Abstract
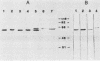
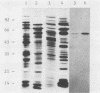
Monoclonal hybridoma antibodies directed against a 65-kilodalton (kDa) mycobacterial protein could detect similarly sized antigens in many other bacterial species. In Pseudomonas aeruginosa, the cross-reacting protein corresponded to a 62-kDa antigen that has been called Common Antigen. The mycobacterial 65-kDa antigen and Common Antigen are similar in that both (i) are highly immunoreactive molecules, (ii) contain species-specific and genus-specific epitopes in addition to the broadly cross-reactive epitopes, (iii) can be isolated as homomultimers of greater than 240 kDa, and (iv) have similar amino acid compositions. In Escherichia coli, the cross-reactive protein corresponded to the GroEL protein. Both the GroEL protein and the mycobacterial 65-kDa protein are expressed as heat shock proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Britton W. J., Garsia R. J., Hellqvist L., Watson J. D., Basten A. The characterization and immunoreactivity of a 70 kD protein common to Mycobacterium leprae and Mycobacterium bovis (BCG). Lepr Rev. 1986 Dec;57 (Suppl 2):67–75. [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Inglis A. S. Immunoreactivity of a 70 kD protein purified from Mycobacterium bovis Bacillus Calmette-Guerin by monoclonal antibody affinity chromatography. J Exp Med. 1986 Sep 1;164(3):695–708. doi: 10.1084/jem.164.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Raison R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J Immunol. 1985 Dec;135(6):4171–4177. [PubMed] [Google Scholar]
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom B. R. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect Immun. 1987 Apr;55(4):1000–1003. doi: 10.1128/iai.55.4.1000-1003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Ono M., Kuwano M., Kung H. Cloning, sequence analysis, and expression of alteration of the mRNA stability gene (ams+) of Escherichia coli. J Bacteriol. 1985 Jan;161(1):446–449. doi: 10.1128/jb.161.1.446-449.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparas S. D. The immunology of mycobacterial infections. Crit Rev Microbiol. 1982;9(2):139–197. doi: 10.3109/10408418209104488. [DOI] [PubMed] [Google Scholar]
- Collins F. M. The immunology of tuberculosis. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):42–49. doi: 10.1164/arrd.1982.125.3P2.42. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Bosmans R., Turneer M., Weckx M., Nyabenda J., Van Vooren J. P., Falmagne P., Wiker H. G., Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987 Jan;55(1):245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayet O., Louarn J. M., Georgopoulos C. Suppression of the Escherichia coli dnaA46 mutation by amplification of the groES and groEL genes. Mol Gen Genet. 1986 Mar;202(3):435–445. doi: 10.1007/BF00333274. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R., Georgopoulos C., Tilly K., Herskowitz I., Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984 Dec;48(4):299–325. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Job C. K. Purification of the 65 kD protein from Mycobacterium gordonae and use in skin test response to Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1987 Mar;55(1):54–62. [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Hindersson P., Petersen C. S., Pedersen N. S., Høiby N., Axelsen N. H. Immunological cross-reaction between antigen Tp-4 of Treponema pallidum and an antigen common to a wide range of bacteria. Acta Pathol Microbiol Immunol Scand B. 1984 Aug;92(4):183–188. doi: 10.1111/j.1699-0463.1984.tb02818.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A., Young R. A., Young D. B. The identification of T cell epitopes in Mycobacterium tuberculosis using human T lymphocyte clones. Lepr Rev. 1986 Dec;57 (Suppl 2):131–137. [PubMed] [Google Scholar]
- Lamb J. R., Young D. B. A novel approach to the identification of T-cell epitopes in Mycobacterium tuberculosis using human T-lymphocyte clones. Immunology. 1987 Jan;60(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Levis W. R., Meeker H. C., Schuller-Levis G. B., Gillis T. P., Marino L. J., Jr, Zabriskie J. Serodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J Clin Microbiol. 1986 Dec;24(6):917–921. doi: 10.1128/jcm.24.6.917-921.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mayer H., Schmidt G. Chemistry and biology of the enterobacterial common antigen (ECA). Curr Top Microbiol Immunol. 1979;85:99–153. doi: 10.1007/978-3-642-67322-1_3. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Mustafa A. S., Oftung F., Gill H. K., Natvig I. Characteristics of human T-cell clones from BCG and killed M. leprae vaccinated subjects and tuberculosis patients. Recognition of recombinant mycobacterial antigens. Lepr Rev. 1986 Dec;57 (Suppl 2):123–130. [PubMed] [Google Scholar]
- Neidhardt F. C., Phillips T. A., VanBogelen R. A., Smith M. W., Georgalis Y., Subramanian A. R. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J Bacteriol. 1981 Jan;145(1):513–520. doi: 10.1128/jb.145.1.513-520.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Husson R., Young R. A., Godal T. Human T cell clones recognize two abundant Mycobacterium tuberculosis protein antigens expressed in Escherichia coli. J Immunol. 1987 Feb 1;138(3):927–931. [PubMed] [Google Scholar]
- Ono M., Kuwano M. A conditional lethal mutation in an Escherichia coli strain with a longer chemical lifetime of messenger RNA. J Mol Biol. 1979 Apr 15;129(3):343–357. doi: 10.1016/0022-2836(79)90500-x. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Klatser P. R., Ivanyi J., Elferink D. G., de Wit M. Y., de Vries R. R. Mycobacterium leprae-specific protein antigens defined by cloned human helper T cells. Nature. 1986 Jan 2;319(6048):66–68. doi: 10.1038/319066a0. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. B., Carlone G. M., Pau C. P., Wilkinson H. W. Purified 60-kilodalton Legionella protein antigen with Legionella-specific and nonspecific epitopes. J Clin Microbiol. 1987 Nov;25(11):2080–2084. doi: 10.1128/jcm.25.11.2080-2084.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Krat C., Schadow S. Isolation and restriction site maps of the genes encoding five Mycobacterium tuberculosis proteins. Infect Immun. 1987 Jul;55(7):1718–1721. doi: 10.1128/iai.55.7.1718-1721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Pedersen V. B., Samra Z. An antigen common to a wide range of bacteria. 2. A biochemical study of a "common antigen" from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Oct;88(5):253–260. doi: 10.1111/j.1699-0463.1980.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Samra Z. An antigen common to a wide range of bacteria. I. The isolation of a 'common antigen' from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Jun;88(3):143–149. doi: 10.1111/j.1699-0463.1980.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]