Abstract
Background and Purpose
Motor recovery after stroke is associated with neuronal reorganization in bilateral hemispheres. We investigated contralesional corticospinal tract remodeling in the brain and spinal cord in rats after stroke and treatment of bone marrow stromal cells.
Methods
Adult male Wistar rats were subjected to permanent right middle cerebral artery occlusion. Phosphate-buffered saline or bone marrow stromal cells were injected into a tail vein 1 day postischemia. An adhesive removal test was performed weekly to monitor functional recovery. Threshold currents of intracortical microstimulation on the left motor cortex for evoking bilateral forelimb movements were measured 6 weeks after stroke. When intracortical microstimulation was completed, biotinylated dextran amine was injected into the left motor cortex to anterogradely label the corticospinal tract. At 4 days before euthanization, pseudorabies virus-152-EGFP and 614-mRFP were injected into left or right forelimb extensor muscles, respectively. All animals were euthanized 8 weeks after stroke.
Results
In normal rats (n=5), the corticospinal tract showed a unilateral innervation pattern. In middle cerebral artery occlusion rats (n=8), our data demonstrated that: 1) stroke reduced the stimulation threshold evoking ipsilateral forelimb movement; 2) EGFP-positive pyramidal neurons were increased in the left intact cortex, which were labeled from the left stroke-impaired forelimb; and 3) biotinylated dextran amine-labeled contralesional axons sprouted into the denervated spinal cord. Bone marrow stromal cells significantly enhanced all 3 responses (n=8, P<0.05).
Conclusions
Our data demonstrated that corticospinal tract fibers originating from the contralesional motor cortex sprout into the denervated spinal cord after stroke and bone marrow stromal cells treatment, which may contribute to functional recovery.
Keywords: bone marrow cell, middle cerebral artery occlusion, neuronal plasticity, rats
Due to limited axonal regeneration in the inhibitory environment of the mammalian central nervous system, patients surviving from stroke often experience long-term devastating and irreversible disability. Depending on the lesion location or the damage extent, some functional recovery may occur spontaneously with time and can be enhanced by rehabilitation training.1 Bone marrow stromal cells (BMSCs) are a mixed cell population, including stem cells and progenitor cells, and are currently a strong candidate for cell-based stroke therapy in animal models and patients to improve neurological outcome.2–4 However, the neuroanatomical mechanisms of how the neuronal connections are re-established to reinnervate the impaired peripheral tissue from the cerebral cortex for functional recovery in chronic stroke are incompletely understood.
The contralesional intact cerebral hemisphere contributes to motor function recovery in patients with chronic stroke and middle cerebral artery occlusion (MCAO) animals, especially in those with an extensive unilateral infarct.5–10 The corticospinal tract (CST) is the main pathway for control of precision voluntary movement, which originates from the pyramidal neurons in the cerebral cortex and connects with motor neurons in the spinal cord directly or indirectly. We and others have also demonstrated that midline crossing CST fibers in the spinal cord originating from the contralesional hemisphere correlate with behavioral outcome after MCAO in adult rodents.11–14 In the present study, we further investigated the contralesional neuronal reorganization in adult rats after experimental ischemic stroke and BMSC administration with intracortical microstimulation (ICMS) and anterograde and retrograde CST tracing. Our results showed an increased innervation of the stroke-impaired forelimb from the contralesional intact cerebral cortex after stroke and subsequent treatment with BMSCs, which may contribute to the improved motor functional recovery.
Materials and Methods
Adult male Wistar rats (n=21, 2 months old, body weight 225 to 250 g; purchased from Charles River Breeding Company, Wilmington, Mass) were used throughout this study. All animals were studied in a blinded fashion for MCAO and BMSC treatment. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Animal Model
Permanent right-sided MCAO was induced using a method of intraluminal vascular occlusion modified in our laboratory.15 Briefly, under Forane (isoflurane) anesthetization, a midline anterior cervical incision was performed to expose the right common carotid artery, external carotid artery, and internal carotid artery. A length of 4–0 monofilament nylon suture (18.5 to 19.5 mm), determined by the animal body weight, with its tip rounded by heating near a flame, was advanced from the external carotid artery into the lumen of the internal carotid artery until it blocked the origin of the middle cerebral artery.
Intravenous Administration of Bone Marrow Stromal Cells
BMSCs were obtained from Theradigm, Inc (Baltimore, Md) and harvested from donor adult male Wistar rats as described previously.16 One day postischemia, randomly selected rats (n=8) received 3×106 BMSCs in 1 mL of phosphate-buffered saline injected into a tail vein.17 Rats injected with phosphate-buffered saline alone were used as MCAO control (n=8). A third group of naive rats without surgery and treatment was used for normal controls (n=5).
Neurological Functional Test
In all animals, a series of adhesive removal tests18 were performed at 1 day after MCAO and weekly thereafter to evaluate functional recovery.
Electrophysiology
To validate the establishment of functional neuronal connections from the left intact cortex to bilateral forelimbs, ICMS was performed 6 weeks after stroke. Rats were anesthetized with ketamine hydrochloride (100 mg/kg, intraperitoneally) and xylazine (5 mg/kg, intraperitoneally), and ketamine (20 mg/kg, intraperitoneally) as supplementary injections when needed. The animal rectal temperature was controlled at 37°C throughout the experiment. The animal was restricted in a Kopf stereotaxic apparatus and a unilateral craniotomy was performed over the left frontal motor cortex with a high-speed drill (Foredom Electric, Bethel, Conn). The exposed cerebral cortex surface was covered with warm silicone oil. A pargyline-coated tungsten stimulating microelectrode (2.0 Megaohms, type TM33A20; WPI Inc., Sarasota, Fla) was descended into the forelimb motor cortex to a depth of 1.5 to 1.7 mm from the cortical surface as to evoke movement at the lowest threshold at 4 points (stereotaxic coordinates: 1 and 2 mm rostral to the bregma, 2.5 and 3.5 mm lateral to the midline).19 The electric stimulus consisted of 20 monophasic cathodal train pulses (60-ms duration at 333 Hz, 0.5-ms pulse duration) of a maximum of 100 μA. For every stimulating point, the lowest threshold value of ICMS that evoked right or left forelimb movement was measured. If no movements were evoked with 100 μA, the current threshold of this point was recorded as 100 μA without additional stimulation at a higher level to avoid cerebral tissue damage. If cortical vessels were encountered at the intended incision site, the site was moved immediately rostral or caudal to avoid the cortical vessel. The average threshold values evoking right or left forelimb movement were calculated from data of 4 stimulation points in each individual animal.
Anterograde Corticospinal Tract Tracing
When the recording was completed, 10% solution of biotinylated dextran amine (BDA, 10 000 MW; Molecular Probes, Eugene, Ore) in phosphate-buffered saline was injected through a finely drawn glass capillary into 4 points in the left frontal motor cortex (100 nL per injection; stereotaxic coordinates as same as ICMS) to anterogradely label the CST originating from this area. The micropipette remained in place for 4 minutes after completion of the injection.
Retrograde Corticospinal Tract Tracing
To confirm the neuronal wiring between the motor cortex and the peripheral target tissues, a pair of transsynaptic tracers, pseudorabies virus (PRV)-152-EGFP and PRV-614-mRFP (gifts from Dr. Lynn Enquist, Princeton University, Princeton, NJ), were used to retrogradely label the cortical pyramidal neurons from the forelimb muscles. Four days before euthanization, a 30-μL total volume of PRV-152-EGFP, which was divided into multiple injections of 1 to 2 μL, was injected into the left forelimb radioulnar extensor muscles, and the same volume of PRV-614-mRFP was delivered into the right forelimb radioulnar extensor muscles with a 50-μL Hamilton syringe under isoflurane anesthetization as described previously. Then the animals were transferred to Biosafety Level 2 room to survive for 102 to 104 hours.
Tissue Preparation and Data Analysis
Animals were euthanized under deep ketamine anesthesia at 8 weeks after MCAO. Rats were perfused transcardially with saline followed by 4% paraformaldehyde. The entire brain and spinal cord were immersed in 4% paraformaldehyde overnight. The brain anterior to the bregma 5 mm was cut into 7 equally spaced (2-mm) coronal blocks using a rat brain matrix (Activational System, Warren, Mich). The first 100 μm of each 500-μm thickness in the frontal 3 brain blocks was cut off using a vibratome for the detection of GFP and/or RFP-positive pyramidal neurons with a Bio-Rad MRC 1024 (argon and krypton) laser scanning confocal imaging system mounted onto a Zeiss microscope (Bio-Rad, Cambridge, Mass). The number of pyramidal neurons labeled with green or orange in the left cerebral cortex were counted and compared among groups at the same location. The remaining brain blocks were embedded in paraffin. A series of adjacent 6-μm-thick sections were cut from each block and stained with hematoxylin and eosin for lesion volume measurement. The sections were traced using a Global Laboratory image analysis system (Data Translation, Marlboro, Mass). Lesion volume was presented as a volume percentage of the lesion area compared with the contralateral hemisphere.
The cervical spinal cord segments of C1 and C4 to 6 were processed for vibratome traverse section (75 μm). Sections were incubated with 0.5% H2O2 for 20 minutes followed with avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, Calif) at 4°C for 48 hours, and BDA-labeled axons were visualized with 3,3′-diaminobenzidine-nickel (Vector) for light microscopy examination. For each animal, the average number of BDA-labeled CST in the dorsal funiculus at C1 level was counted on 3 sections, and the total number of midline-crossing axons in the stroke-impaired side of the ventral gray matter was counted on 50 consecutive sections. To avoid interanimal variation induced by tracing efficiency, the crossed axon number in each animal was corrected with a quotient of mean BDA-labeled CST number in the dorsal funiculus at C1 level divided by the individual CST number.
Statistics
All data were presented as mean±SD. The behavior outcomes were evaluated between the BMSC-treated and control groups with 2-way analysis of variance. Two-sample t test was used to analyze the difference in lesion volume, ICMS threshold current, number of EGFP and mRFP/EGFP-positive pyramidal neurons, and number of BDA-labeled axons between animal groups. A value of P<0.05 was taken as significant. Pearson's correlation coefficients were calculated between functional recovery and anatomic reorganization.
Results
Neurological Functional Outcome and Lesion Volume
The functional recovery of sensorimotor impairments was assessed by the time needed to successfully remove the adhesive tape from the impaired forepaw. Rats with BMSC treatment had significantly improved performance from 2 weeks after stroke compared with the control group (Figure 1A; P<0.05). The lesion volume showed no difference between the control and treatment groups at 8 weeks after stroke (Figure 1B).
Figure 1.
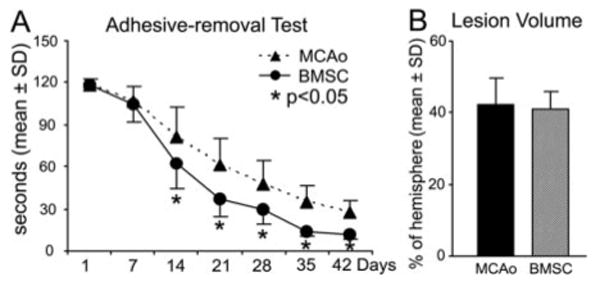
Neurological functional test and lesion volume. The mean time needed to remove a sticky tape from the impaired forepaw was significantly reduced in the BMSC treatment group (n=8) compared with the MCAO control animals (n=8; A, *P<0.05). The lesion volume showed no difference between the control and BMSC treatment groups at 8 weeks after stroke (B).
Electrophysiological Assessment of Functional Reorganization in the Intact Motor Cortex
To test the hypothesis that the contralesional hemisphere may contribute to functional recovery after stroke, threshold current values of direct intracortical stimulations on the forelimb motor area in the contralesional intact hemisphere for eliciting simultaneous movement of contralateral normal and ipsilateral impaired forelimb were compared among normal, MCAO control, and BMSC-treated animals. For contralateral forelimb movement, the current thresholds were comparable in all groups at low level (Figure 2; range 11.5 to 17.5 μA). In normal animals, threshold values evoking ipsilateral movements were much higher than that evoking contralateral movements, including higher currents at some stimulation points with no response evoked by up to 100 μA (mean range, 68.5 to 87.0 μA). However, ipsilateral forelimb thresholds were found to be lower after experimental stroke (range, 38.5 to 64.9 μA; P<0.001). Furthermore, the mean ipsilateral threshold was significantly decreased in the BMSC-treated animals by 23.6% as compared with the MCAO control group (P<0.05). In addition, a high correlation coefficient was observed between the reduction of ipsilateral thresholds and functional outcome (r=0.71, P<0.01).
Figure 2.
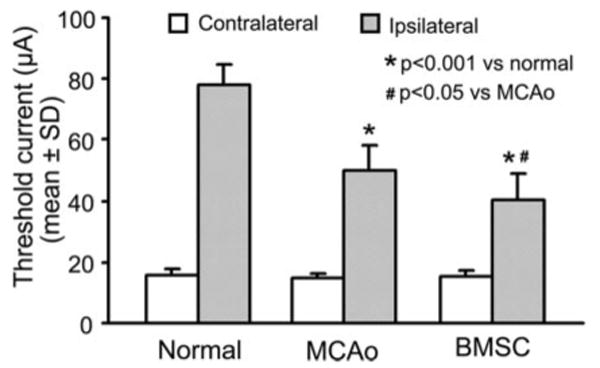
Mean threshold current levels needed to evoke forelimb movements on stimulation of the left cerebral motor cortex. For each animal, the threshold average was calculated from 4 stimulation points. Intracortical microstimulation of the motor cortex in normal adult rats evoked low threshold contralateral forelimb movements and high threshold ipsilateral movements. After stroke, the contralateral movement threshold (for right normal forelimb) was unchanged, whereas the ipsilateral movement threshold was significantly decreased at 6 weeks postischemia (P<0.001). BMSC treatment further reduced the threshold current needed for ipsilateral forelimb movement (P<0.05).
Neural Pathways Between the Contralesional Motor Cortex and the Stroke-Impaired Forelimb Muscles
To investigate the neuronal connections between the contralesional hemisphere and the stroke-impaired peripheral tissue, a pair of retrograde fluorescent tracers of PRV-152-EGFP and 614-mRFP was injected into the forelimb muscles. After 4 days of transsynaptic transport, the cell body and dendrites of most pyramidal neurons in the left intact cerebral cortex were labeled with mRFP (red) injected into the right forelimb, whereas few pyramidal cells were labeled with EGFP (green) injected into the left impaired forelimb in the normal rats (Figure 3A, D). Eight weeks after stroke, number of EGFP (green arrows) and mRFP/EGFP double-labeled pyramidal cells (yellow arrows) were significantly increased in the contralesional hemisphere (Figure 3B, D; P<0.001). The increase of such ipsilateral labeling was widely found in both of caudal forelimb area and rostral forelimb area.19 In the animals with BMSC treatment after stroke, an extensive enhancement in the caudal forelimb area (P<0.01 or 0.05) and a minor enhancement in the rostral forelimb area (P<0.05) were observed compared with that in MCAO control animals.
Figure 3.
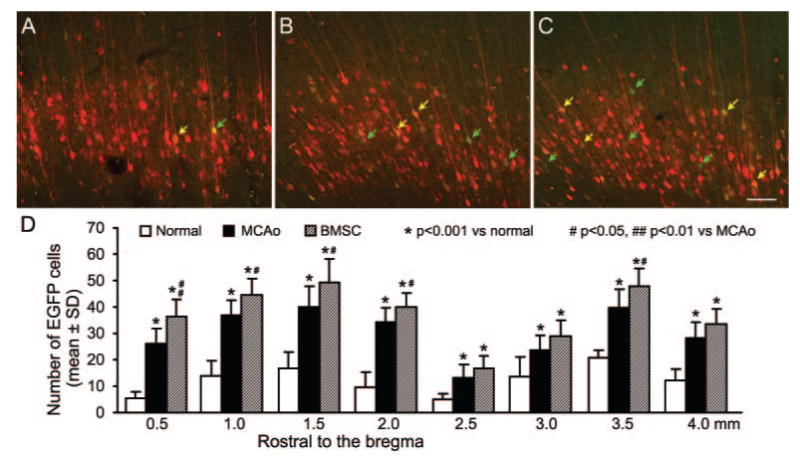
Retrograde transsynaptic tracing of pyramidal neurons in the motor cortical area of left contralesional hemisphere. Single-layer images of confocal microscopy on coronal cerebral sections showing mRFP-positive pyramidal cells labeled from the right forelimb in normal (A) with an increased number of EGFP-positive pyramidal cells labeled from the left forelimb in MCAO control (B, green arrows) and BMSC-treated animals (C) at 8 weeks postischemia. Note that some pyramidal neurons were double-labeled with mRFP and EGFP (yellow arrows). As shown in D, ischemic stroke in the right hemisphere significantly increased the EGFP-labeled pyramidal neurons in the left caudal forelimb area (0.5 to 2.0 mm rostral to the bregma) and rostral forelimb area (2.5 to 4.0 mm rostral to the bregma, P<0.001), which means increased neuronal connections between the contralesional motor cortex and the stroke-impaired forelimb. Such neuronal reorganization was further extensively enhanced in the caudal forelimb area and was moderately enhanced in the rostral forelimb area. Scale bar=100 μm.
Midline-Crossing Corticospinal Tract Axon Sprouting Into the Denervated Side of the Cervical Cord
To measure the hypostatic neural pathway from the contralesional intact motor cortex to reinnervate the denervated side of the spinal cord, cortical pyramidal neurons in the left frontal motor area were labeled with anterograde neuronal tracer BDA. Two weeks after intracortical injection, the CST axons were labeled in the ventral-most part of the right cervical dorsal funiculus (Figure 4A). Interestingly, the labeled CST numbers at the upper cervical level show no difference among the normal, MCAO control, and BMSC treatment groups (Figure 4B). However, at the lower cervical level of C4 to 6, where the spinal motoneurons innervate the forelimb muscles, BDA-labeled CST axons, which originate from the contralesional intact cerebral hemisphere, crossed the midline into the denervated side of the spinal cord (Figure 4C). The number of BDA-labeled axons in the denervated cervical ventral gray matter was significantly increased after ischemic stroke compared with the normal animals (Figure 4D; P<0.001). BMSC treatment further enhanced the number of midline-crossing CST axons by 70.7% compared with MCAO controls (Figure 4D; P<0.01). Such midline-crossing sprouting in the denervated ventral gray matter was highly correlated with behavioral performance after stroke (r=0.67, P<0.05). Otherwise, number of axons in the contralateral side of the ventral horn and the uncrossed ventral CST were essentially unaltered after MCAO and BMSC administration (data not shown).
Figure 4.
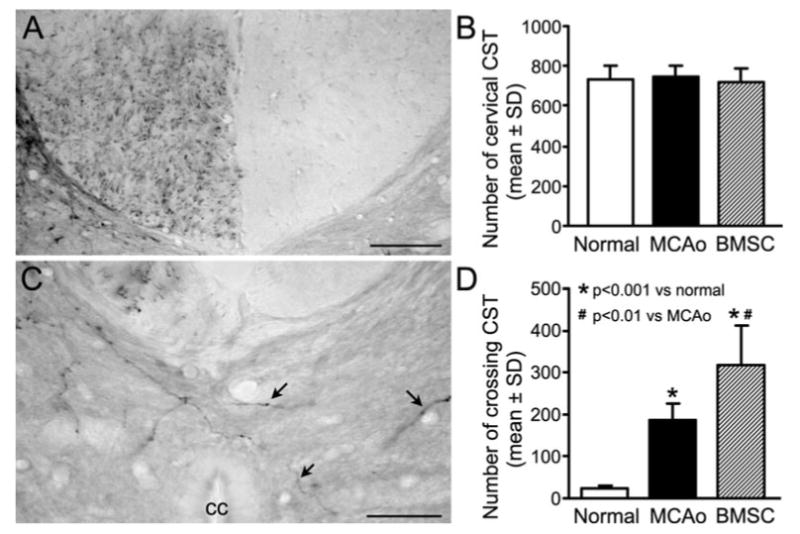
BDA-labeling of CST originating from the contralesional intact hemisphere. A, A representative cervical transverse section showing the BDA-labeled CST in the ventral-most part of the dorsal funiculus at C1 level. B, No differences were found on the mean CST numbers at the upper cervical level among all normal and ischemic animals. C, A representative picture from the cervical cord showing midline-crossing BDA-positive CST axons (arrows) sprouted into denervated side of the ventral gray matter in a rat after stroke. D, Quantitative data showing that only few BDA-labeled axons were found in the opposite side of the cervical cord in normal rats, whereas the numbers of contralesional CST in the denervated cervical gray matter were significantly increased by ischemic stroke (P<0.001 versus normal) and BMSC treatment (P<0.01 versus MCAO control). cc, central canal. Scale bar=100 μm in A and 50 μm in C.
Discussion
The present findings corroborated our prior studies (see reviews2,4) that BMSC treatment significantly improves functional recovery in adult rats after experimental ischemic stroke. A novel finding is that BMSCs enhanced the innervation of stroke-impaired peripheral tissues from the contralesional intact cerebral cortex, suggesting that the neuronal reorganization in the contralesional hemisphere may contribute to the functional improvement.
In this study, the contralesional hemisphere was investigated electrophysiologically using ICMS for functional identification of the neuronal remodeling after stroke. ICMS has been commonly used to define movement representations in the motor cortex20 and to evaluate plastic changes in the motor cortex after motor system lesion21 by applying stimulative current on cortical pyramidal neurons through efferent connections to the spinal motoneurons to elicit limb movements. A previous study demonstrated that ICMS evokes low-threshold contralateral and high-threshold ipsilateral forelimb movements in normal adult rats and abnormal low-threshold ipsilateral movement from the contralesional hemisphere in adult rats after unilateral neonatal cortical lesion.21 Such low-threshold ipsilateral movement can be disrupted by medullary pyramidotomy ipsilateral to the stimulation hemisphere,22 suggesting that these movements, at least in part, are mediated by the CST fibers originating from the contralesional cerebral cortex. High-threshold ipsilateral movements in normal animals may reflect the paucity of ipsilateral projection of CST fibers that are found in the normal rat.23 A recent cortical mapping study also demonstrates that the area evoking ipsilateral movement in the contralesional motor cortex is increased in adult rats after cortical lesion and treatment with Nogo-A antibody IN-1.24 In our study, ICMS was performed only at 4 points in the intact cortex to reduce the potential impact of electrode penetration damage. Our results showed that BMSC treatment significantly decreased the threshold current needed to evoke ipsilateral forelimb movement in the contralesional hemisphere in adult rats after ischemic stroke. Because pyramidal neurons may be activated more effectively by weak currents at a distance from the stimulating electrode,25 the lower threshold current evoking ipsilateral forelimb movement suggested an increased synaptic coupling between the intact motor cortex and the spinal motor neurons in the denervated side of the ventral horn. However, a moderate reduction of the current threshold for ipsilateral movement was observed in the adult MCAO control in our study, differing from a previous report of no threshold change in adult rats after cerebral lesion.21 This discrepancy may be due to the different lesion volume between the experiments. In our MCAO model, a broad lesion area was observed in the cerebral cortex, corpus callosum, striatum, basal ganglia, and thalamus26 in contrast to that the lesion was limited within the cortex in their model.
To further investigate the axonal remodeling in the contralesional hemisphere after stroke and treatment of BMSCs, both anterograde and retrograde neuronal tracing were performed. By using a pair of transsynaptic neuronal tracers, PRV-152-EGFP and 614-mRFP,27 the neuronal pathways between the motor cortex and their peripheral targets were retrogradely labeled. Although there is some evidence of that the rostral forelimb area of the intact hemisphere in rats with neonatal cortical lesion is preferentially developed rather than the caudal forelimb area for the ipsilateral forelimb control,28 our PRV tracing results suggested a wide increased neuronal connection from both rostral forelimb area and caudal forelimb area of the intact hemisphere after stroke in adult rats. Furthermore, the enhancement of neuronal reorganization induced by BMSC treatment more likely occurred in the caudal forelimb area. Direct cortical pyramidal neuron tracing indicated that ischemic stroke and BMSC treatment induced midline-crossing axonal sprouting from the opposite intact CST into the denervated spinal cord, which was innervated unilaterally in normal adult rats, consistent with previous observations in rats with MCAO and treatment of inosine,11 anti-Nogo-A antibody,12 Nogo receptor antagonism,13 or BMSCs.14 In the adult mammal, damaged CST axons are not regenerated. However, we found that the number of BDA-labeled CST axons originating from the contralesional hemisphere at the upper cervical level was not affected by MCAO and BMSC treatment, suggesting that the neuronal remodeling in the contralesional hemisphere after stroke may be, at least partially, attributed to increased arborization of the intact CST axon rather than long-distance regenerative growth.
Recently, Steward et al presented a methodological objection to cortical BDA tracing in a spinal cord injury experiment, that CST axons in the spinal cord can be inaccurately labeled with BDA that leaks into the cerebrospinal fluid in the cerebral ventricle.29 However, the injection volume in our studies of 100 nL for BDA and 80 nL for DiI14 in rats per injection was much lower than that of 0.5 μL used in their mouse study.30 No dye leakage was found outside of the cerebral cortex in our experiments. Therefore, we are confident that all BDA-positive axons appearing in the spinal cord originate from the dye injection area, the contralesional motor cortex. Due to technical limitations, it is difficult to individually identify the physiological function of these axons. Our studies do not exclude the possibility that other neuronal pathways such as ipsilateral ventral CST, corticorubral tract, and other brainstem–spinal systems contribute to functional recovery. However, the limited efficiency of the BAD tracing precludes convincing analysis of these pathways. Based on the finding that the contralesional CST sprouting is highly correlated with motor recovery after stroke, it seems reasonable to propose that such new connections may be, at least, one of underlying substrates for the spontaneous and BMSC treatment-enhanced functional recovery.
After ischemic lesions, the adult central nervous system can induce cellular responses needed for neurite growth and synaptic formation,31 including the expression of growth-promoting factors such as brain-derived neurotrophic factor32 and basic fibroblast growth factor,33 that can promote outgrowth of lesioned CST axons and lead to partial functional recovery. BMSCs transplanted into the ischemic brain secrete an array of neurotrophins and growth factors, including BDNF,34 vascular endothelial growth factor,35 and others in response to the ischemic environment.36 Most importantly, BMSCs stimulate the production of these restorative factors within parenchymal cells, especially in astrocytes after stroke, including BDNF, nerve growth factor, and others.37–39 It is unlikely that the BMSCs can selectively enhance the axonal sprouting of the CST originating from the contralesional cortical hemisphere. However, upregulated expression of axon growth-promoting and attracting guidance factors to reinnervate their targets in the denervated central nervous system tissue40 may partially explain why no significant increase of BDA-labeled axons are present in the intact side of the spinal cord.
Summary
Taken together, the present observations demonstrate that BMSC treatment in adult rats after experimental ischemic stroke significantly enhance neuronal remodeling in the contralesional intact hemisphere through CST axonal sprouting into the impaired side of the spinal cord, which may, at least partially, contribute to the functional recovery.
Acknowledgments
We gratefully acknowledge the gifts of pseudorabies viral tracers from Dr Lynn W. Enquist, Princeton University, Princeton, NJ, and thank Yisheng Cui for technical assistance.
Sources of Funding: This work was supported by National Institute of Neurological Diseases and Stroke grant PO1 NS42345 and the Mandell and Madeleine H. Berman Foundation.
Footnotes
Disclosures: None.
References
- 1.Rijntjes M. Mechanisms of recovery in stroke patients with hemiparesis or aphasia: New insights, old questions and the meaning of therapies. Curr Opin Neurol. 2006;19:76–83. doi: 10.1097/01.wco.0000203886.28068.38. [DOI] [PubMed] [Google Scholar]
- 2.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 4.Chopp M, Li Y. Transplantation of bone marrow stromal cells for treatment of central nervous system diseases. Adv Exp Med Biol. 2006;585:49–64. doi: 10.1007/978-0-387-34133-0_4. [DOI] [PubMed] [Google Scholar]
- 5.Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- 6.Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081–1088. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- 7.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer SC, Crafton KR. Somatotopy and movement representation sites following cortical stroke. Exp Brain Res. 2006;168:25–32. doi: 10.1007/s00221-005-0082-2. [DOI] [PubMed] [Google Scholar]
- 10.Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 11.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiessner C, Bareyre FM, Allegrini PR, Mir AK, Frentzel S, Zurini M, Schnell L, Oertle T, Schwab ME. Anti-Nogo-a antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2003;23:154–165. doi: 10.1097/01.WCB.0000040400.30600.AF. [DOI] [PubMed] [Google Scholar]
- 13.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Li Y, Qu R, Shen L, Gao Q, Zhang X, Lu M, Savant-Bhonsale S, Borneman J, Chopp M. Axonal sprouting into the denervated spinal cord and synaptic and postsynaptic protein expression in the spinal cord after transplantation of bone marrow stromal cell in stroke rats. Brain Res. 2007;1149:172–180. doi: 10.1016/j.brainres.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Chopp M, Zhang ZG, Garcia JH. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1992;12:621–628. doi: 10.1038/jcbfm.1992.86. [DOI] [PubMed] [Google Scholar]
- 16.Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 18.Schallert T, Whishaw IQ. Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav Neurosci. 1984;98:518–540. doi: 10.1037//0735-7044.98.3.518. [DOI] [PubMed] [Google Scholar]
- 19.Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 20.Asanuma H, Rosen I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972;14:243–256. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- 21.Kartje-Tillotson G, Neafsey EJ, Castro AJ. Electrophysiological analysis of motor cortical plasticity after cortical lesions in newborn rats. Brain Res. 1985;332:103–111. doi: 10.1016/0006-8993(85)90393-2. [DOI] [PubMed] [Google Scholar]
- 22.Kartje-Tillotson G, O'Donoghue DL, Dauzvardis MF, Castro AJ. Pyramidotomy abolishes the abnormal movements evoked by intracortical microstimulation in adult rats that sustained neonatal cortical lesions. Brain Res. 1987;415:172–177. doi: 10.1016/0006-8993(87)90283-6. [DOI] [PubMed] [Google Scholar]
- 23.Vahlsing HL, Feringa ER. A ventral uncrossed corticospinal tract in the rat. Exp Neurol. 1980;70:282–287. doi: 10.1016/0014-4886(80)90027-8. [DOI] [PubMed] [Google Scholar]
- 24.Emerick AJ, Neafsey EJ, Schwab ME, Kartje GL. Functional reorganization of the motor cortex in adult rats after cortical lesion and treatment with monoclonal antibody IN-1. J Neurosci. 2003;23:4826–4830. doi: 10.1523/JNEUROSCI.23-12-04826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoney SD, Jr, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol. 1968;31:659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- 26.Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;142:623–635. [PMC free article] [PubMed] [Google Scholar]
- 27.Song CK, Enquist LW, Bartness TJ. New developments in tracing neural circuits with herpesviruses. Virus Res. 2005;111:235–249. doi: 10.1016/j.virusres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Fujimura K, Koga E, Baba S. Neonatal frontal lesion in unilateral hemisphere enhances the development of the intact higher motor cortex in the rat. Brain Res. 2003;965:51–56. doi: 10.1016/s0006-8993(02)04116-1. [DOI] [PubMed] [Google Scholar]
- 29.Steward O, Zheng B, Banos K, Yee KM. Response to: Kim et al, ‘Axon regeneration in young adult mice lacking Nogo-a/b.’ Neuron 38, 187–199. Neuron. 2007;54:191–195. doi: 10.1016/j.neuron.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 31.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 32.Comelli MC, Guidolin D, Seren MS, Zanoni R, Canella R, Rubini R, Manev H. Time course, localization and pharmacological modulation of immediate early inducible genes, brain-derived neurotrophic factor and trkB messenger RNAs in the rat brain following photochemical stroke. Neuroscience. 1993;55:473–490. doi: 10.1016/0306-4522(93)90517-j. [DOI] [PubMed] [Google Scholar]
- 33.Lin TN, Te J, Lee M, Sun GY, Hsu CY. Induction of basic fibroblast growth factor (bFGF) expression following focal cerebral ischemia. Brain Res Mol Brain Res. 1997;49:255–265. doi: 10.1016/s0169-328x(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 34.Dormady SP, Bashayan O, Dougherty R, Zhang XM, Basch RS. Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. J Hematother Stem Cell Res. 2001;10:125–140. doi: 10.1089/152581601750098372. [DOI] [PubMed] [Google Scholar]
- 35.Hamano K, Li TS, Kobayashi T, Kobayashi S, Matsuzaki M, Esato K. Angiogenesis induced by the implantation of self-bone marrow cells: a new material for therapeutic angiogenesis. Cell Transplant. 2000;9:439–443. doi: 10.1177/096368970000900315. [DOI] [PubMed] [Google Scholar]
- 36.Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, Chen X, Chopp M. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 39.Xin H, Li Y, Chen X, Chopp M. Bone marrow stromal cells induce bmp2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006;83:1485–1493. doi: 10.1002/jnr.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wizenmann A, Thies E, Klostermann S, Bonhoeffer F, Bahr M. Appearance of target-specific guidance information for regenerating axons after CNS lesions. Neuron. 1993;11:975–983. doi: 10.1016/0896-6273(93)90126-c. [DOI] [PubMed] [Google Scholar]


