Abstract
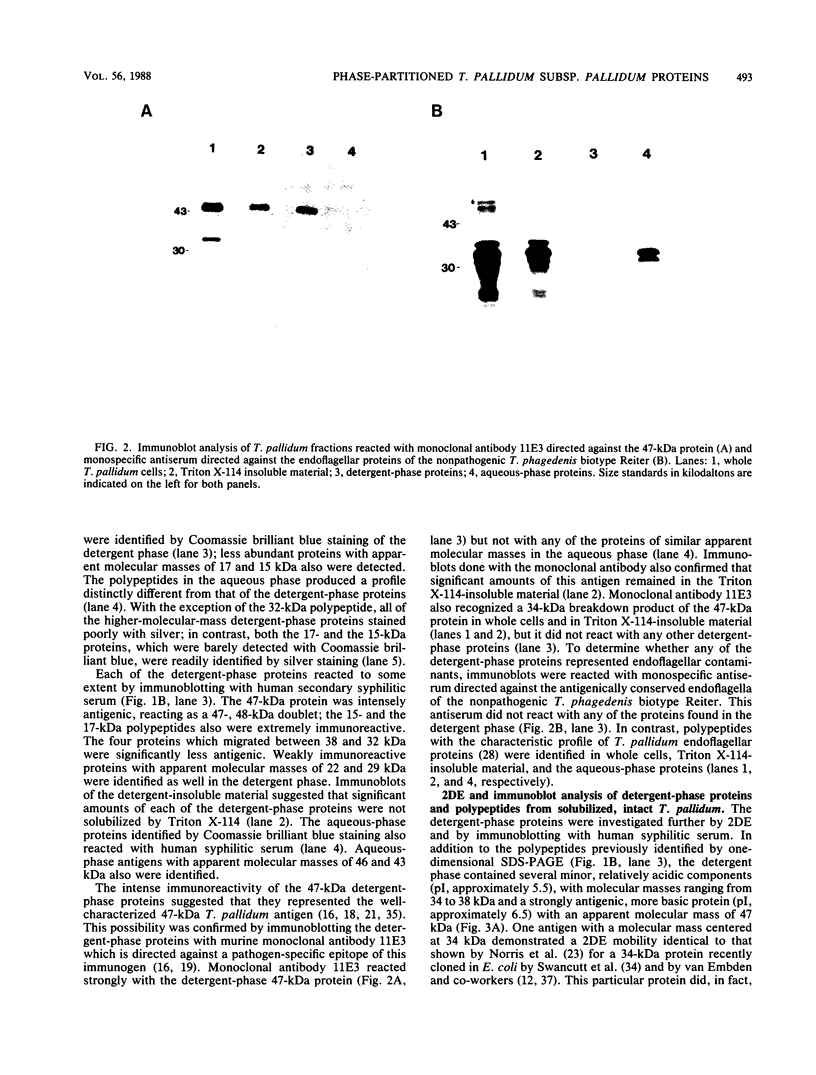
Integral membrane proteins of Treponema pallidum subsp. pallidum (T. pallidum) were identified by phase partitioning with the nonionic detergent Triton X-114; antigens with apparent molecular masses of 47, 38, 36, 34, 32, 17, and 15 kilodaltons (kDa) were identified in the detergent phase. Immunoblotting with murine monoclonal antibodies directed against pathogen-specific 47- and 34-kDa T. pallidum antigens confirmed their presence in the detergent phase. Endoflagellar proteins of T. pallidum were not detected in immunoblots of detergent-phase proteins when monospecific antisera directed against endoflagella of the nonpathogenic T. phagedenis biotype Reiter were used. At detergent concentrations (0.02 and 0.1%) which appeared to solubilize selectively the outer membranes of treponemes radiolabeled with 35S in vitro, limited amounts of detergent-phase proteins were immunoprecipitated. Greater amounts of detergent-phase proteins were extracted at higher detergent concentrations (0.5 and 2.0%) which resulted in both outer membrane solubilization and ultrastructural derangements of the residual cytoplasmic bodies. Furthermore, Triton X-114 extraction of both intact treponemes and organisms without outer membranes yielded detergent phases with similar protein profiles. The results of these experiments indicate that the hydrophobic proteins identified by Triton X-114 are not located exclusively in the T. pallidum outer membrane. The results are also consistent with the hypothesis that the T. pallidum outer membrane is a protein-deficient lipid bilayer.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auran N. E., Johnson R. C., Ritzi D. M. Isolation of the outer sheath of Leptospira and its immunogenic properties in hamsters. Infect Immun. 1972 Jun;5(6):968–975. doi: 10.1128/iai.5.6.968-975.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chamberlain N. R., Radolf J. D., Hsu P. L., Sell S., Norgard M. V. Genetic and physicochemical characterization of the recombinant DNA-derived 47-kilodalton surface immunogen of Treponema pallidum subsp. pallidum. Infect Immun. 1988 Jan;56(1):71–78. doi: 10.1128/iai.56.1.71-78.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K. J., Bienz D., Zahno M. L., Lüscher E. F. Distribution of platelet glycoproteins and phosphoproteins in hydrophobic and hydrophilic phases in Triton X-114 phase partition. Biochim Biophys Acta. 1984 Dec 19;778(3):463–469. doi: 10.1016/0005-2736(84)90395-x. [DOI] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Radolf J. D., Walfield A. M., Cunningham T. M., Miller J. N., Lovett M. A. Native surface association of a recombinant 38-kilodalton Treponema pallidum antigen isolated from the Escherichia coli outer membrane. Infect Immun. 1986 May;52(2):586–593. doi: 10.1128/iai.52.2.586-593.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957 Sep;66(2):160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hensel U., Wellensiek H. J., Bhakdi S. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis immunoblotting as a serological tool in the diagnosis of syphilitic infections. J Clin Microbiol. 1985 Jan;21(1):82–87. doi: 10.1128/jcm.21.1.82-87.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindersson P., Cockayne A., Schouls L. M., van Emden J. D. Immunochemical characterization and purification of Treponema pallidum antigen TpD expressed by Escherichia coli K12. Sex Transm Dis. 1986 Oct-Dec;13(4):237–244. doi: 10.1097/00007435-198610000-00006. [DOI] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ritzi D. M., Livermore B. P. Outer envelope of virulent Treponema pallidum. Infect Immun. 1973 Aug;8(2):291–295. doi: 10.1128/iai.8.2.291-295.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Wachter M. S., Ritzi D. M. Treponeme outer cell envelope: solubilization and reaggregation. Infect Immun. 1973 Feb;7(2):249–258. doi: 10.1128/iai.7.2.249-258.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- Marchitto K. S., Jones S. A., Schell R. F., Holmans P. L., Norgard M. V. Monoclonal antibody analysis of specific antigenic similarities among pathogenic Treponema pallidum subspecies. Infect Immun. 1984 Sep;45(3):660–666. doi: 10.1128/iai.45.3.660-666.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitto K. S., Kindt T. J., Norgard M. V. Monoclonal antibodies directed against major histocompatibility complex antigens bind to the surface of Treponema pallidum isolated from infected rabbits or humans. Cell Immunol. 1986 Sep;101(2):633–642. doi: 10.1016/0008-8749(86)90173-5. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Chamberlain N. R., Swancutt M. A., Goldberg M. S. Cloning and expression of the major 47-kilodalton surface immunogen of Treponema pallidum in Escherichia coli. Infect Immun. 1986 Nov;54(2):500–506. doi: 10.1128/iai.54.2.500-506.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Cloning and expression of Treponema pallidum (Nichols) antigen genes in Escherichia coli. Infect Immun. 1983 Nov;42(2):435–445. doi: 10.1128/iai.42.2.435-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Sell S. Antigenic complexity of Treponema pallidum: antigenicity and surface localization of major polypeptides. J Immunol. 1984 Nov;133(5):2686–2692. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Cockayne A., Bailey M. J. The outer membrane of Treponema pallidum: biological significance and biochemical properties. J Gen Microbiol. 1985 Sep;131(9):2349–2357. doi: 10.1099/00221287-131-9-2349. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Rhodes J. G. Surface-associated antigens of Treponema pallidum concealed by an inert outer layer. Immunology. 1982 May;46(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Blanco D. R., Miller J. N., Lovett M. A. Antigenic interrelationship between endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum (Nichols): molecular characterization of endoflagellar proteins. Infect Immun. 1986 Dec;54(3):626–634. doi: 10.1128/iai.54.3.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Boyer M. J., Wise K. S. Triton X-114 phase fractionation of an integral membrane surface protein mediating monoclonal antibody killing of Mycoplasma hyorhinis. Infect Immun. 1987 May;55(5):1094–1100. doi: 10.1128/iai.55.5.1094-1100.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Bassford P. J., Jr Cellular and extracellular protein antigens of Treponema pallidum synthesized during in vitro incubation of freshly extracted organisms. Infect Immun. 1985 Mar;47(3):799–807. doi: 10.1128/iai.47.3.799-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Hodinka R. L., Wyrick P. B., Bassford P. J., Jr Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect Immun. 1987 Sep;55(9):2255–2261. doi: 10.1128/iai.55.9.2255-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Kerner T. C., Jr, Bankaitis V. A., Bassford P. J., Jr Identification and preliminary characterization of Treponema pallidum protein antigens expressed in Escherichia coli. Infect Immun. 1983 Aug;41(2):709–721. doi: 10.1128/iai.41.2.709-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Twehous D. A., Norgard M. V. Monoclonal antibody selection and analysis of a recombinant DNA-derived surface immunogen of Treponema pallidum expressed in Escherichia coli. Infect Immun. 1986 Apr;52(1):110–119. doi: 10.1128/iai.52.1.110-119.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg R. W., Baseman J. B. Comparison of major protein antigens and protein profiles of Treponema pallidum and Treponema pertenue. Infect Immun. 1983 Nov;42(2):623–627. doi: 10.1128/iai.42.2.623-627.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Walfield A. M., Hanff P. A., Lovett M. A. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982 Apr 30;216(4545):522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- van Embden J. D., van der Donk H. J., van Eijk R. V., van der Heide H. G., de Jong J. A., van Olderen M. F., Osterhaus A. B., Schouls L. M. Molecular cloning and expression of Treponema pallidum DNA in Escherichia coli K-12. Infect Immun. 1983 Oct;42(1):187–196. doi: 10.1128/iai.42.1.187-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]