Abstract
Background
Epidemiological surveillance in Uganda has consistently shown declining HIV prevalence particularly among young antenatal women since the early 1990s, correlated with increased uptake of protective sexual behaviour.
Objective
To describe trends in sexual behaviour nationwide and antenatal HIV prevalence from urban sentinel sites in Uganda (1989–2002).
Methods
Review of antenatal HIV seroprevalence data from the sentinel surveillance system (1989–2002) and data on sexual behavioural indicators from the AIDS module of the National Demographic and Health Surveys (1989, 1995 and 2000/01). Trends in biological and behavioural indicators assessed.
Results
Antenatal HIV seroprevalence in seven urban clinics peaked around 1992 (15–30%) followed by a steady decline by 2002 (5–12%), most markedly among women aged 15–19 and 20–24 years. This coincided with increased primary and secondary abstinence among young people nationwide. Median age at sexual debut increased from 16.5 in 1989 to 17.3 in 2000 for women and from 17.6 in 1995 to 18.3 in 2000 for men. Premarital sex among women and multiple partnerships decreased between 1995 and 2000. There were no significant changes in reporting of extramarital sex among men. Ever use of condoms increased from 1% among women in 1989 and 16% among men in 1995 to 16% and 40% in 2000, respectively. Between 1995 and 2000, condom use at last sex with a non‐regular partner increased from 35% to 59% and 20% to 39% among men and women, respectively.
Conclusion
The ecological correlation between the trends in HIV prevalence and incidence and the increase in protective sexual behaviour during the 1990s makes a compelling case for continuing prevention efforts in Uganda.
Keywords: epidemiology, HIV, sexual behaviour, surveillance, trends
Uganda is among the countries in the African region that have experienced a severe generalised HIV infection epidemic for over two decades. At its peak in 1990–92, the HIV prevalence among women attending urban antenatal clinics ranged between 20% and 30%.1 The epidemic appears to have started to wane in the early 1990s with Uganda being the first country in the region to report significant declines in antenatal HIV prevalence.2 From 1992 to 2002, there was a sustained decline in HIV prevalence among antenatal women, patients with sexually transmitted infections, clients seeking voluntary counselling and HIV testing (VCT),3 as well as in the general population of two rural longitudinal cohorts.4,5
Since 1986, Uganda has piloted and implemented various public health measures against the HIV epidemic including epidemiological surveillance, screening of blood for transfusion, public education and promotion of safer sexual behaviour including condom use and control of other sexually transmitted infections. Since HIV infection in Uganda is mainly sexually transmitted, promoting safer sexual behaviour, in particular sexual abstinence, mutual faithfulness in monogamous relationships and condom use with casual sex partners (the ABC approach) has been emphasised. Young people have been especially targeted, recognising that it is easier to establish safer sexual norms among young people before they begin sexual activity. Interventions have included HIV VCT and prevention of perinatal transmission of HIV (prevention of mother‐to‐child transmission (PMTCT)). Control of HIV/AIDS has involved multisectorial approaches involving individuals, community groups, and public sector, non‐governmental, and faith based organisations in mobilisation of communities. Programme monitoring and evaluation has focused on tracking the magnitude and dynamics of the epidemic through biological surveillance of sexual behaviour as recommended in the World Health Organization (WHO)/Joint United Nations Programme on AIDS (UNAIDS) second generation HIV surveillance guidelines.6 Uganda set up epidemiological surveillance systems earlier than many other African countries.
Between 1992 and 2002, antenatal HIV prevalence (the principal means by which the HIV epidemic is monitored in developing countries) dramatically reduced and practice of protective sexual behaviour in the population increased. In this article, we present more evidence of these trends. In addition, we argue that uptake of protective sexual behaviour that occurred countrywide in Uganda reduced the risk of acquiring HIV, and that this underpins the observed decline in prevalence.
Data and methods
HIV seroprevalence data
HIV seroprevalence data were obtained from the national HIV surveillance system, which is based on annual antenatal HIV serological surveys in selected sentinel clinics. This surveillance system was established in Uganda in 1989, and is based on WHO/TGlobal Programme on AIDS guidelines for HIV sero‐surveillance. The system evolved from initially six antenatal clinics located in Kampala and four other towns to 25 sites by 2002. The progressive increase in sentinel sites was designed to increase geographical coverage, including rural areas. We present data from seven sentinel sites that have annual seroprevalence data for at least 10 years (Nsambya and Rubaga Hospitals in Kampala, Jinja Hospital, 80 km east of Kampala, Mbale Hospital in the east near the Kenyan border, Mbarara Hospital in the southwest, St Mary's Hospital, Lacor in the north, near the border with Sudan, and Tororo Hospital).
Antenatal HIV sentinel surveillance involves collection of a minimum of 300–500 blood samples from all pregnant women at their first antenatal visit at the clinic over a 10–12 week period. The blood is primarily drawn for routine antenatal serological screening for syphilis at the clinic using Rapid Plasma Reagin (RPR) card test. Women found to be infected with syphilis are treated using benzathine penicillin. Residual blood samples are then shipped to the Uganda Virus Research Institute Laboratory in Entebbe for HIV serological tests using anonymous unlinked testing procedures after irreversibly delinking personal identifiers. The tests are based on quality controlled serial enzyme immunoassays (EIAs).
Sexual behavioural data
National data on HIV/AIDS knowledge and sexual behaviour were obtained from three Demographic and Health Surveys (DHS) conducted in 1988/89,7 1995,8 and 2000/01.9 These surveys are based on nationally representative samples of about 7000 women, 15–49 years old, and about 2000 men, 15–54 years old, selected through multistage cluster sample designs. Response rates in all surveys were over 75%. In the DHS extensive information is obtained on demographic and health variables through face to face interviews using structured questionnaires.10 The AIDS module collects data on HIV/AIDS sexual behaviour including reported regular and non‐regular sexual partnerships, abstinence, and condom use. The 1989 survey did not include men and had no module on AIDS. However, it had limited information on sexual behaviour.
Other population surveys with subnational data on sexual behaviour were reviewed in order to inform a general understanding of behavioural trends. Unfortunately, the differences in methodologies that they used do not render their data readily comparable with the DHS as most of them lack sufficient documentation of methods and sampling probabilities to adjust for significant oversampling of certain areas such as towns.
Antenatal HIV seroprevalence
Analysis of trends in HIV seroprevalence was done separately for each clinic owing to the variability in prevalence between clinics and was broken down by age group. Data for rural clinics are available only for a few years and are not presented here. Behavioural data analysis of trends was done where data were available over at least two different time points comparing responses to similar questions (standard programme indicators) between surveys according to sex, age and urban/rural residence. Seroprevalence data are presented for urban areas only, but behavioural data are presented for both urban and rural areas. The χ2 test and tests for linear trend were used as appropriate.
Results
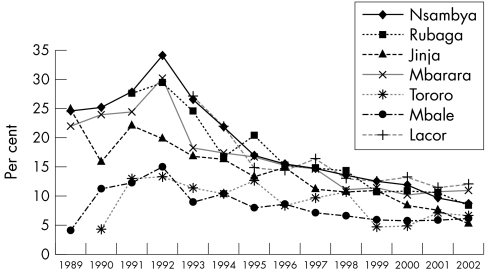
Annual HIV prevalence data for six sentinel sites are presented by age group (table 1) and by site (figure 1). Overall, the prevalence of antenatal HIV infection and the trajectory and magnitude of the decline vary between sites. However, the general trend is that HIV seroprevalence in all clinics peaked in 1991–92, followed by a decline by approximately one half to two thirds between 1992 and 2002. In sentinel clinics located in Kampala (Rubaga and Nsambya hospitals) antenatal HIV seroprevalence peaked at 30% in 1992 then declined to about 8.3% in 2002. In Jinja Hospital, seroprevalence peaked at 22% in 1991 then declined to about 5% by 2002. In Mbarara Hospital, seroprevalence increased from 22% in 1989 to peak at 30% in 1992, followed by a decline and stabilising at about 10% since 2000. In the east, HIV seroprevalence in Mbale and Tororo hospitals was lower than in the other clinics, approximately 15% in 1992, remaining almost stable before declining late in the 1990s to approximately 6%. In St Mary's Hospital, Lacor, Gulu, in the north, an area that has seen prolonged civil strife, antenatal HIV seroprevalence was 27% in 1993 but declined to 12% by 2002.
Table 1 Antenatal HIV sero‐prevalence by clinic and age group: 1990–2002. Values are % (n).
| 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nsambya Hospital | |||||||||||||
| 15–19 years | 20.8 (24) | 28 (82) | 23.3 (43) | U | 16.5 (91) | 9.3 (118) | 10.0 (330) | 10.1 (228) | 8.7 (183) | 7.2 (139) | 9.0 (144) | 8.2 (110) | 4.9 (122) |
| 20–24 years | 33.3 (27) | 35.1 (94) | 31.9 (47) | U | 25 (172) | 18.9 (190) | 17.5 (502) | 13.8 (337) | 12.2 (335) | 9.8 (287) | 12.2 (343) | 6.7 (329) | 9.9 (344) |
| 25–29 years | 60.0 (5) | 19.6 (56) | 34.2 (38) | U | 15.5 (84) | 22.5 (71) | 17.4 (316) | 15.6 (224) | 17.7 (248) | 17.1 (234) | 13.7 (270) | 12.9 (295) | 12.3 (292) |
| 30–34 years | 0 (5) | 26.1 (23) | 5.9 (17) | U | 25.0 (36) | 16.7 (36) | 15.5 (110) | 20.9 (86) | 13.8 (87) | 16.7 (66) | 13.5 (111) | 9.0 (111) | 6.9 (130) |
| 35+ years | 25.0 (4) | 0 (10) | 14.3 (7) | U | 33.3 (12) | 12.5 (16) | 14.3 (35) | 18.5 (27) | 16.7 (30) | 11.8 (34) | 16.7 (48) | 8.3 (36) | 11.8 (34) |
| Rubaga Hospital | |||||||||||||
| 15–19 years | U | U | U | 18.2 (88) | 10.5 (124) | 18.8 (128) | 6.7 (90) | 6.2 (146) | 3.9 (152) | 2.3 (132) | 4.7 (86) | 4.3 (46) | 1.2 (86) |
| 20–24 years | U | U | U | 23.1 (117) | 16.0 (150) | 20.7 (169) | 18.3 (126) | 16.8 (202) | 16.2 (241) | 9.4 (245) | 8.0 (150) | 9.6 (125) | 5.2 (250) |
| 25–29 years | U | U | U | 32.8 (61) | 26.0 (73) | 21.0 (62) | 22.0 (50) | 20.6 (107) | 20.7 (135) | 15.8 (158) | 17.8 (101) | 16 (81) | 8.4 (178) |
| 30–34 years | U | U | U | 25.0 (28) | 23.7 (38) | 17.4 (23) | 7.7 (26) | 20.8 (53) | 18.5 (54) | 10.0 (90) | 12.7 (55) | 13.6 (22) | 11.3 (71) |
| 35+ years | U | U | U | 10.1 (7) | 7.1 (14) | 37.5 (8) | 14.3 (7) | 15.0 (20) | 11.1 (18) | 10.1 (79) | 11.8 (17) | 5.9 (17) | 8.7 (23) |
| Jinja Hospital | |||||||||||||
| 15–19 years | 21.0 105) | U | U | U | 10.7 (112) | 8.5 (118) | 4.5 (66) | 7.5 (53) | 5.4 (74) | 5.1 (59) | 3.1 (64) | U | 3.9 (51) |
| 20–24 years | 16.2 (111) | U | U | U | 17.7 158) | 15.2 132) | 19.7 (66) | 15.6 (45) | 14 (121) | 13.5 (96) | 13.4 (82) | U | 6.2 (81) |
| 25–29 years | 10.1 (79) | U | U | U | 22.3 (94) | 16.7 (84) | 16.7 (42) | 10.3 (29) | 12.1 (58) | 12.1 (58) | 8.3 (48) | U | 7.3 (55) |
| 30–34 years | 7.3 (41) | U | U | U | 12.3 (65) | 16.7 (48) | 20.8 (24) | 16.7 (18) | 18.2 (22) | 10.7 (28) | 9.5 (21) | U | 15.0 (20) |
| 35+ years | 26.3 (19) | U | U | U | 20.0 (15) | 5.9 (17) | 25.0 (12) | 0 (0) | 0 (10) | 10.0 (10) | 9.1 (11) | U | 16.7 (12) |
| Mbarara Hospital | |||||||||||||
| 15–19 years | U | 19.0 (42) | 17.7 (181) | U | 16.9 (82) | 12.3 (96) | 9.1 (44) | 8.8 | 8.3 (60) | U | 1.9 (52) | 11.3 (62) | 6.2 (97) |
| 20–24 years | U | 23.1 (65) | 37.7 (252) | U | 18.9 175) | 16.8 191) | 11.8 (76) | 13.3 | 13.3 135) | U | 11.5 (113) | 6.5 (168) | 6.2 (177) |
| 25–29 years | U | 21.9 (32) | 33.6 122) | U | 15.3 (85) | 15.5 (116) | 17.1 (35) | 17.6 | 8.5 (71) | U | 11.3 (62) | 10.6 (85) | 5.9 (119) |
| 30–34 years | U | 42.9 (14) | 20.3 (59) | U | 15.8 (38) | 27.8 (54) | 10.0 (20) | 30 | 9.5 (21) | U | 17.4 (23) | 21.7 (46) | 10.6 (47) |
| 35+ years | U | 12.5 (8) | 18.2 (22) | U | 16.7 (12) | 18.5 (27) | 11.1 (9) | 0 | 10.1 (12) | U | 0 (8) | 17.4 (23) | 17.6 (17) |
| Mbale Hospital | |||||||||||||
| 15–19 years | 6.6 (61) | U | 11.5 (165) | U | 13.0 (100) | 5.0 (121) | 7.6 (66) | U | 2.8 (71) | U | 1.9 (54) | 1.7 (60) | 5 (159) |
| 20–24 years | 17.6 (51) | U | 11.8 237) | U | 11.6 (164) | 9.5 (147) | 7.1 (99) | U | 5.5 (109) | U | 1.2 (83) | 10.3 (58) | 7.4 (269) |
| 25–29 years | 6.1 (33) | U | 11.7 188) | U | 7.9 (126) | 9.2 (87) | 10.5 (57) | U | 10.1 (69) | U | 15.6 (64) | 4.5 (22) | 6.8 (176) |
| 30–34 years | 23.1 (13) | U | 4.6 (109) | U | 8.8 (80) | 9.3 (54) | 14.3 (49) | U | 6.1 (33) | U | 5.3 (38) | 8.3 (36) | 5.6 (107) |
| 35+ years | 0 (4) | U | 5.9 (34) | U | 6.3 (16) | 4.3 (23) | 0 (11) | U | 8.0 (25) | U | 0 (12) | 0 (17) | 14.3 (63) |
| St Mary's Hospital, Lacor | |||||||||||||
| 15–19 years | U | U | U | 21.5 (284) | 17.2 (587) | 8.7 (483) | 9.6 (281) | 11.5 (828) | 10.2 (1039) | 7.4 (1321) | 7.1 (198) | 6.3 (270) | 5.9 (270) |
| 20–24 years | U | U | U | 31.8 (333) | 24.5 (710) | 17.8 (646) | 15.9 (396) | 17.6 (1000) | 13.0 (1414) | 13.1 (1701) | 14.5 (249) | 10.0 (339) | 10.0 (339) |
| 25–29 years | U | U | U | 28.9 (204) | 25.5 (501) | 20.4 (422) | 14.4 (250) | 20.2 (682) | 16.8 (955) | 15.9 (1164) | 14.0 (171) | 15.4 (201) | 15.4 (201) |
| 30–34 years | U | U | U | 25.7 (109) | 20.9 (258) | 10.2 (197) | 20.4 (422) | 19.2 (308) | 12.5 (432) | 15.2 (566) | 23.9 (88) | 21.1 (152) | 21.1 (152) |
| 35+ years | U | U | U | 15.2 (46) | 15.7 (89) | 10.0 (80) | 11.9 (59) | 11.1 (144) | 6.3 (191) | 9.6 (261) | 7.5 (40) | 9.8 (112) | 9.8 (112) |
| Age missing | U | U | 40.9 (22) | 20.0 (15) | 0.0 (14) | 12.5 (8) | 14.3 (42) | 9.3 (54) | 7.7 (26) | – | – | – | |
U, unavailable.
Figure 1 Prevalence of antenatal HIV infection in seven sentinel clinics (1989–2002).
HIV seroprevalence among young women under 25 years is reflective of recent infection since young women would have been sexually active for a shorter period. Among the 15–19 and 20–24 year olds, there was a consistent decline in HIV seroprevalence in all clinics (table 1). At Nsambya Hospital and St Mary's Hospital, Lacor, for example, prevalence among 15–19 years old declined from 28% in 1991 and 21.5% in 1993, respectively, to 4.9% and 5.9%, respectively, in 2002. Among the 20–24 year olds, it declined from 35.1% in 1991 and 31.8% in 1993 to 9.9% and 10% respectively in 2002.
AIDS related sexual behaviour
Awareness of HIV/AIDS and knowledge of its prevention, although not direct determinants of HIV transmission, are vital for uptake of protective sexual behaviour. National trends in HIV/AIDS knowledge, available from the DHS, are not presented here, however, the general trend is that awareness is almost universal and knowledge of HIV prevention continued to increase throughout this period. Sexual behaviour on the other hand provides the most important means of tracking determinants of HIV transmission, and robust standard indicators have been developed.11 There are limited nationally representative data on sexual behaviour from the 1980s; the data from surveys conducted then were mainly subnational and not directly comparable with DHS.
Youth sexual behaviour
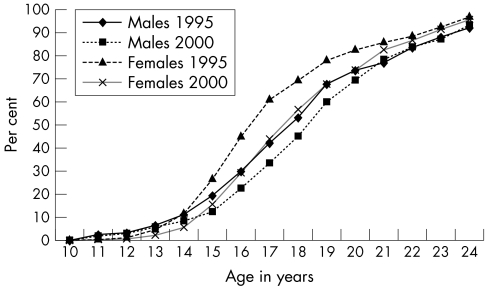
In Uganda, gains have been made in promoting safe youth sexual behaviour as young people appear to be choosing to abstain from sex. The median age at first sex—that is, the age by which half of young people have had penetrative sex among women aged 15–24 years increased from 16.5 years in 1989 to 16.7 years in 1995 and 17.3 years in 2000, and among men from 17.6 years in 1995 to 18.3 years in 2000 (table 2). The proportion of youths aged 15–24 years who had ever had sex at any age, or before age 15, also decreased (fig 2 and table 2).
Table 2 Sexual behaviour indicators from Uganda Demographic and Health Surveys 1989, 1995, and 2000.
| Behavioural characteristic | Women | Men | ||||
|---|---|---|---|---|---|---|
| 1989 | 1995 | 2000 | 1995 | 2000 | ||
| Youth sexual behaviour | ||||||
| Median age at first sex (15–24 year olds)* | ||||||
| Residence | ||||||
| Urban | 16.3 | 17.0 | 17.0 | 17.2 | 17.3 | |
| Rural | 16.5 | 16.7 | 17.3 | 17.6 | 18.4 | |
| All | 16.5 | 16.7 | 17.3 | 17.6 | 18.3 | |
| Youths 15–19 years old who have never had sex | ||||||
| Urban | 34 | 41 | 46 | 42 | 52 | |
| Rural | 38 | 38 | 48 | 54 | 63 | |
| Percent of 15–24 year olds who had sex by age 15 years | ||||||
| 15–19 | 26 | 24 | 14 | 19 | 16 | |
| 20–24 | 31 | 27 | 21 | 20 | 9 | |
| Total | 28 | 26 | 18 | 19 | 13 | |
| Residence | ||||||
| Urban | 26 | 24 | 16 | 28 | 16 | |
| Rural | 29 | 26 | 18 | 18 | 12 | |
| Education | ||||||
| No schooling | 40 | 33 | 30 | 15 | – | |
| Primary | 27 | 27 | 19 | 20 | 13 | |
| Secondary | 11 | 11 | 8 | 19 | 13 | |
| Premarital sex in the past year among single youth | ||||||
| 15–19 | 30 | 17 | 21 | 23 | 22 | |
| 20–24 | 58 | 44 | 55 | 53 | 52 | |
| Residence | ||||||
| Urban | 50 | 27 | 37 | 45 | 47 | |
| Rural | 33 | 21 | 24 | 31 | 27 | |
| Education | ||||||
| No schooling | 28 | 14 | 21 | 22 | – | |
| Primary | 33 | 22 | 22 | 34 | 27 | |
| Secondary | 51 | 25 | 37 | 33 | 39 | |
| Total | 36 | 22 | 27 | 33 | 31 | |
| Median age at first marriage (15–24 years)* | 17.8 | 17.6 | 18.1 | 22.0 | 22.8 | |
| Sexual behaviour of adults | ||||||
| No sex during the past 12 months | ||||||
| Urban | 18 | 31 | 28 | 23 | 27 | |
| Rural | 17 | 22 | 22 | 25 | 26 | |
| Total | 18 | 23 | 23 | 25 | 26 | |
| Had sex with a person other than the spouse in past year | ||||||
| Urban | U | 4 | 3 | 20 | 19 | |
| Rural | U | 2 | 3 | 14 | 11 | |
| Total | U | 3 | 3 | 14 | 12 | |
| One or more non‐marital, non‐cohabiting partner | ||||||
| Urban | U | 22 | 25 | 44 | 49 | |
| Rural | U | 12 | 12 | 26 | 24 | |
| Total | U | 12 | 14 | 29 | 28 | |
| Two or more non‐marital, non‐cohabiting partners | ||||||
| Urban | U | 3 | 2 | 13 | 16 | |
| Rural | U | 2 | 1 | 8 | 6 | |
| Total | U | 2 | 1 | 9 | 8 | |
| Two or more partners in past year among sexually active single respondents | ||||||
| Urban | U | 9 | 7 | 28 | 35 | |
| Rural | U | 11 | 5 | 29 | 29 | |
| Total | U | 11 | 6 | 29 | 31 | |
| Knows where to obtain condoms | ||||||
| Aware of condoms | ||||||
| Urban | 48 | 59 | 85 | 51 | 96 | |
| Rural | 18 | 28 | 47 | 45 | 73 | |
| Total | 22 | 32 | 53 | 46 | 77 | |
| Has ever used a condom | ||||||
| Urban | 4 | 24 | 40 | 41 | 74 | |
| Rural | 1 | 3 | 11 | 12 | 34 | |
| Total | 1 | 6 | 16 | 16 | 40 | |
| Used condoms during last sex with non‐marital, non‐cohabiting partner | ||||||
| Urban | U | 46 | 60 | 62 | 80 | |
| Rural | U | 11 | 31 | 28 | 50 | |
| Total | U | 20 | 39 | 35 | 59 | |
*Age by which one half of youths aged 15–24 years at the time of the survey have had sex or were married.
U, data not available.
Figure 2 Proportion of sexually active men and women (15–24 years): 1995 and 2000.
There was also an overall decline in prevalence of premarital sex, the greatest decline being between 1989 and 1995. Since 1995, premarital sex may have increased slightly among older women 20–24 years, those in urban residence and those educated beyond primary level. Premarital sex is more common among urban than rural residents, women than men, older youth (20–24 years) than teenagers (15–19 years), and single young men and women with educational attainment beyond primary level. Between 1995 and 2000, there was little change overall in the proportion of single young men 15–24 years old who reported having sex during the past year (approximately 31% in both surveys). Because the age of sexual debut increased throughout the decade, the small increases in premarital sex in the late 1990s could reflect an increase in sexual activity among youth who had already initiated sex.
Men and women who become sexually active before marriage expose themselves to risk of HIV infection through multiple casual partnerships. On average, men marry more than four years later than women of the same age and appear to be delaying marriage even further. Between 1995 and 2000, the median age at first marriage among men increased from 22 to 22.8 years, whereas in women there was a modest increase from 17.8 to 18.1 years.
Sexual behaviour of all adults
Primary and secondary abstinence also increased in this period. Of all women surveyed, irrespective of their virginity status, the proportion that reported no sex in the past year increased from 18% in 1989 to 23% in 1995 and 2000. Among non‐virgins, secondary abstinence increased from 8% of women in 1989 to 15% in 1995 and 13% in 2000. The greatest changes in abstinence were seen among urban women, although both groups saw increases in abstinence from 1989 to 1995. About 25% of men reported abstinence during the past year in both surveys.
The pillar of HIV prevention is sexual abstinence until marriage and thereafter mutual faithfulness to one partner. Men are more likely than women to have extramarital sex (12% v 3% in DHS 2000), and, with the exception of some rural areas, the proportion of men reporting extramarital sex has not declined substantially since 1995.
Non‐marital, non‐cohabiting sexual partnerships (non‐regular or casual sex) include all sex among non‐married respondents and extramarital sex, all considered high risk. Whereas there was little change in women and men reporting non‐regular partnerships, there are some urban–rural differences, with a small increase in the proportion of urban men and women having one or more non‐regular partners. With regard to multiple sexual partnerships (that is, two or more sexual partners), fewer women in 1995 and 2000 than men reported multiple partnerships and there may have been a small increase among urban men. Among single, sexually active women, a decline in multiple partnerships has occurred, particularly in rural areas. For single, sexually active men, multiple sexual partners increased slightly.
Condom use
There have been gains made from the promotion of condom use in Uganda since the early 1990s albeit with rural–urban disparities. Knowledge of a source of condoms increased among men and women during the 1990s with almost 50% of women able to name a place from where they could obtain a condom in 2000. The use of condoms also increased from 1% of women in 1989 and 16% of men in 1995 to 16% of women and 40% of men in 2000. Women's reporting of use of a condom at last sex with a casual partner almost doubled to 39% between 1995 and 2000. The increases were greatest among rural women although the level of condom use is still about half that of urban women. Among men, 35% in 1995 and 59% in 2000 reported having used a condom at this last higher risk sex.
Index of sexual behaviour
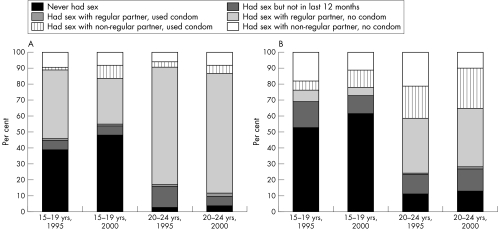
The UNAIDS sexual behaviour index enables simultaneous interpretation of changes in sexual behaviour indicators since the indicators are interrelated and changes in one concomitantly affects the denominator in others. Respondents are categorised according to the sexual behaviour they engaged in during the previous year, from least to most risky. Figure 3 shows the data for national level estimates by age group.
Figure 3 (A) Sexual behaviour of (A) women and (B) men aged 15–19 years and 20–24 years, 1995 v 2000.
Among women aged 15–19 years, the most common behaviour was sex within marriage (43%) and primary abstinence (39%). With increasing age at marriage for women, sex within marriage declined to 29%, replaced mostly by increase in primary and secondary abstinence (48%). There was a slight increase in the reporting of sex outside marriage (either premarital or extramarital); within this group condom use at last sex increased. Among men aged 15–19 years, the most common behaviour in 1995 was primary abstinence (53%), followed by extramarital sex (24%), and of these, most did not use a condom at last sex. By 2000, the proportion of 15–19 year olds practising secondary abstinence grew smaller, replaced mostly by an increase in primary abstinence (61%). The proportion reporting extramarital sex changed little, however, among them, condom use at last sex with a non‐marital partner increased, and extramarital sex without a condom declined.
Older youth (20–24 years) are more likely to be married and the commonest behaviour among them was sex within marriage, and that was commoner among women although the gender gap is narrowing. In 1995, three quarters of women and a third of men in this age group reported that during the previous year, they had sex only with a marital or cohabiting partner. By 2000, the proportion of women with this behaviour changed little, whereas that among men increased to 38%. In most of these relationships, a condom was not used at last sex. Secondary abstinence rates among 20–24 year old women declined from 13% to 6%, replaced by an increase in proportion of women who had sex, distributed evenly among those who had sex within marriage and those who did so outside a marital relationship. The shifts in behaviour among young men involved increased use of condoms during sex with non‐marital partners and subtle increases in primary and secondary abstinence, and about 50% decline in sex without condoms outside marriage.
Discussion
In this article we have presented further evidence of consistent declines in antenatal HIV prevalence among young women attending antenatal clinics in urban areas of Uganda and increased protective sexual behaviour during the 1990s. Previous reports of similar findings covered shorter periods and smaller geographical areas.2,12 Although estimates of HIV incidence would more accurately describe the dynamics of HIV infection, they cannot be directly obtained from a surveillance system. For that matter, estimates of incidence are based on proxy measures such as incidence of HIV among young women recently exposed to sex, which is reported in this article. Increased protective behaviour includes sexual abstinence, reduction in premarital sex, and condom use during risky sex. Preliminary findings of the Uganda HIV serological and sexual behaviour survey appear to indicate that behaviour change trends have continued well into the early part of this decade.13
AIDS mortality and subfertility among HIV infected women could also reduce the prevalence of HIV. Mortality data are not readily available, however, most people in Uganda knew someone who had HIV or who had died of AIDS. Ironically, this can also influence behaviour change and, at the same time, decrease prevalence even in the absence of decline in HIV incidence.14 AIDS mortality can also generate a downward trend in risk behaviour because those people who are at a higher risk are more likely to die, although this is unlikely to have much impact on 15–24 year olds. Reduced fertility among HIV infected women would also selectively bias antenatal based estimates.15 Nonetheless, the magnitude of HIV induced subfertility and AIDS mortality is unlikely to be sufficient to account fully for the magnitude of the observed declines in prevalence. Moreover, such distortions would be least felt among younger women with recent infection.
HIV seroprevalence data from other sentinel clinics, particularly in rural areas have a similar pattern, although trends are less pronounced and data are available for a shorter period. The trends in HIV prevalence from the surveillance system are consistent with trends from elsewhere in Uganda. For instance, HIV prevalence among patients with sexually transmitted diseases attending a Kampala clinic declined to 19% in 2002 from 44.2% in 1989.1 Data from VCT programmes (although it is liable to self‐selection bias, the magnitude of which can change over time) also show a decline in HIV prevalence among first‐time testers aged 15–24 years, particularly after 1992. Among 202 741 asymptomatic clients attending over a nine year period (1992–2000), adjusted HIV prevalence declined from 23% in 1992 to 13% in 2000 (17% to 9% among men and 31% to 18% among women).3 Recent data from the Masaka population cohort show significant declines in HIV prevalence among young adults and in HIV incidence from 8.0/1000 person years in 1990 to 5.2/1000 person years in 2000.5 In the first Rakai cohort 1990–92, overall HIV seroprevalence decreased slightly from 23% in 1990 to 21% in 1992, particularly among young adults aged 15–24 years and among women of reproductive age.4
It is unlikely that errors in ascertainment of HIV sero‐status or sampling techniques affected the observed trends. Firstly, all tests are conducted centrally at the National HIV Reference Laboratory which participates in the WHO external quality control scheme. The serial testing algorithm based on EIAs used did not change throughout this period. Secondly, sampling of mothers throughout this period has not altered and is unlikely to have been affected by selection bias introduced by VCT and PMTCT programmes that were not widespread during this period. In Uganda, over 90% of pregnant women attend antenatal clinics and this was not affected by the health sector reforms that took place during this period.
Limitations
The limitations of the surveillance system include missing data, limited demographic and no behavioural data, and inadequate precision in estimates owing to small denominators. In addition, data on syphilis prevalence are not available. For these reasons, it is not possible to adjust for characteristics of women attending the clinics over time. There is anecdotal evidence of a slight increase in median age of mothers attending antenatal care during this time with increase in age of sexual debut. However, the median age at first marriage for women increased little. At a wider level, there was no massive population movement that could potentially affect the base of mothers attending antenatal clinics during this period except in the north, which has the highest prevalence of HIV.
There are inherent limitations in interpreting behaviour change based on the standard indicators. Firstly, the indicators are interdependent, with shifts in one affecting the others. For instance, condom use indicators are based on respondents who reported sex in a reference period. As casual partnerships decline or abstinence increases, this could result in increase in condom use indicators, even if the number of people reporting condom use has not changed.16 Secondly, some indicators assess behaviours by proxy. For instance, the proportion of sexually active people who used a condom during their last sex with non‐regular partners is used as a reasonable proxy for consistent condom use, yet it does not directly measure consistent condom use. It is only sensitive to changes in consistent condom use; as condom use becomes more consistent, the likelihood that respondents would have used it at their last risky sex increases. The indicator captures changes in both occasional and consistent use. Thirdly, the indicators are based on reported data that are subject to reporting bias, the magnitude of which can change over time.
Another limitation is that detailed exploration of demographic characteristics of the sentinel surveillance sample was not possible due to limited sociodemographic data. It should be noted that seroprevalence data were not linked to behavioural data, and therefore, only ecological‐level analysis was possible and association between HIV prevalence and behavioural covariates could not be ascertained in a more objective manner. Finally, antenatal HIV surveillance does not provide direct HIV prevalence estimates for men. The extent to which antenatal surveillance estimates population level prevalence is a subject of debate.17,18 However, in Uganda, the recent results of the national HIV seroprevalence survey show that the estimates from the two sources are similar.13
Conclusion
The unprecedented decline in antenatal HIV prevalence in Uganda could be due to a variety of factors but the most plausible is uptake of protective sexual behaviour, which reduced acquisition of HIV infection. While it is not possible to quantify the contribution of different behaviours or to attribute the changes conclusively to specific interventions, it is nevertheless clear where and what changes have occurred. Other behaviours, such as reduction in intergenerational sex or widow inheritance, that were not explored could have contributed and are worthy of further study. Nonetheless, these encouraging findings make a compelling case for prevention programmes in Uganda to continue with multipronged efforts.
Acknowledgements
The authors are grateful to the Uganda Ministry of Health for permission to publish this article and to the STD/AIDS Control Programme in the Ministry of Health for providing the surveillance data. The behavioural data were facilitated by the Uganda Bureau of Statistics and ORC Macro.
Authors' contributions
W Kirungi took the lead role in writing the article, collating all pieces together and participated in analysis of the data. J Musinguzi, E Madraa, and N Mulumba participated in the HIV surveillance activity in Uganda throughout this period. T Calleja and P Ghys initiated the idea, provided technical guidelines and reviewed the article at various stages providing insightful comments. R Bessinger was responsible for most of the trend analysis of behavioural data.
Abbreviations
AIDS - acquired immune deficiency syndrome
DHS - Demographic and Health Surveys
EIA - enzyme immunoassay
HIV - human immunodeficiency virus
PMTCT - prevention of mother‐to‐child transmission
VCT - voluntary counselling and testing
Footnotes
Competing interests: none declared
References
- 1.Ministry of Health, STD/AIDS Control Programme HIV/AIDS Surveillance Report. Kampala, Uganda: Ministry of Health, 2003
- 2.Asiimwe‐Okiror G, Opio A, Musinguzi J.et al Change in sexual behaviour and decline in HIV infection among young pregnant women in urban Uganda. AIDS 1997111757–1763. [DOI] [PubMed] [Google Scholar]
- 3.Baryarama F, Bunnell R, Ransom R.et al Using HIV voluntary counseling and testing data for monitoring the Uganda HIV epidemic. J Acquir Immune Defic Syndr 2004371180–1186. [DOI] [PubMed] [Google Scholar]
- 4.Wawer M J, Sewankambo N, Berkley S.et al Incidence of HIV‐1 infection in a rural region of Uganda. BMJ 1994308171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mbulaiteye S M, Mahe C, Whitworth J A G.et al Declining HIV‐1 incidence and associated prevalence over 10 years in a rural population in south‐west Uganda: a cohort study. Lancet 200236041–46. [DOI] [PubMed] [Google Scholar]
- 6.UNAIDS/WHO Guidelines for Second Generation HIV surveillance. Geneva: UNAIDS/WHO, 2000
- 7.Ministry of Health and IRD/Macro Systems, Inc The Uganda Demographic and Health Surveys 1988–89. Kampala, Uganda 1989
- 8.Statistics Department, Ministry of Finance, Planning and Economic Development and Macro International Inc The Uganda Demographic and Health Survey 1995. Kampala, Uganda 1996
- 9.Uganda Bureau of Statistics and ORC Macro The Uganda Demographic and Health Survey 2000‐02, Kampala, Uganda 2001
- 10. HIV/AIDS Survey Indicators Database at www.measuredhs.com/hivdata/start , September 2005
- 11.UNAIDS National AIDS Programmes; A Guide to Monitoring and Evaluation. Geneva: UNAIDS, 2006
- 12.Kilian A, Gregson S, Ndayanabangi B.et al Reductions in risk behaviour provide the most consistent explanation for declining HIV‐1 prevalence in Uganda. AIDS 199913391–398. [DOI] [PubMed] [Google Scholar]
- 13. Ministry of Health and ORC Macro Intrernational: Uganda HIV Sero‐behavioural survey 2004–05: draft preliminary report. Kampala, Uganda, 2005
- 14.Wawer M, Serwadda D, Gray R.et al Trends in HIV‐1 prevalence may not reflect trends in incidence in mature epidemics: data from the Rakai population‐based cohort, Uganda. AIDS 1997111023–1030. [DOI] [PubMed] [Google Scholar]
- 15.Gray R H, Wawer M J, Serwadda D.et al Population based study of fertility in women with HIV‐1 infection in Uganda. Lancet 199835198–103. [DOI] [PubMed] [Google Scholar]
- 16.Cleland J, Boerma T, Careal M.et al Monitoring sexual behaviour in general populations: a synthesis of lessons from the past decade. Sex Transm Infect 200480(suppl)ii1–ii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glynn J R, Buve A, Carael M.et al Factors influencing the difference in HIV prevalence between antenatal clinics and general population in Sub Saharan Africa. AIDS 2001151717–1725. [DOI] [PubMed] [Google Scholar]
- 18.Zaba B, Boerma T, White R. Monitoring the AIDS epidemic using HIV prevalence data among young women attending antenatal clinics: prospects and problems. AIDS 2000141633–1645. [DOI] [PubMed] [Google Scholar]