Abstract
Background
Pseudoxanthoma elasticum (PXE) is a heritable connective tissue disorder caused by mutations in the ABCC6 gene. Fragmentation of elastic fibres and deposition of proteoglycans result in a highly variable clinical picture. The altered proteoglycan metabolism suggests that enzymes from this pathway function as genetic co‐factors in the severity of PXE. Therefore, we propose the XYLT genes encoding xylosyltransferase I (XT‐I) as the chain‐initiating enzyme in the biosynthesis of proteoglycans and the highly homologous XT‐II as potential candidate genes.
Methods
We screened all XYLT exons in 65 German PXE patients using denaturing high performance liquid chromatography and analysed the influence of the variations on clinical characteristics.
Results
We identified 22 variations in the XYLT genes. The missense variation p.A115S (XT‐I) is associated with higher serum XT activity (p = 0.005). The amino acid substitution p.T801R (XT‐II; c.2402C>G) occurs with significantly higher frequency in patients under 30 years of age at diagnosis (43% v 26%; p = 0.04); all PXE patients with this variation suffer from skin lesions compared to only 75% of the wild type patients (p = 0.002). c.166G>A, c.1569C>T, and c.2402C>G in the XYLT‐II gene were found to be more frequent in patients with higher organ involvement (p = 0.04, p = 0.01, and p = 0.02, respectively).
Conclusions
Here we show for the first time that variations in the XYLT‐II gene are genetic co‐factors in the severity of PXE. Furthermore, the higher XT activity in patients with the exchange p.A115S (XT‐I) indicates that this polymorphism is a potential marker for increased remodelling of the extracellular matrix.
Keywords: DHPLC, polymorphisms, proteoglycan, pseudoxanthoma elasticum, xylosyltransferase
Pseudoxanthoma elasticum (PXE) is a systemic degenerative disorder of connective tissue characterised by progressive mineralisation and fragmentation of elastic fibres and increased deposition of proteoglycans. These alterations in the extracellular matrix lead to a loss of elasticity in the skin, the eyes, and the cardiovascular system.1
PXE is caused by mutations in the ABCC6 gene (ATP‐binding cassette transporter subfamily C member 6) encoding the protein MRP6 (transmembrane transporter protein multidrug resistance‐associated protein 6).2,3,4 To date, more than 120 PXE causing mutations have been identified.3,4,5,6,7,8,9
The central hallmark of PXE is degenerative calcification with subsequent disintegration and destruction of elastic tissue in the skin, eyes, and cardiovascular system. PXE shows considerable clinical heterogeneity. In severe PXE calcification leads to contraction of the blood vessels, which in young patients can result in hypertension, arteriosclerosis, or angina pectoris and increases the risk of myocardial infarction.10
However, PXE is also characterised by collagen fibril abnormalities and massive accumulation of proteoglycans in the extracellular matrix of affected tissue.11,12 It has been hypothesised that the involvement of these components influences the highly variable clinical picture.10
Fibroblasts from PXE affected patients produce proteoglycan populations with stronger anionic properties, increased hydrodynamic size, abnormal hydrophobic interactions, and differences in content and distribution of heparan sulfate.13,14,15,16 Alterations in proteoglycan metabolism result in changes to urinary glycosaminoglycans (GAGs). A significant decrease in the amount of chondroitin sulfate and a significant increase in heparan sulfate was described. This resulted in a 34% reduction in total polysaccharides in the urine of PXE affected patients. Furthermore, the grade of sulfatation of heparan sulfate and chondoitin sulfate was changed in PXE patients.17
Proteoglycans are polyanionic macromolecules composed of GAG chains covalently bound to a protein core. The different types of proteoglycans are distinguished by their various core proteins and the structures of their polysaccharide chains. The GAG chains are responsible for the various biological functions of the proteoglycans.18 The glycosylation of core protein in heparan sulfate, heparin, chondroitin sulfate, and dermatan sulfate containing proteoglycans is initiated by xylosyltransferase I (XT‐I, EC 2.4.2.26). XT‐I catalyses the transfer of xylose from UDP‐xylose to selected serine residues in the proteoglycan core protein. This is the initial and apparently rate‐limiting step in the formation of the common tetrasaccharide linkage region (‐GlcA‐Gal‐Gal‐Xyl‐Ser), to which many disaccharide repeats are attached as part of the biosynthesis of proteoglycans.18 The disaccharide elongation of the linker is followed by extensive modification of GAGs through a variety of different enzymes. This leads to the presence of highly negatively charged sulfate and carboxyl groups.18
The XYLT‐II gene codes for a protein highly homologous to XT‐I. The C‐terminal regions of both proteins, where the catalytic domain was found to be located in glycosyltransferases, are highly conserved.19,20,21 These findings have led us to conclude that the XYLT‐II gene encodes another xylosyltransferase, although the catalytic activity and the biologic function of XT‐II are not yet known.20 The gene for XYLT‐I is located on chromosome 16p13.1, consists of 12 exons, and extends over 300 kb. In contrast, the XYLT‐II gene is localised on chromosome 17q21.3–22, comprises 11 exons, and spans only 15 kb. The observed modifications in proteoglycan metabolism in PXE patients reflect altered biosynthesis of the extracellular matrix. We propose that XT‐I, as the initial and most important enzyme in the biosynthesis of GAG containing proteoglycans, and XT‐II, as a highly homologous protein, might influence the clinical characteristics of PXE. As most XT‐I is secreted into the extracellular matrix, XT‐I activity was proposed as a diagnostic marker for the determination of enhanced proteoglycan biosynthesis and of tissue destruction.22 We have shown previously that serum XT‐I activity is a confirmed biochemical marker for the determination of fibrotic activity in systemic sclerosis23,24 and of increased proteoglycan biosynthesis in PXE patients, especially those with hypertension.25 Based on these observations, the present study was carried out to comprehensively investigate the implications of genetic variations in the XYLT genes for the highly variable PXE phenotype.
Methods
Subjects
A total of 65 German patients with PXE (18 males, 47 females) were examined for genetic variations in the human XYLT genes. The clinical characteristics of the patients are listed in table 1.
Table 1 Clinical characteristics of German PXE patients.
| Male | Female | p value | |
|---|---|---|---|
| n | 18 | 47 | – |
| Age (years) | 52.8±13.3 | 46.0±14.3 | 0.09 |
| Age at PXE diagnosis (years) | 37.1±16.2 | 30.0±15.8 | 0.12 |
| Smoking | 3 (16.7%) | 10 (21.3%) | 1.0 |
| Organ involvement | |||
| Mean number | 3.6±1.6 | 3.0±1.5 | 0.15 |
| Eyes | 17 (94.4%) | 41 (87.2%) | 0.66 |
| Skin | 15 (83.3%) | 43 (91.5%) | 0.39 |
| Heart | 7 (38.9%) | 4 (8.5%) | 0.01 |
| Vascular tissue | 8 (44.4%) | 13 (27.7%) | 0.24 |
| Hypertension | 5 (27.8%) | 15 (31.9%) | 0.80 |
| Kidney/urinary tract | 4 (22.2%) | 3 (6.4%) | 0.09 |
| Gastrointestinal tract | 3 (16.7%) | 5 (10.6%) | 0.67 |
| XT‐I activity (mU/l) | 0.98±0.44 | 0.89±0.37 | 0.39 |
Data are means±SD, total number (percentage), or n.
For control purposes, the detected variations were also determined in DNA samples obtained from 104 to 240 blood donors (44.7% males), 18–68 years of age (mean age±SD, 39.7±19.3 years). Through extensive clinical examination we excluded impaired organ function and skin lesions in the controls. The PXE patients included in this study were clinically examined by medical specialists in internal medicine, dermatology, and ophthalmology and in addition thoroughly questioned about their personal disease and organ involvement in the Krankenhaus Bethesda, Freudenberg, Germany; disease was diagnosed based on international criteria.26 The status of PXE in each patient was determined by the presence of ocular findings and dermal lesions and was histologically confirmed by observation of calcification in the elastic fibres in skin biopsies from lesional regions after von Kossa staining. The experimental design was approved by the institutional ethics committee, and all patients gave their informed consent. Genomic DNA was isolated from peripheral blood leucocytes.
Mutational analysis and genotyping
Polymorphism screening and genotyping of the XYLT genes of PXE patients was performed by denaturing high performance liquid chromatography (DHPLC). The 23 exons of the XYLT genes were amplified from genomic DNA by PCR with primers located in the flanking intron regions and the products were analysed by DHPLC as described previously.27 DNA samples with an elution profile which differed from the wild type DNA were further analysed by double stranded sequencing. Seventeen of the detected variations (seven XYLT‐I and ten XYLT‐II) were initially genotyped in a control group by DHPLC or restriction fragment length polymorphism (RFLP) (table 2). The size of the control cohort for the determination of the allelic frequencies of each of the variations was dependent on its location and potential effect on gene function. The cohort size was defined before analyses were performed and no retrospective changes were allowed.
Table 2 Allelic frequencies of detected XYLT variations in PXE patients and blood donors, their effect on the amino acid sequence, and their location in the gene.
| Gene | Polymorphism*/† | rs number‡ | Amino acid | Region | Genotyping | Allelic frequency in | |
|---|---|---|---|---|---|---|---|
| PXE patients | Blood donors | ||||||
| XYLT‐I | IVS1‐5C>G | 5′‐UTR | ND§ | ND | ND | ||
| c.343G>T | p.A115S | Exon 1 | NmuCI¶ | 4.5% | 3.4% | ||
| c.1077C>T | Exon 4 | Eco72I¶ | 7.6% | 4.5% | |||
| c.1216C>T | p.R406W | Exon 5 | DHPLC | 0.8% | 0% | ||
| c.1284C>G | Exon 5 | DHPLC | 15.9% | 17.9% | |||
| c.1989T>C | rs 12708815 | Exon 9 | BsuRI¶ | 32.6% | 34.5% | ||
| c.1994C>T | p.T665M | Exon 9 | AatII¶ | 0.8% | 0% | ||
| c.2631C>T | Exon 12 | DHPLC | 12.9% | 17.5% | |||
| XYLT‐II | IVS1‐86delG | 5′‐UTR | ND | ND | ND | ||
| IVS1‐84G>A | rs 9912067 | 5′‐UTR | ND | ND | ND | ||
| IVS1‐72G>C | 5′‐UTR | ND | ND | ND | |||
| IVS1‐35G>C | 5′‐UTR | ND | ND | ND | |||
| c.166G>A | p.D56N | Exon 2 | Eco47I¶ | 2.3% | 2.0% | ||
| c.177A>G | rs 739990 | Exon 2 | Sequencing | 73.5% | 77.1% | ||
| c.342T>C | rs 739989 | Exon 2 | BsaWI** | 73.5% | 77.1% | ||
| c.344C>T | p.P115L | Exon 2 | DHPLC | 0.8% | 0% | ||
| c.914C>G | rs 12451299 | p.T305R | Exon 4 | Bsu36I** | 75% | 78.9% | |
| IVS6‐9T>C | Intron 5 | Sequencing | 3.8% | 1.1% | |||
| IVS6‐14_IVS6‐13insG | Intron 5 | HpyF10VI¶ | 3.8% | 1.1% | |||
| c.1253C>T | p.P418L | Exon 6 | BfuAI** | 2.3% | 2.2% | ||
| c.1569C>T | rs 4794136 | Exon 8 | MslI** | 48.5% | 52.7% | ||
| c.2402C>G | rs 6504649 | p.T801R | Exon 11 | DHPLC | 34.1% | 40.2% | |
Mutation numbering refers to the cDNA sequences with the A of the ATG translation initiation start site as nucleotide +1.
*Numbering of the XT‐I variations is based on human cDNA sequence (GenBank accession number NM_022166). There are sequence discrepancies between the cDNA and the genomic DNA (GenBank accession numbers AC109446, AC099494, AC009152, AC109495). We detected in our samples at the following positions only the nucleotide of the genomic sequence: IVS1‐14: g; c.489: c; c.2850: g; c.2853: a.
†Numbering of the XT‐II variations was based on human cDNA sequence (GenBank accession number NM_022167). ‡rs number according to data banks (http://www.ensembl.org and http://snpper.chip.org).
§ND, not determined.
¶MBI Fermentas, Burlington, Canada.
**New England BioLabs, Frankfurt am Main, Germany.
XT‐I activity assay
The method for the determination of XT‐I activity is based on the incorporation of [14C]‐D‐xylose into an acceptor protein. Activity in the serum of PXE patients was investigated in a previous study.25
Statistical analysis
The distribution of the alleles of each single nucleotide polymorphism (SNP) was tested for Hardy‐Weinberg equilibrium. Fisher's two‐tailed exact p test was used to compare polymorphism frequencies between subjects and controls. Values of p<0.05 were considered statistically significant. In consideration of multiple testing, p values were corrected according to the Bonferroni or Sidák method where appropriate. For normally distributed clinical characteristics, a comparison between the groups was performed by an unpaired Student's t test. For non‐normally distributed variables, a Mann‐Whitney U test was used.
Results
Mutational analysis
To identify genetic variations in the XYLT‐I and XYLT‐II genes that contribute to the highly variable clinical picture of PXE, we examined DNA samples from 65 Caucasian German PXE patients. We screened all 23 exons, exon/intron junctions, and parts of the 5′‐ and 3′‐untranslated regions (UTR) applying the DHPLC method. In total 9.4 kb were screened in each PXE patient. This first mutational analysis of German PXE patients revealed a total of 22 different sequence variations (eight variations in XYLT‐I and 14 in XYLT‐II) (table 2). These variations included a single nucleotide deletion in the 5′‐UTR and an insertion in the intron of XT‐II. All other variations were SNPs. Five of them are located in the introns or in the 5′‐UTR. Eight of the 15 variations in the coding region cause a substitution of the amino acid (three in XT‐I and five in XT‐II), while the others are synonymous variations. Nineteen of the detected variations had recently been described by our group,27,28 whereas three missense variations were newly identified in PXE patients (p.R406W and p.T665M in XT‐I, and p.P115L in XT‐II). Based on their location and potential effect on gene function, 17 variations (seven XYLT‐I and ten XYLT‐II) were initially genotyped in a control group by DHPLC or RFLP (table 2). Genotype distributions for the 17 SNPs studied in our population were all within Hardy‐Weinberg equilibrium.
Comparison between PXE patients and controls
The determined allele frequencies of the 17 polymorphisms in XYLT‐I and XYLT‐II show no statistically significant differences between PXE patients and controls. Each of the three newly described missense variations was only detected in one PXE patient in the heterozygous state; all control subjects were negative for the base substitutions.
Initial analyses of controls and non‐PXE affected individuals (n = 165) revealed six variations (five in the XYLT‐I gene and one in the XYLT‐II gene) which were not detected in PXE patients.27,28 As these variations were unique, with the exception of one which was found twice in the heterozygous state, they were classified as having no significance for disease association (data not shown).
Genotype‐phenotype correlations in PXE patients
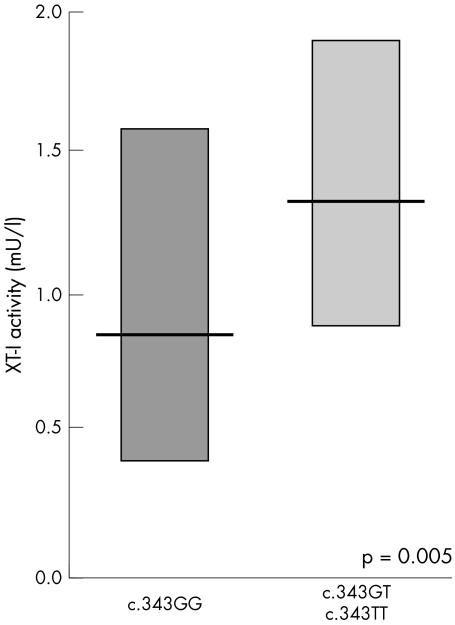
To analyse whether the discovered SNPs with a frequency of over 2% (five in XYLT‐I and nine in XYLT‐II) are associated with altered XT‐I activity and organ involvement, we compared characteristics between homo‐ and heterozygous patients and wild type patients. We detected an association between the amino acid substitution alanine 115 to serine in exon 1 of XT‐I (c.343G>T) and serum XT‐I activity. Patients with the T allele have significantly increased serum XT‐I activity compared with homozygous wild type patients (fig 1).
Figure 1 Serum XT‐I activity of PXE patients according to their genotype at position c.343G>T in the XYLT‐I gene (GG v GT+TT). Mean values and 90% ranges are shown. Serum XT‐I activity in PXE patients with the T allele in the homo‐ and heterozygous state is significantly elevated in comparison to patients carrying the wild type G allele in the homozygous state (p = 0.005).
Furthermore, we showed that patients with the amino acid exchange from threonine 801 to arginine in XT‐II more often suffered from skin involvement (p = 0.002). All 37 carriers (100%) of this variation in the homo‐ or heterozygous state have typical PXE skin lesions compared to only 21 of the 28 wild type patients (75%).
We subdivided the patients into two groups depending on age at diagnosis and number of organs involved. We compared the allelic frequencies between these groups to see whether the genetic variations influence the severity of disease.
Comparison of the allele frequencies revealed that the coding SNP c.2402C>G (p.T801R) in exon 11 of the XYLT‐II gene is significantly more frequent (43% v 26%; p = 0.04) in patients with an early age of diagnosis (7–28 years of age; 30 patients) than in those with a later age of diagnosis (30–65 years of age; 33 patients).
Three polymorphisms in the XYLT‐II gene (table 3) were detected with a significantly higher frequency in PXE patients with more organ involvement (four to seven organs; 23 patients) than in patients with lesser organ involvement (one to three organs; 42 patients). Analyses of the haplotypes revealed that these variations were neither in linkage disequilibrium with each other nor with another haplotype linkage block.
Table 3 Allelic frequencies of XYLT‐II SNPs in PXE patients.
| XYLT‐II variation | Amino acid | 1–3 organs | 4–7 organs | p value |
|---|---|---|---|---|
| c.166G>A | p.D56N | 0/84 | 3/46 | 0.04 |
| c.1569C>T | 34/84 | 30/46 | 0.01 | |
| c.2402C>G | p.T801R | 23/84 | 22/46 | 0.02 |
Discussion
PXE is a rare heritable disease of connective tissue caused by mutations in the ABCC6 gene.2,3,4 The course of the disease is very variable and, therefore, it was thought that additional factors could be involved in the pathogenesis of PXE.10 It has been proposed that PXE patients have altered proteoglycan metabolism, which results in increased accumulation of proteoglycans in the extracellular space of affected tissue and in changes to the composition of urinary proteoglycans.11,12,17 Furthermore, PXE patients have elevated serum XT‐I activity.25 On the basis of these observations we suggest a connection between the severity of the disease and genetic variations in the XYLT genes.
The first systematic mutational analysis of XYLT genes in PXE patients revealed 22 different genetic variations in the exons and the flanking intron regions. The frequencies of the 17 genotyped variations detected in PXE patients are similar in patients and controls and the same applies for the six alterations detected only in controls. Therefore, this study showed that genetic variations in the XYLT genes are neither risk factors nor protective factors in the development of PXE. The course of the disease in those patients carrying one of the three newly identified unique and heterozygous amino acid substitutions did not differ from the other patients; it seems that these substitutions have no major clinical relevance.
Genotype‐phenotype analysis of the polymorphisms revealed that PXE patients with the amino acid exchange from alanine 115 to serine in XT‐I have significantly higher serum XT‐I activity than those patients with the wild type allele. This exchange is located in the stem region of type II transmembrane protein XT‐I. It was previously shown that the first 260 amino acids of XT‐I are not necessary for enzymatic activity.29 The stem region included the signal sequences for the intracellular localisation and the potential proteinase restriction site for the soluble XT‐I form.30 This amino acid substitution could change the signal sequence directly or indirectly through the building of a new potential O‐glycoslylation site. Comparison of the amino acids in these positions with those of XT in Mus musculus, Pan troglodytes, Canis familiaris, and Xenopus laevis revealed a conserved alanine in all species. The higher serum XT‐I activity in carriers of the c.343T allele might be due to accelerated shedding of this mutant from the Golgi surface and subsequent release into the blood stream. Furthermore, it is possible that this mutant increases the turnover rate of the enzyme. This would lead to increased proteoglycan biosynthesis as XT‐I catalyses the apparent rate‐limiting step in the posttranslational biosynthesis of the glycosaminoglycan chains. As XT‐I is secreted together with the proteoglycan this would also result in elevated serum XT‐I activity. Further in vitro studies with the altered protein would be useful to investigate how substitution of the unpolar and aliphatic amino acid alanine for the more reactive and hydrophilic amino acid serine might influence the Golgi retention or the catalytic mechanism.
The amino acid exchange p.T801R in XT‐II in PXE patients is significantly associated with more skin lesions, earlier age at diagnosis, and the involvement of more organs. Furthermore, the variations c.166G>A and c.2402C>G in the XYLT‐II gene are also correlated with a more severe course of the disease. To understand the effect of these two amino acid exchanges and the one synonymous variation located over the whole XYLT‐II gene (exons 2, 8, and 11), it is necessary to identify the physiological function and the region of the catalytic domain of XT‐II. Whether XT‐II is an XT with a limited substrate specificity or an XT with a different peptide sequence specificity, or whether this XT requires substrates that already carry a xylose residue at another site, is unknown. Probably, the two variations within the N‐ and C‐terminal regions of the enzyme might not be directly responsible for the catalytic activity but are necessary for correct conformation.
Use of these three variations as prognostic markers might be advantageous. Until now, only symptomatic therapy has been possible, but avoiding potential risk factors such as smoking and fatty food could slow the progression of the disease, in particular cardiovascular impairment.31 Former studies supplied evidence indicating that low‐calcium foodstuffs influence the course of the disease in a positive way.32
Our mutational analyses of the XYLT genes of German PXE patients revealed for the first time that genetic variations in the XYLT‐II gene function as genetic co‐factors for the severity of PXE. Additional multi‐centre studies are required to evaluate the clinical relevance of our initial findings for risk assessment in PXE patients.
Acknowledgments
We thank Alexandra Adam for her technical assistance and Sarah L Kirkby for her linguistic advice. We are very grateful to all the PXE patients, whose cooperation made this study possible. Furthermore, we thank Peter Hof, chairman of the Selbsthilfegruppe für PXE‐Erkrankte Deutschlands e.V., and the staff at the Bethesda hospital in Freudenberg, Germany.
Electronic‐database information
The Ensembl web site is at http://www.ensembl.org and the CHIP Bioinformatics Tools at http://snpper.chip.org
Abbreviations
ABCC6 - ATP‐binding cassette transporter subfamily C member 6
DHPLC - denaturing high performance liquid chromatography
GAG - glycosaminoglycan
MRP6 - transmembrane transporter protein multidrug resistance‐associated protein 6
PXE - pseudoxanthoma elasticum
RFLP - restriction fragment length polymorphism
SNP - single nucleotide polymorphism
UTR - untranslated region
XT - xylosyltransferase (protein)
XYLT - xylosyltransferase (gene)
Footnotes
Competing interests: none declared
The Ensembl web site is at http://www.ensembl.org and the CHIP Bioinformatics Tools at http://snpper.chip.org
References
- 1.Neldner K H. Pseudoxanthoma elasticum. Int J Dermatol 19882798–100. [DOI] [PubMed] [Google Scholar]
- 2.Bergen A A, Plomp A S, Schuurman E J, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Baas F, ten Brink J B, de Jong P T V M. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet 200025228–231. [DOI] [PubMed] [Google Scholar]
- 3.Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, Pasquali‐Ronchetti I, Pope F M, Richards A, Terry S, Bercovitch L, de Paepe A, Boyd C D. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 200025223–227. [DOI] [PubMed] [Google Scholar]
- 4.Ringpfeil F, Lebwohl M G, Christiano A M, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP‐binding cassette (ABC) transporter. Proc Natl Acad Sci U S A 2000976001–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Struk B, Cai L, Zach S, Ji W, Chung J, Lumsden A, Stumm M, Huber M, Schaen L, Kim C A, Goldsmith L A, Viljoen D, Figuera L E, Fuchs W, Munier F, Ramesar R, Hohl D, Richards R, Neldner K H, Lindpaintner K. Mutations of the gene encoding the transmembrane transporter protein ABC‐C6 cause pseudoxanthoma elasticum. J Mol Med 200078282–286. [DOI] [PubMed] [Google Scholar]
- 6.Götting C, Schulz V, Hendig D, Grundt A, Dreier J, Szliska C, Brinkmann T, Kleesiek K. Assessment of rapid‐cycle PCR assay for the identification of the recurrent c.3421C>T mutation in the ABCC6 gene in pseudoxanthoma elasticum patients. Lab Invest 200484122–130. [DOI] [PubMed] [Google Scholar]
- 7.Hendig D, Schulz V, Eichgrün J, Szliska C, Götting C, Kleesiek K. New ABCC6 gene mutations in German pseudoxanthoma elasticum (PXE) patients. J Mol Med 200583140–147. [DOI] [PubMed] [Google Scholar]
- 8.Schulz V, Hendig D, Szliska C, Götting C, Kleesiek K. Novel mutations in the ABCC6 gene of German patients with Pseudoxanthoma elasticum. Hum Biol 200577367–384. [DOI] [PubMed] [Google Scholar]
- 9.Miksch S, Lumsden A, Guenther U P, Foernzler D, Christen‐Zach S, Daugherty C, Ramesar R K, Lebwohl M, Hohl D, Neldner K H, Lindpaintner K, Richards R I, Struk B. Molecular genetics of pseudoxanthoma elasticum: type and frequency of mutations in ABCC6. Hum Mutat 20055235–248. [DOI] [PubMed] [Google Scholar]
- 10.Lebwohl M, Halperin J, Phelps R G. Brief report: occult pseudoxanthoma elasticum in patients with premature cardiovascular disease. N Engl J Med 19933291237–1239. [DOI] [PubMed] [Google Scholar]
- 11.Pasquali‐Ronchetti I, Volpin D, Baccarani‐Contri M, Castellani I, Peserico A. Pseudoxanthoma elasticum. Biochemical and ultrastructural studies. Dermatologica 1981163307–325. [PubMed] [Google Scholar]
- 12.Pasquali‐Ronchetti I, Baccarani‐Contri M, Pincelli C, Bertazzoni G M. Effect of selective enzymatic digestions on skin biopsies from pseudoxanthoma elasticum: an ultrastructural study. Arch Dermatol Res 1986278386–392. [DOI] [PubMed] [Google Scholar]
- 13.Longas M O, Wisch P, Lebwohl M G, Fleischmajer R. Glycosaminoglycans of skin and urine in pseudoxanthoma elasticum: evidence for chondroitin 6‐sulfate alteration. Clin Chim Acta 1986155227–236. [DOI] [PubMed] [Google Scholar]
- 14.Sakuraoka K, Tajima S, Nishikawa T, Seyama Y. Biochemical analyses of macromolecular matrix components in patients with pseudoxanthoma elasticum. J Dermatol 19942198–101. [DOI] [PubMed] [Google Scholar]
- 15.Tiozzo‐Costa R, Baccarani‐Contri M, Cingi M R, Pasquali‐Ronchetti I, Salvini R, Rindi S, de Luca G. Pseudoxanthoma elasticum (PXE): ultrastructural and biochemical study on proteoglycan and proteoglycan‐associated material produced by skin fibroblasts in vitro. Coll Relat Res 1988849–64. [DOI] [PubMed] [Google Scholar]
- 16.Passi A, Albertini R, Baccarani‐Contri M, de Luca G, de Paepe A, Pallavicini G, Pasquali‐Ronchetti I, Tiozzo R. Proteoglycan alterations in skin fibroblast cultures from patients affected with pseudoxanthoma elasticum. Cell Biochem Funct 199614111–120. [DOI] [PubMed] [Google Scholar]
- 17.Maccari F, Gheduzzi D, Volpi N. Anomalous structure of urinary glycosaminoglycans in patients with pseudoxanthoma elasticum. Clin Chem 200349380–388. [DOI] [PubMed] [Google Scholar]
- 18.Kjellén L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem 199160443–475. [DOI] [PubMed] [Google Scholar]
- 19.Field M C, Wainwright L J. Molecular cloning of eucaryotic glycoprotein and glycolipid glycosyltransferases: a survey. Glycobiology 19955463–472. [DOI] [PubMed] [Google Scholar]
- 20.Götting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K. Molecular cloning and expression of human UDP‐D‐xylose: proteoglycan core protein β‐D‐xylosyltransferase and its first isoform XT‐II. J Mol Biol 2000304517–528. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn J, Götting C, Schnölzer M, Kempf T, Brinkmann T, Kleesiek K. Isolation of human UDP‐D‐xylose: proteoglycan core protein β‐D‐xylosyltransferase secreted from cultured JAR choriocarcinoma cells. J Biol Chem 20012764940–4947. [DOI] [PubMed] [Google Scholar]
- 22.Weilke C, Brinkmann T, Kleesiek K. Determination of xylosyltransferase activity in serum with recombinant human bikunin as receptor. Clin Chem 19974345–51. [PubMed] [Google Scholar]
- 23.Götting C, Sollberg S, Kuhn J, Weilke C, Huerkamp C, Brinkmann T, Krieg T, Kleesiek K. Serum xylosyltransferase: a new biochemical marker of the sclerotic process in systemic sclerosis. J Invest Dermatol 1999112919–924. [DOI] [PubMed] [Google Scholar]
- 24.Götting C, Kuhn J, Sollberg S, Huerkamp C, Brinkmann T, Krieg T, Kleesiek K. Elevated serum xylosyltransferase activity correlates with a high level of hyaluronate in patients with systemic sclerosis. Acta Derm Venerol 20008060–61. [DOI] [PubMed] [Google Scholar]
- 25.Götting C, Hendig D, Adam A, Schön S, Schulz V, Szliska C, Kuhn J, Kleesiek K. Elevated xylosyltransferase I activities in pseudoxanthoma elasticum (PXE) patients as a marker of stimulated proteoglycan biosynthesis. J Mol Med 200583984–992. [DOI] [PubMed] [Google Scholar]
- 26.Lebwohl M, Neldner K, Pope F M, De Paepe A, Christiano A M, Boyd C D, Uitto J, McKusick V A. Classification of pseudoxanthoma elasticum: report of a consensus conference. J Am Acad Dermatol 199430103–107. [DOI] [PubMed] [Google Scholar]
- 27.Schön S, Prante C, Müller S, Schöttler M, Tarnow L, Kuhn J, Kleesiek K, Götting C. Impact of polymorphisms in the genes encoding xylosyltransferase I and a homologue in type 1 diabetic patients with and without nephropathy. Kidney Int 2005681483–1490. [DOI] [PubMed] [Google Scholar]
- 28.Schön S, Huep G, Prante C, Müller S, Christ R, Hagena F W, Kuhn J, Kleesiek K, Götting C. Mutational and functional analyses of xylosyltransferases and their implication in osteoarthritis. Osteoarthritis Cartilage. 2005 Dec 21; [Epub ahead of print] [DOI] [PubMed]
- 29.Müller S, Schöttler M, Schön S, Prante C, Brinkmann T, Kuhn J, Götting C, Kleesiek K. Human xylosyltransferase I: functional and biochemical characterization of cysteine residues required for enzymatic activity. Biochem J 2005386227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Götting C, Kuhn J, Brinkmann T, Kleesiek K. Xylosylation of alternatively spliced isoforms of Alzheimer APP by xylosyltransferase. J Protein Chem 199817295–302. [DOI] [PubMed] [Google Scholar]
- 31.Neldner K H, Struk B. Pseudoxanthoma elasticum. In: Royce P, Steinmann B, eds. Connective tissue and its heritable disorders: molecular, genetic, and medical aspects 2nd ed. New York: Wiley‐Liss, 2002561–583.
- 32.Renie W A, Pyeritz R E, Combs J, Fine S L. Pseudoxanthoma elasticum: high calcium intake in early life correlates with severity. Am J Med Genet 198419235–244. [DOI] [PubMed] [Google Scholar]