Abstract
The combined lactic acid, monolaurin, and nisin effects on time-to-detection (optical density at 600 nm) extension were greater (P < 0.05) than any single or paired combination effect, which demonstrates a synergistic interaction among the antimicrobials. Monolaurin exposure caused C12:0 cell membrane incorporation. Lactic acid caused increased monolaurin C12:0 membrane incorporation, while nisin had no influence. We postulate that lactic acid-enhanced monolaurin C12:0 incorporation into the cell membrane increased membrane fluidity resulting in increased nisin activity.
Listeria monocytogenes is a well-known food-borne human pathogen associated with the consumption of ready-to-eat foods that is ubiquitous in the natural environment (15). One method to control L. monocytogenes in ready-to-eat foods is through the use of antimicrobial interventions. Kabara and Marshall (6) suggested that future antimicrobial applications in foods would be more likely to be not through the invention of novel antimicrobials but rather through effective combinations of existing ones by using the multiple-hurdle concept to achieve additive or synergistic effects. The effectiveness of fatty acid monoesters (such as monolaurin [ML]) against gram-positive bacteria, especially L. monocytogenes, is well documented (6, 13). Low-molecular-weight organic acids (such as lactic acid [LA]) have a long history of being used to control microbial growth (3), and their synergistic interaction with ML has been previously shown (14). Bacteriocins (such as nisin [NI]) derived from LA bacteria have been investigated for use as potential antimicrobial treatments (1, 11, 16). Synergistic interactions of NI-LA (12) and NI-ML (8) combinations have been reported.
The ability of bacteria to multiply under suboptimal conditions (low pH or in the presence of toxic chemicals) requires membrane fluidity modification, a phenomenon known as homeoviscous adaptation (15). Membrane fluidity can be altered through membrane fatty acid profile modification (18). Several reports have described fatty acid profile changes in L. monocytogenes that were caused by growth temperature (7, 9), NI (7, 9), and individual fatty acids (5). To our knowledge, no one has described the combined effects of LA, ML, and NI on growth inhibition of L. monocytogenes. In addition, we are unaware of reports demonstrating how these agents may act together to alter membrane fluidity of the bacterium. Therefore, the present study was designed to define the interaction between these agents and to elucidate how they may influence the cell membrane fluidity of L. monocytogenes.
MATERIALS AND METHODS
Culture preparation.
NI-sensitive Listeria monocytogenes ATCC 7644 was maintained at 4°C on Trypticase soy agar plus 0.6% yeast extract (BD Diagnostic Systems, Sparks, MD) slants with biweekly transfers. Working culture preparation involved streaking slant cultures onto Trypticase soy agar plates (35°C, 24 h), followed by individual colony inoculation into 30 ml Trypticase soy broth plus 0.6% yeast extract (TSB; BD Diagnostic Systems) that was incubated at 35°C for 12 h with 200-rpm shaking to yield 109 CFU/ml. One-half milliliter of broth was centrifuged for 20 s at 20,800 × g (centrifuge model 5417C; Eppendorf AG, Hamburg, Germany), and the pellet was resuspended in 1 ml of TSB. A one-half-milliliter aliquot of this resuspension was diluted in 9.5 ml of TSB to prepare a 108-CFU/ml working culture.
Antimicrobial preparation.
LA broth was prepared by dissolving 0.6 mg/ml (broth pH 6.7) and 2.4 mg/ml (broth pH 5.5) of LA (85% racemic; ThermoFisher Scientific, Fairlawn, NJ) in 50 ml of TSB (unacidified; pH 7.2), followed by autoclaving (15 min, 121°C) and pH measurement. ML (Sigma Chemical Co., St. Louis, MO) stock solution was prepared in 95% ethanol and diluted in TSB to obtain a 125-μg/ml working solution. NI (2.5% with NaCl and milk solids; Sigma) stock solution was prepared in 0.02 N HCl, followed by filter sterilization (0.2 μM) and dilution in TSB to obtain a 312.5-IU/ml NI working solution.
Combined antimicrobial activity assays.
Eight antimicrobial combinations (LA-ML-NI, LA-NI, LA-ML, LA alone, ML-NI, NI alone, ML alone, and no drugs) were analyzed in triplicate by using microtiter plate growth assays in 96-well plates (4, 10). Each well contained 240 μl TSB with antimicrobials and was inoculated with 10 μl of 1:100-diluted working culture to yield 105 CFU/ml. Antimicrobial concentrations in the final volume were 0.6 mg/ml LA, 2.5 μg/ml ML, and/or 12.5 IU/ml NI, individually or combined. Selected sublethal antimicrobial concentrations were chosen based on preliminary experiments (results not shown) that allow for growth of L. monocytogenes yielding measurable turbidity. Inoculated plates were incubated at an optimum growth temperature of 35°C for 40 h, and the optical density at 600 nm (OD600) of each well was monitored every 30 min after an automatic 10-s shake (SpectraMax 250; Molecular Devices, Sunnyvale, CA). Nine growth curves for each treatment (three replications) were fitted into the Gompertz equation (SigmaPlot 5.0; Systat Software, Inc., San Jose, CA) describing bacterial growth (10):
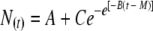 |
where N(t) is the absorbance at time t, A is the absorbance at time zero, C is the difference between absorbance at inoculation and absorbance at the stationary phase, B is the maximum relative growth rate, t is time, and M is the time at which exponential growth is maximal.
The time-to-detection value (T2D) was calculated for each curve by using the following equation (10): T2D = M − 1/B.
Extension of T2D was calculated by subtracting treatment T2D from control T2D. Triple antimicrobial effect was calculated by summing the effects of the three individual antimicrobial treatments, the double-combination treatments plus the third treatment alone, and the triple-combination treatment. Two of the nine curves for the triple-combination treatment were omitted from this calculation due to no growth.
Antimicrobial effects on membrane fatty acids.
Individual (LA, ML, NI) and selected dual combinations (ML-LA and ML-NI) of antimicrobials were assessed for their impact on changes in L. monocytogenes membrane fatty acid profiles. Treatments (30 ml sterile TSB in Erlenmeyer flasks) contained 2.4 mg/ml LA alone (pH 5.5), 5 μg/ml ML alone, 2.25 IU/ml NI alone, 2.5 μg/ml ML alone, 2.4 mg/ml LA and 5 μg/ml ML combined (pH 5.5), and 2.5 μg/ml ML and 2.25 IU/ml NI combined. Each treatment and positive control flask was inoculated with 12 μl working culture yielding 105 CFU/ml. All flasks were incubated in an environmental shaker under aerobic conditions at 35°C and 200 rpm for 24, 48, or 52 h. The experiment was replicated three times on different days.
After appropriate incubation, two 5-ml aliquots from each flask were centrifuged for 7 min at 1,380 × g to obtain two cell pellets with a cell mass of approximately 40 mg each. Each pellet was subjected to fatty acid extraction, identification, and quantification exactly as described by Yuk and Marshall (17). Results showed no presence of fatty acids in the uninoculated broth (40 μl, with or without LA, ML, or NI), proving that fatty acids were derived from the cells and not from the broth. Fatty acid profiles were reported as percentages of fatty acids present compared to the total area under the curves of chromatograms. Minor fatty acids (<1%) were not reported. Fatty acids extracted in this study were considered bioconcentrated by L. monocytogenes either on the cell surface, in the cell membrane, or inside the cell. Because the majority of lipids in bacterial cells are present in the cell membrane, it was assumed that all identified fatty acids derived from the cell membrane.
Statistical analysis.
Statistical analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, NC). Treatment effects (individual and combined antimicrobials) on T2D (h) and T2D extension (h) of L. monocytogenes growth and the relative percentage of individual fatty acids extracted from an L. monocytogenes biomass, were determined using one-way analysis of variance, with means separated by using Fisher's least significant difference test (P < 0.05).
RESULTS AND DISCUSSION
Combined antimicrobial effects.
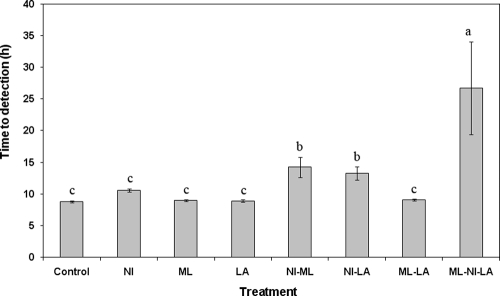
Average T2D values for all treatments are shown in Fig. 1, with the two no-growth ML-NI-LA samples omitted. Significant delay (P < 0.05) in T2D was observed following treatment with NI-ML (14.2 h), NI-LA (13.3 h), and ML-NI-LA (26.7 h) compared to the positive control (8.8 h). T2D values of the ML-LA treatment and treatments with individual compounds did not differ (P > 0.05) from those from the control (Fig. 1).
FIG. 1.
Mean T2D of individual, dual-combination, or triple-combination antimicrobials for growth of Listeria monocytogenes at 35°C. Means of the antimicrobial treatments with the same letters above their bars are not significantly different (P > 0.05).
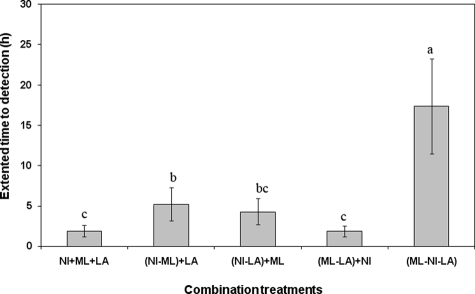
The sum of the individual antimicrobial T2D extensions (2.4 h) was not significantly different (P > 0.05) from the sum of ML-LA plus NI and NI-LA plus ML treatments, which suggests an additive interaction (Fig. 2). The combined effect of the three antimicrobials (ML-NI-LA) on T2D extension was significantly greater (P < 0.05) than the sum of the individual effects of the three antimicrobial compounds alone or any combination of two compounds plus the effect of using a third compound alone (Fig. 2), which suggests a synergistic interaction.
FIG. 2.
Extension of T2D of individual, dual-combination, or triple-combination antimicrobials compared to the sum of individual effects for growth of Listeria monocytogenes at 35°C. Means of the antimicrobial treatments with the same letters above their bars are not significantly different (P > 0.05).
Influence of LA and ML on membrane fatty acid profiles.
Incubation time of some of the individual and combined treatments was extended to allow for sufficient production of biomass for fatty acid analyses. Control, LA, and ML treatments yielded sufficient L. monocytogenes growth (OD600, ∼1.0) after 24 h at 35°C. Combined ML-LA treatment showed sufficient growth only after 48 h of incubation.
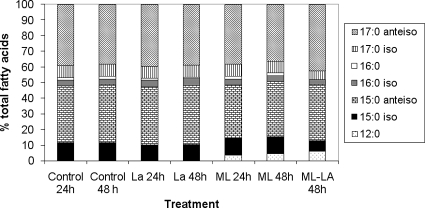
Fatty acid profiles of cells treated with LA alone, ML alone, and ML-LA are shown in Fig. 3. Treatment with LA alone did not change fatty acid profiles of the bacterium after 24 or 48 h of incubation. This observation confirms a previous finding by Juneja et al. (5), who showed no fatty acid profile changes and no change in the C15/C17 membrane fluidity ratio of L. monocytogenes grown in the presence of LA. It is well known that short-chain aliphatic organic acids do not possess antimicrobial activity by a simple reduction in pH or by altering membranes but, rather, by crossing the bacterial membrane in undissociated form and dissociating in the cell cytoplasm, causing intracellular pH drop and cell death (3).
FIG. 3.
Fatty acid profiles of Listeria monocytogenes grown at 35°C in the presence of 2.4 mg/ml LA, 5 μg/ml ML, and an LA-ML combination. Each bar represents 100% of all identified fatty acids, while bar sections represent the average percentages of individual fatty acids.
There was a notable fatty acid profile change when L. monocytogenes was grown in the presence of ML; namely, the appearance of lauric acid (C12:0) was observed in the membrane (Fig. 3). Juneja et al. (5) reported that L. monocytogenes grown in the presence of exogenous fatty acids (C14:0, C16:0, C18:0, and C18:1) resulted in significant increases in the quantities of these acids in the cell membrane. In the present study, C12:0 membrane incorporation was dependent upon time and ML concentration. With a monolaurin concentration of 2.5 μg/ml, C12:0 incorporation average percentages were 2.06 and 2.38% after 24 and 48 h of incubation, respectively (these means were not significantly different [P > 0.05]). With a monolaurin concentration of 5 μg/ml, they were 3.63 and 4.36% after 24 and 48 h of incubation, respectively. ML is known to produce highly ordered membranes, which is thought to disrupt membrane function by affecting signal transduction due to blockage of promoters, uncoupling of energy systems, altered respiration, and altered amino acid uptake (6). When ML was combined with LA, greater incorporation of C12:0 into the cell membrane was observed than with ML alone after 48 h of incubation (Table 1). Thus, it appears that LA improved the uptake of lauric acid into the membrane, which probably affects membrane function and, furthermore, leads to measurable synergism of the combined antimicrobial treatment (14). Membrane fluidity as measured by the C15/C17 ratio and by the (C15-C12)/C17 adjusted fluidity index showed no major changes when cells were grown in the presence of LA alone, ML alone, or the combined LA-ML treatment (Table 2). However, the (C15-C12)/C17 adjusted fluidity index was greater than the C15/C17 ratio for the same treatment when ML was present, which suggests that L. monocytogenes incorporates C12:0 at the expense of shorter-chained C15:0 but not C17:0 (Fig. 3). This observation implies that the adjusted fluidity index may better indicate membrane fluidity than does the C15/C17 ratio. Therefore, it would appear that antimicrobial synergy between LA and ML might be related to changes in both membrane function and fluidity.
TABLE 1.
Changes in mean C12:0 percentages and fluidity indices of cell membranes of Listeria monocytogenes due to exposure to LA (2.4 mg/ml), ML (5 μg/ml), and ML-LA during growth for 24 to 48 h
| Treatment | Incubation time (h) | Change ind:
|
||
|---|---|---|---|---|
| Mean C12:0 %a | C15/C17 ratiob | (C12-C15)/C17 indexc | ||
| Control | 24 | 0 | 1.00 | 1.00 |
| 48 | 0 | 1.07 | 1.07 | |
| LA | 24 | 0 | 1.01 | 1.01 |
| 48 | 0 | 1.04 | 1.04 | |
| ML | 24 | 3.63 | 0.97 | 1.05 |
| 48 | 4.36 | 1.05 | 1.15 | |
| ML-LA | 24 | NG | NG | NG |
| 48 | 6.35 | 0.90 | 1.03 | |
All four values for treatments with control and LA are not significantly different from each other. Both values for ML treatment are not significantly different (P > 0.05).
All values for treatment with control and LA and the value for ML treatment at 48 h incubation are not significantly different from each other. The value for control treatment at 24 h, that for ML treatment at 24 h, and that for ML-LA treatment at 48 h are not significantly different from each other (P > 0.05).
The values for control treatment at 48 h and ML treatment at 24 and 48 h are not significantly different from each other. Both values for control treatment, both for LA treatment, the value for ML treatment at 24 h, and that for ML-LA treatment at 48 h are not significantly different from each other (P > 0.05).
NG, no growth.
Influence of ML and NI on membrane fatty acid profiles.
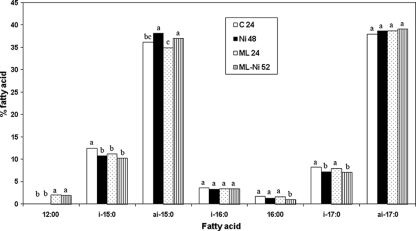
Control and ML treatments showed substantial growth after 24 h, and cells were successfully harvested for fatty acid analysis after 24 and 48 h. NI- and ML-NI-treated cells showed growth and were harvested after 48 and 52 h (OD600, ∼1.0), respectively. NI alone had little influence on fatty acid profiles, with a notable exception of an increase (P < 0.05) in anteiso-15:0 and decreases (P < 0.05) in iso-15:0 and iso-17:0 fatty acid amounts compared to control (Fig. 4). More importantly, NI had no influence on C12:0 incorporation caused by ML. Both control and NI-treated L. monocytogenes cells had no C12:0 present in their membranes. ML-treated cells had the same amount (P > 0.05) of C12:0 (2.06%) as did ML-NI-treated cells (1.92%).
FIG. 4.
Fatty acid profiles of Listeria monocytogenes grown to sufficient biomass (24, 48, and 52 h) at 35°C in the presence of 2.5 μg/ml ML, 2.25 IU/ml NI, and an ML-NI combination. Means for the same fatty acids with the same letters above their bars are not significantly different (P > 0.05).
NI causes pore formation in bacterial membranes, which causes leakage of intracellular fluids and disruption of proton motive force (2). It is generally believed that NI is more active against L. monocytogenes at lower temperatures, when membrane fluidity is higher (9). Mazotta and Montville (9) also noted that NI-resistant L. monocytogenes strains had a modified fatty acid profile, which was associated with a more rigid membrane.
Given the above observations, the following model of synergistic ML-NI-LA inhibition is proposed. LA enhanced ML C12:0 incorporation into the cell membrane, which subsequently increased membrane fluidity resulting in increased NI activity. NI alone did not have the ability to increase C12:0 incorporation into L. monocytogenes cell membranes caused by ML. In applications where NI-resistant strains of L. monocytogenes may be expected, perhaps the addition of LA and ML to the food product formulation may help retain NI activity.
Acknowledgments
The manuscript has been approved for publication as journal article no. J-11363 of the Mississippi Agricultural and Forestry Experiment Station.
This work was supported in part by USDA-ARS and by the Mississippi Agricultural and Forestry Experiment Station under project MIS-371070.
Footnotes
Published ahead of print on 26 September 2008.
REFERENCES
- 1.Benkerroum, N., and W. E. Sandine. 1988. Inhibitory action of nisin against Listeria monocytogenes. J. Dairy Sci. 71:3237-3245. [DOI] [PubMed] [Google Scholar]
- 2.Demel, R. A., T. Peelen, R. J. Siezen, B. De Kruijff, and O. P. Kuipers. 1996. Nisin Z, mutant nisin Z and lacticin 481 interactions with anionic lipids correlate with antimicrobial activity: a monolayer study. Eur. J. Biochem. 235:267-274. [DOI] [PubMed] [Google Scholar]
- 3.Doores, S. 2005. Organic acids, p. 91-142. In P. M. Davidson, J. N. Sofos, and J. L. Brannen (ed.), Antimicrobials in foods, 3rd ed. CRC Press, Taylor & Francis Group, Boca Raton, FL.
- 4.Dufour, M., R. S. Simmonds, and P. J. Bremer. 2003. Development of a method to quantify in vitro the synergistic activity of “natural” antimicrobials. Int. J. Food Microbiol. 85:249-258. [DOI] [PubMed] [Google Scholar]
- 5.Juneja, V. K., and P. M. Davidson. 1993. Influence of altered fatty acid composition on resistance of Listeria monocytogenes to antimicrobials. J. Food Prot. 56:302-305. [DOI] [PubMed] [Google Scholar]
- 6.Kabara, J. J., and D. L. Marshall. 2005. Medium-chain fatty acids and esters, p. 327-360. In P. M. Davidson, J. N. Sofos, and J. L. Brannen (ed.), Antimicrobials in foods, 3rd ed. CRC Press, Taylor & Francis Group, Boca Raton, FL.
- 7.Li, J., M. L. Chikindas, R. D. Ludescher, and T. J. Montville. 2002. Temperature- and surfactant-induced membrane modifications that alter Listeria monocytogenes nisin sensitivity by different mechanisms. Appl. Environ. Microbiol. 68:5904-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansour, M., and J. B. Milliere. 2001. An inhibitory synergistic effect of nisin-monolaurin combination on Bacillus sp. vegetative cell in milk. Food Microbiol. 18:87-94. [Google Scholar]
- 9.Mazzotta, A. S., and T. J. Montville. 1997. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10 degrees C and 30 degrees C. J. Appl. Microbiol. 82:32-38. [DOI] [PubMed] [Google Scholar]
- 10.McEntire, J. C., T. J. Montville, and M. L. Chikindas. 2003. Synergy between nisin and selected lactates is due to the metal cations. J. Food Prot. 66:1631-1636. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed, G. E., A. Seaman, and M. Woodbine. 1984. Food antibiotic nisin comparative effect on Erysipelothrix and Listeria. Proceedings of the 4th International Symposium on Antibiotics in Agriculture. Blackwell Publishing, Ltd., London, England.
- 12.Nykänen, A., K. Weckman, and A. Lapvetelainen. 2000. Synergistic inhibition of Listeria monocytogenes on cold-smoked rainbow trout by nisin and sodium lactate. Int. J. Food Microbiol. 61:63-72. [DOI] [PubMed] [Google Scholar]
- 13.Oh, D. H., and D. L. Marshall. 1992. Effect of pH on the minimum inhibitory concentration of monolaurin against Listeria monocytogenes. J. Food Prot. 55:449-450. [DOI] [PubMed] [Google Scholar]
- 14.Oh, D. H., and D. L. Marshall. 1994. Enhanced inhibition of Listeria monocytogenes by glycerol monolaurate with organic acids. J. Food Sci. 59:1258-1261. [Google Scholar]
- 15.Sinensky, M. 1974. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 71:522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas, L. V., and J. Delves-Broughon. 2005. Nisin, p. 237-274. In P. M. Davidson, J. N. Sofos, and J. L. Brannen (ed.), Antimicrobials in foods, 3rd ed. CRC Press, Taylor & Francis Group, Boca Raton, FL.
- 17.Yuk, H. G., and D. L. Marshall. 2003. Heat adaptation alters Escherichia coli O157:H7 membrane lipid composition and verotoxin production. Appl. Environ. Microbiol. 69:5115-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuk, H. G., and D. L. Marshall. 2004. Adaptation of Escherichia coli O157:H7 to pH alters membrane lipid composition, verotoxin secretion, and resistance to simulated gastric fluid acid. Appl. Environ. Microbiol. 70:3500-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]