Abstract
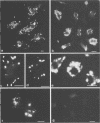
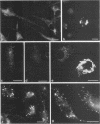
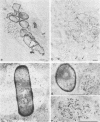
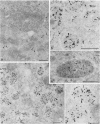
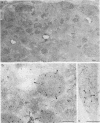
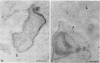
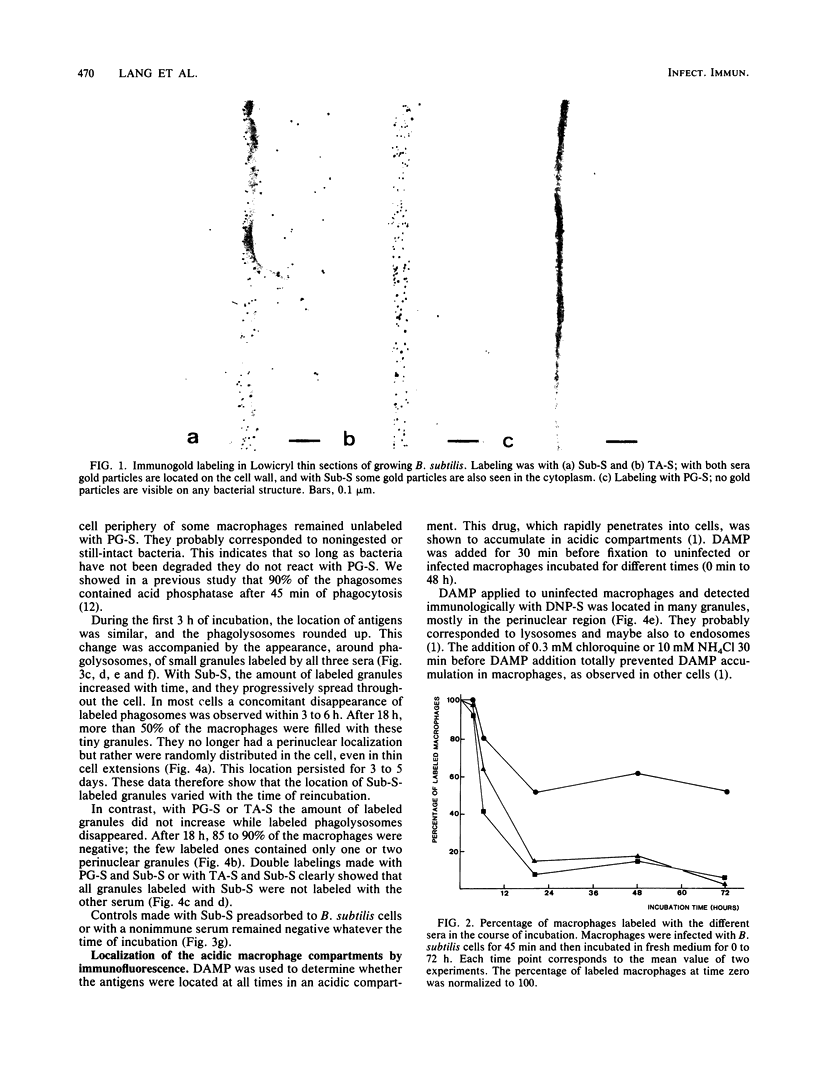
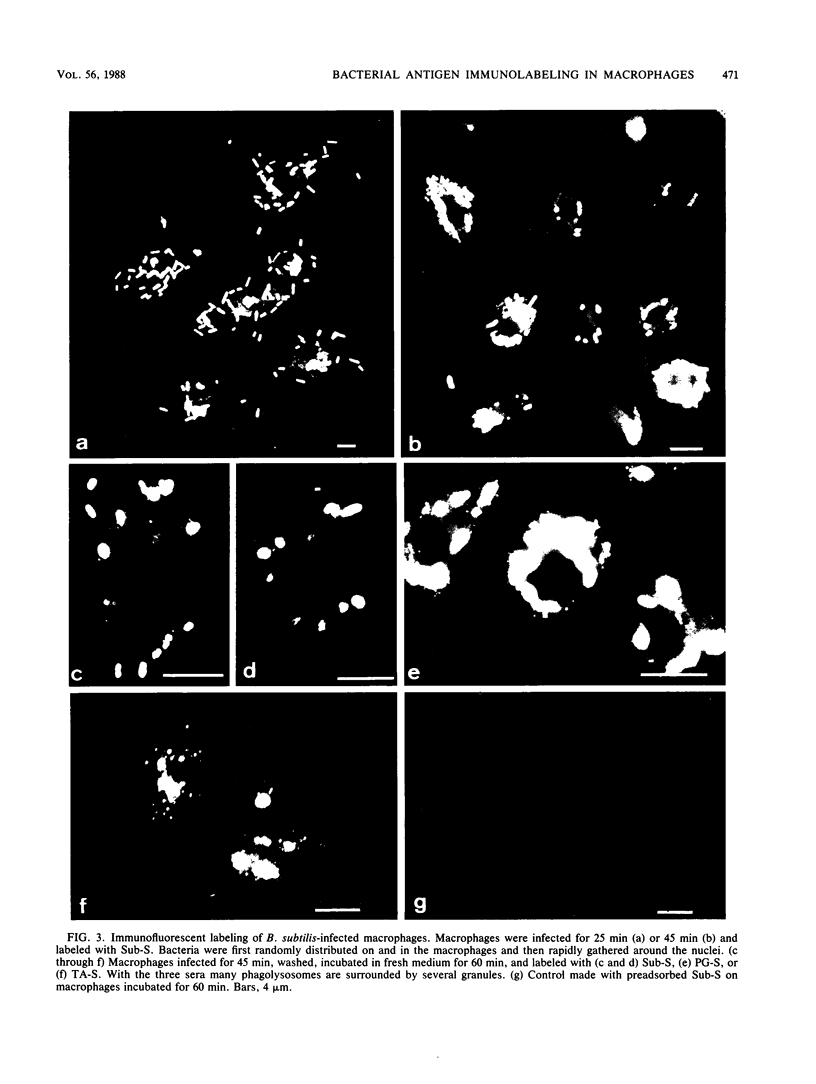
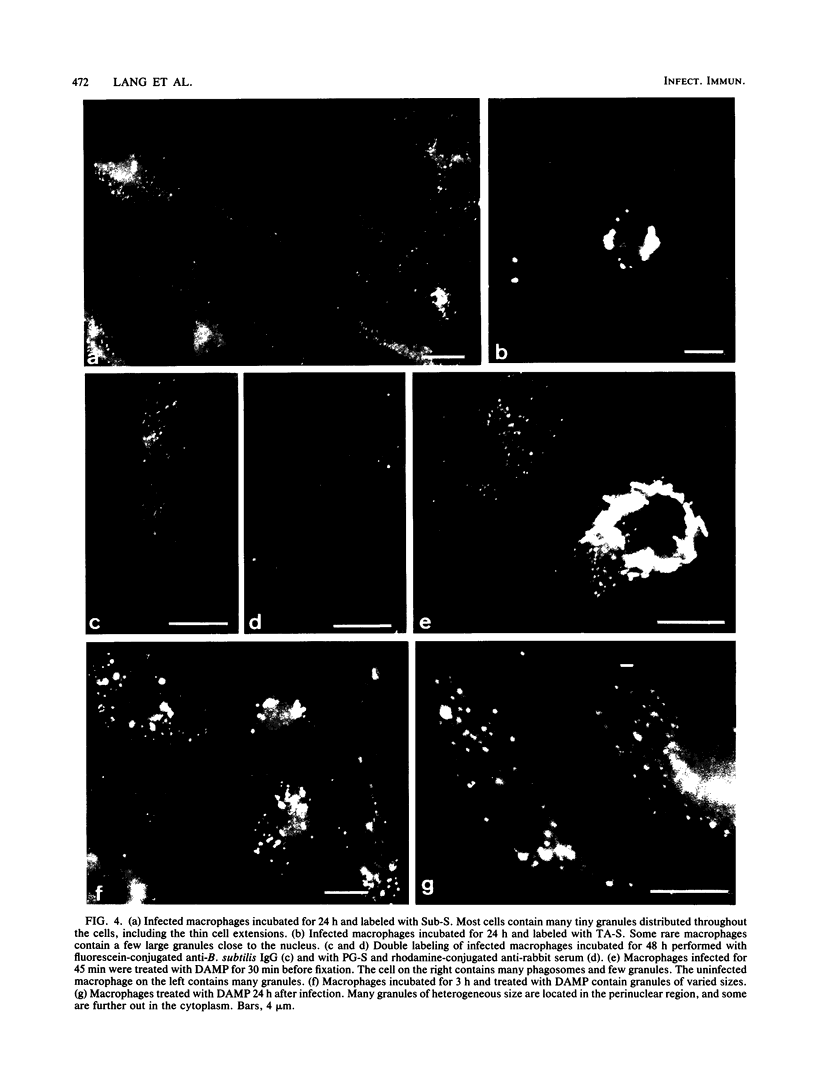
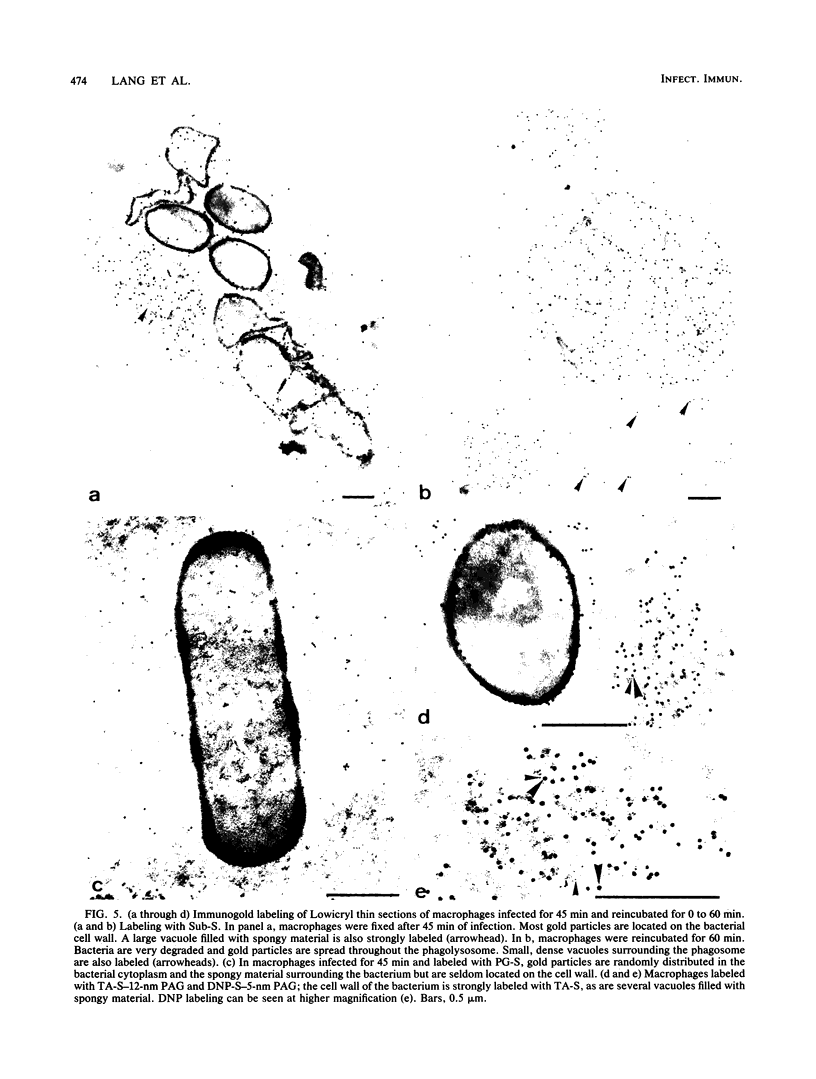
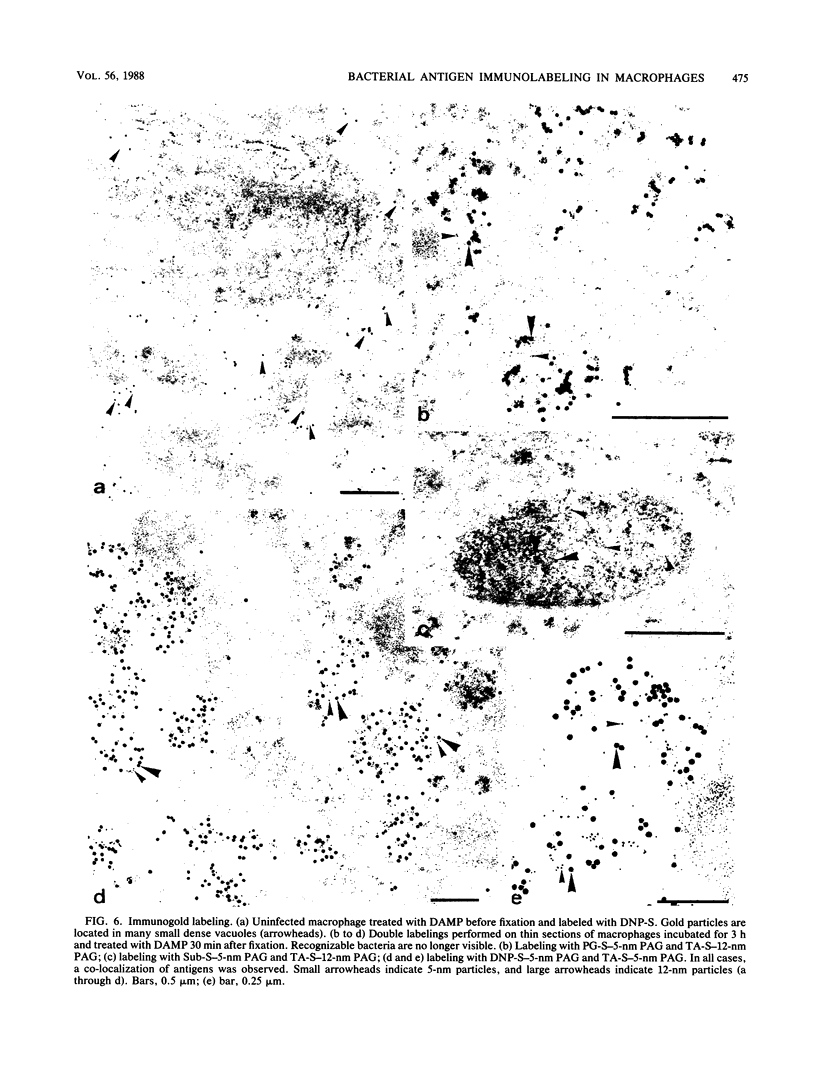
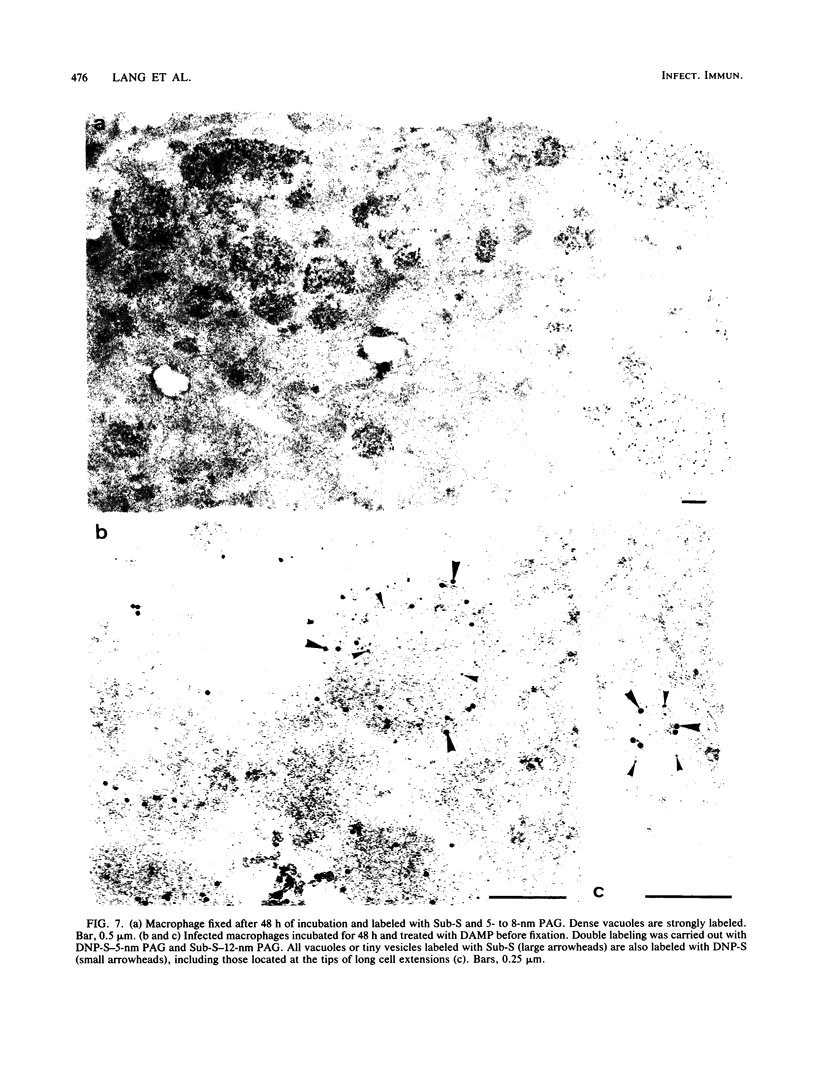
After phagocytosis of Bacillus subtilis 168 by bone marrow-derived macrophages, the intracellular pathway followed by different antigens was studied by immunofluorescence and immunoelectron microscopy. Three different rabbit antisera were used: (i) an antiserum to B. subtilis whole cells mainly recognizing the cell wall constituents, (ii) an antiserum to teichoic acid, and (iii) an antiserum to peptidoglycan recognizing the disaccharide tetrapeptide molecules resulting from peptidoglycan degradation. During the first 3 h after phagocytosis of B. subtilis, the three antisera were confined to the same vacuolar compartments, as follows. They were first found in phagosomes gathered in the perinuclear region. Upon bacterial degradation, the three antisera colocalized in an increasing number of small dense vesicles, located in the perinuclear region, that seemed to result from the fragmentation of phagolysosomes. These vesicles correspond to an acidic compartment since they also stained for 3-(2,4-dinitroanilino)-3'-amino-N-methyldipropylamine, a drug known to accumulate in the acidic compartments of cells. At later time points, the antigens recognized by the three antisera followed different pathways. After 18 h, teichoic acid and peptidoglycan were no longer detectable in macrophages whereas an antigen(s) labeled with antiserum to B. subtilis whole cells remained stocked for several days in small acidic vesicles randomly distributed throughout the macrophage. This compartment appeared to be different from the one labeled during the first 3 h after ingestion of bacteria. These results suggest that the transport rate and the compartments implicated in antigen processing differ according to the antigen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Falck J. R., Goldstein J. L., Brown M. S. Visualization of acidic organelles in intact cells by electron microscopy. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4838–4842. doi: 10.1073/pnas.81.15.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Bahr G. M., Eshhar Z., Ben-Yitzhak R., Modabber F. Z., Arnon R., Sela M., Chedid L. Monoclonal antibodies to the synthetic adjuvant muramyl dipeptide: characterization of the specificity. Mol Immunol. 1983 Jul;20(7):745–752. doi: 10.1016/0161-5890(83)90052-4. [DOI] [PubMed] [Google Scholar]
- Barnard M., Holt S. C. Effects of peptidoglycans from periodontal pathogens on selected biological activities of CD-1 murine peritoneal macrophages. Can J Microbiol. 1985 Feb;31(2):161–172. doi: 10.1139/m85-031. [DOI] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. II. The modification of intracellular degradation. J Exp Med. 1963 Jan 1;117:43–53. doi: 10.1084/jem.117.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. L., Jr, Hoffman J., Tesh V. L., Morrison D. C. Immunologic activity of lipopolysaccharides released from macrophages after the uptake of intact E. coli in vitro. J Immunol. 1986 Apr 15;136(8):2924–2929. [PubMed] [Google Scholar]
- Duncan R. L., Jr, Morrison D. C. The fate of E. coli lipopolysaccharide after the uptake of E. coli by murine macrophages in vitro. J Immunol. 1984 Mar;132(3):1416–1424. [PubMed] [Google Scholar]
- Elsbach P., Pettis P., Beckerdite S., Franson R. Effects of phagocytosis by rabbit granulocytes on macromolecular synthesis and degradation in different species of bacteria. J Bacteriol. 1973 Aug;115(2):490–497. doi: 10.1128/jb.115.2.490-497.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Chesnut R. W., Shimonkevitz R., Marrack P., Kappler J. Mechanisms of antigen processing and presentation. Immunobiology. 1984 Dec;168(3-5):202–212. doi: 10.1016/S0171-2985(84)80111-4. [DOI] [PubMed] [Google Scholar]
- Heimer G. V., Taylor C. E. Improved mountant for immunofluorescence preparations. J Clin Pathol. 1974 Mar;27(3):254–256. doi: 10.1136/jcp.27.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L. C., Johnson M. M., Khera V. R., Barrow W. W. Macrophage uptake and retention of radiolabeled glycopeptidolipid antigens associated with the superficial L1 layer of Mycobacterium intracellulare serovar 20. Infect Immun. 1986 Oct;54(1):133–141. doi: 10.1128/iai.54.1.133-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Huy H., Nauciel C., Wermuth C. G. Immunochemical study of the peptidoglycan of gram-negative bacteria. Eur J Biochem. 1976 Jun 15;66(1):79–84. doi: 10.1111/j.1432-1033.1976.tb10427.x. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Ohtomo T., Ichiman Y., Chomarat M., Yoshida K. Comparison of cell wall teichoic acid fractions isolated from three different encapsulated strains of Staphylococcus epidermidis. Ann Microbiol (Paris) 1984 May-Jun;135A(3):353–365. doi: 10.1016/s0769-2609(84)80077-0. [DOI] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- Ryter A. Relationship between ultrastructure and specific functions of macrophages. Comp Immunol Microbiol Infect Dis. 1985;8(2):119–133. doi: 10.1016/0147-9571(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Shibaev V. N., Duckworth M., Archibald A. R., Baddiley J. The structure of a polymer containing galactosamine from walls of Bacillus subtilis 168. Biochem J. 1973 Oct;135(2):383–384. doi: 10.1042/bj1350383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Tereletsky M. J., Barrow W. W. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983 Sep;41(3):1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilo L. Quantification of endocytosis-derived membrane traffic. Biochim Biophys Acta. 1985 Sep 9;822(2):243–266. doi: 10.1016/0304-4157(85)90010-3. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Vermeulen M. W., Gray G. R. Processing of Bacillus subtilis peptidoglycan by a mouse macrophage cell line. Infect Immun. 1984 Nov;46(2):476–483. doi: 10.1128/iai.46.2.476-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981 Jun;45(2):211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse R. L., Benichou J. C., Couture-Tosi E., Schenkman S., Ryter A. Immunolabelling of bacteriophage lambda receptor protein (LamB) on thin sections of E. coli embedded in Lowicryl. Biol Cell. 1984;51(3):389–394. doi: 10.1111/j.1768-322x.1984.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Williams K. M., Sacci J. B., Anthony R. L. Identification and recovery of Leishmania antigen displayed on the surface membrane of mouse peritoneal macrophages infected in vitro. J Immunol. 1986 Mar 1;136(5):1853–1858. [PubMed] [Google Scholar]
- Ziegler H. K. The processing and presentation of Listeria monocytogenes antigens by macrophages. Clin Invest Med. 1984;7(4):269–272. [PubMed] [Google Scholar]
- de Chastellier C., Lang T., Ryter A., Thilo L. Exchange kinetics and composition of endocytic membranes in terms of plasma membrane constituents: a morphometric study in macrophages. Eur J Cell Biol. 1987 Aug;44(1):112–123. [PubMed] [Google Scholar]