspension was filtered onto a Whatman apparatus (Polycarp 75 SPF; Whatman International, Maidstone, England) and yeast extract (0.3%, wt/vol), sucrose, glucose, and maltose (1.5%, wt/vol, final concentration) were added. The WFH was sterilized by filtration on 0.22-μm membrane filters (Millipore Corporation, Bedford, MA) and stored at 4°C before use. The pH of the WFH was ca. 5.6. WFH was chosen as the substrate because it is representative of the chemical composition of wheat flour.
Sourdough fermentation.
Cells of lactic acid bacteria were cultivated in MRS at 30°C until the late exponential phase of growth was reached (10 h). After growth, cells were harvested by centrifugation at 10,000 × g for 10 min at 4°C and washed with 20 mM phosphate buffer, pH 7.0. Strains were individually used for sourdough fermentation. The characteristics of the flour (Triticum aestivum cv. Appulo) used for sourdough fermentation were as follows: moisture, 14.2%; protein (N × 5.70), 11.5% of dry matter (d.m.); fat, 1.6% of d.m.; ash, 0.6% of d.m.; total soluble carbohydrates, 1.5% of d.m. Ten grams of wheat flour and 23 ml of tap water (dough yield of 330), containing a lactic acid bacterial cell density of ca. 108 CFU/g of dough, were used to produce 33 g of dough. Sourdoughs were incubated for 24 h at 37°C while being stirred (ca. 200 rpm). At the end of the fermentation, 1 g of dough was diluted with 4 ml of 50 mM Tris-HCl (pH 8.8), held at 4°C for 1 h with vortexing at 15-min intervals, and centrifuged at 20,000 × g for 20 min. The water-soluble extract was used for antifungal assays.
Fermentation of buckwheat, rye, spelt (Triticum spelta), corn, and wheat whole-meal flours was carried out under the same conditions.
Water-soluble extracts of cheeses and vegetables.
Seventeen varieties of cheeses that had the potential to contain biologically active peptides (26) were used. Water-soluble extracts of cheeses were prepared according to the method of Kuchroo and Fox (16).
Water-soluble extracts of vegetables were prepared as described by Schmourlo et al. (27), with some modifications. Fifty grams of each vegetable was homogenized with 50 ml of distilled water in a blender (PBI International, Milan, Italy). The ground material was added to 50 ml of boiling distilled water, incubated for 10 min at room temperature while being stirred, and centrifuged at 14,000 × g for 20 min to recover the supernatants for antifungal activity assays.
Assays for antifungal activity.
Three different methods were used to determine antifungal activity. The agar diffusion assay was carried out as previously described by Ye and Ng (39), with some modifications. Specifically, petri plates (90-mm diameter) containing 10 ml of PDA (Oxoid) were inoculated with the fungus. After the mycelial colony had developed, sterile blank paper disks (0.5-cm diameter) were placed at a distance of ca. 0.5 cm away from the rim of the mycelial colony. Ten microliters of the water-soluble extract to be tested was added to the disks. Plates were incubated at 25°C for 72 h until the mycelial growth overlaid the paper disk containing the control (without water-soluble extract addition). At the same time, zones of inhibition were evident on corresponding paper disks containing water-soluble extracts with inhibitory activity. Inhibitory activity was scored visually as follows: −, no inhibition; +, very weak inhibition; ++, low inhibition with a little clear zone near the rim of the colony; +++, strong inhibition with a large clear zone near the rim of the colony; ++++, very strong inhibition with no growth near the rim of the colony.
The effect of inhibitory compounds on the germination of conidia was also determined (14). After growth for 7 days on PDA plates, conidia of P. roqueforti DPPMAF1 were harvested in sterile water containing 0.05% (vol/vol) Tween 80. A fixed number of ca. 106 to 107 conidia/ml was added to 5 ml of the mixture of WFH and water-soluble extract (ratio of ca. 3:1). The mixture was incubated in 60-mm petri dishes for 24 h at 25°C while being stirred. WFH alone and WFH with 0.3% (wt/vol) calcium propionate added were used as the controls. To determine the percentage of germinated conidia (length/width ratio, ≥2), slides of the suspension were examined under a microscope (×40 magnification) at 4-h intervals. For each inhibitory compound and the controls, three separate replications of at least 100 conidia were assayed.
To determine MICs, concentrated water-soluble extracts and fractions were serially diluted in WFH. The mixture was poured into 60-mm petri dishes, inoculated with a suspension of conidia (106 to 107 conidia/ml, final density), and incubated for 12 h at 25°C while being stirred. After 12 h, slides of the suspension were examined under a microscope and the germinated spores were counted as described above. The MIC was defined as the lowest concentration of water-soluble extract that inhibited fungal growth for 12 h at 25°C. The concentrations of peptides and proteins in the water-soluble extracts and fractions were determined by the o-phthaldialdehyde and Bradford methods (4, 7), respectively.
Mycelial dry weight was determined as described by Gourama (14), with some modifications. After incubation for 48 h at 25°C in WFH with or without water-soluble extracts, the mycelial biomasses were collected by filtration through a Whatman apparatus (0.22-μm membrane filter). After the filters were dried at room temperature, dry weight was measured until it became constant (24 to 48 h). All assays for antifungal activity were carried out at least in triplicate.
Proteolysis and heat stability of antifungal compounds.
Water-soluble extracts were treated with trypsin (EC 3.4.21.4; Sigma Aldrich Co.) as described by Atanassova et al. (3). Trypsin was dissolved in 0.25 M Tris HCl, pH 8 (1%, wt/vol, final concentration). One hundred microliters of the supernatant containing antifungal compounds and 100 μl of the buffered enzyme solution were mixed. After 5 h of incubation at 25°C, the reaction was stopped by boiling the mixture for 3 min. After treatment, the pH of the solution was adjusted to 6.0 and the residual activity was determined by agar diffusion assay. The heat stability of the water-soluble extracts was determined by heating them for 5 min at 100°C. After treatments, the residual activity was determined by agar diffusion assay.
Purification of antifungal compounds.
First, water-soluble extract of P. vulgaris cv. Pinto was fractionated by ultrafiltration (Ultrafree-MC centrifugal filter units; Millipore) by using three different membrane sizes with 50-, 30-, and 10-kDa cutoffs. Aliquots of 400 μl of the water-soluble extract were centrifuged at 10,000 × g for 60 min. After ultrafiltration, fractions were used for agar diffusion assay. The fraction containing polypeptides in the range 50 to 30 kDa was further subjected to reversed-phase fast-performance liquid chromatography (RP-FPLC) with a Resource RPC column and an ÄKTA FPLC apparatus with the UV detector operating at 280 nm (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Aliquots containing 2.5 mg/ml protein, as determined by the Bradford method (4), were centrifuged at 10,000 × g for 10 min. The supernatant was filtered through a 0.22-μm filter membrane and loaded onto the column. Gradient elution was performed at a flow rate of 0.3 ml/min with a mobile phase consisting of 50 mM K-phosphate buffer and 0.15 M NaCl, pH 7.0. Collected fractions were freeze dried, redissolved in sterile water, and subjected to agar diffusion assay. Polypeptides from the fraction with antifungal activity were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the Laemmli procedure (17) and subjected to mass spectrometry analysis.
Water-soluble extract of sourdough was directly fractionated by FPLC with an RP Resource column with the UV detector of the ÄKTA FPLC apparatus operating at 214 nm. Aliquots containing 0.875 mg/ml peptide were added to 0.05% (vol/vol) trifluoroacetic acid and centrifuged at 10,000 × g for 10 min. The supernatant was filtered through a 0.22-μm-pore-size filter and loaded onto the column. Gradient elution was performed at a flow rate of 1 ml/min with a mobile phase composed of water and acetonitrile (CH3CN) containing 0.05% (vol/vol) trifluoroacetic acid. The CH3CN concentration was increased linearly from 5 to 46% between 16 and 62 min and from 46 to 100% between 62 and 72 min. Solvents were removed from collected fractions by freeze drying. The fractions were redissolved in sterile water and subjected to agar diffusion assay. The freeze-dried preparation of the fraction with antifungal activity was used for identification.
A mixture of organic acids (lactic, acetic, caproic, propionic, butyric, n-valeric, and formic acids) with presumptive antifungal activity that might be synthesized by lactic acid bacteria during sourdough fermentation (8) was analyzed by RP-FPLC under the same conditions.
Identification of antifungal proteins and peptides.
Identification of proteins and peptides was carried out at the Proteome Factory (Proteome Factory AG, Berlin, Germany) by using nano liquid chromatography-electrospray ionization-tandem mass spectrometry (nano-LC-ESI-MS/MS). The MS system consisted of an Agilent 1100 nanoLC system (Agilent, Boeblingen, Germany), a PicoTip emitter (New Objective, Woburn, MA), and an Esquire 3000 plus ion trap MS (Bruker, Bremen, Germany). Protein bands cut from gels were digested in gel with trypsin (Promega, Mannheim, Germany) and subjected to nano-LC-ESI-MS/MS. Trapping and desalting of the peptides were carried out on an enrichment column (Zorbax SB C18, 0.3 by 5 mm; Agilent) with a 3% acetonitrile-0.1% formic acid solution for 5 min. Peptides were separated on a Zorbax 300 SB C18 column (75 μm by 150 mm; Agilent) with an acetonitrile-0.1% formic acid gradient of 5 to 40% acetonitrile within 40 min. Following the manufacturer's instrument settings for nano-LC-ESI-MS/MS analyses, MS spectra were automatically taken by the Esquire 3000 plus. MS/MS spectra were processed with a DataAnalysis 3.2 (Bruker, Bremen, Germany) generating peak lists suitable for database searches. Proteins and peptides were identified by MS/MS ion searching the NCBI protein database (National Center for Biotechnology Information, Bethesda, MD) with the Mascot search engine (Matrix Science, London, England).
For identification of peptides, the following parameters were considered: species (T. aestivum and other cereal species belonging to the genera Oryza, Hordeum, and Secale); enzyme, none; instrument type, ESI-trap; peptide mass tolerance, ±0.1%; fragment mass tolerance, ±0.5 Da. Peptide identification results were subjected to a manual evaluation, as described by Chen et al. (5), and the validated peptide sequences explained all of the major peaks in the MS/MS spectrum.
Detection of antifungal activity by SDS-PAGE and synthesis of peptides.
Duplicate samples of the purified fraction from the water-soluble extract of P. vulgaris cv. Pinto and molecular mass markers were subjected to SDS-PAGE (17). Following electrophoresis, the gel was cut vertically. One part was stained with Coomassie blue R-250 to determine the protein molecular masses. The other part was fixed with 25% (vol/vol) isopropanol and 10% (vol/vol) acetic acid for 30 min, washed twice with double-distilled water for 1 h, and overlaid in a petri dish containing the suspension of P. roqueforti DPPMAF1 conidia in PDA at ca. 50°C. The PDA plates were incubated at 25°C for 72 to 96 h until the zone of inhibition was visible (6).
All of the peptides identified in the water-soluble extract of sourdough were chemically synthesized by NeoSystem Laboratoire (Strasbourg, France). The purity of the synthesized peptides was higher than 95% as determined by high-performance liquid chromatography analysis and certified by the manufacturer.
Bread making.
Cells of L. brevis AM7 grown to the exponential phase were used to prepare sourdough. After fermentation for 18 h at 37°C, the sourdough harbored a cell density of presumptive lactic acid bacteria of ca. 5 × 109 CFU/g. Five different breads were manufactured at the pilot plant of the Department of Plant Protection and Applied Microbiology according to the formulas in Table 1. Doughs were mixed (60 × g for 5 min) with a continuous high-speed mixer (Chopin and Co., Boulogne, Seine, France), allowed to ferment for 2.5 h at 30°C, and baked at 220°C for 40 min (Combo 3; Zucchelli, Verona, Italy). Two slices of each bread were cut. Each slice was 12 cm high and 1.5 cm wide. One slice was inoculated by nebulization with a suspension of 102 P. roqueforti DPPMAF1 conidia/ml, and the other slice was left without inoculum. Slices were then packed in polyethylene bags to maintain constant moisture and incubated at room temperature for 21 days. pH values were determined by a Foodtrode electrode (Hamilton, Bonaduz, Switzerland). Moisture was determined according to the standard American Association of Cereal Chemists method (2).
TABLE 1.
Formulas used for bread makingc
| Ingredient (unit of measurement) | Bread 1 | Bread 2 | Bread 3 | Bread 4 | Bread 5 |
|---|---|---|---|---|---|
| Wheat flour (g) | 180 | 180 | 124.2 | 180 | 124.2 |
| Tap water (ml) | 114 | 114 | 79.8 | 34.2 | |
| Sourdougha (g) | 90 | 90 | |||
| Baker's yeast (g) | 6 | 6 | 6 | 6 | 6 |
| Water-soluble extract of P. vulgaris cv. Pinto (ml) | 79.8b | 79.8 | |||
| Calcium propionate (g) | 0.9 |
Sourdough contained 60% flour, 30% water (dough yield of 160), and 108 CFU/ml L. brevis AM7.
The protein concentration of the water-soluble extract of P. vulgaris cv. Pinto was 5 mg/ml.
The dough yield of all of the breads was 160.
Statistical analysis.
Data were submitted to analysis of variance, and means were compared by using the Tukey HSD test (30).
RESULTS
Antifungal activities from different sources.
The concentrations of peptides in water-soluble extracts of sourdoughs were 1.2 to 1.9 mg/ml. As shown by the agar diffusion assay, only eight water-soluble extracts of wheat sourdoughs showed antifungal activity toward the indicator P. roqueforti DPPMAF1. Water-soluble extracts of wheat sourdoughs fermented with L. brevis AM7 and L. paraplantarum 4DE had the highest inhibitory activity. Water-soluble extracts of wheat sourdoughs fermented with L. rossiae 6H, L. sanfranciscensis E9, L. farcimins DSM 20184, and L. pontis DSM 8745 had low activity, causing only small clearing zones near the rim of the colony. The 27 sourdough lactic acid bacterial strains were also used for fermentation of buckwheat, rye, spelt, corn, and wheat whole-meal flours, and similar results were obtained. Water-soluble extracts of wheat sourdoughs fermented with L. brevis AM7 and L. paraplantarum 4DE were freeze-dried, concentrated two- to fourfold, and assayed. Under these conditions, the inhibitory activity of both strains resulted in a clear zone near the rim of the colony. The combined use of the two strains to ferment wheat sourdough did not further increase the inhibitory activity.
Water-soluble extracts of cheeses generally exhibited little inhibition, although pecorino piedmontese and crescenza cheeses showed slight activity against the colony of P. roqueforti DPPMAF1.
The concentrations of proteins in the water-soluble extracts of vegetables were 0.8 to 6.7 mg/ml. P. vulgaris cv. Pinto showed very strong inhibitory activity, with no growth near the colony (Table 2). Marked inhibition was also found with extract of Vigna unguiculata.
TABLE 2.
Inhibitory activity on P. roqueforti DPPMAF1 of water-soluble extracts from various vegetable species as determined by agar diffusion assay
| Botanical name | Part(s) of plant | pH of extract | Inhibitory activitya |
|---|---|---|---|
| Cucurbita pepo | Fruit | 6.35 | − |
| Phaseolus vulgaris | Beans, seeds | 6.07 | − |
| Phaseolus vulgaris cv. Pinto | Seeds | 6.36 | ++++ |
| Vigna unguiculata | Seeds | 6.50 | +++ |
| Pisum sativum | Seeds | 6.94 | − |
| Vicia faba | Seeds | 6.49 | − |
| Brassica oleracea cv. Botrytis cauliflaura | Bloom | 6.31 | − |
| Spinacia oleracea | Leaves | 6.83 | − |
| Asparagus officinalis | Bloom, shaft | 5.69 | + |
| Solanum melongena | Fruit | 4.85 | − |
| Aloe arborescens | Leaves | 4.10 | + |
| Camellia sinensis | Leaves | 5.10 | + |
Inhibitory activity was scored as follows: −, no inhibition; +, very weak inhibition; ++, low inhibition with a little clear zone near the rim of the colony; +++, strong inhibition with a large clear zone near the rim of the colony; ++++, very strong inhibition with no growth near the rim of the colony.
Characterization of antifungal activity.
After incubation for 12 h at 25°C in WFH, the mean percentage of spore germination of P. roqueforti DPPMAF1 was 10.1%. Incubation in the presence of the water-soluble extracts of the sourdough fermented with L. brevis AM7 and P. vulgaris cv. Pinto, alone or in combination, induced a moderate but significant (P ≤ 0.001) decrease in spore germination to values below 8.0%. The inhibitory activity of 0.3% (wt/vol) calcium propionate was similar (data not shown).
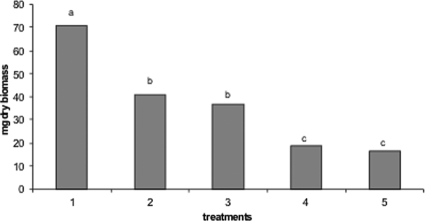
After incubation for 48 h at 25°C in WFH, the dry biomass of P. roqueforti DPPMAF1 was ca. 70 mg (Fig. 1). When 0.3% (wt/vol) calcium propionate was added, the dry biomass significantly (P ≤ 0.001) decreased to ca. 40 mg. Almost the same value was found in the presence of the water-soluble extract of the sourdough fermented with L. brevis AM7. The water-soluble extract of P. vulgaris cv. Pinto alone or in a mixture with the water-soluble extract of sourdough L. brevis AM7 caused the lowest values of mycelial dry biomass (ca. 18.6 and 16.3 mg, respectively).
FIG. 1.
Dry biomass (milligrams) of P. roqueforti DPPMAF1 incubated in WFH for 48 h at 25°C under different conditions. Treatments were as follows: 1, WFH alone (control); 2, WFH with 0.3% (wt/vol) calcium propionate added; 3, WFH with 30% (vol/vol, 1.71 mg of peptide/ml) water-soluble extract of sourdough L. brevis AM7 added; 4, WFH with 27% (vol/vol, 5 mg of protein/ml) water-soluble extract of P. vulgaris cv. Pinto added; 5, WFH with a mixture of 30% (vol/vol, 1.71 mg of peptide/ml) water-soluble extract of sourdough L. brevis AM7 and 27% (vol/vol, 5 mg of protein/ml) water-soluble extract of P. vulgaris cv. Pinto added. Data are the mean of three independent experiments, and the values marked with different letters are statistically significantly different (P ≤ 0.001).
To determine the MIC, water-soluble extracts of sourdough L. brevis AM7 and P. vulgaris cv. Pinto were freeze-dried and redissolved in WFH, obtaining a range of concentrations of 0.875 to 45 mg of peptide/ml and 5 to 35 mg of protein/ml, respectively. The MICs were 40 mg peptide/ml and 30.9 mg protein/ml for the water-soluble extracts of sourdough fermented by L. brevis AM7 and P. vulgaris cv. Pinto, respectively.
Inhibitory spectrum.
The activities of the water-soluble extracts of sourdough L. brevis AM7, P. vulgaris cv. Pinto, and a mixture of the two were assayed against several fungi isolated from bakeries (Table 3). Overall, the inhibitory spectrum of water-soluble extract of P. vulgaris cv. Pinto was larger and also the inhibitory activity was stronger than that of the water-soluble extract of sourdough L. brevis AM7. Compared to the water-soluble extract of P. vulgaris cv. Pinto alone, the inhibitory activity of the water-soluble extracts used in a mixture slightly increased only toward P. polonicum CBS 112490, P. brevicompactum CBS 28997, and E. herbarioum CBS 117336. Among the 12 species of fungi tested, only A. versicolor CBS 117286 was resistant to both of the water-soluble extracts.
TABLE 3.
Inhibitory spectra of the water-soluble extracts from sourdough L. brevis AM7 (1.71 mg of peptide/ml), P. vulgaris cv. Pinto (5 mg of protein/ml), and a mixture of the two as determined by agar diffusion assays
| Fungus | Source of isolation | Inhibitory activitya
|
||
|---|---|---|---|---|
| L. brevis AM7 | P. vulgaris cv. Pinto | Mixture | ||
| Penicillium polonicum CBS 112490 | Bread, Italy | + | +/− | + |
| Penicillium chrysogenum CBS 111214 | Bread, England | − | +++ | ++ |
| Penicillium paneum CBS 101032 | Rye bread, Denmark | + | +++ | ++ |
| Penicillium bialowiezense CBS 110102 | Bread, Italy | − | ++ | + |
| Penicillium brevicompactum CBS 28997 | Cake, Denmark | +++ | ++ | +++ |
| Penicillium albocoremium CBS 109582 | Cake factory, Denmark | +/− | + | + |
| Penicillium chermesinum CBS 117279 | Bakery plant, The Netherlands | ++ | +++ | +++ |
| Penicillium carneum CBS 112297 | Rye bread, Denmark | + | ++ | +/++ |
| Eurotium herbarioum CBS 117336 | Chocolate cake, The Netherlands | +++ | +++ | ++++ |
| Eurotium rubrum CBS 150.92 | Cake | + | ++ | + |
| Aspergillus parasiticus CBS 971.97 | Indian sweets, India | + | + | + |
| Aspergillus versicolor CBS 117286 | Wall in bakery, The Netherlands | − | − | − |
Inhibitory activity was scored as follows: −, no inhibition; +, very weak inhibition; ++, low inhibition with a little clear zone near the rim of the colony; +++, strong inhibition with a large clear zone near the rim of the colony; ++++, very strong inhibition with no growth near the rim of the colony.
Proteolysis and heat stability of antifungal compounds.
After digestion with trypsin, the antifungal activity of the water-soluble extract of P. vulgaris cv. Pinto was completely lost. In contrast, the antifungal activity of the water-soluble extract of sourdough L. brevis AM7 persisted. Protection from the activity of trypsin by other proteinaceous and nonantifungal compounds also contained in the water-soluble extract of sourdough L. brevis AM7 could not be excluded. The inhibitory activities of the water-soluble extracts of sourdough L. brevis AM7 and P. vulgaris cv. Pinto were unaffected by heating for 5 min at 100°C.
Identification of antifungal compounds.
As shown by SDS-PAGE, the water-soluble extract of P. vulgaris cv. Pinto contained protein bands in a molecular mass range of 200 to 10 kDa (Fig. 2). First, the water-soluble extract was fractionated by ultrafiltration showing antifungal activity only in a molecular mass range of 50 to 30 kDa. This preparation was further purified through size exclusion FPLC. Sixteen fractions were collected, and antifungal activity toward P. roqueforti DPPMAF1 was mainly found in one fraction. As shown by SDS-PAGE, the profile of this fraction consisted of three major bands having molecular masses of ca. 45 kDa for bands A and B and ca. 30 kDa for band C (Fig. 2). The identification of the above polypeptides was carried out by nano-LC-ESI-MS/MS, followed by an MS/MS ion search with the Mascot search engine. Bands A and B corresponded to two phaseolins (gi 130169 and gi 403594) with molecular masses of 49.2 and 47.5 kDa and pIs of 5.25 and 5.42, respectively (Table 4). Band C of the SDS-PAGE (Fig. 2) was identified as erythroagglutinating phytohemagglutinin precursor (PHA-E) (gi 130007, 29.7 kDa, pI 5.15). The antifungal activity of the fraction containing the three proteins was also assayed by SDS-PAGE. All three proteins showed almost the same inhibitory activity toward P. roqueforti DPPMAF1.
FIG. 2.
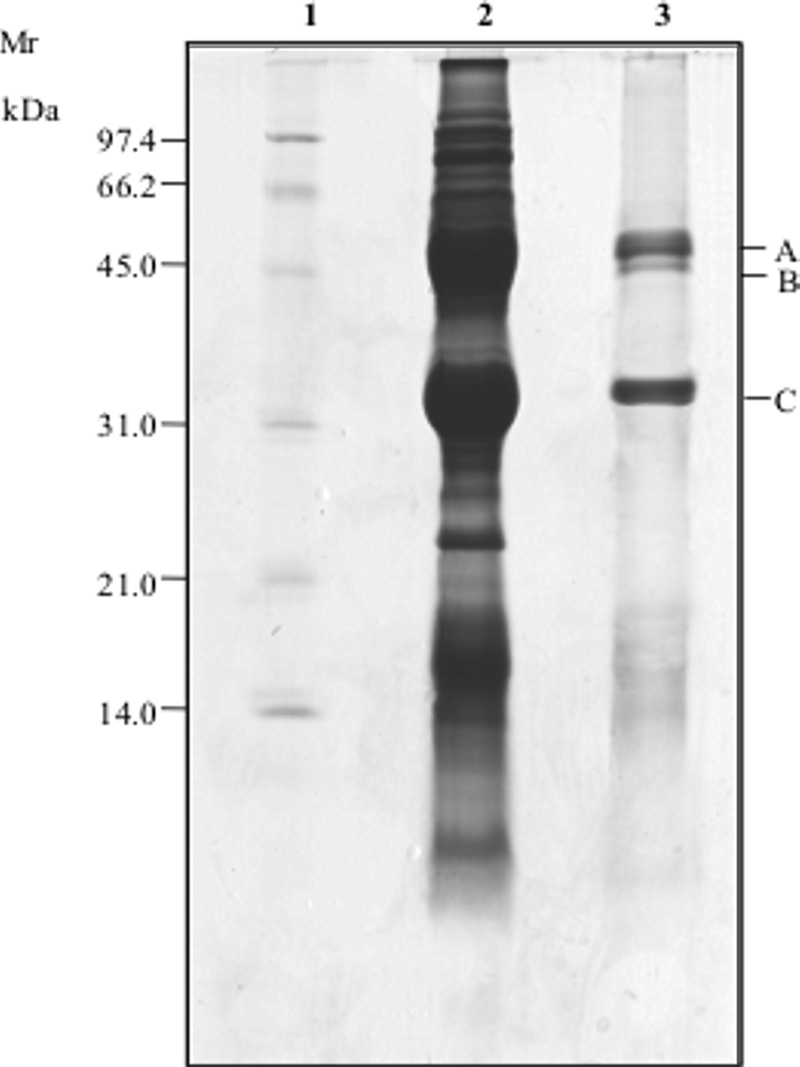
SDS-PAGE of the water-soluble extract of P. vulgaris cv. Pinto. Lanes: 1, protein standard (Bio-Rad, Hercules, CA); 2, crude water-soluble extract; 3, polypeptides after FPLC purification. Identified polypeptides are indicated. Mr, molecular mass.
TABLE 4.
Antifungal polypeptides identified in the water-soluble extract of P. vulgaris cv. Pinto by nano-LC-ESI-MS/MS
| Proteina | NCBI accession no. | Theoretical mass (kDa) | pI | % Coverage | Score |
|---|---|---|---|---|---|
| A, phaseolin, alpha-type precursor | gi 130169 | 49.24 | 5.25 | 44 | 871 |
| B, phaseolin | gi 403594 | 47.52 | 5.42 | 54 | 898 |
| C, PHA-E | gi 130007 | 29.73 | 5.15 | 53 | 734 |
The letters A, B, and C correspond to those in Fig. 2.
The water-soluble extract of sourdough L. brevis AM7 was purified by RP-FPLC. Thirty-seven fractions were collected. Antifungal activity toward P. roqueforti DPPMAF1 was mainly found in two fractions eluted in the early (hydrophilic) and late (hydrophobic) zones of the acetonitrile gradient, respectively. As expected, the mixture of organic acids used as the standard also eluted in the same hydrophilic fraction of the acetonitrile gradient. Therefore, the inhibitory activity of this fraction was presumptively attributed to organic acids synthesized by L. brevis AM7 during sourdough fermentation. Peptides contained in the other fraction were identified by nano-LC-ESI-MS/MS. A mixture comprising various-sized peptides was identified (Table 5): DPVAPLQRSGPEIP, PRSGNVGESGLID, ESVSLVA, PHAVAAVPPVLR, LLGWGHKGSSIID, HCNDPEKKNL, PILQSLIRFDGGACSSF, and RSQIKREQYTPQDVEMLFSSF. All of the peptides were chemically synthesized and assayed at a concentration of 0.875 to 45 mg of peptide/ml. When used singly, DPVAPLQRSGPEIP, PHAVAAVPPVLR, LLGWGHKGSSIID, PILQSLIRFDGGACSSF, and RSQIKREQYTPQDVEMLFSSF confirmed the inhibitory activity. The range of MICs was 3.5 to 8.2 mg/ml. The other synthesized peptides did not show appreciable inhibition at the highest concentration. Among all possible combinations, the lowest MIC (ca. 0.95 mg of peptide/ml) was found when DPVAPLQRSGPEIP and PHAVAAVPPVLR were used in a mixture.
TABLE 5.
Antifungal polypeptides identified in the water-soluble extract of L. brevis AM7 sourdough by nano-LC-ESI-MS/MS
| Peptide sequencea | Score | Charge |
Mr
|
Delta | Source protein NCBI accession no. | |
|---|---|---|---|---|---|---|
| Expected | Calculated | |||||
| DPVAPLQRSGPEIP | 49 | 2 | 1,263.1854 | 1,262.6983 | 0.49 | gi|231540 |
| PRSGNVGESGLID | 45 | 2 | 1,300.0854 | 1,299.6419 | 0.44 | gi|123957 |
| ESVSLVA | 38 | 1 | 703.5727 | 703.3752 | 0.19 | gi|115483781 |
| PHAVAAVPPVLR | 30 | 2 | 1,226.065448 | 1,225.7295 | 0.33 | gi|125587095 |
| LLGWGHKGSSIID | 31 | 2 | 1,376.165448 | 1,375.7170 | 0.45 | gi|115457014 |
| HCNDPEKKNL | 26 | 2 | 1,197.0454 | 1,196.5608 | 0.48 | gi|125541005 |
| PILQSLIRFDGGACSSF | 26 | 3 | 1,896.3382 | 1,896.9404 | 0.60 | gi|115438727 |
| RSQIKREQYTPQDVEMLFSSF | 20 | 3 | 2,588.3482 | 2,588.2693 | 0.08 | gi|38344927 |
Single-letter amino acid code is used.
Bread making.
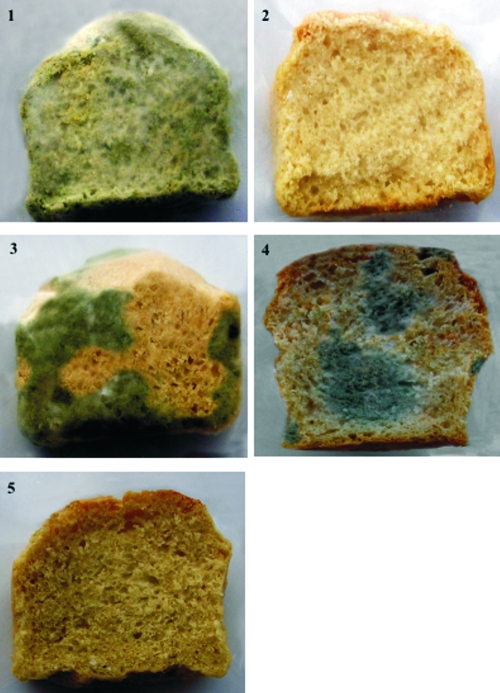
Five different breads were manufactured at the pilot plant according to the formulas in Table 1. The pHs of the breads were 5.70 (breads 1 and 2), 4.57 (bread 3), 5.65 (bread 4), and 4.58 (bread 5). After baking, two slices of each bread were cut; one slice was inoculated with a suspension (102 conidia/ml) of P. roqueforti DPPMAF1, and the other slice was left without inoculum. Slices were packed with polyethylene film and stored at room temperature. The moisture of all of the slices was in the range of 35.5 to 37.0% throughout 21 days of storage. After 4 days of storage, the slice of bread 1 (control) inoculated with the suspension of spores was almost completely colonized by P. roqueforti DPPMAF1. On the contrary, only a very few zones of fungal growth appeared on breads 3 (fermented with L. brevis AM7), 4 (water-soluble extract of P. vulgaris cv. Pinto added), and 5 (fermented with L. brevis AM7 with water-soluble extract of P. vulgaris cv. Pinto added). No growth of P. roqueforti DPPMAF1 was found on bread 2, containing 0.3% calcium propionate. After 7 days, mycelial growth appeared on the slice of bread 1 without inoculum of the spore suspension. No fungal growth was evident on any of the other breads. At 21 days, the contamination by fungi was almost complete on the slice of bread 1, while it was lower on breads 3 and 4. At the same time, no contamination by fungi was visible on breads 2 and 5 (Fig. 3). After 21 days of storage, the moisture of the slices gradually decreased, indicating that the lack of growth of P. roqueforti DPPMAF1 was probably due to a decrease in water activity.
FIG. 3.
Slices of different breads stored at room temperature for 21 days without fungal spore inoculum. Bread 1, control; bread 2, with 0.3% (wt/wt) calcium propionate added; bread 3, fermented with sourdough L. brevis AM7; bread 4, with 27% (vol/wt, 5 mg of protein/ml) P. vulgaris cv. Pinto water-soluble extract added; bread 5, fermented with sourdough L. brevis AM7 with 27% (vol/wt, 5 mg of protein/ml) P. vulgaris cv. Pinto water-soluble extract added. The formulas used for bread making are shown in Table 1.
DISCUSSION
Fungal contamination is a major problem for the long-term shelf life of baked goods. Several natural antimicrobials have been identified, including proteins and peptides from plants and phenyllactic acid, cyclic dipeptides, and short- or medium-chain fatty acids from lactic acid bacteria (8, 18, 19, 21, 28, 29, 31). The inhibitory activity of these compounds was not assayed under realistic bread-making conditions and during long incubations. With the goal of identifying new natural preservatives with marked antifungal activity, we screened the potential of water-soluble extracts of different food matrices, identified the antifungal compounds, and confirmed the inhibitory activity during bread making at the pilot plant scale and long-term storage.
The water-soluble extract of P. vulgaris cv. Pinto showed marked antifungal activity. Three polypeptides, phaseolin alpha-type precursor, phaseolin, and PHA-E, were identified. When their antifungal activity was assayed on SDS-PAGE, all three proteins confirmed the inhibitory activity. Phaseolin alpha-type precursor and phaseolin belong to the group of phytoalexins (phaseolins), while PHA-E corresponds to a lectin with hemagglutinating activity. Other polypeptides such as the 32-kDa novel protein, 28-kDa chitinase-like protein, and 31-kDa galactose-specific lectin have also been characterized as the antifungal compounds from P. vulgaris cv. Pinto (22, 37). Phytoalexins are involved in the mechanisms of plant disease resistance and show a large spectrum of inhibitory activity toward fungi, especially those pathogenic to beans (34). Lectins have been investigated for antitumor, anti-insect, antifungal, antibacterial, anti-human immunodeficiency virus, and mitogenic activities (22), although potential toxicity has also been reported (34, 36). Hemagglutinating activity is markedly sensitive to heat treatment (e.g., 90 to 100°C for a few minutes) (36) so that the risk of potential lectin hemagglutinating activity is minimal after bread making (ca. 100°C for 15 min).
Antifungal activity was found in the water-soluble extract of sourdough fermented with L. brevis AM7. A mixture of eight peptides was identified in the partially purified fraction. When chemically synthesized, only five showed antifungal activity (Table 5). The peptide PILQSLIRFDGGACSSF was 60% identical to a region within the defensin-like protein of pear (40). Peptides DPVAPLQRSGPEIP and PHAVAAVPPVLR contained the tripeptides VAP and VPP, which correspond to well-known antihypertensive and antimicrobial sequences encrypted in caseins (13). As previously shown (8, 23), complex and synergistic activities between organic acids and peptides were found to be responsible for the antifungal activity of sourdough lactic acid bacteria. A mixture of organic acids, especially lactic and acetic acids, was also presumptively synthesized during sourdough fermentation by L. brevis AM7.
Three in vitro assays were used to investigate the antifungal activity, including the determination of spore germination and mycelial dry biomass. The pH of the WFH medium used for both assays was ca. 5.6, comparable to the pH of a baker's yeast bread. As previously shown (18), calcium propionate at a concentration of 0.3% (wt/vol) had very poor spore germination-inhibiting activity. Almost the same was found for the water-soluble extracts of sourdough L. brevis AM7 and P. vulgaris cv. Pinto. On the contrary, the latter water-soluble extract alone or especially in combination with the water-soluble extract of sourdough L. brevis AM7 markedly decreased the mycelial dry biomass. This suggested a presumptive fungistatic activity. The mixture of the two water-soluble extracts was also inhibitory toward some of the most important bread contaminants (32), such as P. brevicompactum CBS 289.97, P. chermesinum CBS 117279, and E. herbariorum CBS 15092 (Table 3).
The MICs of the water-soluble extracts of sourdough L. brevis AM7 and P. vulgaris cv. Pinto were 40 mg peptide/ml and 30.9 mg protein/ml, respectively. As shown by SDS-PAGE (Fig. 2) and FPLC (data not shown) analyses, these values are clearly overestimates due to the presence in the crude extracts of other peptides and/or proteins. After FPLC purification, the MICs of the two fractions from the water-soluble extracts of sourdough L. brevis AM7 and P. vulgaris cv. Pinto were 12.0 and 2.65 mg/ml, respectively. Further, the MIC of the mixture of the two chemically synthesized peptides DPVAPLQRSGPEIP and PHAVAAVPPVLR was 0.95 mg of the peptides/ml. These values are comparable to the antifungal activities of similar proteinaceous substances (31, 38) and phenyllactic acid (19) toward P. roqueforti.
Bread making at the pilot plant did not consider the addition of the water-soluble extract of L. brevis AM7 sourdough but simply the use of the sourdough as the ingredient (Table 1). The bread made with sourdough and addition of the water-soluble extract (27%, vol/wt, 5 mg of protein/ml) from P. vulgaris cv. Pinto delayed the growth of P. roqueforti DPPMAF1 at least until 21 days of storage at room temperature, showing the same activity as 0.3% (wt/wt) calcium propionate. Our preliminary internal panel tests have shown that the use of the water-soluble extract of P. vulgaris cv. Pinto in sourdough bread making does not interfere with bread's sensory and rheological properties (data not shown).
The use of the water-soluble extract of P. vulgaris cv. Pinto combined with sourdough fermentation by selected lactic acid bacteria seems to have the same efficacy as chemical preservatives (e.g., calcium propionate) for preventing contamination by fungi in bakery products. Furthermore, it satisfies the consumer request for preservative-free baked goods with extended shelf life.
Acknowledgments
This work was supported by Puracor N.V., Groot-Bijgaarden, Belgium.
Footnotes
Published ahead of print on 10 October 2008.
REFERENCES
- 1.Abellana, M., L. Torres, V. Sanchis, and A. J. Ramos. 1997. Caracterización de diferentes productos de bollería industrial. II. Estudio de la microflora. Alimentaria 287:51-56. [Google Scholar]
- 2.American Association of Cereal Chemists. 1983. Methods 08-01, 44-15 A, 46-13, and 54-20. Approved methods of the American Association of Cereal Chemists, 8th ed. American Association of Cereal Chemists, St. Paul, MN.
- 3.Atanassova, M., Y. Choiset, M. Dalgalarrondo, J. M. Chobert, X. Dousset, I. Ivanova, and T. Haertle. 2003. Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. Int. J. Food Microbiol. 87:63-73. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., S. W. Know, S. C. Kim, and Y. Zhao. 2005. Integrated approach for manual evaluation of peptides identified by searching protein sequence databases with tandem mass spectra. J. Proteome Res. 4:998-1005. [DOI] [PubMed] [Google Scholar]
- 6.Cherif, A., H. Ouzari, D. Daffonchio, H. Cherif, K. Ben Slama, A. Hassen, S. Jaoua, and A. Boudabous. 2001. Thuricin 7: a novel bacteriocin produced by Bacillus thuringiensis BMG1.7, a new strain isolated from soil. Lett. Appl. Microbiol. 32:237-247. [DOI] [PubMed] [Google Scholar]
- 7.Church, F. C., H. E. Swaisgood, D. H. Porter, and G. L. Catignani. 1983. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins J. Dairy Sci. 66:1219-1227. [Google Scholar]
- 8.Corsetti, A., M. Gobbetti, J. Rossi, and P. Damiani. 1998. Antimould activity of sourdough lactic acid bacteria: identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl. Microbiol. Biotechnol. 50:253-256. [DOI] [PubMed] [Google Scholar]
- 9.Dal Bello, F., C. I. Clarke, L. A. M. Ryan, H. Ulmera, T. J. Schobera, K. Strom, J. Sjogren, D. van Sinderen, J. Schnurer, and E. K. Arendt. 2007. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 45:309-318. [Google Scholar]
- 10.Dantigny, P., A. Guilmart, F. Radoi, M. Bensoussan, and M. Zwietering. 2005. Modelling the effect of ethanol on growth rate of food spoilage moulds. Int. J. Food Microbiol. 98:261-269. [DOI] [PubMed] [Google Scholar]
- 11.European Union. 1995. European Parliament and Council directive no. 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners, p. 53. Office for Official Publications of the European Communities, Luxembourg. http://europa.eu.int/eur-lex/en/consleg/pdf/1995/en_1995L0002_do_001.pdf.
- 12.Gobbetti, M., A. Corsetti, and J. Rossi. 1994. The sourdough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of carbohydrates. Appl. Microbiol. Biotechnol. 41:456-460. [DOI] [PubMed] [Google Scholar]
- 13.Gobbetti, M., F. Minervini, and C. G. Rizzello. 2007. Bioactive peptides in dairy products, p. 489-517. In Y. H. Hui (ed.), Handbook of food products manufacturing. Wiley-Interscience, Hoboken, NJ.
- 14.Gourama, H. 1997. Inhibition of growth and mycotoxin production of Penicillium by Lactobacillus species. Lebensm.-Wiss. Technol. 30:279-283. [Google Scholar]
- 15.Guynot, M. E., S. Marín, V. Sanchis, and A. J. Ramos. 2005. An attempt to optimize potassium sorbate use to preserve low pH (4.5-5.5) intermediate moisture bakery products by modeling Eurotium spp., Aspergillus spp. and Penicillium corilophilum growth. Int. J. Food Microbiol. 101:169-177. [DOI] [PubMed] [Google Scholar]
- 16.Kuchroo, C. N., and P. F. Fox. 1982. Soluble nitrogen in cheddar cheese: comparison of extraction procedures. Milchwissenschaft 37:331-335. [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Lavermicocca, P., F. Valerio, A. Evidente, S. Lazzaroni, A. Corsetti, and M. Gobbetti. 2000. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 66:4084-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavermicocca, P., F. Valerio, and A. Visconti. 2003. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 69:634-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnusson, J., and J. Schnürer. 2001. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 67:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnusson, J., K. Ström, S. Roos, J. Sjögren, and J. Schnürer. 2003. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol. Lett. 219:129-135. [DOI] [PubMed] [Google Scholar]
- 22.Ng, T. B. 2004. Antifungal proteins and peptides of leguminous and non-leguminous origins. Peptides 25:1215-1222. [DOI] [PubMed] [Google Scholar]
- 23.Niku-Paavola, M., L. A. Laitila, T. Mattila-Sandholm, and A. Hikara. 1999. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 86:29-35. [DOI] [PubMed] [Google Scholar]
- 24.Pattison, T. L., D. Lindsay, and A. von Holy. 2004. Natural antimicrobial as potential replacements for calcium propionate in bread. S. Afr. J. Sci. 100:339-342. [Google Scholar]
- 25.Ponte, J. G., and C. C. Tsen. 1987. Bakery products, p. 233-267. In L. R. Beuchat (ed.), Food and beverage mycology, 2nd ed. AVI Van Nostrand Reinhold, New York, NY.
- 26.Rizzello, C. G., I. Losito, M. Gobbetti, T. Carbonara, M. D. De Bari, and P. G. Zambonin. 2005. Antibacterial activities of peptides from the water-soluble extracts of Italian cheese varieties. J. Dairy Sci. 88:2348-2360. [DOI] [PubMed] [Google Scholar]
- 27.Schmourlo, G., R. R. Mendoça-Filho, C. Sales Alviano, and S. S. Costa. 2005. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J. Ethnopharmacol. 96:563-568. [DOI] [PubMed] [Google Scholar]
- 28.Schnürer, J., and J. Magnusson. 2005. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 16:70-78. [Google Scholar]
- 29.Sjögren, J., J. Magnusson, A. Broberg, J. Schnürer, and L. Kenne. 2003. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 69:7554-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snedecor, G. W., and W. G. Cochran. 1980. Statistical methods. The Iowa State University Press, Ames.
- 31.Ström, K., J. Sjögren, A. Broberg, and J. Schnürer. 2002. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(l-Phe-l-Pro) and cyclo(l-Phe-l-trans-4-OH-l-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 68:4322-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suhr, K. I., and P. V. Nielsen. 2004. Effect of weak acid preservatives on growth of bakery product spoilage fungi at different water activities and pH values. Int. J. Food Microbiol. 95:67-78. [DOI] [PubMed] [Google Scholar]
- 33.Valerio, F., P. Lavermicocca, M. Pascale, and A. Visconti. 2004. Production of phenyllactic acid by lactic acid bacteria: an approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol. Lett. 233:289-295. [DOI] [PubMed] [Google Scholar]
- 34.Van Damme, E. J. M., W. J. Peumans, A. Pusztai, and S. Bardocz. 1998. Plant lectins in mammalian nutrition, immunology, metabolism and as oral therapeutic and immune agents, p. 31-50. In E. J. M. Van Damme (ed.), Handbook of plant lectins: properties and biomedical applications. Wiley, London, United Kingdom.
- 35.VanEtten, H. D. 1973. Differential sensitivity of fungi to pisatin and to phaseollin. Phytopathology 63:1477-1482. [Google Scholar]
- 36.Weder, J. K. P., L. Telek, M. Vozari-Hampe, and H. S. Saini. 1997. Antinutritional factors in anasazi and other pinto beans (Phaseolus vulgaris L.). Plant Foods Hum. Nutr. 51:85-98. [DOI] [PubMed] [Google Scholar]
- 37.Wong, J. H., C. C. Wong, and T. B. Ng. 2006. Purification and characterization of a galactose-specific lectin with mitogenic activity from pinto beans. Biochim. Biophys. Acta 1760:808-813. [DOI] [PubMed] [Google Scholar]
- 38.Xia, L., and T. B. Ng. 2005. An antifungal protein from flageolet beans. Peptides 26:2397-2403. [DOI] [PubMed] [Google Scholar]
- 39.Ye, X. Y., and T. B. Ng. 2001. Hypogin, a novel antifungal peptide from peanuts with sequence similarity to peanut allergen J. Peptide Res. 57:330-336. [DOI] [PubMed] [Google Scholar]
- 40.Zhou, Y., N. Norioka, S. Li, and S. Norioka. 2003. Nucleotide sequence of pdn3 gene encoding a plant defensin-like protein in pollen grains of Japanese pear (Pyrus pyrifolia). J. Plant Physiol. Mol. Biol. 29:360-361. [Google Scholar]