Abstract
Here, we show that commensal bacteria can stimulate intestinal epithelial cells to secrete insulin in response to glucose. Commensal strains were engineered to secrete the insulinotropic proteins GLP-1 and PDX-1. Epithelia stimulated by engineered strains and glucose secreted up to 1 ng ml−1 of insulin with no significant background secretion.
Two proteins, GLP-1 (glucagon-like peptide 1) and PDX-1 (pancreatic and duodenal homeobox gene 1), have recently been shown to stimulate intestinal epithelial cells to synthesize insulin in response to glucose (14) and irrespective of glucose levels (15), respectively. GLP-1 is secreted by intestinal epithelia of the distal small bowel in response to glucose and other nutrients (2). It has a very short half-life, and its degradation by dipeptidylpeptidase IV (DPP-IV) occurs in the blood vessels draining the intestinal mucosa (7). GLP-1 activates insulin synthesis in pancreatic β cells by binding to the membrane receptor GLP-1R and has been suggested as a therapeutic for treating both type 1 (14) and type 2 (2) diabetes.
Suzuki and coworkers demonstrated that intestinal epithelial cells in both neonatal and adult rats injected intraperitoneally with GLP-1 became glucose-responsive, insulin-secreting cells (14). In addition, they found that surgical implantation into mice of epithelial cells stimulated in vitro with GLP-1 resulted in reversal of diabetes mellitus in mice receiving the implants.
The transcriptional activator PDX-1 has been shown to stimulate insulin secretion in both β cells and intestinal epithelia (10, 11). Koizumi and coworkers have shown that when pancreatic epithelia are virally transfected with pdx-1 and concurrently stimulated with exogenous GLP-1 they become insulin-secreting cells (10). The same group demonstrated that intestinal epithelia (mouse ileal loops) express insulin when transfected with pdx-1, although in that paper, no data on the addition of GLP-1 to these cells were presented (11).
Supplemental gut bacteria are widely available as “probiotics” and are generally regarded as safe by the Food and Drug Administration (1). Potential advantages of using commensal strains for in vivo recombinant gene expression include their compatibility with the host (particularly the host's immune system), their controllable persistence in the gut, and their ability to be orally dosed. Commensal bacterial expression of various recombinant cytokines and antigens in animal models has been reported (4, 6, 8, 9). We recently engineered Escherichia coli Nissle 1917 (an over-the-counter probiotic strain, hereinafter referred to as Nissle) for the expression of a Vibrio cholerae quorum-sensing signal, creating a potential prophylactic for cholera (5).
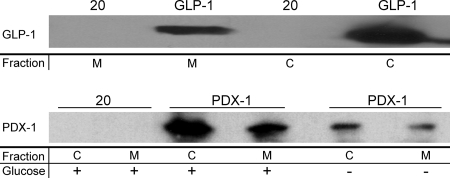
In this work, Nissle was engineered to secrete either GLP-1 (amino acids 1 through 37) or the full-length PDX-1 protein by using the fliC secretion tag (13). PDX-1 was secreted as a fusion with a cell-penetrating peptide (CPP) (12) to facilitate rapid entry into the epithelia postsecretion. Western blots of secreted GLP-1 and PDX-1-CPP in the Nissle supernatant and in the Nissle cell pellet are shown in Fig. 1. It was clear from these data that both proteins were being secreted. PDX-1 was secreted under the control of a glucose-responsive promoter element that had little observed leaky expression (Fig. 1; also see Fig. S2 in the supplemental material).
FIG. 1.
Secretion of recombinant insulinotropic proteins by E. coli Nissle. Nissle was engineered to secrete either GLP-1 under the control of the fliC promoter or PDX-1-CPP under the control of a glucose-responsive element. Western blots for secreted proteins GLP-1 (top blot) and PDX-1-CPP (bottom blot) are shown. Cells were grown for 6 to 8 h, normalized to an optical density at 600 nm of 1, and centrifuged. The pellets were lysed, and the amount of each protein was determined (fraction “C”). The supernatant was preserved and similarly analyzed (fraction “M”). For cells expressing PDX-1-CPP, a comparison was made between cells grown in medium containing glucose (0.4%) or glycerol (0.4%). Cells expressing the empty plasmid (20) were used as a negative control.
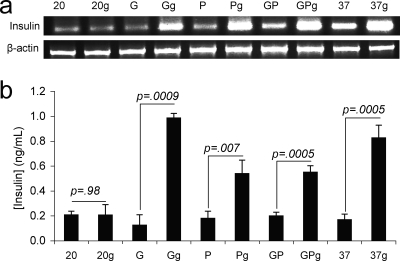
To test if the engineered Nissle strains could induce insulin secretion in human epithelial cells, Caco-2 cells were cultured with cell-free medium (CFM) from overnight cultures of Nissle strains expressing either PDX-1-CPP, GLP-1, or a 20-amino-acid sequence tag as a negative control. The overnight cultures were grown in F-12K medium (Mediatech, Manassas, VA) without glucose (with the exception of PDX-1 strains, which required glucose to produce PDX-1). Culturing of the Caco-2 cells in a 1:1 mixture of fresh F-12K medium without glucose and CFM from overnight cultures of Nissle secreting PDX-1-CPP, GLP-1, a 20-amino-acid sequence tag, or a 1:1 combination of PDX-1-CPP CFM and GLP-1 CFM ran for 16 h before the medium was removed and the Caco-2 cells were cultured in medium with either glucose (0.4%) or glycerol (0.4%) for 2 h. Following the glucose challenge, each sample was analyzed for insulin secretion and transcription. As a positive control, Caco-2 cells were incubated in fresh F-12K medium (without glucose) and purchased GLP-1 (amino acids 1 through 37) for the same 16-h time period before being cultured with glucose (0.4%) or glycerol (0.4%) for 2 h.
Both transcription and enzyme-linked immunosorbent assay data indicated that human epithelia incubated with CFM from GLP-1 and PDX-1-CPP either together or separately were stimulated to produce insulin (Fig. 2). The most insulin production was consistently seen for incubations with GLP-1 (amino acids 1 through 37) CFM. PDX-1-CPP CFM stimulated glucose-responsive insulin secretion whether added by itself or with GLP-1. Both GLP-1- and PDX-1-mediated insulin secretions occurred in response to glucose. The negative-control epithelia cultured with CFM from the 20-amino-acid sequence tag overnight exhibited no glucose-responsive insulin production (Fig. 2).
FIG. 2.
Stimulation of insulin secretion in epithelial cells. Caco-2 epithelial cells were incubated either with CFM from overnight cultures of E. coli Nissle expressing GLP-1 (G), PDX-1-CPP (P), both GLP-1 and PDX-1-CPP (GP), or a control plasmid (samples denoted “20”) or with synthesized GLP-1 (amino acids 1 to 37; samples denoted “37”) for 16 h before being challenged with glucose or glycerol. (a) Reverse transcription-PCR of Caco-2 cells incubated with CFM from the indicated cell line or protein and subsequent stimulation with either glucose (“g”) or glycerol. (b) Enzyme-linked immunosorbent assay of insulin secretion by stimulated Caco-2 cells. Error bars represent 1 standard deviation for at least three experiments. P values are from a Student t test (n = 3).
That PDX-1-CPP treatment resulted in glucose-responsive insulin secretion in the Caco-2 cells (Fig. 2) was unexpected. Yoshida and coworkers reported that PDX-1 stimulates constitutive insulin production in IEC-6 (rat) epithelial cells, but only when these cells are also treated with betacellulin (15). More-recent work by Koizumi et al. showed that mice transfected with PDX-1 in vivo expressed insulin from their small intestines, but that study did not specifically determine the cells responsible for the secretion and did not determine their glucose responsiveness (11). Our results imply a distinct difference between human and rat epithelial cells with respect to their responses to PDX-1.
It was estimated (calculations and assumptions in are shown in Fig. S2 in the supplemental material) that insulin levels in the blood would be 164 fmol liter−1 to 164 pmol liter−1 for Nissle survivability levels ranging from 106 to 109 CFU ml−1, respectively. Given that postprandial serum insulin concentrations can be as high as 400 pmol liter−1 for adult nondiabetics (3), we are encouraged that our unoptimized engineered bacteria could stimulate an insulin release at least within the same order of magnitude as would be required for normal metabolism.
These results point to a promising and easily implemented treatment for type 1 diabetes. With simple oral dosing, no significant background expression, and glucose responsiveness, the use of recombinant commensal strains may significantly reduce or even eliminate the need for insulin injection and could help to reduce the long-term complications exhibited by diabetics by replacing host insulin synthesis. This system is currently being investigated in vivo.
Supplementary Material
Acknowledgments
Benita Westerlund-Wikström of the University of Helsinki generously provided the fliC construct for peptide secretion in E. coli.
This work was supported in part by a Cornell University innovation grant and by the Hartwell Foundation.
Footnotes
Published ahead of print on 3 October 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Ahmed, F. E. 2003. Genetically modified probiotics in foods. Trends Biotechnol. 21:491-497. [DOI] [PubMed] [Google Scholar]
- 2.Baggio, L. L., and D. J. Drucker. 2007. Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131-2157. [DOI] [PubMed] [Google Scholar]
- 3.Basu, R., C. Dalla Man, M. Campioni, A. Basu, G. Klee, G. Toffolo, C. Cobelli, and R. A. Rizza. 2006. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 55:2001-2014. [DOI] [PubMed] [Google Scholar]
- 4.Daniel, C., A. Repa, C. Wild, A. Pollak, B. Pot, H. Breiteneder, U. Wiedermann, and A. Mercenier. 2006. Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy 61:812-819. [DOI] [PubMed] [Google Scholar]
- 5.Duan, F., and J. C. March. 2008. Interrupting Vibrio cholerae infection of human epithelial cells with engineered commensal bacterial signaling. Biotechnol. Bioeng. 101:128-134. [DOI] [PubMed] [Google Scholar]
- 6.Farrar, M. D., T. R. Whitehead, J. Lan, P. Dilger, R. Thorpe, K. T. Holland, and S. R. Carding. 2005. Engineering of the gut commensal bacterium Bacteroides ovatus to produce and secrete biologically active murine interleukin-2 in response to xylan. J. Appl. Microbiol. 98:1191-1197. [DOI] [PubMed] [Google Scholar]
- 7.Hansen, L., C. F. Deacon, C. Orskov, and J. J. Holst. 1999. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140:5356-5363. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann, M., A. Westendorf, J. Buer, and F. Gunzer. 2004. E-coli Nissle 1917 as a vehicle for intestinal expression of therapeutic molecules: construction of an E-coli a hemolysin based expression vector. Int. J. Med. Microbiol. 294:198. [Google Scholar]
- 9.Hazebrouck, S., L. Pothelune, V. Azevedo, G. Corthier, J. M. Wal, and P. Langella. 2007. Efficient production and secretion of bovine beta-lactoglobulin by Lactobacillus casei. Microb. Cell Fact. 6:6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koizumi, M., R. Doi, K. Fujimoto, D. Ito, E. Toyoda, T. Mori, K. Kami, Y. Kawaguchi, G. K. Gittes, and M. Imamura. 2005. Pancreatic epithelial cells can be converted into insulin-producing cells by GLP-1 in conjunction with virus-mediated gene transfer of pdx-1. Surgery 138:125-133. [DOI] [PubMed] [Google Scholar]
- 11.Koizumi, M., K. Nagai, A. Kida, K. Kami, D. Ito, K. Fujimoto, Y. Kawaguchi, and R. Doi. 2006. Forced expression of PDX-1 induces insulin production in intestinal epithelia. Surgery 140:273-280. [DOI] [PubMed] [Google Scholar]
- 12.Liang, J. F., and V. C. Yang. 2005. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem. Biophys. Res. Commun. 335:734-738. [DOI] [PubMed] [Google Scholar]
- 13.Majander, K., L. Anton, J. Antikainen, H. Lang, M. Brummer, T. K. Korhonen, and B. Westerlund-Wikstrom. 2005. Extracellular secretion of polypeptides using a modified Escherichia coli flagellar secretion apparatus. Nat. Biotechnol. 23:475-481. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki, A., H. Nakauchi, and H. Taniguchi. 2003. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc. Natl. Acad. Sci. USA 100:5034-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida, S., Y. Kajimoto, T. Yasuda, H. Watada, Y. Fujitani, H. Kosaka, T. Gotow, T. Miyatsuka, Y. Umayahara, Y. Yamasaki, and M. Hori. 2002. PDX-1 induces differentiation of intestinal epithelioid IEC-6 into insulin-producing cells. Diabetes 51:2505-2513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.