Abstract
Twin studies of personality are consistent in attributing approximately half of the variance in personality to genetic effects, with the remaining variance attributed to environments that make people within the same families different. Such conclusions, however, are based on quantitative models of human individual differences that estimate genetic and environmental contributions as constants for entire populations. Recent advances in statistical modeling allow for the possibility of estimating genetic and environmental contributions contingent on other variables, allowing the quantification of phenomena that have traditionally been characterized as gene-environment interaction and correlation. We applied these newer models to understand how adolescents’ descriptions of their relationships with their parents might change or moderate the impact of genetic and environmental factors on personality. We documented notable moderation in the domains of positive and negative emotionality, with parental relationships acting to both enhance and diminish both genetic and environmental effects. We discuss how genetic and environmental contributions to personality might be more richly conceptualized as dynamic systems of gene-environment interplay – systems that are not captured by classical concepts, such as the overall heritability of personality.
Keywords: Twin study, Behavior Genetics, Interpersonal Relationships
Individual differences are heritable, by which we mean that genetic influences make a substantial contribution to individual differences in peoples’ observable characteristics (or “phenotypes”). Indeed, this finding is so universal that Turkheimer (2000) enshrined it as the “first law” of behavior genetics. Turkheimer (2000) went on to propose second and third laws as well. The “second law” states that being raised in the same family has a smaller effect on individual differences than genetic effects. The “third law” is that a nontrivial portion of individual differences can be attributed to effects unique to each individual person, beyond genetic differences, and also beyond being raised in the same family.
These laws are well-supported by an extensive literature, and they clearly apply to personality as much as they apply to other individual differences (for recent reviews of the behavior genetics of personality, see, e.g., Krueger, Johnson & Kling, 2006; Krueger & Johnson, in press). Indeed, these laws have had a fundamental impact on thinking in scientific psychology. Our late colleague David Lykken described the impact of behavior genetic studies as “rearranging the furniture in psychology's house.” The “furniture,” or the topics of interest in psychology, was not fundamentally changed by behavior genetic research. For example, the phenotypic, observable structure of personality (Goldberg, 1993) would be a topic of basic importance, regardless of the etiology of personality. Nevertheless, the “rearrangement” – understanding that the etiology of personality is partly genetic – requires one to think about personality in a different way from the “radical environmentalist” viewpoint that dominated much of psychology throughout its history (e.g., Watson, 1930). A person's personality is not solely a product of environmental forces acting “from the outside”; personality is also the result of a genetic blueprint leading the person to actively seek out and interpret external environments in unique ways (Bouchard, 1997).
Given that we are at the point that scientific “laws” are being proposed based on an extensive literature (Turkheimer, 2000), have behavior genetic studies of personality and other individual differences outlived their usefulness? Our answer is “no,” and we arrived at this answer because the three behavior genetic laws strike us as vague, albeit of fundamental historical importance. The “laws” provided a needed corrective to the idea that people are puppets with strings pulled by the external environment. Nevertheless, the laws are derived from an approach to behavior genetic inquiry that estimates genetic and environmental effects on people in general. For example, when researchers conclude that “the heritability of extraversion is 50%,” they are concluding that 50% of the total variance in extraversion in their sample is associated with genetic influences. They can not conclude that a specific person's extraversion level is “50% genetic;” the concept of heritability applies not to individuals, but rather, to differences among many individuals. Stated in statistical terms, heritability applies to the variance of a set of observations, rather than to a single specific observation.
We can think about the meaning of heritability by drawing an analogy to the average score on a variable (the mean). Consider estimating the mean level of aggression for a group of people that includes both men and women. If we compute the mean for the overall group, collapsing across gender, we will understand the typical level of aggression in the group. This estimate will be accurate and meaningful, but very general – it applies to people in general, as opposed to people with specific characteristics (e.g., men vs. women). If we consider the possibility that gender affects (moderates) aggression, and examine mean aggression levels for men and women separately, we will get a more nuanced, less general understanding of aggression, finding that the mean for men is higher than the mean for women (Campbell, 2002). It is not that the overall mean of aggression for human beings is somehow “inaccurate;” rather, a more nuanced understanding is achieved by considering how gender moderates aggression levels.
This is exactly the situation with behavior genetic inquiry into the etiology of human individual differences. The “three laws of behavior genetics” are not inaccurate, they are simply highly general, being based on estimates that apply to people in general, as opposed to persons with specific characteristics. Fortunately, recent developments in statistical methodology allow for more fine-grained estimates of the impact of genetic and environmental factors on individual differences. These developments go well beyond incorporating coarse, ordinal moderator variables (e.g., dichotomous moderator variables such as gender). Newer models can also handle continuous moderator variables with theoretically compelling implications for understanding personality, such as peoples’ perceptions of their relationships with important others.
Before we delve into the nature of these modeling developments and apply them to understanding the etiology of personality, we first turn to describe the basic model for human individual differences that forms the foundations of research in behavior genetics. Some understanding of this basic model is needed to appreciate recent modeling refinements that allow us to go beyond the “three laws,” to achieve a more nuanced understanding of how genetic and environmental factors interact and correlate to produce individual differences in personality.
The ACE model of Individual Differences
Turkheimer's (2000) three laws are statements about the typically-observed values of three quantities that can be estimated from genetically-informative data, such as data on twins. These quantities are the effects of additive genetic factors (abbreviated A), environments that make people in the same families similar (“common” environments, abbreviated C), and environments that make people in the same families different (“non-shared” or “unique” environments, abbreviated E). Genetically-informative data, such as data collected from twins, can be used to estimate A, C, and E (for a standard textbook account, see Neale & Cardon, 1992). Stated in terms of a formal quantitative model for human individual differences,
| (1) |
In Equation (1), p2 represents the total observed (phenotypic, hence “p”) variance in a trait. This is the same variance statistic described in all standard introductory statistics textbooks, i.e., the average squared difference between the observed scores on a variable and the mean of that variable. The variance is an index of how “spread out” the scores are, or how different people tend to be from one another. As we described earlier, Turkheimer's (2000) three laws are actually statements about the typical values of a2, c2 and e2 found in behavior genetic research. For personality traits, a2 tends to be around 50% of p2, c2 tends to be around 0% of p2, and e2 tends to be around 50% of p2 (Bouchard & Loehlin, 2001). Typical values of a2 are the basis for Turkheimer's first law (genetic effects are non-trivial for all individual differences), typical c2 values correspond with the second law (being raised in the same family has a smaller effect on individual differences than genetic effects), and e2 corresponds with the third law (effects unique to each individual person, beyond a2 and c2, are non-trivial).
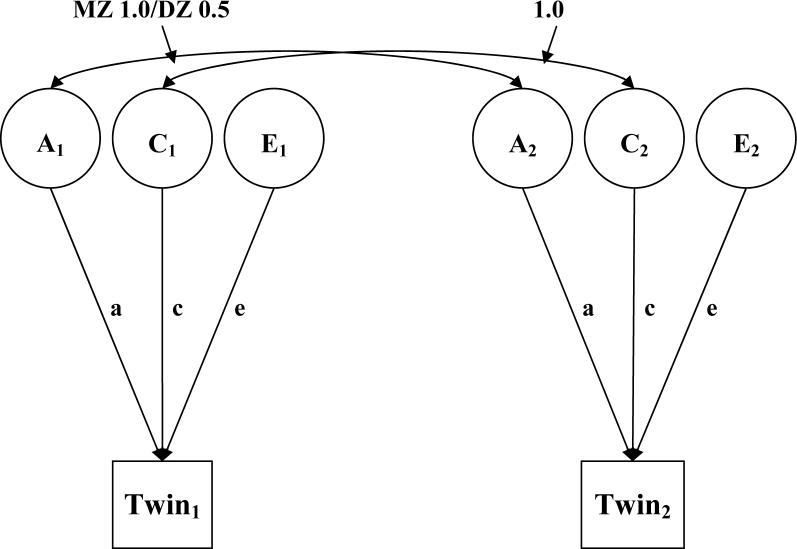
The ACE model in Equation 1 is illustrated graphically as a path diagram in Figure 1, which portrays the ACE model as applied to data on identical (monozygotic, or MZ) and fraternal (dizygotic, or DZ) twin pairs. Following standard tracing rules for path diagrams (see, e.g., Loehlin, 2004), and standardizing the variances of the latent A, C, and E variables (the circles at the top of Figure 1) to 1.0, the diagram shows how the model in Figure 1 corresponds to Equation 1. This is shown by the paths pointing at phenotypes (labeled “Twin 1” and “Twin 2”) observed in the first and second twins in the pairs of twins in the study. The variance in those phenotypes is the sum of the ACE effects because, following the tracing rules for path diagrams, deriving the variances for Twin 1 and Twin 2 requires traversing up each path and back down to the observed phenotypes for each effect, multiplying paths involved in each individual effect, and summing the effects to get the total model predicted variances. The phenotypic variables have the same predicted variance because the paths pointing at them are set up to have the same coefficients (a, c, and e), so both have predicted variance = p2 (moreover, there is no reason that the Twin 1 and Twin 2 variables should differ in any way that would affect their variances). Traversing the A path produces a (up to A) × a (back down to P) = a2, traversing the C path produces c2, traversing the E path produces e2, and the sum of the three effects is a2 + c2 + e2 = p2.
Figure 1.
Standard model for the etiology of individual differences in a single observable characteristic measured in twin pairs. A= additive genetic variance, C=shared environmental variance, E=nonshared environmental variance.
Figure 1 also shows how twin data define the A, C, and E effects. These definitions are provided by curved lines at the top part of the Figure, which represent model-defined correlations between the A, C, and E effects, across halves of twin pairs. The A effect is defined by the distinct biological processes that produce MZ and DZ twins. MZ twins share the same genotype, and hence the correlation between the A effects across the 1st and 2nd twins in the MZ twins is 1.0. By comparison, DZ twins share, on average, half of the genes that differ from person to person. Accordingly, their A correlation is .5 (for a fascinating study that verifies the accuracy of the .5 value via modern molecular genetic methods, see Visscher et al., 2006). Expressing a2 as a proportion of p2 gives the well-known heritability statistic, the extent to which observable, phenotypic individual differences are attributable to genetic differences.
Shared environmental (C) effects are defined by a correlation of 1.0 across the halves of the twin pairs for both MZ and DZ twins. This predicted 1.0 correlation derives from the definition of a shared environmental effect – the extent to which growing up in the same family makes people the same, independent of genetic similarity. If shared environmental effects are a major explanation for individual differences, then everyone growing up in the same family should turn out similar, regardless of their genetic similarity, and c2 should be a substantial proportion of p2.
Nonshared environmental (E) effects are defined by a correlation of 0.0 across the halves of the twin pairs, for both MZ and DZ twins. Like shared environmental effects, this predicted correlation derives from the definition of a nonshared environmental effect. This effect is the extent to which people are distinct (uncorrelated, or no more similar than two randomly paired people), in spite of sharing genetic material within families, and growing up together. If nonshared environmental effects are a major explanation for individual differences, then everyone should turn out relatively uniquely, regardless of their genetic similarity or the fact that some people grew up in the same families, and e2 should be a substantial proportion of p2. Nonshared environmental variance terms reflect the variance remaining after the effects of additive genetic and shared environmental variance have been estimated, and therefore random error variance is included in this variance component.
Expanding the ACE Model to Encompass Interaction and Correlation
The model in Figure 1 has been highly generative in personality research, forming the basis for numerous studies that yield a consistent picture of the etiology of personality as consisting of mostly genetic and nonshared environmental effects (Krueger & Johnson, in press). Although fundamental, the model in Figure 1 also has some notable limitations, owing to its simplicity. First, the model is written for only a single phenotype, yet we live in a complex, multivariate world. Along these lines, the model defines the A, C and E effects for only a single variable. That is, the model lacks a way of handling correlations between genetic and environmental effects on multiple variables. Second, as described earlier, the model defines the effects of A, C, and E as independent and as applying equally to everyone in the sample. That is, the model lacks a way of handling moderation (a phenomenon that is also sometimes termed “gene-environment interaction”), as well as a way of handling genetically influenced exposure to different environments (gene-environment correlation).
Although these limitations are notable, the way forward is to develop more sophisticated models that overcome these limitations by trying to capture some additional complexities that might exist in nature, that are glossed over by the basic model in Figure 1. Such modeling developments form an active area of inquiry in behavior genetics (see e.g., Eaves & Erkanli, 2003; Johnson, 2007; Purcell, 2002). The developments we will explore here involve defining the ACE model for two phenotypes, and in ways that allow the phenotypes in the model to transact, thereby providing a more nuanced understanding than can be achieved from evaluating only the a, c and e values from Figure 1.
In the model we will use, one phenotype acts to moderate the other phenotype, and the correlation between the phenotypes is also modeled directly. Simulation studies of this model were provided by Purcell (2002), some recent empirical applications of the model in personality research can be seen in Johnson and Krueger (2005, 2006), and Johnson (2007) describes the broad relevance of this model for providing a richer understanding of how genes and environments transact in human behavior.
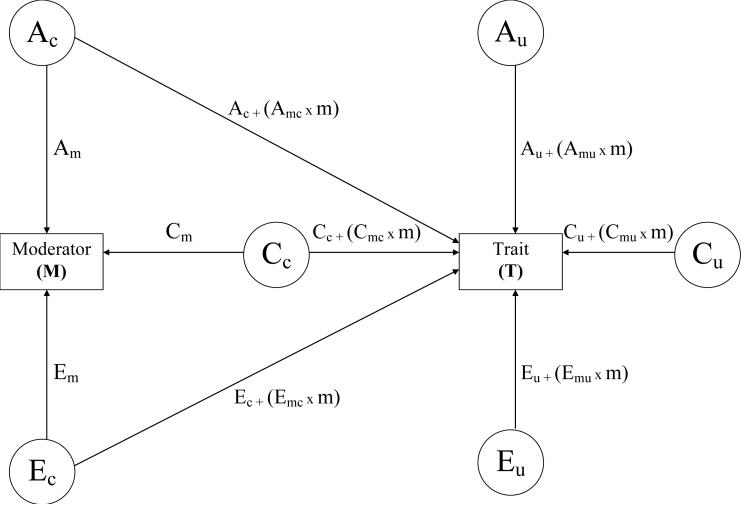
The model is portrayed in Figure 2. For simplicity, the model in Figure 2 is drawn for one twin in each pair only; the A, C and E correlations across the two members of each pair, although not shown, are defined in exactly the same way as in Figure 1. Although Figure 2 may look daunting, it is a logical extension of the model in Figure 1, made possible by recent technical developments in model fitting. The key development allowing the model in Figure 2 to be fit to real data involves the ability to model the data obtained from all the persons in a study directly, as opposed to having to first summarize the data. Traditionally, the model in Figure 1 (and other statistical models) have been fit to summary data, such as the means, variances, and covariances of a set of variables. This is a perfectly reasonable thing to do if these summary data contain all the interesting information in a dataset. For example, the model in Figure 1 was traditionally fit to the within twin variances and cross-twin covariances of the single phenotype, estimated separately for MZ and DZ twin pairs. But summarizing the data in this manner means the ACE effects apply to people in general, and this is a limitation that would be hard to overcome in a summary statistics framework. Consider, for example, the possibility that the ACE values for a personality trait differ based on how adolescents perceive their relationships with their parents. If perceived parenting is assessed continuously and has, e.g., 25 distinct values, one would need to model 25 covariance matrices for both MZ and DZ twins (so 50 matrices total)! Clearly, this approach becomes unwieldy quickly. Fortunately, the problem can be circumvented by predicting not the summary statistics for the data, but the values of the raw data directly. Indeed, this kind of raw data modeling has opened up exciting conceptual possibilities in a number of areas of individual differences research (see, e.g., Krueger, Markon, Patrick, & Iacono, 2005, for an example of how raw data modeling can distinguish categorical and dimensional models of individual differences).
Figure 2.
Model for the etiology of individual differences in a trait allowing for potential moderation. A= additive genetic variance, C=shared environmental variance, E=nonshared environmental variance.
The model in Figure 2 involves the same A, C, and E effects as in Figure 1, but also involves two fundamental extensions of Figure 1. First, the model now involves two phenotypes, as opposed to one phenotype in Figure 1, and it allows for both genetic and environmental connections between the phenotypes, via the ACE “common” effects (labeled Ac, Cc, and Ec on the Figure, the c suffix denoting effects in common). Second, the model allows for the possibility that the level of the first phenotype (the Moderator, M) moderates the ACE components of the second phenotype (the Trait, T). Some of these moderating effects are in common between M and T (those involving the c suffix, in the middle of the Figure), whereas others are unique to T (those involving the u suffix, to denote unique effects, on the right side of the Figure). Each of the ACE pathways impacting T has the form “effect + (moderated effect × level of the moderator)”. That is, each of these paths contains an overall coefficient that is separate from the level of the moderator (e.g., for A effects in common between M and T, this is denoted as Ac), and a coefficient that indexes how much the effect is changed (moderated) by the level of the moderator (e.g., for A effects in common between M and T, this is denoted Amc, and it is multiplied by m because it represents how much the level of M changes the impact of the genetic effects on T that are in common between M and T).
As a result of this algebraic arrangement, each of the ACE effects on T is not static, but can vary dynamically as a function of the level of M. This allows for the possibility of “gene × environment interaction,” in the sense that the genetic and environmental aspects of T are not single static values estimated for the entire population, but rather, interact with M. In addition, the possibility of “gene-environment correlation” is handled in the Figure 2 model via the genetic and environmental connections between M and T. M and T are allowed to correlate, via both genetic (A) and environmental (C and E) pathways.
Personality and Relationships with Parents in Late Adolescence
The availability of these new biometrical moderation models allow us to test whether the heritability of personality may vary as a function of different environmental variables. Developmental psychologists have long acknowledged the role that family environment, particularly parenting style, parental monitoring, and the nature of the parent-child relationship, has in the development of temperament and personality (Thomas, Chess, Birch, Hertzig, & Korn, 1963). While some have questioned the importance of parental influence on later psychological outcomes (Harris, 1995, 1998), recent reviews provide empirical evidence of the links between parenting and children's behavioral outcomes (Bates & McFadyen-Ketchum, 2000; Gallagher, 2002; Putnam, Sanson, & Rothbart, 2002). Negative, inappropriate, uninvolved, or unskilled parenting variables appear to play a particularly important role in the development of externalizing behaviors, while warm and supportive parenting behaviors seem to act as protective factors (Bates, Petit, Dodge, & Ridge, 1998; Belsky, Hsieh, & Crnic, 1998; Rubin, Burgess, Dwyer, & Hastins, 2003; Stoolmiller, 2001). Currently, there appears to be a growing consensus that parenting may influence adolescent development by acting as a moderator of other effects, such as genetic effects (Collins, Maccoby, Steinberg, Hetherington, & Bornstein, 2000). Thus, we hypothesized that an adolescent's perception of parenting behaviors would moderate genetic influences on personality.
Specifically, we applied the model in Figure 2 to data on adolescents’ personality traits and their accounts of their relationships with their parents. The low C (shared environmental) values obtained for personality traits mean that people are not similar in their personalities within families, beyond the similarity predicted by knowing the genetic relationships among people within families. One interpretation of this finding is that parents do not have much impact on the personalities of their offspring (Harris, 1998). This interpretation is reasonable if the only way in which parents impact offspring is by making them more similar to each other than they would be based on their genetic endowments. Another possibility is that the impact of parents is not an entirely shared environmental phenomenon. For example, parenting could act to make children within the same family different, in ways that are not correlated with genetic endowments. In this scenario, parenting is a nonshared environmental phenomenon, encompassed by the E component -- a component of variation that, for personality, is as large as the A component (Krueger & Johnson, in press).
Unfortunately, attempts to link the E variance in personality with specific measured psychological variables have not borne much fruit. Much research has sought to characterize E in psychological terms, particularly with regard to adolescent development, yet there has been little success in identifying systematic sources of E variation (Reiss, Neiderhiser, Hetherington, & Plomin, 2000). Indeed, with regard to personality, some of E might actually be idiosyncratic, as opposed to being relevant to understanding consistent individual differences in behavioral propensities that transcend perspectives. For example, combining self and peer-reports of personality leads to larger estimates of A, and smaller estimates of E, when compared with modeling self and peer-reports of personality separately (Riemann et al., 1997).
In addition, parenting behaviors need not be purely environmental phenomena. A number of studies have shown that peoples’ reports of how they approach the task of parenting are partly heritable (Kendler, 1996; Losoya et al., 1997; Perusse, Neale, Heath, & Eaves, 1994). Nevertheless, Spinath and O'Connor (2003) also showed how the personalities of parents and their reports of how they approach parenting are connected primarily through environmental mechanisms. This literature suggests a complex system of intertwining genetic and environmental influences on parenting (cf. McGue, Elkins, Walden, & Iacono, 2005).
In the current research, rather than thinking about parenting as linked to shared or nonshared environments, we conceptualized parenting as an emergent phenotype, with both genetic and environmental aspects. In addition, we assessed parenting from the perspective of the persons receiving the parenting, our adolescent twin research participants. That is, we asked our participants to independently describe their unique relationships with their parents. We treated these parental relationship measures as potential moderators of genetic and environmental effects on personality, and also as phenotypes that may be correlated with personality, through both genetic and environmental processes. Specifically, our adolescent twins’ reports of their relationships with their parents served as the “M” variable in the model in Figure 2, and their personality traits served as the “T” variable
Method
Research Participants
Our participants were male and female twin pairs who participated in the Minnesota Twin Family Study (MTFS), an ongoing population-based, longitudinal study of twins born in the state of Minnesota and their families. More than 90% of twin births from 1971 through 1985 were located from birth records and public databases. Families were excluded from the study if either twin had a cognitive or physical handicap that would prevent them from completing our daylong, in-person assessment, or if the family lived more than one day's drive from our laboratory at the University of Minnesota in Minneapolis. Of the eligible families, 83% agreed to participate. There were no significant differences between participating and nonparticipating families in socioeconomic status and self-reported mental health problems, but parents in participating families had slightly, albeit significantly, more education (0.25 years) than parents in nonparticipating families (Iacono, Carlson, Taylor, Elkins, & McGue, 1999). Reflecting the population of Minnesota at the time of the twins’ birth, approximately 98% of the participants were Caucasian. Children gave informed assent, while parents gave informed consent for themselves and their children. The research protocol was approved by the University of Minnesota Institutional Review Board. Further information regarding all aspects of MTFS recruitment is detailed elsewhere (Iacono et al., 1999; Iacono, McGue, & Krueger, 2007).
The MTFS utilizes an accelerated longitudinal design, with twins first visiting the study at age 11 or 17 years and returning for follow-up assessments approximately every three years thereafter. The 11-year-old-intake cohort consisted of 756 same-sex, reared-together monozygotic (MZ) and dizygotic (DZ) twin pairs: 376 male (254 MZ; 122 DZ) and 380 female pairs (233 MZ; 147 DZ). The 17-year-old-intake cohort consisted of 626 same-sex twin pairs: 289 male (188 MZ; 101 DZ) and 337 female (223 MZ; 114 DZ) pairs. For the purposes of the current study, we utilized data from both cohorts at the overlapping assessment point of age 17 years: the older cohort at intake and the younger cohort at their second follow-up visit. The sample thereby included all 1,252 individuals from the older cohort at the intake assessment, and 1,320 twins from the younger cohort who completed the second follow-up assessment (87% of the younger cohort). From this total possible sample size of 2,572 individuals, participants were excluded if they were missing data on all of the personality variables and all of the parenting variables (N=155) and if co-twin data were entirely missing (N=97). This brought the final sample size to 2,320 total persons who provided data on either personality or parenting. This sample included 556 male twin pairs (368 MZ; 188 DZ) and 604 female twin pairs (390 MZ; 214 DZ). There were more MZ than DZ twins in the present sample as a result of an overrepresentation of MZ twins in the population from which the sample was drawn, as well as a somewhat higher participation rate of families with MZ twins (Hur, McGue, & Iacono, 1995). Participants had a mean age of 17.76 (SD=.63) when they completed the measures used in the current project.
Zygosity Diagnosis
The MTFS combines three estimates to determine twin zygosity. First, parents complete a standard zygosity questionnaire. Second, MTFS staff evaluate physical similarity, including visage, hair color, and face and ear shape. Finally, ponderal and cephalic indices and fingerprint ridge count are measured. A previous validation study (N=50) demonstrated 100% accuracy of zygosity determination when these three estimates agree. When these three estimates do not agree, a blood sample is requested and a serological analysis is performed.
Assessment of Personality
Personality was measured with a shortened (198-item) version of the Multidimensional Personality Questionnaire (MPQ; Tellegen & Waller, in press) developed for the MTFS. The MPQ is a self-report personality instrument developed through factor analysis to assess a broad range of personality characteristics in normal populations. Internal consistency reliabilities for the MPQ scales range from .76 to .90, and 30-day test-retest reliabilities range from .82 to .92 (Tellegen & Waller, in press). In the current research, we focused on three higher-order factors indexed by the MPQ: Positive Emotionality (PEM; a broad measure of positive well-being and tendency to view life as a pleasurable experience), Negative Emotionality (NEM; a propensity to experience psychological distress) and Constraint (CN; a tendency to endorse traditional values and act in a cautious manner). Positive Emotionality subsumes the lower order scales of Well Being (cheerful, happy), Achievement (likes to work hard and strives for goals), Social Potency (likes to lead others), and Social Closeness (sociable, likes others). Negative Emotionality is comprised of Aggression (hurts others for own advantage), Alienation (suspiciousness, thinks others intend harm), and Stress Reaction (proneness to negative emotions, tensions, and mood lability). Finally, Constraint is a composite of Traditionalism (conservative, endorses high moral standards), Control (careful and planful), and Harm Avoidance (avoids danger in favor of safe activities).
All families were mailed the MPQ prior to the assessment. Participants were asked to bring the completed MPQ with them to their in-person visit. If the MPQ was not completed upon their arrival for their laboratory assessment or by the end of the day-long visit, participants were asked to complete it at home and return it by mail. MPQ data were available for 2,134 persons (men=1037, women=1097).
Relationship with Parents
The Parental Environment Questionnaire (PEQ) was administered to tap perceptions of mother-twin and father-twin relationships. The twins independently rated their relationships with each parent on 50 items assessing aspects of their relationship on a 4-point scale (1 = definitely true, 2 = probably true, 3 = probably false, and 4 = definitely false). Because ratings about mom and dad were highly correlated (> .80; McGue et al., 2005), we combined these ratings.
Elkins, McGue, and Iacono (1997) provided a description of the development, theoretical rationale, and psychometric properties of the scale. Briefly, the PEQ was developed by the MTFS because the standard measures of family environment available when the study began typically sought to assess the overall family climate rather than dyadic relationships within the family. The PEQ scales were organized around the two broad domains of conflict (vs. nurturance/warmth) and control, and correlate significantly and in the expected direction with an alternative measure of the family environment (Elkins et al., 1997).
Scores were prorated for scales missing ratings for 10% or fewer of their items (i.e., the average of the other items was used as the missing item's score); if more than 10% of the scale's constituent items were missing the scale was considered missing. For the present investigation, we utilized scores for the Parent-Child Conflict Scale (12 items: e.g., my parent often loses her/his temper with me; alpha = .82), the Regard for Parent Scale (8 items: e.g., I want to be like my parent in a number of ways; alpha = .75), and the Parent Regard for Twin Scale (5 items: e.g., my parent is proud of me; alpha = .69; McGue et al., 2005). The correlation between the two Regard Scales was large (r = .65), so to simplify our analyses, scores for the two Regard Scales were combined to form one summary score, interpretable as mutual Regard.
All families were mailed the PEQ prior to the assessment. Participants were asked to bring the completed PEQ with them to their in-person visit. If the PEQ was not completed upon their arrival for their laboratory assessment or by the end of the day-long visit, participants were asked to complete it at home and return it by mail. One telephone prompt was made if a PEQ was still not received. PEQ data were available for 1,990 individuals for Conflict (men=939, women=1051) and 1,983 individuals for Regard (men=934, women=1049). Although the Regard and Conflict scales were negatively correlated (r = −.66), we analyzed them separately because we wanted to consider the possibility that they would have different moderating effects.
Biometrical Analytic Approach
Statistical models for twin data (biometrical models) were fit to raw MPQ and PEQ data using the software package Mx (Neale, Boker, Xie, & Maes, 2002). Because some MPQ and PEQ data were missing on some participants, we used full-information maximum-likelihood raw data techniques. When data are missing, this approach uses all available information to impute a value and then adjusts for the imprecision of the imputed value. The fit of the model shown in Figure 2 was judged relative to the fit of a model in which the moderation parameters were fixed at zero. That is, the parameters Amc, Cmc, Emc, Amu, Cmu, and Emu were fixed at zero, so that the paths contributing to the variance in the Trait become constants for the entire sample. For example, the unique A effect on the trait becomes Au + (0 × m) = Au, and the contribution of the Au effect to the variance in the trait is no longer affected by the level of the Moderator (m is multiplied by zero). Comparing the fit of the two models (with and without moderator coefficients) thereby produces a statistical test of moderation. If the model with the moderation coefficients (Amc, Cmc, Emc, Amu, Cmu, and Emu) fits better than the model with those coefficients fixed at zero, then the ACE contributions to the Trait are not constant across levels of M, and moderation effects are important in understanding the etiology of the Trait.
Two indices were used to evaluate model fit: (1) the likelihood-ratio test (LRT; distributed as χ2, and computed as the difference in the −2 log-likelihood values for the two models); and (2) Akaike's Information Criterion (AIC; Akaike, 1987). Improvements in the model's fit, from adding or omitting parameters, can be assessed by noting the statistical significance of the LRT. Like the LRT, the AIC considers goodness of fit in the likelihood sense (how well the model reproduces the observed data), but also penalizes model complexity, preferring models that capture the data both accurately and parsimoniously. Lower AIC values are associated with better fitting models.
In the interests of focusing our analyses specifically on personality – parenting associations, all measured phenotypes were adjusted to be independent of age and sex effects (i.e., age, age2, age × gender, and age2 × gender were regressed out of the variables, and the standardized residuals from these regressions were used in our analyses; McGue & Bouchard, 1984). In theory, the other option here would be to model age and gender, but this creates analytic complexities that are difficult to render tractable in practice (e.g., multi-way interactions that cannot be modeled readily), and that are also tangential to our current aims. In addition, to promote clarity in interpreting effects, the personality variables were recoded as necessary so that they would be positively correlated with the parenting variables, which remained scored in the same direction for all models, with higher levels referring to greater conflict or greater regard (e.g., our raw PEM variable was negatively correlated with Conflict, so it was reversed so the correlation would be positive).
Results
Phenotypic Correlations
We first sought to understand how personality traits are associated with perceived relationships with parents. We did this by estimating the observed, phenotypic associations between the parental relationship variables and the personality variables. Specifically, we estimated correlations using the MLR estimator in the software package M+ (Muthén & Muthén, 1998-2006), because by using this estimator, the confidence intervals around the correlations are adjusted for the non-independence of twins within pairs. Conflict was significantly correlated with PEM (r =− .14, 95% CI = −.09, −.20), NEM (r = .41, 95% CI= .37, .46), and CN (r = −.27, 95% CI=−.22, −.32). Similarly, Regard was significantly correlated with PEM (r = 0.29, 95% CI=.23, .35), NEM (r = −.28, 95% CI=−.23, −.32), and CN (r = .24, 95% CI=.19, .29). As these correlations show, PEM and CN were associated with more positive perceived relationships with parents, whereas NEM was associated with more negative perceived relationships with parents.
Biometric Analyses
We next sought to understand how these phenotypic relationships between personality and relationships with parents are mediated genetically and environmentally. We did this by fitting the model in Figure 2 to each combination of the two relationship variables (treated as M variables) and the three personality variables (treated as T variables), for a total of six models where the moderation parameters were freely estimated. We also fit the six models to the same variables, fixing the moderating effects to zero (i.e., the parameters Amc, Cmc, Emc, Amu, Cmu, and Emu were set to zero). Comparing the fit of the pairs of models (with and without moderation) provides an evaluation of whether the moderation effects improve the ability of the model to capture our data. Table 1 gives the fit indices for these 12 models (6 with moderation, 6 without moderation).
Table 1.
Fit Statistics from Biometrical Models with and without Moderation Coefficients
| Model | Moderating Variable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regard |
Conflict |
|||||||||||
| −2lnL | df | χ2 | Δdf | p | AIC | −2lnL | df | χ2 | Δdf | p | AIC | |
| Positive Emotionality | ||||||||||||
| No Moderation | 9229.59 | 3471 | 2287.59 | 9457.97 | 3503 | 2451.97 | ||||||
| Only A Moderation | 9195.36 | 3469 | 34.23 | 2 | 0.0000 | 2257.36 | 9440.14 | 3501 | 17.83 | 2 | 0.0001 | 2438.14 |
| Only C Moderation | 9198.43 | 3469 | 31.16 | 2 | 0.0000 | 2260.43 | 9436.26 | 3501 | 21.72 | 2 | 0.0000 | 2434.26 |
| Only E Moderation | 9203.46 | 3469 | 26.13 | 2 | 0.0000 | 2265.46 | 9439.50 | 3501 | 18.48 | 2 | 0.0001 | 2437.50 |
| A and C Moderation (no E) | 9191.80 | 3467 | 37.79 | 4 | 0.0000 | 2257.80 | 9435.36 | 3499 | 22.62 | 4 | 0.0002 | 2437.36 |
| A and E Moderation (no C) | 9185.37 | 3467 | 44.22 | 4 | 0.0000 | 2251.37 | 9433.03 | 3499 | 24.95 | 4 | 0.0001 | 2435.03 |
| C and E Moderation (no A) | 9191.59 | 3467 | 38.00 | 4 | 0.0000 | 2257.59 | 9429.13 | 3499 | 28.85 | 4 | 0.0000 | 2431.13 |
| A, C, and E Full Moderation | 9183.77 | 3465 | 45.82 | 6 | 0.0000 | 2253.77 | 9428.86 | 3497 | 29.11 | 6 | 0.0001 | 2434.86 |
| Negative Emotionality | ||||||||||||
| No Moderation | 9186.75 | 3471 | 2244.75 | 9191.35 | 3503 | 2185.35 | ||||||
| Only A Moderation | 9168.74 | 3469 | 18.01 | 2 | 0.0001 | 2230.74 | 9176.97 | 3501 | 14.38 | 2 | 0.0008 | 2174.97 |
| Only C Moderation | 9175.65 | 3469 | 11.09 | 2 | 0.0039 | 2237.65 | 9174.61 | 3501 | 16.74 | 2 | 0.0002 | 2172.61 |
| Only E Moderation | 9174.66 | 3469 | 12.09 | 2 | 0.0024 | 2236.66 | 9180.33 | 3501 | 11.02 | 2 | 0.0040 | 2178.33 |
| A and C Moderation (no E) | 9167.48 | 3467 | 19.27 | 4 | 0.0007 | 2233.48 | 9171.42 | 3499 | 19.93 | 4 | 0.0005 | 2173.42 |
| A and E Moderation (no C) | 9168.23 | 3467 | 18.52 | 4 | 0.0010 | 2234.23 | 9176.40 | 3499 | 14.95 | 4 | 0.0048 | 2178.40 |
| C and E Moderation (no A) | 9170.24 | 3467 | 16.51 | 4 | 0.0024 | 2236.24 | 9172.56 | 3499 | 18.79 | 4 | 0.0009 | 2174.56 |
| A, C, and E Full Moderation | 9166.10 | 3465 | 20.64 | 6 | 0.0021 | 2236.10 | 9170.87 | 3497 | 20.48 | 6 | 0.0023 | 2176.87 |
| Constraint | ||||||||||||
| No Moderation | 9186.73 | 3471 | 2244.73 | 9310.86 | 3503 | 2304.86 | ||||||
| A, C, and E Full Moderation | 9181.28 | 3465 | 5.46 | 6 | 0.4867 | 2251.28 | 9297.09 | 3497 | 13.773 | 6 | 0.0323 | 2303.09 |
Note. −2lnL = −2 log likelihood; AIC = Akaike's Information Criterion; χ2 and associated p values correspond to the 6 df likelihood-ratio test comparing the −2lnL values for No Moderation and Moderation models. The selected model is shown in bold.
Table 1 shows that full moderation effects were notable for five of the six possible combinations of personality and parent relationship variables, according to both the LRT and AIC. The strongest were the moderating effects of Regard on PEM (χ2=45.82, df=6, p<.001) and Conflict on PEM (χ2=29.11, df=6, p<.001). For CN, the moderating effects of Regard could be removed without a significant decrease in fit. Similarly, the model fit improvement for moderation of CN by Conflict just reached significance, (χ2=13.77, df=6, p<.05) suggesting caution in interpreting this result.
Robustness to non-normality of moderators
Conflict was skewed such that more people reported relatively less conflict than would be the case if Conflict were normally distributed (skew = 0.43), whereas Regard was skewed such that more people reported relatively more Regard than would be the case if Regard were normally distributed (skew = −1.27). We therefore transformed the Conflict and Regard variables, and re-estimated the models in Table 1. Conflict was first reverse scored and both variables were subjected to a square transformation.
The results using transformed scores for Conflict and Regard generally replicated the results using raw scores. However, consistent with this being the weakest significant effect in Table 1, when transformed variables were used in the biometric moderation models, the moderating effect of Conflict on CN was no longer significant according to the LRT (χ2=11.40, df=6, ns). In addition, for Conflict and CN, AIC indicated marginally better fit for the no moderation model (2327.57 for no moderation, vs. 2328.16 for moderation). Moderation of NEM by Conflict was still significant according to the LRT (χ2=14.83, df=6, p=<.025) but the AIC for the No Moderation model was slightly lower (2196.94) when compared with the moderation model (2201.11) for this combination of variables. The other three significant moderation effects in Table 1 (PEM and Conflict, NEM and Regard, and PEM and Regard) remained significant by LRT, and for these three effects, the moderation models also showed better fit by AIC when transformed scores were modeled.
In addition, we truncated the raw data for the parenting variables, to ensure that more extreme observations were not the major reason we observed the effects in Table 1. Regard and Conflict were trimmed such that anyone more than three standard deviations from the mean was recoded to these boundaries. All five significant effects found with the untrimmed data in Table 1 were replicated with trimmed data.
Interpretation of moderator models
With these robustness results in mind, we limit our interpretation of moderation effects to the four most consistent effects shown in Table 1: NEM and PEM moderated by Regard and Conflict. Having established that the full moderation model with all moderation parameters freely estimated provided a better fit to the data than the no moderation models for four of the six combinations of parenting and personality variables, we then sought to establish which moderation parameters were driving the effect. That is, we wished to determine whether all of the three types of variance (genetic, shared environmental, unique environmental) were moderated by the parenting variables, or whether moderation occurred for some of these variance components and not others. Starting from the no moderation, baseline model, we added moderation for each of the A, C, and E paths and their combinations in turn. In sum, a total of six models (in addition to the no moderation and full moderation models) were run: 1) only A moderation (no C and E); 2) only C moderation (no A and E); 3) only E moderation (no A and C); 4) A and C moderation (no E); 5) A and E moderation (no C); and 6) C and E moderation (no A). The results for this full series of models are discussed in turn below. In Table 1, the moderation models we selected are highlighted in bold.
Table 2 presents the A, C and E estimates derived from the no moderation model and the best fitting moderation models. For each combination of personality and parenting variable, the top row reports ACE estimates from models that do not consider moderation. These values do not differ as functions of the moderator because they are derived from a model in which moderation is not possible. These results are what one would interpret from the “standard approach” to modeling twin data, an approach that does not consider moderation. In that sense, these estimates are useful to compare with the results from the moderation models. The existence of moderation means that the ACE effects on personality vary continuously as a function of the level of the parental relationship variables. Hence, the ACE estimates for the personality traits in the moderation models are given at five levels of the moderator, scaled in standard deviation units (z-scored): −2, −1, 0, 1, and 2 standard deviations away from the mean of the moderator.
Table 2.
Estimates of Variance Components and Proportions of Variance in Personality and the Moderating Variables of Parental Conflict and Regard and their Genetic and Environmental Correlations
| Moderating Variable |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variance components | Proportions of variance | Correlations with moderator | Variance components | Proportions of variance | Correlations with moderator | |||||||||||||
| A |
C |
E |
A(%) |
C(%) |
E(%) |
rA |
rC |
rE |
A |
C |
E |
A(%) |
C(%) |
E(%) |
rA |
rC |
rE |
|
| Regard |
Conflict |
|||||||||||||||||
| Positive Emotionality | ||||||||||||||||||
| No Moderation Model | 0.52 | 0.02 | 0.46 | 0.52 | 0.02 | 0.46 | 0.33 | 1.00 | 0.16 | 0.46 | 0.08 | 0.46 | 0.46 | 0.08 | 0.46 | −0.04 | 1.00 | 0.12 |
| Positive Emotionality at Level of Moderator | ||||||||||||||||||
| −2 | 0.38 | 0.02 | 0.69 | 0.35 | 0.02 | 0.64 | −0.01 | 1.00 | 0.25 | 0.41 | 0.49 | 0.37 | 0.32 | 0.39 | 0.29 | 0.01 | 0.76 | 0.33 |
| −1 | 0.42 | 0.02 | 0.57 | 0.42 | 0.02 | 0.57 | 0.27 | 1.00 | 0.24 | 0.41 | 0.23 | 0.40 | 0.39 | 0.22 | 0.38 | 0.01 | 0.86 | 0.23 |
| 0 | 0.53 | 0.02 | 0.46 | 0.52 | 0.02 | 0.46 | 0.50 | 1.00 | 0.22 | 0.41 | 0.09 | 0.44 | 0.44 | 0.09 | 0.47 | 0.01 | 0.99 | 0.14 |
| 1 | 0.71 | 0.02 | 0.37 | 0.65 | 0.02 | 0.34 | 0.65 | 1.00 | 0.20 | 0.41 | 0.06 | 0.49 | 0.43 | 0.06 | 0.51 | 0.01 | 0.73 | 0.05 |
| 2 | 0.95 | 0.02 | 0.28 | 0.76 | 0.01 | 0.23 | 0.75 | 1.00 | 0.17 | 0.41 | 0.14 | 0.54 | 0.37 | 0.13 | 0.50 | 0.01 | 0.14 | −0.03 |
| Negative Emotionality | ||||||||||||||||||
| No Moderation Model | 0.40 | 0.01 | 0.51 | 0.43 | 0.02 | 0.55 | 0.32 | 1.00 | 0.18 | 0.41 | 0.00 | 0.52 | 0.44 | 0.00 | 0.56 | 0.51 | 1.00 | 0.30 |
| Negative Emotionality at Level of Moderator | ||||||||||||||||||
| −2 | 0.21 | 0.02 | 0.51 | 0.28 | 0.03 | 0.69 | 0.17 | 1.00 | 0.22 | 0.73 | 0.10 | 0.50 | 0.55 | 0.08 | 0.37 | 0.67 | −0.73 | 0.31 |
| −1 | 0.30 | 0.02 | 0.51 | 0.35 | 0.03 | 0.62 | 0.29 | 1.00 | 0.22 | 0.54 | 0.02 | 0.50 | 0.52 | 0.01 | 0.47 | 0.62 | −0.72 | 0.31 |
| 0 | 0.41 | 0.02 | 0.51 | 0.43 | 0.02 | 0.55 | 0.38 | 1.00 | 0.22 | 0.39 | 0.01 | 0.50 | 0.44 | 0.01 | 0.56 | 0.56 | 0.79 | 0.31 |
| 1 | 0.54 | 0.02 | 0.51 | 0.50 | 0.02 | 0.48 | 0.44 | 1.00 | 0.22 | 0.27 | 0.07 | 0.50 | 0.32 | 0.09 | 0.59 | 0.46 | 0.75 | 0.31 |
| 2 | 0.69 | 0.02 | 0.51 | 0.56 | 0.02 | 0.42 | 0.48 | 1.00 | 0.22 | 0.18 | 0.22 | 0.50 | 0.20 | 0.25 | 0.56 | 0.31 | 0.75 | 0.31 |
Note. The variance components are raw and thus do not necessarily sum to 1.00. The proportions of variance sum to 1.00.
Three types of ACE values are given in Table 2, for both the moderation and no moderation models: (1) the raw A, C, and E estimates (variance components); (2) A, C, and E estimates expressed as proportions of the total variance in T (e.g., A% = A / A+C+E); and (3) correlations between the A, C, and E effects on M and T (rA, rC, and rE). The raw ACE estimates are also plotted on Figures 3-6. We next turn to interpret the results of the four moderator situations (PEM and NEM with Regard and Conflict) in turn.
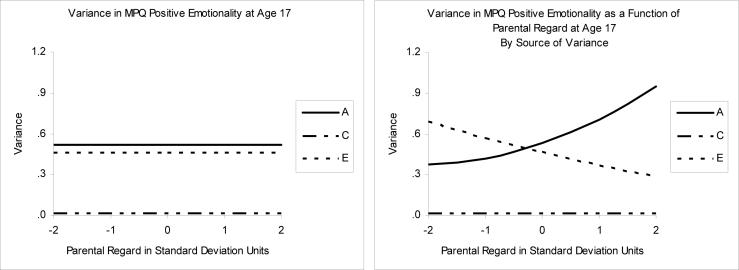
Figure 3.
Variance in Positive Emotionality across fixed and varying levels of Parental Regard. A= additive genetic variance, C=shared environmental variance, E=nonshared environmental variance.
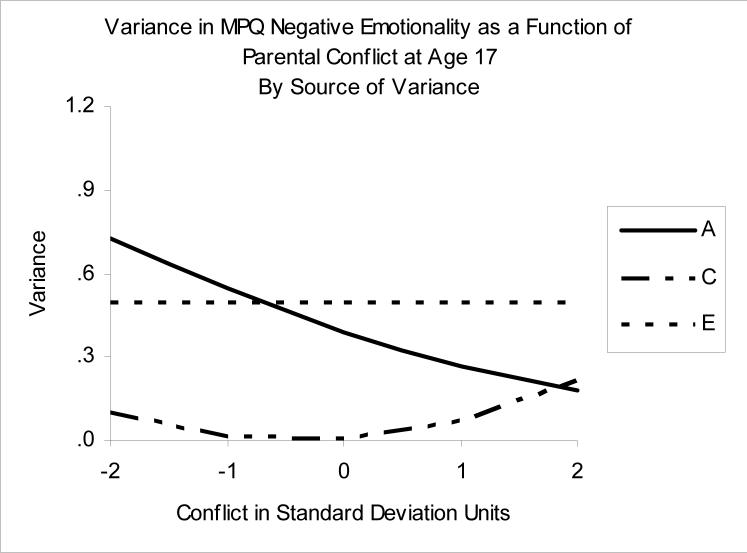
Figure 6.
Variance in Negative Emotionality as a function of Parental Conflict. A= additive genetic variance, C=shared environmental variance, E=nonshared environmental variance.
Association between Increased Parental Regard and Increased Positive Emotionality
As shown in Table 1, the best-fitting model for moderation of Positive Emotionality by Regard included moderation on the A and E paths. The values for the variance components for this model and for the no moderation model, as shown in Table 2, are also displayed graphically in two plots in Figure 3. The first panel corresponds with the no moderation model, and the second with the best-fitting moderation model. In the first panel, the ACE effects are constant (flat lines) across level of Regard, whereas in the second panel, the A and E effects vary continuously as a function of Regard (there was no moderation of C, so this line stays flat). The first panel portrays the results from the traditional approach to ACE decomposition (the values in the no moderation line in Table 2), whereas the second panel portrays the decomposition taking into account moderation (the values of PEM at different levels of Regard in Table 2). As described earlier, the model fit results in Table 1 indicate that, in spite of the additional complexity, the portrayal on the right side of Figure 3 provides a better fit to our data.
Note that the values on the left side of Figure 3 are very consistent with the typical results for personality derived from previous twin studies, with a heritability (A%) for PEM of 52%, a minor contribution from the shared environment (C% = 2%), and the remainder of the variation from the nonshared environment (E% = 46%). By contrast, the right side of Figure 3 shows that the impact of A and E on PEM varies as a function of the level of Regard perceived by the adolescent. At low levels of Regard, the etiology of PEM is more attributable to nonshared environmental factors (e.g., at −2 SD, E% = 64% and A% = 35%; see Table 2), whereas at high levels of Regard, the etiology is more genetic (e.g., at +2 SD, E% = 23% and A% = 76%).
This shift in the etiology of PEM as a function of Regard is also accompanied by an enhanced genetic link between PEM and Regard. This is revealed by examining the genetic and environmental correlations between PEM and Regard (rA and rE in Table 2). Like the AE variance components, in the moderator model (Figure 2), these correlations vary continuously as a function of Regard, whereas in the no moderator model, they are constants. (Shared environmental factors (C) are not a substantial contributor to the etiology of PEM at any level of Regard, so the rC correlations of 1.0 are not useful to interpret, as they represent correlations between trivial variance components. These are presented simply for the sake of completeness.) Table 2 shows how the rA correlations between Regard and PEM increase notably as a function of Regard, increasing monotonically from −.01 at −2 SD on Regard, to .75 at +2 SD on Regard.
Taken together, the AE variance components and correlations portray a dynamic system where adolescents who perceive high levels of Regard express the level of PEM consistent with their genotype (A% is larger), and the etiologies of Regard and PEM become genetically intertwined (rA is larger). At lower levels of Regard, this system is disrupted (rA is reduced). The heritability of PEM is not the fixed 52% derived from the no moderation model, but rather, is better modeled dynamically as a function of Regard, and ranges from 35% to 76%.
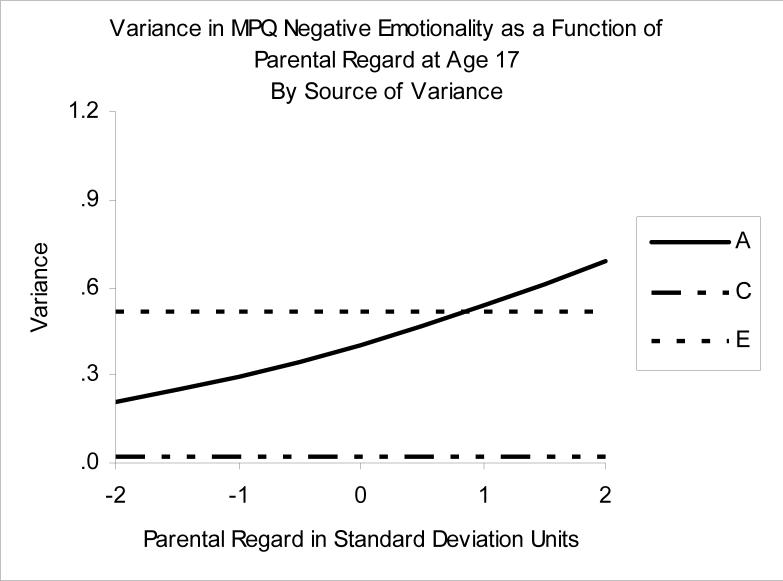
Association between Increased Parental Regard and Reduced Negative Emotionality
The best-fitting model for the relationship between Regard and lower NEM was one in which moderation was present only on the genetic variance component (A). At higher levels of Regard, genetic influences were relatively greater, whereas at low levels of Regard, nonshared environmental influences were relatively more important (see Figure 4 and the values in Table 2, which show the decomposition of NEM as a function of Regard, derived from the best-fitting moderation model). The pattern of genetic correlations linking NEM and Regard in the moderation model closely mirrored the pattern for PEM and Regard. As Regard increased, so did the genetic link between Regard and lower NEM, with rA ranging from .17 (−2 SD) to .48 (+2SD). Again, the picture that emerges is one of dynamic interplay between the perceived parental relationship and personality. Adolescents who perceive high levels of Regard express the level of NEM consistent with their genotypes (A% is larger), and the etiologies of Regard and lower NEM become genetically intertwined (rA is larger). At lower levels of Regard, the etiology of NEM separates from Regard, and becomes more associated with nonshared environments. The heritability of NEM is not 43% for everyone in the sample; rather, it varies from 28% − 56%, depending on the person's perceived Regard (see the bottom of Table 2).
Figure 4.
Variance in Negative Emotionality as a function of Parental Regard. A= additive genetic variance, C=shared environmental variance, E=nonshared environmental variance.
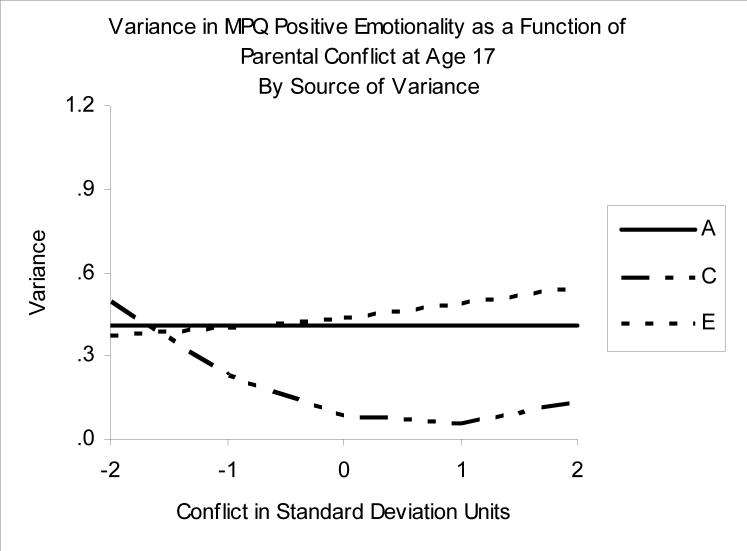
Association between Increased Parental Conflict and Reduced Positive Emotionality
Figure 5 and the values in Table 2 show the ACE decomposition of PEM as a function of Conflict. Shown graphically in Figure 5 is the best-fitting moderation model, in which the values of shared and unique environmental variance (C and E components) vary as a function of conflict. Throughout most of the range of Conflict, the impact of the shared environment is modest, but there is a curvilinear pattern in the C values, such that they are highest at lower levels of Conflict, and start to rise slightly at high levels of Conflict. Genetic effects did not vary as a function of Conflict, and the genetic correlation between PEM and Conflict (rA) was consistently negligible (rA values all essentially zero). To a large extent, the etiologies of PEM and Conflict are relatively distinct at the genetic level.
Figure 5.
Variance in Positive Emotionality as a function of Parental Conflict. A= additive genetic variance, C=shared environmental variance, E=nonshared environmental variance.
Association between Increased Parental Conflict and Increased Negative Emotionality
As shown in Table 1, two of the moderation models were very close in fit—only C moderation, and A and C moderation. We graphed the variance components of both models to compare, and found a notable effect on A in the A and C moderation model. Therefore, while the only C moderation model fit slightly better according to AIC, we decided to present the A and C moderation model in order to capture this A effect. Figure 6 and the values in Table 2 show the ACE decomposition of NEM as a function of Conflict. The picture is one of constant contributions from the nonshared environment (E) (the best fitting model included no moderation of E), but dynamic changes in the relative impact of shared environment (C) vs. genetic contributions (A). Again, similar to PEM and Conflict, we find a curvilinear pattern in the C values, such that they are greatest at lower and higher levels of Conflict. Along with this, there is a notable decrease in genetic (A) contributions to NEM at high levels of Conflict, such that the impact of the shared environment (C) slightly exceeds the impact of genetic factors (A) at high levels of Conflict.
This picture is enriched by also considering the ACE correlations linking NEM and Conflict. In particular, the genetic overlap between NEM and Conflict (rA) is notable at all levels of Conflict, although somewhat lower at the highest levels of Conflict (rA=0.31 at +2SD). Unlike the situation with PEM and Conflict, this suggests a consistent common genetic etiology to NEM traits and perceptions of conflict with parents. Nevertheless, genetic influences are less important when conflict is perceived, with shared, family-level environmental factors (C) being more important when the adolescent perceives conflict with their parents.
Discussion
The goal of the current study was to evaluate the extent to which genetic and environmental contributions to personality are relatively constant, as opposed to contingent on other circumstances. We pursued this issue in the context of adolescent personality, asking if genetic and environmental contributions to personality traits in late adolescence are moderated by adolescents’ perceptions of their relationships with their parents. We found notable moderating effects of adolescents’ accounts of their relationships with their parents on the broad personality domains of positive and negative emotionality. In circumstances where adolescents perceived high levels of Regard, genetic influences on personality were relatively more important, whereas low levels of Regard were associated with diminished genetic influences, and a relatively greater contribution of environmental contributions that made our participants different from their twin brothers or sisters (nonshared environment, “E”). High levels of Conflict were associated with relatively diminished genetic contributions and enhanced contributions from environments that made our participants more similar to their twin brothers or sisters (shared environment, “C”), especially for negative emotionality. Indeed, at high levels of perceived conflict, the etiology of negative emotionality was as attributable to shared environmental factors as it was to genetic factors (see Figure 6).
We focus here on the general implications of these findings for conceptualizing the etiology of personality, acknowledging that there are limitations to our results based on study measures and design. We used a measure that assessed how adolescents perceive their parents to be parenting, a perspective distinct from parent, observer, or laboratory-based measures of parenting. We recommend that the findings reported here be extended to other perspectives on parenting (see e.g., Reiss et al., 2000). We also acknowledge that this was a cross-sectional study of personality and parenting. We conceptualized parenting as a socialization process that plays a causal role in the development of personality, but we also recognize that the relationship is most likely bidirectional. Indeed, in a companion paper, South, Krueger, Johnson & Iacono (submitted) we examined this question using the same data set and found that personality also moderates genetic and environmental influences on parenting behavior. Future research that examines the transaction between personality and parenting longitudinally will be important in teasing apart the gene-environment interplay between these variables.
Implications for Understanding the Etiology of Personality
Why do people differ in their characteristic ways of thinking, feeling, and behaving? Twin studies of personality have been influential in addressing this question because they provide a powerful method of disentangling genetic and environmental contributions to personality. The conclusions that emerge from twin studies are highly consistent: individual differences in personality are attributable to contributions of genes and nonshared environments, with minimal contributions from the shared environment.
This portrayal of the origins of personality is based on a model that estimates genetic and environmental contributions to personality in general. The model characterizes the origins of personality by collapsing across all the persons in a sample. This model is useful, but limited because it cannot accommodate the ways in which the unique characteristics of specific persons might moderate the influence of genes and environments on personality (Johnson, 2007). When we applied a model capable of handling moderation (Figure 2) to our twin data on perceptions of parental relationships and personality, the relevance of moderation effects in better capturing the data was revealed (Table 1).
These findings have implications for how to think about “the heritability of a personality trait.” The concept of an overall heritability for a specific individual-differences variable is meaningful only in a very general sense. It is akin to estimating, e.g., the average yearly temperature for a wide region, such as North America. The average yearly temperature in North America is not meaningless, it is simply very general. For example, examining historical trends in the average yearly temperature yields data relevant to phenomena that are posited to have a very broad impact (e.g., global warming). Nevertheless, the average yearly temperature collapses across the diverse climates that are found in North America, and provides no information about more specific areas or times of the year that are relevant for more specific considerations. For example, the average temperature in Minnesota in the winter is relevant to a cross-country skiing enthusiast contemplating moving to Minnesota, whereas the average yearly North American temperature is too general to provide any relevant guidance to our hypothetical skier.
This is the kind of comparison portrayed in Table 2. Comparing the average temperature over a wide region to the average temperatures in several more specific regions is akin to comparing the parameters derived from the biometrical model with no moderation (Figure 1, No Moderation model lines in Table 2) to the biometrical model taking into account potential moderating effects (Figure 2, Moderation model lines in Table 2). The No Moderation components in Table 2 collapses across all the twins in our sample to get ACE estimates for positive and negative emotionality, and is highly consistent with the existing literature. The heritability (A%) of personality is 43%−44% for negative emotionality and 46%−52% for positive emotionality, with the remaining variance attributable almost entirely to the nonshared environment (E), values very similar to other heritabilities for personality traits reported in the literature (Bouchard & Loehlin, 2001). In addition, there is notable genetic overlap between personality and relationship perceptions (rA; cf. Spotts et al., 2005), with the exception of the independence of positive emotionality and Conflict. Nevertheless, this picture collapses across segments of the data that, when unpacked, reveal nontrivial differences in the etiology of personality. These differences pertain to a person's specific circumstances – perceptions of the parental relationship in our case. For example, depending on an adolescent's perceived relationship with his or her parents, the heritability of negative and positive emotionality ranged from 20%−76% (the range of A% values in Table 2). Discussing and conceptualizing personality in terms of its overall heritability is not incorrect, but it is rather limited in its information value because it collapses across diverse circumstances that act to both diminish and enhance genetic and environmental effects.
At this point, it may be useful to comment on the meaning of environmental moderation. Significant moderation of unique environmental effects (E) was found for positive emotionality. It is tempting to conclude from our results that low levels of perceived regard and high levels of perceived conflict in the parent-adolescent relationship are associated with bona fide enhancement of environmental effects, unique to each participant because they are perceptions of their own relationship with their parents, as opposed to measurement error . We must be cautious in this interpretation, however, because unique environmental effects do contain “error” variance, due to imperfect reliability of all assessment measures and normal fluctuations in how we perceive ourselves and our world. If we had found only moderation of the E parameter, we would be particularly concerned that we were simply modeling changes in error. However, for positive emotionality, we also found moderation of genetic effects in the case of regard, and of shared environmental effects in the case of conflict, indicating moderation of more than simply psychometric error.
Turning to the shared environment (C), one of the more interesting findings in Table 2 pertains to the relevance of these effects. As noted consistently in the literature, the effect of shared environments on personality is almost always estimated to be zero (Bouchard & Loehlin, 2001). This finding has typically proven controversial because it is often interpreted as a limit on the ability of families to influence the characteristics of their offspring (Rowe, 1994). Nevertheless, we identified moderate shared environmental effects on personality in the context of diminished genetic effects, but only in very specific circumstances, i.e., when the adolescent perceived unusual levels of conflict in the family. For example, at unusually high levels of Conflict, negative emotionality levels were as attributable to shared family environment (C) as they were to genetic effects (A; see Figure 6). A reasonable interpretation of this finding is that, when an adolescent perceives conflict with his or her parents, his or her negative emotionality level is less strongly the result of genotype because it is also impacted by family relations that, at least from the adolescent's viewpoint, are troubled. By definition, conflict involves interactions with others in the family, which may be part of the reason conflict moderates family-level, shared environmental (C) effects. In sum, the family-level or shared environment is not universally irrelevant to understanding the etiology of personality. Rather, its influence only appears in unusual circumstances (cf. Scarr & McCartney, 1983). Interestingly, in our data, this applied also when Conflict was unusually low. In those circumstances, the etiology of positive emotionality was as attributable to shared environmental factors as it was to genetic factors (see Figure 5).
Clearly, these findings of shared environmental influence require replication before we make too much of them, but they do suggest a new vantage point for trying to understand how environments affect personality, made possible by the modeling advances portrayed in Figure 2 (the biometrical moderator model). The literature to date is not “wrong” in documenting the trivial impact of shared environments on personality in general. For most adolescents, in families with normative levels of conflict, personality does result from genetic factors (A) and environments that make people different from their family members (E; see Table 2). Nevertheless, our understanding can be enhanced by examining adolescent personality in unusual family circumstances -- in our case, unusually conflicted or unconflicted relationships with parents – where the impact of the family-level, shared environment (C) on personality can be seen.
Implications of a Contingent Understanding of Heritability for Personality Genetics
In contemplating limitations on interpreting the overall heritability of a personality trait, it is also instructive to compare the ACE values for the same personality domain, seen from the vantage point of the two different moderators, Regard and Conflict. In our data, the specific lens through which a personality characteristic was viewed impacted its etiology, and non-contingent statements about etiology gloss over a fairly wide range of etiologic scenarios. Consider a question such as, “what contributions do genes make to positive emotionality?” Genetic factors (A) make a moderate contribution that is fairly consistent across levels of Conflict (see Figure 5), such that examining only Conflict as a potential moderator would lead to the conclusion that positive emotionality is always moderately heritable. Yet the impact of genetic factors on positive emotionality varied notably depending on the level of Regard (see Figure 3). Identifying the right lens (moderator variable) appears to be important in understanding the etiology of personality, such that we might sharpen our thinking by framing the question as: “how do genes contribute to positive emotionality for persons in specific circumstances?”
This contingent perspective on heritability has implications for research that aims to identify the specific molecular-level polymorphisms that underlie genetic effects on personality. In general, progress in understanding the molecular genetics of personality has been slow. The problem has been one of small effect sizes and associated difficulty in replicating effects from study to study (Ebstein, 2006). These scientific challenges are not limited to the study of personality; they extend into molecular genetic research on other complex human individual differences, such as common medical disorders, for which specific genes of relevance have often been hard to identify reliably (Hemminki, Bermejo, & Försti, 2006).
One potential reason for the difficulty in linking specific polymorphisms to personality and other individual differences may lie with the contingent nature of genetic contributions we have documented here (Ebstein, 2006). These phenomena are especially well-documented in literatures where the research subjects are not free-living humans, but rather, are animals whose genotypes and environments can be more directly manipulated (McClearn, 2006). The phenomenon of contingent genetic effects has also become generative in human molecular behavioral genetics. For example, a recent meta-analysis supports the relevance of the monoamine oxidase A gene to antisocial behavior, contingent on the stressor of maltreatment (Kim-Cohen et al., 2006). In general, the search for connections between molecular polymorphisms and behavior will likely be enhanced by taking a contingent perspective on gene expression, looking for specific molecular genetic effects in circumstances where quantitative genetic effects are greater.
Broadening Conceptions of Gene-Environment Interplay
Phenomena such as those we observed here have typically been subsumed by the rubric of “gene × environment interaction.” What researchers typically mean when they use this term is that specific environments trigger genetic susceptibilities. Many researchers have been examining this type of interplay outside the area of personality for some time, particularly in the areas of additions research (see e.g., Dick et al., 2007; Heath & Nelson, 2002; Rose & Dick, 2004-2005) and conduct disorder and aggressive behavior (e.g., Cadoret, Yates, Troughton, Woodworth, & Stewart, 1995; Riggins-Caspers, Cadoret, Knutson, & Langbehn, 2003). However, the application of this approach in the study of personality is relatively new (Krueger & Johnson, in press).
In spite of the intuitive appeal of the “gene × environment interaction” rubric, the limitation of the concept is that it does not capture all the forms of interplay that we observed between parenting and personality. Thinking about the current results, one might be tempted to think of relationships with parents as the “external environment” impacting “the genetics of personality”. However, consistent with the seminal work of David Rowe on the genetics of putatively “environmental measures” (Rowe, 1994), parental relationships were not entirely environmental in our research (as discussed in greater detail by McGue et al., 2005 and South et al., submitted), and the aspects of personality affected by parenting were not just the genetic aspects, but also the environmental aspects.
This broadened conception of gene-environment interplay may prove generative in thinking about contingent gene-environment relations. For example, the putative “environment” in gene × environment interaction and correlation could be a genetically-influenced characteristic of the person's intra-psychic experience (e.g., personality moderating genetic and environmental contributions to parenting; South et al., submitted), as opposed to only “the external environment.” Indeed, even putatively objective external environments might be more profitably conceptualized in terms of individual perceptions, since this level of conceptualization is closer to the individual's experience of “the environment.” Personality psychologists have been aware of this distinction between more objective and subjective environments for some time (Murray, 1938), and pursuing this distinction in personality genetics might enhance our ability to better understand the meaning of genetic and environmental effects on behavior.
Acknowledgments
This work was supported in part by USPHS grants AA00175, AA09367, and DA05147.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Bates JE, McFadyen-Ketchum S. Temperament and parent-child relations as interacting factors in children's behavioral adjustment. In: Molfese VJ, Molfese DL, editors. Temperament and personality development across the life span. Lawrence Erlbaum Associates; Mahwah, NJ: 2000. pp. 141–176. [Google Scholar]
- Bates JE, Petit GS, Dodge KA, Ridge B. Interaction of temperamental resistance to control and restrictive parenting in the development of externalizing behavior. Developmental Psychology. 1998;34:982–995. doi: 10.1037//0012-1649.34.5.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Hsieh K, Crnic K. Mothering, fathering, and infant negativity as antecedents of boys’ externalizing problems and inhibition at age 3: Differential susceptibility to rearing influence? Development and Psychopathology. 1998;10:301–319. doi: 10.1017/s095457949800162x. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ., Jr. Experience producing drive theory: How genes drive experience and shape personality. Acta Paediatrica. 1997;422:60–64. doi: 10.1111/j.1651-2227.1997.tb18347.x. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, Jr., Loehlin JC. Genes, evolution, and personality. Behavior Genetics. 2001;31:243–273. doi: 10.1023/a:1012294324713. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Yates W, Troughton E, Woodworth G, Stewart M. Genetic-environmental interaction in the genesis of aggressivity and conduct disorders. Archives of General Psychiatry. 1995;52:916–924. doi: 10.1001/archpsyc.1995.03950230030006. [DOI] [PubMed] [Google Scholar]
- Campbell A. A mind of her own: The evolutionary psychology of women. Oxford University Press; Oxford: 2002. [Google Scholar]
- Collins WA, Maccoby EE, Steinberg L, Hetherington EM, Bornstein MH. Contemporary research on parenting: The case for nature and nurture. American Psychologist. 2000;55:218–232. [PubMed] [Google Scholar]
- Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of Abnormal Psychology. 2007;116:213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves L, Erkanli A. Markov Chain Monte Carlo approaches to analysis of genetic and environmental components of human developmental change and G X E interaction. Behavior Genetics. 2003;33:279–299. doi: 10.1023/a:1023446524917. [DOI] [PubMed] [Google Scholar]
- Ebstein RP. The molecular genetic architecture of human personality: Beyond self report questionnaires. Molecular Psychiatry. 2006;11:427–445. doi: 10.1038/sj.mp.4001814. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent-son relationships: Evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33:351–363. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Gallagher KC. Does child temperament moderate the influence of parenting on adjustment? Developmental Review. 2002;22:623–643. [Google Scholar]
- Goldberg LR. The structure of phenotypic personality traits. American Psychologist. 1993;48:26–34. doi: 10.1037//0003-066x.48.1.26. [DOI] [PubMed] [Google Scholar]
- Harris JR. Where is the child's environment? A group socialization theory of development. Psychological Review. 1995;102:458–489. [Google Scholar]
- Harris JR. The nurture assumption: Why children turn out the way they do. Free Press; New York: 1998. [Google Scholar]
- Heath AC, Nelson EC. Effects of the interaction between genotype and environment: Research into the genetic epidemiology of alcohol dependence. Alcohol Research & Health. 2002;26:193–201. [PMC free article] [PubMed] [Google Scholar]
- Hemminki K, Bermejo JL, Försti A. The balance between heritable and environmental aetiology of human disease. Nature Reviews: Genetics. 2006;7:958–965. doi: 10.1038/nrg2009. [DOI] [PubMed] [Google Scholar]
- Hur Y-M, McGue M, Iacono WG. Unequal rate of monozygotic and like-sex dizygotic twin birth: Evidence from the Minnesota Twin Family Study. Behavior Genetics. 1995;25:337–340. doi: 10.1007/BF02197282. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor JT, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development & Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M, Krueger RF. Minnesota center for twin and family research. Twin Research and Human Genetics. 2007;9:978–984. doi: 10.1375/183242706779462642. [DOI] [PubMed] [Google Scholar]
- Johnson W. Genetic and environmental influences on behavior: Capturing all the interplay. Psychological Review. 2007;114:423–440. doi: 10.1037/0033-295X.114.2.423. [DOI] [PubMed] [Google Scholar]
- Johnson W, Krueger RF. Higher perceived life control decreases genetic variance in physical health: Evidence from a national twin study. Journal of Personality and Social Psychology. 2005;88:165–173. doi: 10.1037/0022-3514.88.1.165. [DOI] [PubMed] [Google Scholar]
- Johnson W, Krueger RF. How money buys happiness: Genetic and environmental processes linking finances and life satisfaction. Journal of Personality and Social Psychology. 2006;90:680–691. doi: 10.1037/0022-3514.90.4.680. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Parenting: A genetic-epidemiological perspective. American Journal of Psychiatry. 1996;153:11–20. doi: 10.1176/ajp.153.1.11. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: New evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Johnson W, Kling KC. Behavior genetics and personality development. In: Mroczek D, Little T, editors. Handbook of personality development. Erlbaum; Mahwah, NJ: 2006. pp. 81–108. [Google Scholar]
- Krueger RF, Johnson W. Behavioral genetics and personality. In: Pervin LA, John OP, Robins RW, editors. Handbook of Personality: Theory and Research. 3rd Ed. Guilford; New York: (in press) [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehlin JC. Latent variable models: An introduction to factor, path, and structural equation analysis. Erlbaum; Mahwah, NJ: 2004. [Google Scholar]
- Losoya SH, Callor S, Rowe DC, Goldsmith HH. Origins of familial similarity in parenting: A study of twins and adoptive siblings. Developmental Psychology. 1997;33:1012–1023. doi: 10.1037//0012-1649.33.6.1012. [DOI] [PubMed] [Google Scholar]
- McClearn GE. Contextual genetics. Trends in Genetics. 2006;22:314–319. doi: 10.1016/j.tig.2006.04.005. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Walden B, Iacono WG. Perceptions of the parent-adolescent relationship: A longitudinal investigation. Developmental Psychology. 2005;41:971–984. doi: 10.1037/0012-1649.41.6.971. [DOI] [PubMed] [Google Scholar]
- Murray HA. Explorations in Personality. Oxford University Press; New York: 1938. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Fourth Edition Muthén & Muthén; Los Angeles, CA: 19982006. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th Edition Department of Psychiatry.; VCU Box 900126, Richmond, VA 23298: 2002. [Google Scholar]
- Neale MC, Cardon LL, 11 contributing authors . Methodology for genetic studies of twins and families. Kluwer Academic; 1992. [Google Scholar]
- Perusse D, Neale MC, Heath AC, Eaves LJ. Human parental behavior: Evidence for genetic influence and potential implication for gene-culture transmission. Behavior Genetics. 1994;24:327–335. doi: 10.1007/BF01067533. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance component models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Sanson AV, Rothbart MK. Child temperament and parenting. In: Bornstein MH, editor. Handbook of parenting: Vol. 1. Children and parenting. 2nd ed. Lawrence Erlbaum Associates; Mahwah, NJ: 2002. pp. 255–277. [Google Scholar]
- Reiss D, Neiderhiser JM, Hetherington EM, Plomin R. The relationship code: Deciphering genetic and social influences on adolescent development. Harvard; Cambridge, MA: 2000. [Google Scholar]
- Riemann R, Angleitner A, Strelau J. Genetic and environmental influences on personality: A study of twins reared together using the self- and peer-report NEO-FFI scales. Journal of Personality. 1997;65:449–475. [Google Scholar]
- Riggins-Caspers KM, Cadoret RJ, Knutson JF, Langbehn D. Biology-environment interaction and evocative biology-environment correlation: Contributions of harsh discipline and parental psychopathology to problem adolescent behaviors. Behavior Genetics. 2003;33:205–220. doi: 10.1023/a:1023434206261. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM. Gene-environment interplay in adolescent drinking behavior. Alcohol Research & Health. 20042005;28:222–229. [Google Scholar]
- Rowe DC. The limits of family influence: Genes, experience, and behavior. Guilford; New York: 1994. [Google Scholar]
- Rubin KH, Burgess KB, Dwyer KM, Hastings PD. Predicting preschoolers’ externalizing behaviors from toddler temperament, conflict, and maternal negativity. Developmental Psychology. 2003;39:164–176. [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype -> environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- South SC, Krueger RF, Johnson W, Iacono WG. Gene-environment interactions and correlations between personality and parenting: The role of personality in shaping parent relationship quality. (submitted) [DOI] [PMC free article] [PubMed]
- Spinath FM, O'Connor TG. A behavioral genetic study of the overlap between personality and parenting. Journal of Personality. 2003;71:785–808. doi: 10.1111/1467-6494.7105004. [DOI] [PubMed] [Google Scholar]
- Spotts EL, Lichtenstein P, Pedersen N, Niederhiser JM, Hansson K, Cederblad M, Reiss D. Personality and marital satisfaction: A Behavioural genetic analysis. European Journal of Personality. 2005;19:205–227. [Google Scholar]
- Stoolmiller M. Synergistic interaction of child manageability problems and parent-discipline tactics in predicting future growth in externalizing behavior for boys. Developmental Psychology. 2001;37:814–825. doi: 10.1037//0012-1649.37.6.814. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire (MPQ) University of Minnesota Press; Minneapolis: (in press) [Google Scholar]
- Thomas A, Chess S, Birch H, Hertzig M, Korn S. Behavioral individuality in early childhood. New York University Press; New York: 1963. [Google Scholar]
- Turkheimer E. Three laws of behavior genetics and what they mean. Current Directions in Psychological Science. 2000;9:160–164. [Google Scholar]
- Visscher PM, Medland SE, Ferreira MAR, Morley KI, Zhu G, Cornes BK, Montgomery GW, Martin NG. Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genetics. 2006;2:316–325. doi: 10.1371/journal.pgen.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB. Behaviorism. University of Chicago Press; Chicago: 1930. [Google Scholar]