Abstract
Eradication of HCV infection requires a complex and coordinated interplay between innate and adaptive immune responses that, when it fails, leads to chronic infection. Increasing evidence suggest that defects in innate immune recognition and in innate immunity-induced activation of adaptive immune responses play a critical role in failure of HCV clearance. The evolutionarily preserved receptors of viral recognition in immune cells and in hepatocytes sense invading pathogens that results in induction of Type I IFNs, the central players in antiviral immunity. In this review the innate immune mechanisms by which HCV is sensed and by which HCV undermines host defense are discussed. The critical role of dendritic cells in antigen presentation/T cell activation and IFNα-production as well as interference of HCV with innate immune cell functions are reviewed. Finally, current and emerging therapeutic approaches targeting innate immune pathways will be evaluated.
Keywords: Toll-like receptors, RNA Helicases, Dendritic cell, Macrophages, NK cell, Inflammation
Innate immunity – the first line of defense in HCV infection
Innate immunity is the first line of defense in the host response to invading viral, bacterial, or fungal pathogens and hepatitis C, a single stranded RNA virus, is no exception (1,2). Cells participating in the innate immune response include monocytes, macrophages, dendritic cells, leukocytes, natural killer cells (NK) and NKT cells which are all equipped with pathogen sensing receptors and are present in the liver (Fig 1) (1). Monocytes, macrophages and leukocytes are effectors and regulators of inflammation owing to their capacity to take up pathogens, produce reactive oxygen radicals and pro- and anti-inflammatory cytokines. Dendritic cells are sophisticated in antigen presentation and induction of T cell activation through their expression of co-stimulatory molecules and cytokine production while NK cells provide interaction with virus infected cells, T lymphocytes, and DCs (1,3). Recognition of viral pathogens by a coordinated interaction of the cells of the innate immune system will lead to activation of adaptive immunity targeting viral-specific antigens for pathogen elimination. Of the various pattern recognition receptors, Toll-like receptors and RNA helicase receptors play an important role in sensing viral RNA and in induction of the initial Type I IFN production. Double-stranded (ds) RNA is recognized by both TLR3 expressed in the endosomes and RIG-I and MDA5 localized in the cytosol while single stranded (ss) RNA is sensed by TLR7 and TLR8, and by RIG-I in some viruses (Fig 2 and Fig 3) (3–5).
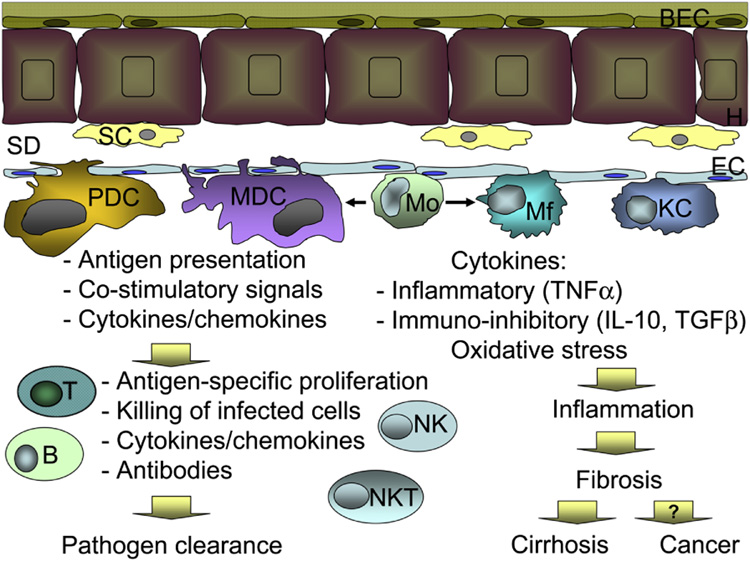
Figure 1. Schematic representation of the immune cells in the liver.
Hepatocytes (H) are lined with biliary endothelial cells (BEC) on the portal facet and stellate cells (SC) in the space of Disse (SD). Endothelial cells (EC) separate the space of Disse from the blood flow. Dendritic cells of plasmacytoid (PDC) and myeloid (MCD) origin, monocytes (Mo), macrophages (Mf) and Kupffer cells (KC) are located in close proximity to endothelial cells. Blood monocytes are immature and can give rise to macrophages or myeloid dendritic cells, depending on the environment. Upon encountering pathogens, derived from either blood stream or infected hepatocytes, MDC and PDCs are primarily responsible for antigen presentation to adaptive immune cells and creation of favorable milieu for antigen presentation, including production of cytokines and availability of co-stimulatory molecules. In turn, adaptive immune cells, including T and B-lymphocytes, natural killer (NK) and NKT cells react with antigen-specific proliferation, cytotoxicity, and production of soluble mediators, such as antibodies or cytokines. Collectively, these events lead to pathogen elimination. Monocytes, macrophages and Kupffer cells also recognize pathogens, however in contrast to dendritic cells, they have a less pronounced effect on the adaptive immunity. Mo, Mf and KCs are potent producers of inflammatory and immunoregulatory cytokines and are powerful sources of free radical with oxidative capacity, thus lead to initiation and maintenance of tissue inflammation. Chronic inflammation, in addition to direct pathogen recognition, activates stellate cells, which in turn produce collagen and favor development of liver fibrosis. At the end stage liver disease, chronic inflammation and fibrosis drive progression to cirrhosis and possibly favor neoplastic transformation and liver cancer.
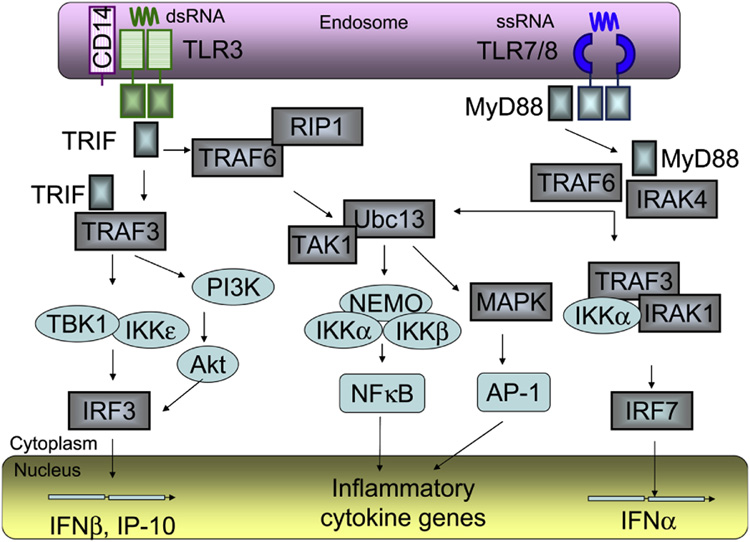
Figure 2. TLR3 and TLR7/8 signaling.
Following stimulation with single stranded (ss) RNA, TLR7/8 recruit MyD88, IRAKs and TRAF6 to activate 2 distinct pathways. When MyD88, IRAKs and TRAF6 recruit Ubc13/TAK, the TAK1 complex then activates the IKK complex, composed of IKKα, IKKβ and IKKγ/NEMO, which catalyzes phosphorylation of IκB proteins and subsequent translocation of NFκB to the nucleus. TAK1 also activates the MAPK pathway, which mediates AP-1 activation. NFκB and AP-1 control the expression of genes encoding inflammatory cytokines. When MyD88, IRAKs and TRAF6 recruit TRAF3, IRAK1 and IKKε into a complex, IRF7 is directly phosphorylated by IRAK1 IKKε , and then translocated to the nucleus to induce expression of IFNα and IFN-inducible genes. Upon interaction with double stranded (ds) RNA, TLR3 recruited TRIF and interacts with TBK1 and IKKε, or activates the PI3K/Akt complex, which both mediate phosphorylation of IRF3. The phosphorylated IRF3 dimerizes and is translocated to the nucleus to induce expression of IFNβ and IFN-inducible genes, including IP-10. TRIF also interacts with TRAF6 and RIP1, which mediate NFκB activation. TLR3 and TLR7/8 are located in endosomes, all signaling events occur in the cytoplasm, while activated transcription factors IRF3, IRF7, NFκB and AP-1 act on the genes within the nucleus. The cellular compartments are not scaled.
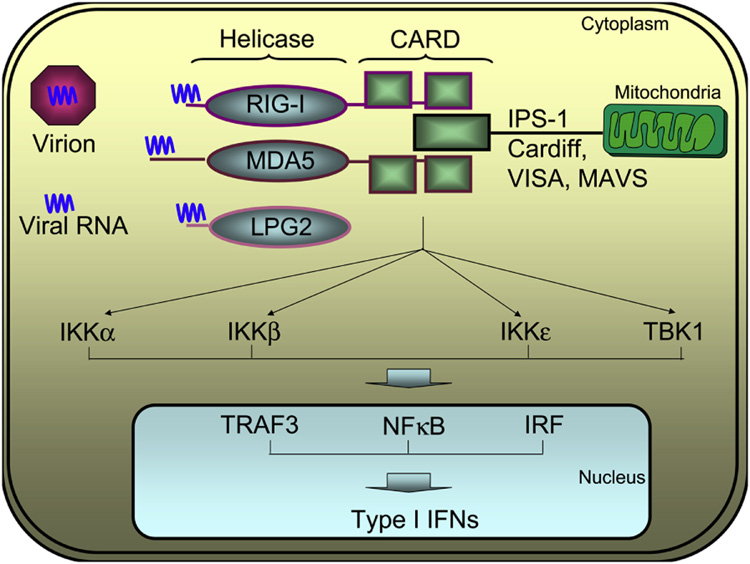
Figure 3. Intracytoplasmic viral recognition and antiviral pathways.
The RNA derived from virions is recognized at the helicase domain of the RIG-I, MDA5 or LPG2 helicases. Upon ligand interaction, the tandem caspase activation and recruitment (CARD) domains of RIG-I and MDA5 engage the CARD domain of mitochondria-bound IPS-1 and trigger activation of a series of kinases including IKKα, IKKβ, IKKε and TBK1. These signaling events activate TRAF3, NFκB and IRF, leading to their translocation to the nucleus and initiation of the type 1 IFN production. LPG2 is CARD-less and does not trigger the signaling events, however it modulates the activity of RIG-I by blocking RIG-I self-association, owed to disrupting homotypic CARD/helicase domain and/or C terminus interactions, by disrupting assembly of the RIG-I-containing signaling complex on IPS-1 and by sequestering the viral dsRNA.
Toll-like receptor 3
Ligand-engagement of TLR3 results in recruitment of the adapter molecule, TRIF (TLR domain-containing adapter inducing IFNβ) (Fig 2) (6,7). TRIF interacts with a number of signaling molecules such as TRAF6, which in turn activates nuclear regulatory factor κB (NFκB) and mitogen-activated protein kinases (MAPK). Important to viral infection, TLR3 stimulation leads to activation of TBK/IKKe that phosphorylate Interferon regulatory factor 3 (IRF3) allowing its dimerization and nuclear translocation. Activated IRF3 binds to the promoter of Type I IFNs and triggers antiviral innate immune activation (6,7).
RNA helicases
Recently, three homologous DExD/H box RNA helicases emerged as cytoplasmic sensors of virally derived RNA (Fig 3). Upon activation with viral dsRNA, two helicases, retinoic-acid-inducible gene I (RIG-I; also called DDX58) and melanoma-differentiation-associated gene 5 (MDA5; also called Helicard), cooperate in induction of antiviral type I IFN (5,8). The third helicase, LGP2, prevents viral-induced activation, most likely through sequestration of dsRNA from RIG-I or MDA5 (8–10). Although RIG-I and MDA5 seem to share structural and functional similarities, their recognition capacity of viral-derived RNA is distinct. RIG-I, but not MDA5, senses Japanese encephalitis virus, Newcastle disease virus, VSV, Sendai virus and influenza virus, while MDA5 mounts antiviral responses against the picornavirus encephalomyocarditis virus (11). To date it is unknown how RIG-I undergoes activation only with viral RNA and not with cellular RNA. The direct implication of either RIG-I or MDA5 in recognition of ssRNA hepatotropic viruses, such as HCV, has been suggested (12). Of interest, RIG-I-deficiency strongly affects antiviral responses in conventional dendritic cells and fibroblasts, however no differences are observed in plasmacytoid dendritic cells, suggesting tissue preference of these helicases. RIG-I, MDA5 as well as LGP2 are all expressed in hepatocytes (13,14). Interestingly, decreased expression levels of RIG-I and MDA5 were detected in Huh7 and huh7.5 cells that are permissive to HCV replicons and in vitro infection with the replication efficient JFH1 and JFH/J6 HCV strains (15).
RIG-I and MDA-5 share structural similarity in the caspase recruitment domains (CARDs) and RNA helicase domains, while LGP2 lacks the CARD domain (10,11). RIG-I and MDA5 use their CARDs to signal downstream events via an adaptor named CARD adaptor inducing IFN-beta (Cardif), MAVS, IPS-1 or VISA (16–19). IPS-1 is anchored with its C terminus to the outer mitochondrial membrane, while its N-terminal CARD domain interacts with both RIG-I and MDA5. Mitochondrial localization renders IPS-1 functional, since cytoplasmic of endoplasmic reticulum-bound IPS-1 no longer mediates downstream IRF and NFκB activation (18). The details of the signaling pathway downstream of IPS-1 are currently under scrutiny. Once activated by dsRNA, the IPS-1 most probably recruits appropriate signaling intermediates, such as IKKs (namely IKKα, IKKβ, IKKε and TBK1) to activate NFκB, TRAF3 and IRF transcription factors (20). All these pathways induce production of type 1 IFNs, however the kinetics of the differential production of IFNα and IFNβ upon RLH activation is yet to be defined.
It has recently been demonstrated that RIG-I but not MDA5 efficiently binds to secondary structured HCV RNA to confer induction of IFNβ expression (21). LGP2 is a functional negative regulator of host defense and it binds HCV (21). In resting cells, RIG-I is maintained as a monomer in an auto-inhibited state but during virus infection and RNA binding it undergoes conformational changes that promote self-association and CARD interactions with the IPS-1 adaptor protein to signal IRF3 and NFκB-responsive genes (9,10,21). This interaction is regulated by an internal repressor domain, which controls RIG-I multimerization and recruitment of IPS-1. An analogous regulatory domain in LGP2 interacts with RIG-I to ablate self-association and signaling. Thus, RIG-I is a cytoplasmic sensor of HCV and it is governed by regulatory domain interactions that area shared with LGP2 as an on/off switch controlling innate defenses (21).
Toll-like receptors 7 and 8
TLR7 and TLR8 are expressed in the endosome and recognize a number of ssRNA viruses (Fig 2) (22). TLR7 was initially identified as a receptor able to recognize imidazoquinoline derivatives with antiviral activity (23). Subsequently, guanosine or uridine rich ssRNA derived from HIV-1 and influenza virus, synthetic poly U RNA and certain small interfering RNAs were identified as ligands for TLR7 (23,24). TLR7 is expressed in plasmacytoid DCs and TLR7 mRNA was detected in hepatocytes (25–28). TLR8 is functional in humans but not in mice and it is expressed in myeloid DCs, monocytes, macrophages and regulatory T cells (29,30). Human TLR8 mediates recognition of HIV-derived ssRNA and chemical ligand R848 and its role in HCV infection is currently unknown (31). Recent studies revealed functional differences between human TLR7 and TLR8, where TLR7 agonists primarily activated PDCs while TLR8 agonists activated MDC, monocytes and macrophages (32). In addition, the cytokine production profile of TLR7 was dominated by IFNα induction, while TLR8 triggered predominantly the pro-inflammatory cytokines and chemokines, such as TNFα, IL-12 and MIP-1α (32). TLR7/8 agonists impair monocyte-derived dendritic cell differentiation and maturation; it is intriguing that the phenotype of TLR7/8 ligand-treated DCs is similar to DC defects found in HCV infected patients (33,34).
The pattern recognition site for the leucine rich repeats of TLR7/8 molecule is contained within the endosome, while the Toll/Interleukin 1 receptor (TIR) domain is exposed to the cytoplasm, where it transduces intracellular signals by recruitment of the Myeloid differentiation primary response gene 88 (MyD88), a common TLR adaptor protein (3,6). MyD88 further forms complexes with members of the IRAK family (IRAK1 and IRAK4) and TRAF6, which in turn activate TAK1 and result in NFκB activation. Type I IFN induction after TLR7 activation is independent of IRF3, suggesting the possible involvement of other IRF family members in this pathway. IRF7 is structurally similar to IRF3 and while its expression is low in most cell types, it is constitutively expressed in PDCs (35,36). IRF7 is able to form a signaling complex with MyD88, IRAK1, IRAK4 and TRAF6, where IRAK1 is capable of phosphorylating IRF7 (37). Activated IRF7 homodimerizes allowing this complex to translocate into the nucleus and bind to the Interferon Stimulated Response Element, promoter site (ISRE) (38). Type I interferons, composed of IFNα and IFNβ, are released in response to TLR7 or TLR8 signaling.
Immune response and the outcome of HCV infection
Studies relating to innate immune response changes during viral clearance are limited. However, it is clear that the interaction between HCV viral components and the immune system ultimately determines whether the balance tilts in the favor of the virus, leading to chronic infection, or the host, conditioning viral clearance. Evidence points to both innate and adaptive immune responses as key determinants of the outcome. A strong, multi-specific T lymphocyte response, including both CD4+ and CD8+ cells, produced early in infection is associated with viral clearance and disease resolution, whereas a narrowly focused and delayed response is associated with chronic infection (39–43). However even in patients who develop chronic infection, T cell responses were briefly or episodically vigorous rather than absent at the onset of infection, indicating that substantive CD4+ T cell response must also be maintained in order to facilitate clearance (44). Further, CD8+ T cells lose recognition of one or more HCV epitopes originally targeted in primary infection in patients who progressed to chronic infection, thus leading to almost 3/4 reduction in the magnitude of the response (45). Importantly, no new epitope-specificity is developed in T cells of patients with persistent HCV infection (45,46.) These results indicate that adaptive responses cannot overcome HCV aggressiveness alone and suggest that the adaptive immune system fails to receive support from innate immune cells in order to provide a long-lasting defense. The information in regards to the function of innate immunity during early HCV infection is scarce. In one study, resolution of acute HCV infection was associated with a single nucleotide polymorphism in the haplotype region of IL-10 and IL-19/IL-20 genes in African Americans but not in European Americans (47).
HCV interferes with innate immune recognition
Increasing evidence suggest that HCV can interfere with innate immune activation at multiple levels. The non-structural proteins of HCV, particularly NS3-4A have been found to interact with various host adaptor molecules to disrupt type I IFN induction pathways. Foy et al found that NS3-4A serine protease blocked HCV-induced activation of IRF3 in the human hepatoma cell line, Huh-7 (48). It has been reported that NS3-4A protease also targets and cleaves the interferon promoter stimulator (IPS-1) adaptor protein from the mitochondria to ablate signaling to IFN a/β immune defenses (49, 50).
Inhibition of RIG-I-dependent signaling to the IFN pathway has been found in HCV infection by the HCV NS3/4A protease activity (51,52). This inhibition was localized to upstream of the non-canonical IKK-related kinases IKKε and TBK1, which phosphorylate IRF3, at the level of the TLR adapter proteins, TRIF/ICAM1 (51,52). In the replicon system IKKε over-expression inhibited HCV expression even in the presence of neutralizing antibodies to IFN receptors or in the presence of a dominant negative STAT mutant (53) suggesting that IKKε expression is important for rapid activation of the cellular antiviral response in HCV infection. In HCV patients’ liver biopsies expression of IKKε and the RNA helicases RIG-I/MDA5/LGP2 were significantly reduced whereas expression of TBK1 and Cardif was not significantly altered (53). These observations support the contention that HCV interferes with host pathways directed at viral elimination.
While the HCV NS3-4a-mediated cleavage of the adaptor molecules TRIF (adaptor to TLR3) and Cardif/IPS-1 (adaptor to RIG-I and MDA5) has been reported in the human hepatoma cells Huh7 and Huh7.5, recent studies performed in the non-neoplastic human hepatocyte PH5CH8 cells showed a retained and robust TRIF- and Cardif-mediated pathway activation. Dansako et al found that more robust induction of IFNβ in PH5CH8 cells compared to Huh7 and NS3-4a failed to suppress TIRF-mediated IFNβ production in these cells. Cardif-mediated IFNβ production was still suppressed by NS3-4a by cleaving Cardif at the Cys508 residue (54)
Further analysis of the HCV NS3-4A protease defined the site of its interaction with host targets. NS3 mutants lacking the helicase domain retained the ability to control virus signaling initiated by RIG-I or MDA5 and suppressed downstream activation of IRF3 and NFκB through the targeted proteolysis of IPS-1 (55). However, truncation of the NS3 protease domain or point mutation ablated the protease activity providing evidence for the active site of NS3 interaction with the host immune recognition pathways.
A recent study found modulation of TLR-mediated signaling in a macrophage cell line expressing HCV proteins (Abe et al 2007). Various genotypes of NS5A protein bound the common TLR adaptor, MyD88, and thereby inhibited the recruitment of interleukin-1 receptor associated kinase 1 (IRAK1) to MyD88 and impaired cytokine production in response to TLR ligands (56). Transfection of NS3, NS3/4, NS5B or NS5A into mouse macrophages resulted in inhibition of IL-6 induction by various TLR ligands (56). While this observation indicates a novel possible way of interaction of HCV with innate immunity, results of this in vitro study are in contrast with previous observations from human monocytes/macrophages form individuals with chronic HCV infection where increased pro-inflammatory cytokine induction was observed in response to TLR4 or TLR2 stimulation (57,58). An additional consideration is whether macrophages are infected with HCV and/or whether these cells support HCV replication. Positive strand RNA has been detected in PBMCs and blood monocytes by several groups, however, observation of negative–strand RNA, which would indicate active viral replication, in monocytes/macrophages has been found only in few studies (59). A recent study found presence of HCV genomic RNA in circulating DCs and at even higher expression level in monocytes of HCV patients (60). In this study, infection of DCs with HCV was associated with impaired expression of IL-12 and TNFα (60).
In the liver, dendritic cells as well as resident and recruited macrophages play an important role in elimination of damaged cells. In in vitro studies, engulfment of apoptotic blood mononuclear cells expressing HCV proteins resulted in differential chemokine expression and STAT signaling in DCs (61) suggesting that virus-infected hepatocytes may modulate phagocytic cell functions even in the absence of their direct infection with HCV.
Increased expression of TLR2, TLR3 and TLR6 mRNA were found in peripheral blood mononuclear cells of patients with chronic HCV infection that correlated with sustained virological response (62). In HCV treatment trials the number of genes that were up- or down-regulated by pegylated interferon and ribavirin treatment was fewer in patients with a poor response than in those with an intermediate or marked viral response (63). The induction of IFN-inducible genes (2’5’-oligonucleotide synthetase, MX1, IRF7, and TLR7 genes was lower in patients with poor response compared to patients with marked or intermediate response suggesting that blunted IFN signaling and TLR signaling is associated with the lack of response to IFN therapy (63). A recent study found association between TLR7 single nucleotide polymorphism (SNP) and protection from advanced inflammation and fibrosis in male patients with chronic HCV infection (64). The C1-120G TLR7 allele was found to offer protection from the development of inflammation and fibrosis (64).
HCV interferes with activation of adaptive immune responses by innate immune cells
Effects on dendritic cells
In addition to recognition of invading pathogens, dendritic cells play a central role in activation of naïve T lymphocytes to initiate virus-specific T cell responses. Dendritic cells, including circulating myeloid DCs, monocyte-derived DCs and plasmacytoid DCs have been studied in chronic HCV infection by several groups of investigators. Plasmacytoid DCs are the major producers of IFNα and are specifically equipped to sense viral nucleic acids via their expression of TLR7 and TLR9 (65). Most investigators found decreased frequency, reduced IFNα production and impaired T cell stimulatory capacity of circulating plasmacytoid DCs (66,67). Interestingly the expression of CD123 and BDCA2, markers of PDCs, were expressed at higher levels in livers of HCV infected patients raising the possibility of sequestration of PDCs in HCV infected livers (67). Indeed, enrichment for DCs within the intrahepatic compartment was recently reported (68), possibly due to HCV E2/CD81-mediated induction of RANTES (69). Despite the immuno-tolerogenic environment in the liver (70,71.), myeloid DCs (MDC) from HCV-infected liver demonstrated higher expression of MHC class II, CD86 and CD123, were more efficient stimulators of allogeneic T-cells and secreted less IL-10 compared to controls (72,73). In contrast, pDCs were present at lower frequencies in HCV-infected liver however they expressed higher levels of the regulatory receptor BDCA-2 (72,73) and showed increased ability to prime T cells compared to controls (74).
While the active HCV infection of dendritic cells is uncertain (75,76), the HCV quasispecies sequences cloned from DCs bear an internal ribosome entry site with poor efficiency for translation in cells of liver, lymphoid, or DC origin (77), suggesting that passage through dendritic cells significantly affects the function of the HCV virion. However, there are clear indications that HCV-derived proteins expressed in vivo affect immune functions. Studies from mice expressing HCV non-structural protein showed decreased capacity of a mixed PDC and MDC population to activate T cells (78), while overexpression of structural proteins leads to impaired major histocompatibility complex class-I presentation during dendritic cell maturation (79)
In human studies, findings related to myeloid DC functions are controversial. Complex defects, namely decreased T cell stimulatory capacity, overproduction of the immunoregulatory cytokine IL-10 and deficiency in co-stimulatory molecules were detected in myeloid dendritic cells (mDC) of patients with chronic HCV infection by some investigators (80–85), while others failed to identify any mDC abnormalities (86–90). Such discrepancies most possibly derive from different patients cohorts, but also assessment of non-human primate models of HCV infection, different experimental approaches, and distinct read-outs.
Anti-HCV treatment also may affect dendritic cell functions. Combination alpha interferon-ribavirin therapy alters the cytokine profile of maturing DCs by suppressing IL-10 production but maintaining IL-12(p70) and TNFα production, a pattern that would favor viral elimination through downstream effects on T cells (91). Myeloid DCs from HCV infected patients are impaired in their ability to drive Th1 in response to IFNα (92
Natural killer cells
Natural killer (NK) cells constitute a potent, rapid part of the innate immune response to viral infections but also to neoplastic cells, and also participate in priming of adaptive immunity (93). NK cells are capable of performing cytolysis as well as cytokines and chemokines release (93). NK cells mount an anti-HCV response and can be triggered by HCV-derived proteins or HCV-infected cells. In vitro NK cells were capable of inducing an HCV-associated, perforin/granzyme-dependent lysis of human hepatoma cells in a direct cellular contact-dependent but MHC class I–independent manner (94,95). However such potent cytolytic effect is only observed in cytokine-primed NKs, suggesting that either HCV-infected cells are poor triggers of NK activity, or such effect is more potent in the context of established HCV-specific immune response. Further, inhibition of NK cell CD16-mediated cytotoxicity following engagement of CD81 molecules on NK cells with HCV E2 protein was reported (96,97). Impaired NK cell triggering owing to reduced expression of NKG2D ligands (MIC-A and MIC-B) on mature DC as well as increased NK cell inhibition through increased CD94/NKG2A expression and TGFβ and IL-10 production have been suggested to occur in vivo during HCV infection (98101). More recently, increased expression of NKp30 and NKp46, the specialized NK-triggering receptors involved in non-MHC-restricted natural cytotoxicity, was identified in NK cells from HCV-infected patients (102). Freshly separated NK cells from HCV patients showed significant production of IL-10 and normal concentrations of IFNγ upon direct cell contact-mediated triggering (102). Thus, skewed NK receptor expression during HCV infection combined with increased production of immunoregulatory cytokines could contribute to an inefficient NK-DC and NK/T cells crosstalk leading to inefficient subsequent adaptive immune responses and lack of virus control (103).
HCV infection results in inflammatory cell activation
Chronic HCV infection is associated with activation of the inflammatory cell and cytokine cascade including recruitment of inflammatory cells to the HCV infected liver, increased liver and serum levels of pro-inflammatory cytokines and evidence of monocyte/macrophage activation (104). Several mechanisms may account for this inflammatory activation including pattern recognition receptor activation as a result of HCV infection and amplification of the cytokine cascade by endogenous mediators or HCV-derived products.
In addition to TLRs that recognize viral nucleic acid sequences, surface expressed TLRs have been shown to sense HCV viral proteins and thereby induce pro-inflammatory pathways in inflammatory cells. TLR2-mediated activation of monocytes and macrophages is induced by HCV core and NS3 proteins to result in activation of the inflammatory cascade including activation of IRAK1 kinase, NFκB, MAPK and TNFα production (34,58,67,80,105). In addition, monocytes of patients with chronic HCV infection respond to TLR4 stimulation with an augmented pro-inflammatory response compared to non-infected controls (67,80). Increased levels of TLR2 and TLR4 expression were observed in peripheral blood monocytes of patients with chronic HCV infection and increased expression of TLR2 in particular was associated with increased circulating TNFα levels and hepatic inflammatory activity (106). In support of the role of TLR2 in HCV infection, a recent study found association between homozygous TLR2 Arg753Gln polymorphism and allograft failure and mortality after liver transplantation for chronic HCV while there was no association found for TLR4 (Asp299Gly and Thr399Ile) polymorphism (107).
It has recently been proposed that the increased activation of inflammatory cascade activation in HCV could be related to a loss of TLR tolerance in chronically HCV infected patients’ monocytes via multiple TLR signals such as circulating HCV core proteins that stimulate TLR2, low levels of circulating endotoxin in these patients (TLR4), as well as the presence of increased levels of IFNγ that can amplify inflammatory cell activation and promote loss of TLR tolerance (58,108). In addition to HCV proteins, some investigators found cell activation by HCV lipopeptides via TLR2 and TLR4 (57).
In children, chronic HCV infection was associated with increased expression of TLR2 and TLR4 in neutrophil leukocytes compared to HCV negative controls or HCV antibody positive individuals who spontaneously cleared HCV infection (109). TLR2 and TLR4 mRNA and protein expression were also increased in the liver of children with chronic HCV infection compared to controls without viral infection (110).
In adult HCV-infected cirrhotic livers, gene expression changes involved activation of the innate antiviral immune response genes that was in contrast with the gene activation pattern in livers with alcoholic cirrhosis (111). The link between activation of inflammatory cell and mediators and stellate cell activation and fibrosis is well established (112,113). Recent evidence suggests that persistent inflammation also predisposes to cancer. Consistent with this, biomarkers of oxidative stress and inflammation were associated with hepatocellular carcinoma (HCC) in HCV infected livers (114). Another study found that chronic inflammation associated with HCV infection shifts hepatocytic TGFβ signaling from tumor suppression to fibrogenesis, which can accelerate both liver fibrosis and the risk of HCC (115). These observations lend support to the role of inflammation in the increased frequency of HCC in HCV-infected livers.
Innate immunity as a therapeutic target in HCV infection
Potent activation of antiviral immune pathways though selective TLR activation provides an attractive therapeutic target in HCV treatment. In support of this contention, recent studies found promising results with TLR7 and TLR9 agonists. The TLR7 and TLR9 activation strategy is based on increasing endogenous IFNα production in dendritic cells, however, additional immunomodulatory effects of TLR9 and/or TLR7 are yet to be evaluated. Isatoribine, an agonist of TLR7, reduced plasma virus concentrations in chronic HCV infection (116). Oral resiquimod, a TLR7 and TLR8 agonist, showed promising antiviral effects in phase IIa safety and efficacy trials (117,118). IFNα levels correlated with decreases in viral titer and lymphocyte counts in the treatment group (117,118). In another study, administration of CPG10101, a TLR9 agonist, showed safety and efficacy in normal volunteers (119). While flu-like symptoms were the reported side effects, CPG10101 induced interferons, cytokines and chemokines in vivo suggesting its potential in HCV therapy. At this time, however, further clinical trials with both the TLR7 and TLR9 agonists are on hold due to concerns related to some of their side effects.
Analysis of various TLR ligands on induction of antiviral molecules in human peripheral blood mononuclear cells (PBMC) revealed that agonists of TLRs 3, 4, 7, 8, and 9 were potent inducers of antiviral activity including induction of IFNα and IFN-induced 2’,5’-oligoadenylate synthase (120). However, TLR4 and TLR8 stimulation also induced high levels of TNFα and IL-1β (120).
Recent localization of the functional site of HCV NS3-4A protease to the protease domain in cleaving IPS-1 that is essential for blocking RIG-I signaling to IRF3 and NFκB provided another potential therapeutic target for HCV (55). In vitro studies suggest that TLR ligands may overcome some of the immune defects associated with HCV infection. Yonkers et al reported that TLR3, TLR7/8 and TLR9 ligands could enhance both myeloid DC and PDC activation of naïve CD4+ T cells (66). However, PDCs from HCV infected patients had reduced expression of activation markers (HLA-DR) and IFNα production upon TLR7/8 stimulation and showed decreased activation of CD4+ T cells (66). These data indicate that stimulation of certain TLRs may have benefit on restoration of innate and adaptive immunity in chronic HCV infection.
Summary
Increasing evidence suggests that HCV can interfere with innate immune activation at multiple levels. First, HCV, through its viral proteins can undermine viral recognition by cleaving pivotal adaptor proteins in TLR and RIG-I/MDA5 signaling. Second, HCV directly or indirectly modulates key antigen presenting functions of various dendritic cell types, contributing to impaired virus-specific T cell activation. Third, IFNα production by PDCs, the main cell type producing IFNα, is drastically reduced in chronic HCV infection. Fourth, chronic HCV infection results in activation of pro-inflammatory pathways and mediators in inflammatory cells that contribute not only to aberrant innate-adaptive immune interactions but also to activation of liver fibrosis and a microenvironment that may support cancer formation. Therapeutic strategies to counteract innate immune alterations in chronic HCV provide a promising target and need further investigation.
Acknowledgments
Funding support: This work was supported by PHS grant AA014372 to GS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERNCES
- 1.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 2.Pawlotsky JM. Virology of hepatitis B and C viruses and antiviral targets. J Hepatol. 2006;44 1 Suppl:S10–S13. doi: 10.1016/j.jhep.2005.11.005. Epub 2005 Nov 21. [DOI] [PubMed] [Google Scholar]
- 3.Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20(5):393–405. [PubMed] [Google Scholar]
- 4.Barton GM. Viral recognition by Toll-like receptors. Semin Immunol. 2007;19(1):33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Bowie AG, Fitzgerald KA. RIG-I: tri-ing to discriminate between self and non-self RNA. Trends Immunol. 2007;28(4):147–150. doi: 10.1016/j.it.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur J Immunol. 2007;37(2):306–309. doi: 10.1002/eji.200637009. [DOI] [PubMed] [Google Scholar]
- 8.Yoneyama M, Fujita T. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem. 2007;282(21):15315–15318. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]
- 9.Rothenfusser S, Goutagny N, DiPerna G, et al. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175(8):5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 10.Yoneyama M, Kikuchi M, Matsumoto K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175(5):2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 11.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Hirai R, Loo YM, et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci U S A. 2007;104(2):582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li K, Chen Z, Kato N, et al. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J Biol Chem. 2005;280(17):16739–16747. doi: 10.1074/jbc.M414139200. [DOI] [PubMed] [Google Scholar]
- 14.Dansako H, Ikeda M, Kato N. Limited suppression of the interferon-beta production by hepatitis C virus serine protease in cultured human hepatocytes. FEBS J. 2007;274(16):4161–4176. doi: 10.1111/j.1742-4658.2007.05942.x. [DOI] [PubMed] [Google Scholar]
- 15.Binder M, Kochs G, Bartenschlager R, et al. Hepatitis C virus escape from the interferon regulatory factor 3 pathway by a passive and active evasion strategy. Hepatology. 2007 August 1; doi: 10.1002/hep.21829. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Meylan E, Curran J, Hofmann K, et al. Cardif is an adaptor in the RIG-1 antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 17.Xu LG, Wang XY, Han KJ, et al. VISA is an adaptor protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NFkappaB and IRF 3. Cell. 2005 Sep 9;122(5):669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Takahashi K, Sato S, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 20.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442(7098):39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 21.Saito T, Hirai R, Loo YM, et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci U S A. 2007;104(2):582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sioud M. Innate sensing of self and non-self RNAs by Toll-like receptors. Trends Mol Med. 2006;12:167–176. doi: 10.1016/j.molmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 24.Diebold SS, Kaisho T, Hemmi H, et al. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 25.Hornung V, Guenthner-Biller M, Bourquin C, et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005:11. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Gallo D, Green A, et al. Role of TLRs in changes in gene expression and NFκB activation in mouse hepatocytes stimulated with LPS. Infect Immun. 2002;70:3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human TLRs and related genes. Biol Pharm Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- 28.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology. 2006;44:287–298. doi: 10.1002/hep.21308. [DOI] [PubMed] [Google Scholar]
- 29.Ito T, Amakawa R, S F. Roles of Toll-like receptors in natural interferon-producing cells as sensors in immune surveillance. Hum Immunol. 2002;63:1120–1125. doi: 10.1016/s0198-8859(02)00750-4. [DOI] [PubMed] [Google Scholar]
- 30.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 32.Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 33.Assier E, Marin-Esteban V, Haziot A, et al. TLR7/8 agonists impair monocyte-derived dendritic cell differentiation and maturation. J Leukoc Biol. 2007;81:221–228. doi: 10.1189/jlb.0705385. [DOI] [PubMed] [Google Scholar]
- 34.Dolganiuc A, Kodys K, Kopasz A, et al. Hepatitis C virus core and NS3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615–5624. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 35.Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991;351:355–356. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 36.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 37.Umeatsu S, Sato S, Yamamoto M, et al. IRAK-1 plays an essential role for TLR7-and TLR9-mediated IFN-α induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato M, Hata N, Asagiri M, et al. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto K, Stadanlick J, Ikeda F, et al. Influence of ethnicity in the outcome of hepatitis C virus infection and cellular immune response. Hepatology. 2003;37(3):590–599. doi: 10.1053/jhep.2003.50103. [DOI] [PubMed] [Google Scholar]
- 40.Thimme R, Oldach D, Chang KM, et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194(10):1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang KM, Thimme R, Melpolder JJ, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33(1):267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 42.Chang KM, Gruener NH, Southwood S, et al. Identification of HLA-A3 and -B7-restricted CTL response to hepatitis C virus in patients with acute and chronic hepatitis C. J Immunol. 1999;162(2):1156–1164. [PubMed] [Google Scholar]
- 43.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436(7053):946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 44.Thimme R, Bukh J, Spangenberg HC, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99(24):15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox AL, Mosbruger T, Lauer GM, et al. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42(1):104–112. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper S, Erickson AL, Adams EJ, et al. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10(4):439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 47.Oleksyk TK, Thio CL, Truelove AL, et al. Single nucleotide polymorphisms and haplotypes in the IL-10 region associated with HCV clearance. Genes Immun. 2005;6(4):347–357. doi: 10.1038/sj.gene.6364188. [DOI] [PubMed] [Google Scholar]
- 48.Foy E, Ferreon JC, Nakamura M, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102(8):2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Li X-D, Sun L. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci USA. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meylan E, Curran J, Hofmann K, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 51.Breiman A, Vitour D, Vilasco M, et al. A hepatitis C virus (HCV) NS3/4A protease-dependent strategy for the identification and purification of HCV-infected cells. J Gen Virol. 2006;87:3587–3598. doi: 10.1099/vir.0.82214-0. [DOI] [PubMed] [Google Scholar]
- 52.Breiman A, Grandvaux N, Lin R, et al. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKepsilon. J Virol. 2005;79(7):3969–3978. doi: 10.1128/JVI.79.7.3969-3978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vilasco M, Larrea E, Vitour D, et al. The protein kinase IKKepsilon can inhibit HCV expression independently of IFN and its own expression is downregulated in HCV-infected livers. Hepatology. 2006;44(6):1635–1647. doi: 10.1002/hep.21432. [DOI] [PubMed] [Google Scholar]
- 54.Dansako H, Ikeda M, Kato N. Limited suppression of the interferon-beta production by hepatitis C virus serine protease in cultured human hepatocytes. FEBS J. 2007;274(16):4161–4176. doi: 10.1111/j.1742-4658.2007.05942.x. [DOI] [PubMed] [Google Scholar]
- 55.Johnson CL, Owen DM, Gale M., Jr Functional and therapeutic analysis of hepatitis C virus NS3.4A protease control of antiviral immune defense. J Biol Chem. 2007;282(14):10792–10803. doi: 10.1074/jbc.M610361200. [DOI] [PubMed] [Google Scholar]
- 56.Abe T, Kaname Y, Hamamoto I, et al. Hepatitis C virus nonstructural protein 5A modulates the toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J Virol. 2007;81(17):8953–8966. doi: 10.1128/JVI.00649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duesberg U, von dem Bussche A, Kirschning C, et al. Activation by synthetic lipopeptides of the hepatitis C virus (HCV)--core protein is mediated by toll like receptors (TLRs) 2 and 4. Immunol Lett. 2002;84(2):89–95. doi: 10.1016/s0165-2478(02)00178-5. [DOI] [PubMed] [Google Scholar]
- 58.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210(2–4):237–247. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Pham TN, MacParland SA, Mulrooney PM. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J Virol. 2004;78(11):5867–5874. doi: 10.1128/JVI.78.11.5867-5874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodrigue-Gervais IG, Jouan L, Beaule G, Sauve D, Bruneau J, et al. Poly(I:C) and lipopolysaccharide innate sensing functions of circulating human myeloid dendritic cells are affected in vivo in hepatitis C virus-infected patients. J Virol. 2007 Jun;81(11):5537–5546. doi: 10.1128/JVI.01741-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wertheimer AM, Polyak SJ, Leistikow R, et al. Engulfment of apoptotic cells expressing HCV proteins leads to differential chemokine expression and STAT signaling in human dendritic cells. Hepatology. 2007;45(6):1422–1432. doi: 10.1002/hep.21637. [DOI] [PubMed] [Google Scholar]
- 62.He Q, Graham CS, Durante Mangoni E, et al. Increased expression of TLR2, TLR3 and TLR6 mRNA were found in peripheral blood mononculear cells of patients with chronic HCV infection which correlated with sustained virological response. Liver Int. 2006;26:1100–1110. doi: 10.1111/j.1478-3231.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 63.Taylor MW, Tsukahara T, Brodsky L, et al. Changes in gene expression during pegylated interferon and ribavirin therapy of chronic hepatitis C virus distinguish responders from nonresponders to antiviral therapy. J Virol. 2007;81(7):3391–3401. doi: 10.1128/JVI.02640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schott E, Witt H, Neumann K, et al. A Toll-like receptor 7 single nucleotide polymorphism protects from advanced inflammation and fibrosis in male patients with chronic HCV-infection. J Hepatol. 2007;47(2):203–211. doi: 10.1016/j.jhep.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Cao W, Liu YJ. Innate immune functions of plasmacytoid dendritic cells. Curr Opin Immunol. 2007;19(1):24–30. doi: 10.1016/j.coi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Yonkers NL, Rodriguez B, Milkovich KA, et al. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J Immunol. 2007;178(7):4436–4444. doi: 10.4049/jimmunol.178.7.4436. [DOI] [PubMed] [Google Scholar]
- 67.Dolganiuc A, Chang S, Kodys K, et al. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177(10):6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 68.Wertheimer AM, Bakke A, Rosen HR. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology. 2004;40(2):335–345. doi: 10.1002/hep.20306. [DOI] [PubMed] [Google Scholar]
- 69.Nattermann J, Zimmermann H, Iwan A, et al. Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44(4):945–954. doi: 10.1002/hep.21350. [DOI] [PubMed] [Google Scholar]
- 70.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol. 2005;26(10):512–517. doi: 10.1016/j.it.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 72.Lai WK, Curbishley SM, Goddard S, et al. Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol. 2007;47(3):338–347. doi: 10.1016/j.jhep.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 73.Lai WK, Sun PJ, Zhang J, et al. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles. Am J Pathol. 2006;169(1):200–208. doi: 10.2353/ajpath.2006.051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123(1):40–49. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsubouchi E, Akbar SM, Horiike N, et al. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J Gastroenterol. 2004;39(8):754–762. doi: 10.1007/s00535-003-1385-3. [DOI] [PubMed] [Google Scholar]
- 76.Pachiadakis I, Pollara G, Chain BM, et al. Is hepatitis C virus infection of dendritic cells a mechanism facilitating viral persistence? Lancet Infect Dis. 2005;5(5):296–304. doi: 10.1016/S1473-3099(05)70114-6. [DOI] [PubMed] [Google Scholar]
- 77.Laporte J, Bain C, Maurel P, et al. Differential distribution and internal translation efficiency of hepatitis C virus quasispecies present in dendritic and liver cells. Blood. 2003;101(1):52–57. doi: 10.1182/blood-2002-03-0818. [DOI] [PubMed] [Google Scholar]
- 78.Aloman C, Gehring S, Wintermeyer P, et al. Chronic ethanol consumption impairs cellular immune responses against HCV NS5 protein due to dendritic cell dysfunction. Gastroenterology. 2007;132(2):698–708. doi: 10.1053/j.gastro.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 79.Hiasa Y, Takahashi H, Shimizu M, et al. Major histocompatibility complex class-I presentation impaired in transgenic mice expressing hepatitis C virus structural proteins during dendritic cell maturation. J Med Virol. 2004;74(2):253–261. doi: 10.1002/jmv.20164. [DOI] [PubMed] [Google Scholar]
- 80.Dolganiuc A, Kodys K, Kopasz A, et al. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170(11):5615–5624. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 81.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97(10):3171–3176. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 82.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162(9):5584–5591. [PubMed] [Google Scholar]
- 83.Bain C, Fatmi A, Zoulim F, et al. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120(2):512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 84.Gelderblom HC, Nijhuis LE, de Jong EC, et al. Monocyte-derived dendritic cells from chronic HCV patients are not infected but show an immature phenotype and aberrant cytokine profile. Liver Int. 2007;27(7):944–953. doi: 10.1111/j.1478-3231.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 85.MacDonald AJ, Semper AE, Libri NA, et al. Monocyte-derived dendritic cell function in chronic hepatitis C is impaired at physiological numbers of dendritic cells. Clin Exp Immunol. 2007;148(3):494–500. doi: 10.1111/j.1365-2249.2007.03367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larsson M, Babcock E, Grakoui A, et al. Lack of phenotypic and functional impairment in dendritic cells from chimpanzees chronically infected with hepatitis C virus. J Virol. 2004;78(12):6151–6161. doi: 10.1128/JVI.78.12.6151-6161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Longman RS, Talal AH, Jacobson IM, et al. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192(3):497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 88.Longman RS, Talal AH, Jacobson IM, et al. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103(3):1026–1029. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 89.Piccioli D, Tavarini S, Nuti S, et al. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42(1):61–67. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 90.Rollier C, Drexhage JA, Verstrepen BE, et al. Chronic hepatitis C virus infection established and maintained in chimpanzees independent of dendritic cell impairment. Hepatology. 2003;38(4):851–858. doi: 10.1053/jhep.2003.50426. [DOI] [PubMed] [Google Scholar]
- 91.Barnes E, Salio M, Cerundolo V, et al. Impact of alpha interferon and ribavirin on the function of maturing dendritic cells. Antimicrob Agents Chemother. 2004;48(9):3382–3389. doi: 10.1128/AAC.48.9.3382-3389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyatake H, Kanto T, Inoue M, et al. Impaired ability of interferon-alpha-primed dendritic cells to stimulate Th1-type CD4 T-cell response in chronic hepatitis C virus infection. J Viral Hepat. 2007;14(6):404–412. doi: 10.1111/j.1365-2893.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 93.Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28(6):252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Larkin J, Bost A, Glass JI, et al. Cytokine-activated natural killer cells exert direct killing of hepatoma cells harboring hepatitis C virus replicons. J Interferon Cytokine Res. 2006;26(12):854–865. doi: 10.1089/jir.2006.26.854. [DOI] [PubMed] [Google Scholar]
- 95.Larkin J, Jin L, Farmen M, et al. Synergistic antiviral activity of human interferon combinations in the hepatitis C virus replicon system. J Interferon Cytokine Res. 2003;23(5):247–257. doi: 10.1089/107999003321829962. [DOI] [PubMed] [Google Scholar]
- 96.Crotta S, Ronconi V, Ulivieri C, et al. Cytoskeleton rearrangement induced by tetraspanin engagement modulates the activation of T and NK cells. Eur J Immunol. 2006;36(4):919–929. doi: 10.1002/eji.200535527. [DOI] [PubMed] [Google Scholar]
- 97.Crotta S, Stilla A, Wack A, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195(1):35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jinushi M, Takehara T, Tatsumi T, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173(10):6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 99.Jinushi M, Takehara T, Tatsumi T, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. Immunol. 2003;171(10):5423–5424. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 100.Lechmann M, Woitas RP, Langhans B, et al. Decreased frequency of HCV core-specific peripheral blood mononuclear cells with type 1 cytokine secretion in chronic hepatitis C. J Hepatol. 1999;31(6):971–978. doi: 10.1016/s0168-8278(99)80307-9. [DOI] [PubMed] [Google Scholar]
- 101.Godkin A, Jeanguet N, Thursz M, et al. Characterization of novel HLA-DR11-restricted HCV epitopes reveals both qualitative and quantitative differences in HCV-specific CD4+ T cell responses in chronically infected and non-viremic patients. Eur J Immunol. 2001;31(5):1438–1446. doi: 10.1002/1521-4141(200105)31:5<1438::AID-IMMU1438>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 102.De Maria A, Fogli M, Mazza S, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37(2):445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 103.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 2006;12(3):363–372. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 104.Szabo G, Dolganiuc A. HCV immunopathogenesis: virus-induced strategies against host immunity. Clin Liver Dis. 2006;10(4):753–771. doi: 10.1016/j.cld.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 105.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82(3):479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 106.Riordan SM, Skinner NA, Kurtovic J, et al. Toll-like receptor expression in chronic hepatitis C: correlation with pro-inflammatory cytokine levels and liver injury. Inflamm Res. 2006;55(7):279–285. doi: 10.1007/s00011-006-0082-0. [DOI] [PubMed] [Google Scholar]
- 107.Eid AJ, Brown RA, Paya CV, et al. Association between toll-like receptor polymorphisms and the outcome of liver transplantation for chronic hepatitis C virus. Transplantation. 2007;84(4):511–516. doi: 10.1097/01.tp.0000276960.35313.bf. [DOI] [PubMed] [Google Scholar]
- 108.Dolganiuc A, Oak S, Kodys K, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127(5):1513–1524. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 109.Wisniewska-Ligier M, Wozniakowska-Gesicka T, Glowacka E, et al. Involvement of innate immunity in the pathogenesis of chronic hepatitis C in children. Scand J Immunol. 2006;64(4):425–432. doi: 10.1111/j.1365-3083.2006.01800.x. [DOI] [PubMed] [Google Scholar]
- 110.Mozer-Lisewska I, Sluzewski W, Kaczmarek M, et al. Tissue localization of Toll-like receptors in biopsy specimens of liver from children infected with hepatitis C virus. Scand J Immunol. 2005;62(4):407–412. doi: 10.1111/j.1365-3083.2005.01670.x. [DOI] [PubMed] [Google Scholar]
- 111.Lederer SL, Walters KA, Proll S, et al. Distinct cellular responses differentiating alcohol- and hepatitis C virus-induced liver cirrhosis. Virol J. 2006;3:98. doi: 10.1186/1743-422X-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117(3):539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DeMinicis S, Bataller R, Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenerology. 2006;131(1):272–275. doi: 10.1053/j.gastro.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 114.Maki A, Kono H, Gupta M, et al. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol. 2007;14(3):1182–1190. doi: 10.1245/s10434-006-9049-1. [DOI] [PubMed] [Google Scholar]
- 115.Matsuzaki K, Murata M, Yoshida K, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46(1):48–57. doi: 10.1002/hep.21672. [DOI] [PubMed] [Google Scholar]
- 116.Horsmans Y, Berg T, Desager JP, et al. Isatoribine, an agonist of TLR7, reduces plasma virus concentration in chronic hepatitis C infection. Hepatology. 2005;42(3):724–731. doi: 10.1002/hep.20839. [DOI] [PubMed] [Google Scholar]
- 117.Pockros PJ, Schiff ER, Shiffman ML, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46(2):324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 118.Pockros PJ, Guyader D, Patton H, et al. Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J Hepatol. 2007;47(2):174–182. doi: 10.1016/j.jhep.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 119.Vicari AP, Schmalbach T, Lekstrom-Himes J, et al. Safety, pharmacokinetics and immune effects in normal volunteers of CPG 10101 (ACTILOM), an investigational synthetic toll-like receptor 9 agonist. Antivir Ther. 2007;12(5):741–751. [PubMed] [Google Scholar]
- 120.Thomas A, Laxton C, Rodman J, et al. Investigating Toll-like receptor agonists for potential to treat hepatitis C virus infection. Antimicrob Agents Chemother. 2007;31(8):2969–2978. doi: 10.1128/AAC.00268-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meylan E, Burns K, Hofmann K, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5(5):503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]